Abstract
Background
This study aimed to investigate the potential influence of microRNA-451 (miR-451) in drug resistances of the Paclitaxel-resistant breast cancer cell line by transfecting miR-451 mimics and miR-451 inhibitors to MCE-7, MCF-7/EPI, and MCF-7/DOC.
Material/Methods
Real-time quantitative PCR (qRT-PCR) was performed for detecting whether transfected miR-451 mimics and miR-451 inhibitors could regulate the expression of miR-451 effectively. The apoptosis of the 3 cell lines was measured by applying Annexin V-APC/PI staining. Western blot was used for the detection of the protein expression of Bcl-2 and Caspase 3 after the transfection of miR-451 mimics /inhibitors. Bioinformatics analysis demonstrated that Bcl-2 protein is a potential target gene for miR-451.
Results
In comparison to the control group, after transfection with miR-451 mimics, there was a significant increase in miR-451 expression in MCF-7, MCF-7/EPI, and MCF-7/DOC. Cells in the three cell lines had increased apoptosis, Bcl-2 protein expression decreased significantly, and Caspase protein expression increased obviously. After the transfection with miR-451 inhibitors, miR-451 expression was significantly decreased and apoptosis in the 3 cell lines had no significant decrease compared with the control group.
Conclusions
Increased miR-451 expression may negatively regulate Bcl-2 mRNA and protein expression, followed by affecting the protein expression of caspase 3, and accelerate the apoptosis in breast cancer, indicating that miR-451 might influence the drug resistances of the Paclitaxel-resistant breast cancer cell line.
MeSH Keywords: bcl-2 Homologous Antagonist-Killer Protein; Caspase 3; Drug Resistance, Bacterial; MCF-7 Cells
Background
Breast cancer, the most common malignant tumor affecting women, with over 1 million new diagnosed cases each year, is ranked second in causing cancer deaths in women worldwide [1–3]. In 2013, 232 340 US women were diagnosed with invasive breast cancer and there were 39 620 deaths due to this disease [4]. Breast cancer is a group of diseases with heterogeneous features encompassing numerous entities differing in biological behavior, morphology, response to therapy, and clinical outcome [5]. According to the molecular profiling, it can be divided into 3 subtypes: luminal, basal-like, and human epidermal growth factor receptor-2 (HER2)-positive [6].
The recognized molecular mechanisms for drug resistance include DNA repair pathway alteration, resistance to the initiation of apoptotic pathway, cellular target mutations, and the development of signaling pathways constitutively activated [7–9]. Recently, many microRNAs (miRNAs) such as miR-155, miR-34, and miR-195 have been characterized and identified as indispensable regulators and/or biomarkers in breast cancer development, including initiation, metastasis, and therapy resistance [2,8,10,11]. miR-451, which is mapped to chromosome 17 at 17q11.2 where the miR-144 gene is located 100 bp downstream, is dysregulated in many human malignant tumors, including breast cancer, playing a crucial role in oncogenesis [12]. This miRNA has been documented to be associated with the drug resistance mechanisms of multiple cancers, such as non-small-cell lung cancer (NSCLC) and colon carcinoma [13,14] In 2008, a study explored the role of miR-451 in the chemotherapeutic drug resistance of MCF-7 cells to doxorubicin, revealing that overexpressed miR-451 was capable of sensitizing MCF-7 cells to doxorubicin therapy through directly targeting the multi-drug resistance 1 (mdr1) gene [15]. However, whether and how the regulation of miR-451 influences the drug resistance of other agents, such as docetaxel and epirubicin, has not been fully determined. In the present study, we aimed to determine the influence of miR-451 on drug resistances of the paclitaxel-resistant breast cancer cell line via transfecting and regulating the levels of miR-451 and to explore the mechanisms of this influence.
Material and Methods
Cell lines and cell culture
MCF-7 human breast adenocarcinoma cell line and its multidrug-resistant cell lines (MCF-7/DOC and MCF-7/EPI) were obtained from the Affiliated Shengjing Hospital of China Medical University. MCF-7/DOC and MCF-7/EPI, members of the MCF-7 cell line, were seeded in RPMI-1640 (GIBCO, USA) medium supplemented with 10% fetal bovine serum (FBS, GIBCO, USA) at 37°C/5% CO2 atmosphere, and then transferred into the culture flask. The medium was changed every 3 day. Cells were passaged upon reaching 90% confluency (proliferation density). The adherent cells were detached by digesting in 0.25% trypsin/0.1% EDTA (Cellgro®, VA), centrifuged for 5 min at 1000 rpm, and then the original medium was replaced with a fresh complete medium, incubated at 37°C/5% CO2. Cells during logarithmic growth phase were collected, washed with PBS (3 times) after the removal of the medium, digested with 0.25% trypsin for 1~2 min, and added to fresh RPMI-1640 medium. The adherent cells were released by repeated pipetting with a mouth-operated micropipette, and the cell suspension was put into a new centrifugal tube (1000 r/min; 10 min). We discarded the supernatant, added RPMI 1640 medium with 10% FBS, pre-cooled it for 10 min at 4°C, and added sterile glycerol. Subsequently, cells were put in frozen pipes and kept in a nitrogen canister. Slow freezing and rapid thawing was performed for the cell recovery.
Establishment of drug-resistant cell lines
For the establishment of drug-resistant cell lines, parental cells MCF-7 during logarithmic growth phase were collected, digested with 0.25% trypsin (Gibco, USA) and centrifuged, and then a cell suspension (1×109 cells/L) was obtained. Then, 10 mL cell suspension was seeded to a culture bottle for 24 h. Subsequently, medium containing Epirubicin (EPI, 10 ng/mL, Pfizer, Inc.) was added. After culturing for 48 h, the medium containing drugs was discarded. Then fresh medium was supplemented for a further 48 h of culturing. Repeated fluid replacement and cell passage were performed and gradient concentration of EPI (10 ng/ml – 50 ng/ml – 100 ng/ml – 200 ng/ml – 500 ng/ml) was used for intermittent induction to obtain a 500 ng/mL EPI-resistant cell line (breast cancer drug-resistant cell strain MCF-7/EPI). With the same method, 500 ng/mL Docetaxel (DOC, Qilu Pharmaceutical Factory, Jinan, China) was used to obtain breast cancer drug-resistant cell strain MCF-7/DOC. MCF-7/EPI and MCF-7/DOC were cultured further in complete culture medium containing 10 ng/mL EPI and DOC.
Transfection and apoptosis assay
Twenty-four hours before the transfection, the MCF-7 cell line, MCF-7/DOC and MCF-7/EPI were collected during logarithmic growth phase. After trypsinization and centrifugation, cell suspension was obtained, and seeded in a 24-well plate chamber (medium without antibiotic) at a density of 2×105 cells/well. Cells were grown to 50% confluency and miR-415 mimics or inhibitors were transiently transfected using Lipofectamine 2000 (Invitrogen, USA) according to the specifications of the manufacturer. Six hours after the transfection, the medium containing miRNA-lipo2000 mixture was replaced with fresh complete medium, and the plate was placed in an incubator with 37°C/5% CO2 atmosphere. Cells transfected with empty plasmid served as a control group. The cells were collected for miRNA detection at 24 h after transfection and cells were harvested for protein analyses at 36 h after the transfection. The cell apoptosis assay was performed at 72 h after the transfection using flow cytometry. The Annexin V-APC/PI Apoptosis Detection Kit (Invitrogen, USA) was used for the detection of cell apoptosis. For each group, this experiment was repeated 3 times.
qRT-PCR for miR-451
Total RNA was extracted from serum sample and cell line sample using the miRNeasy Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. The concentration and purity of RNA samples were determined using a UV spectrophotometer (NanoDrop 2000). Agarose gel electrophoresis (1%) was applied to observe the integrity of RNA samples. Then complementary DNA (cDNA) was synthesized utilizing 5 μg of purified RNA by using the miScript II RT Kit (QIAGEN, Hilden, Germany) based on the manufacturer’s recommendations. For the detection of miR-451 expression in different cell lines, quantitative RT-PCR (qRT-PCR) was performed using miScript SYBR Green PCR Kit (QIAGEN, Hilden, Germany). U6 was amplified as the internal control. The designed PCR primers were provided by Shanghai Sangon Biotechnology Co., Ltd., China and are presented in Table 1. Total volume of qRT-PCR reaction included: 5×RT Buffer (4.0 μL), Enzyme mix (2.0 μL), Template total RNA (4.0 μL), Enzyme mix (2.0 μL), Template total RNA (4.0 μL), SYBR Green master mix (10.0 μL), PCR primer mix (2.0 μL), and Diluter cDNA template (8.0 μL). qRT-PCR reaction conditions were: pre-denaturing at 95°C for 10 min, denaturing at 95°C for 10 s, and annealing at 60°C for 1 min. Total 40 cycles were conducted in this qRT-PCR reaction. Relative expression of miR-451 was determined using the 2–ΔΔCt method: ΔΔCT=ΔCTstudy group miRNA–ΔCTcontrol group miRNA, ΔCT=CTmiR-451–CTU6 RNA. For each group, this experiment was repeated 5 times.
Table 1.
Primer sequences used in study.
| Primers | Sequences | |
|---|---|---|
| Target gene | 451 RT primer | 5′GCGCGTGAGCAGGCTGGAGAAATT3′ |
| 451 F | 5′CCTAGCAGCACAGAAA3′ | |
| 451 R | 5′GAGCAGGCTGGAGAA3′ | |
| Internal control gene | U6 RT primer | 5′CGCTTCACGAATTTGCGTGTCAT3′ |
| U6 F | 5′CTCGCTTCGGCAGCACATA3′ | |
| U6 R | 5′CGCTTCACGAATTTGCGTG3′ | |
451 – microRNA-451; RT – reserve transcription; F – forward; R – reverse.
Western blot for protein expressions of Bcl-2 and Caspase 3
After being washed with PBS, total cells were added to RIPA buffer provided by Beijing Puli Lai Gene Technology Co., Ltd., Beijing, China, digestion at 4°C for 15 min, and the supernatant was obtained by centrifugation. The protein levels of Bcl-2 and caspase 3 were detected with BCA protein assay. The soluble proteins (30 μg) were electrophoresed using 10% SDS-PAGE, electro-transferred onto PVDF and blocked with PBS containing 5% dried skimmed milk for 2 h. The primary antibodies (p-Bcl-2 and p-Caspase 3, both 1:1000 dilution) were added and incubated with the membrane overnight at 4°C, then washed with TBS. Then goat anti-rabbit IgG labeled by horseradish peroxidase (HRP) was added as a second antibody and incubated at room temperature for 2 h. Finally, the membranes were rinsed with PBS and the bands were visualized using the ECL detection kit (Amersham International plc) and exposed to a Kodak X-Omat film. The same membrane was stripped and re-blotted with an antibody specific to β-actin (Sigma, 1:5000 dilution). Bands of BCL-2 and capase-3 were semi-quantified by densitometry using Scanimage software and normalized by β-actin levels. For each group, this experiment was repeated 5 times.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) and statistical analyses were performed using SPSS 18.0 software. Comparison between variables was analyzed with analysis of variance (ANOVA). Data were considered statistically significant at a value of P<0.05.
Results
Bcl-2 as a direct target of miR-451
The miRBase database was used to analyze the basic miR-451 information, suggesting that miR-451 was located near chromosome 17 centromere, and formed a gene cluster with miR-144 at l00 bp upstream of miR-451. We also found that the 72 nt precursor miR-451, perfectly conserved among vertebrates, became 23 nt mature miR-451 through the direct cleavage of Ago2, but not Dicer cleavage, to exert its biological function. Bioinformatics analysis based on TargetScan target prediction software, PITA target prediction software, and miRanda database, demonstrated Bcl-2 protein as a potential target gene for miR-451.
miR-451 expression in MCF-7, MCF-7/EPI, and MCF-7/DOC cells
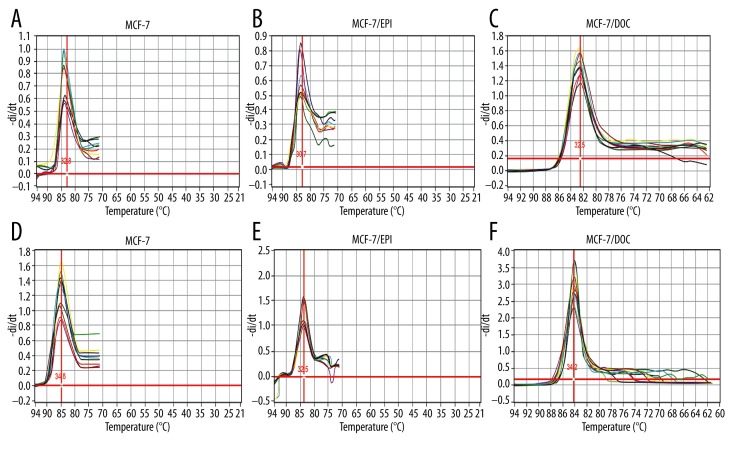
In order to explore the expression of miR-451 in MCF-7, MCF-7/EPI, and MCF-7/DOC cells after transfection by miR-451 mimics and miR-451 inhibitors, qRT-PCR was used. The melting peaks of miR-451 and internal reference U6 were Tm, which represents the temperature of 50% opening of base pairs of double-stranded DNA. The unimodal melting curves of miR-451 and U6 revealed a good specificity of PCR amplification, and Tm values of miRNA between 30°C and 35°C at the single peak. Results showed that miR-451 expression increased obviously in all 3 types of cells after transfection with miR-451 mimics, as compared with the control group (P<0.01). The miR-451 expression in all these 3 types of cells transfected with miR-451 inhibitors was lower than that in the control group (Figure 1).
Figure 1.
The melting curve of miR-451 and U6 after MCF-7, MCF-7/EPI, and MCF-7/DOC cells transfecting with miR-451 mimics and miR-451 inhibitors. The melting peaks of miR-451 and internal reference U6 were Tm, which represents the temperature of 50% opening of base pairs of double-stranded DNA. The unimodal melting curves of miR-451 and U6 revealed a good specificity of PCR amplification, and Tm values of miRNA between 30°C and 35°C at the single peak. (A–C), solubility curves of miRNA-451; (D–F) solubility curves of U6.
Apoptosis after transfection
Figure 2 displays the apoptosis of MCF-7, MCF-7/EPI, and MCF-7/DOC cells transfected with miR-451 mimics and miR-451 inhibitors by flow cytometry analysis. As compared with the control group, the apoptotic capability of all MCF-7, MCF-7/EPI, and MCF-7/DOC cells evidently enhanced in varying degrees after being transfected with miR-451 mimics (P<0.01), as shown in Figure 2. However, no observable deceasing tendency of cell apoptosis was detected between the miR-451 inhibitors group and the control group (Figure 2).
Figure 2.
Apoptosis of MCF-7, MCF-7/EPI and MCF-7/DOC cells after transfection with miR-451 mimics and miR-451 inhibitors. * Compared with control group, P<0.05.
Western blot for protein expressions of Bcl-2 and caspase 3 after transfection
After MCF-7, MCF-7/EPI, and MCF-7/DOC cells were transfected with miRNA-451 mimics and miRNA-451 inhibitors, the protein expression of Bcl-2 was detected by using Western blotting. After transfecting miR-451 mimics into MCF-7, MCF-7/EPI, and MCF-7/DOC cells, the expression levels of Bcl-2 protein in the 3 types of cells all obviously decreased in comparison with the negative control group, which presented a statistical significance with P values less than 0.01. However, no significant difference was found in the Bcl-2 protein expression between the miR-451 inhibitors group and the control group (Figure 3). After MCF-7, MCF-7/EPI, and MCF-7/DOC cells were transfected with miRNA-451 mimics and miRNA-451 inhibitors, the protein expression of caspase 3 was detected by using Western blotting. Results showed that caspase 3 protein expression in the 3 kinds of cells increased obviously as compared with negative control groups after transfections (all P<0.01). Nevertheless, transfection with miR-451 inhibitors into MCF-7, MCF-7/EPI, and MCF-7/DOC cells made no obvious difference in caspase 3 protein expression from the control group (Figure 4).
Figure 3.
Expression of Bcl-2 protein in MCF-7, MCF-7/EPI, and MCF-7/DOC cells after transfection with miR-451 mimics and miR-451 inhibitors. * compared with control group, P<0.05.
Figure 4.
Expression of caspase 3 protein in MCF-7, MCF-7/EPI, and MCF-7/DOC cells after transfection with miR-451 mimics and miR-451 inhibitors. * Compared with control group, P<0.05.
Discussion
Previous studies indicated that miR-451 acts as a tumor suppressor oncogene in several cancers, including breast cancer [16–18]. Our study reveals that the expression of miR-451 may be regulated after the transfection of miR-451 mimics and miR-451 inhibitors, and the properties of these 2 synthetic oligonucleotides might contribute to the outcomes. The specific results of our study indicate that the expression of miR-451 was up-regulated relative to the control group after the transfecting miR-451 mimics, and was down-regulated to some extent after transfecting miR-451 inhibitors in MCF-7, MCF-7/EPI, and MCF-7/DOC cells, but no without statistical significance, which may lead to the inherent low level of miR-451 expression in breast cancer cells.
Results of the present study also show that miR-451 might influence the sensitivity to neo-adjuvant chemotherapy through regulating cell apoptosis. After transfection of miR-451 mimics, the apoptosis of MCF-7, MCF-7/EPI, and MCF-7/DOC cells increased by various extents, suggesting that the up-regulation of miR-451 expression may promote the apoptosis of tumor cells. Some toxic drugs can induce changes in DNA sequence, including mutations, deletions, and rearrangements, which may lead to the initiation of signaling that will result in cell death or the deactivation of several cancer suppressor genes [19,20]. Moreover, cell death in response to DNA damage in most cases has been shown to result from cell apoptosis [21,22]. Consequently, we believe that regulation of apoptosis-associated proteins is an important approach to alter the drug-resistance in cancer cells. miR-451 may modulate cell apoptosis to make tumor cells have drug-resistances and then influence the sensitiveness to neo-adjuvant chemotherapy.
According to our results, miR-451 could down-regulate the expression of Bcl-2 proteins via negatively regulating the levels of Bcl-2 mRNA, which might impact the caspase 3 expression, thereby playing an effective role in apoptosis in human breast cancer cells. The Bcl-2 family, proteins of which are key regulators in the process of apoptosis, comprises both prosurvival and proapoptotic proteins, and the balance shifting toward the first has been elucidated as a mechanism by which cancer cells evade apoptosis [23,24]. Evidence has shown that an elevated Bcl-2 level could contribute to the docetaxel-involved chemotherapy efficacy in breast cancer, indicating that Bcl-2 expression may be regarded as a predictive biomarker for the sensitivity to this chemotherapy [25]. Based on these conditions, we considered that serum miR-451 might impact the drug resistance of neoadjuvant chemotherapy in breast cancer by regulating Bcl-2 expression. As has been noted by Nan et al., miR-451 can affect the invasion and survival of cells via the regulation of target proteins, including Bcl-2, suggesting that the miR-451 could lead to an inhibition of Bcl-2 mRNA translation through the binding of its 3′ untranslated region, thus down-regulating the expression of Bcl-2 protein [18].
Caspase-3, a major protein that executes the caspase signaling pathway, is a downstream effector caspase playing a critical role in apoptosis induction [26,27]. Its activation has been considered as the terminal event before cell death, while its deficiency in tumor cells or stroma may result in significant sensitivity to radiotherapy in mouse or xenograft tumors [28]. In general, caspase activation is strongly associated with the release of cytochrome c (cyt-c), both of which could be impaired in tumors involving overexpression of Bcl-2 [29–31]. Additionally, a heightened ratio of Bax/Bcl-2 may enhance the activity of caspase-3, and the up-regulation of miR-451 may increase the caspase-3-dependent apoptosis [32]. Thus, we conclude that miR-451 could influence the apoptosis of breast cancer cells via regulating and impacting the expression of Bcl-2 mRNA and caspase 3 protein.
Conclusions
The up-regulation of serum miR-451 could negatively regulate the expression of Bcl-2 mRNA and Bcl-2 its protein to impact the expression of caspase 3 protein and consequently play an effective role in the apoptosis of human breast cancer cells, which might explain how serum miR-451 influenced the multidrug resistance in breast cancer. Our research provides a basis for further studying the influence and mechanism of the regulation of miR-451 on the expression of Bcl-2 and caspase 3 in multidrug resistance in breast cancer cells. However, our study still has some limitations, such as no Bcl-2 transfection after miR-451 transfection to ensure the uniqueness of Bcl-2 in miR-451-induced cell apoptosis, which may influence the reliability of our results; therefore, further research with more rigorous study design is needed.
Acknowledgments
We would like to acknowledge the helpful comments on this paper received from our reviewers.
Footnotes
Competing interests
The authors have declared that no competing interests exist.
Source of support: This study was supported by the Science and Technology Research Project of Liaoning Province (No. 2012225016)
References
- 1.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 2.Liu H. MicroRNAs in breast cancer initiation and progression. Cell Mol Life Sci. 2012;69:3587–99. doi: 10.1007/s00018-012-1128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spanheimer PM, Carr JC, Thomas A, et al. The response to neoadjuvant chemotherapy predicts clinical outcome and increases breast conservation in advanced breast cancer. Am J Surg. 2013;206:2–7. doi: 10.1016/j.amjsurg.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 5.Hurley J, Reis IM, Rodgers SE, et al. The use of neoadjuvant platinum-based chemotherapy in locally advanced breast cancer that is triple negative: retrospective analysis of 144 patients. Breast Cancer Res Treat. 2013;138:783–94. doi: 10.1007/s10549-013-2497-y. [DOI] [PubMed] [Google Scholar]
- 6.Khoury T, Ademuyiwa FO, Chandrasekhar R, et al. Aldehyde dehydrogenase 1A1 expression in breast cancer is associated with stage, triple negativity, and outcome to neoadjuvant chemotherapy. Mod Pathol. 2012;25:388–97. doi: 10.1038/modpathol.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marquette C, Nabell L. Chemotherapy-resistant metastatic breast cancer. Curr Treat Options Oncol. 2012;13:263–75. doi: 10.1007/s11864-012-0184-6. [DOI] [PubMed] [Google Scholar]
- 8.Yang G, Wu D, Zhu J, et al. Upregulation of miR-195 increases the sensitivity of breast cancer cells to Adriamycin treatment through inhibition of Raf-1. Oncol Rep. 2013;30:877–89. doi: 10.3892/or.2013.2532. [DOI] [PubMed] [Google Scholar]
- 9.Coley HM. Mechanisms and strategies to overcome chemotherapy resistance in metastatic breast cancer. Cancer Treat Rev. 2008;34:378–90. doi: 10.1016/j.ctrv.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Gasparini P, Lovat F, Fassan M, et al. Protective role of miR-155 in breast cancer through RAD51 targeting impairs homologous recombination after irradiation. Proc Natl Acad Sci USA. 2014;111:4536–41. doi: 10.1073/pnas.1402604111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang S, Li Y, Gao J, et al. MicroRNA-34 suppresses breast cancer invasion and metastasis by directly targeting Fra-1. Oncogene. 2013;32:4294–303. doi: 10.1038/onc.2012.432. [DOI] [PubMed] [Google Scholar]
- 12.Pan X, Wang R, Wang ZX. The potential role of miR-451 in cancer diagnosis, prognosis, and therapy. Mol Cancer Ther. 2013;12:1153–62. doi: 10.1158/1535-7163.MCT-12-0802. [DOI] [PubMed] [Google Scholar]
- 13.Wang XC, Tian LL, Jiang XY, et al. The expression and function of miRNA-451 in non-small cell lung cancer. Cancer Lett. 2011;311:203–9. doi: 10.1016/j.canlet.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Bitarte N, Bandres E, Boni V, et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells. 2011;29:1661–71. doi: 10.1002/stem.741. [DOI] [PubMed] [Google Scholar]
- 15.Kovalchuk O, Filkowski J, Meservy J, et al. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–59. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 16.Bergamaschi A, Katzenellenbogen BS. Tamoxifen downregulation of miR-451 increases 14-3-3zeta and promotes breast cancer cell survival and endocrine resistance. Oncogene. 2012;31:39–47. doi: 10.1038/onc.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gits CM, van Kuijk PF, Jonkers MB, et al. MicroRNA expression profiles distinguish liposarcoma subtypes and implicate miR-145 and miR-451 as tumor suppressors. Int J Cancer. 2014;135:348–61. doi: 10.1002/ijc.28694. [DOI] [PubMed] [Google Scholar]
- 18.Nan Y, Han L, Zhang A, et al. MiRNA-451 plays a role as tumor suppressor in human glioma cells. Brain Res. 2010;1359:14–21. doi: 10.1016/j.brainres.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 19.Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119:3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- 20.Mates JM, Segura JA, Alonso FJ, Marquez J. Oxidative stress in apoptosis and cancer: an update. Arch Toxicol. 2012;86:1649–65. doi: 10.1007/s00204-012-0906-3. [DOI] [PubMed] [Google Scholar]
- 21.Dwyer DJ, Camacho DM, Kohanski MA, et al. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol Cell. 2012;46:561–72. doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roos WP, Kaina B. DNA damage-induced cell death: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332:237–48. doi: 10.1016/j.canlet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 24.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–24. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakes SR, Vaillant F, Lim E, et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci USA. 2012;109:2766–71. doi: 10.1073/pnas.1104778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun X, Wu Y, Chen B, et al. Regulator of calcineurin 1 (RCAN1) facilitates neuronal apoptosis through caspase-3 activation. J Biol Chem. 2011;286:9049–62. doi: 10.1074/jbc.M110.177519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suffert G, Malterer G, Hausser J, et al. Kaposi’s sarcoma herpesvirus microRNAs target caspase 3 and regulate apoptosis. PLoS Pathog. 2011;7:e1002405. doi: 10.1371/journal.ppat.1002405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snigdha S, Smith ED, Prieto GA, Cotman CW. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci Bull. 2012;28:14–24. doi: 10.1007/s12264-012-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouaziz C, Sharaf el dein O, Martel C, et al. Molecular events involved in ochratoxin A induced mitochondrial pathway of apoptosis, modulation by Bcl-2 family members. Environ Toxicol. 2011;26:579–90. doi: 10.1002/tox.20581. [DOI] [PubMed] [Google Scholar]
- 30.Morales-Cruz M, Figueroa CM, Gonzalez-Robles T, et al. Activation of caspase-dependent apoptosis by intracellular delivery of cytochrome c-based nanoparticles. J Nanobiotechnology. 2014;12:33. doi: 10.1186/s12951-014-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seervi M, Joseph J, Sobhan PK, et al. Essential requirement of cytochrome c release for caspase activation by procaspase-activating compound defined by cellular models. Cell Death Dis. 2011;2:e207. doi: 10.1038/cddis.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bian HB, Pan X, Yang JS, et al. Upregulation of microRNA-451 increases cisplatin sensitivity of non-small cell lung cancer cell line (A549) J Exp Clin Cancer Res. 2011;30:20. doi: 10.1186/1756-9966-30-20. [DOI] [PMC free article] [PubMed] [Google Scholar]