Abstract
Streptococcus pneumoniae relies exclusively on carbohydrates as a carbon source and devotes 30% of all transport mechanisms to carbohydrate import. Pneumococci utilize at least 32 carbohydrates in vitro; yet, some proposed substrates are not human-derived making it unclear where they are encountered in the host niche while other substrates remain unidentified. The majority of transporter loci are conserved, arguing against redundancy and instead for distinct roles during pathogenesis. Despite this, expression and regulation of carbohydrate transporters in vivo remains ill-defined. Recent work has also demonstrated multiple ABC transporters share an ATPase; whether this evolved for genome minimization or for transporter regulation remains unknown. Continued efforts to understand carbohydrate import may reveal novel vaccine and therapeutic targets and increase understanding of pneumococcal pathogenesis.
Keywords: Streptococcus pneumoniae, carbohydrate, phosphotransferase system, ATP-binding cassette transport
Carbohydrate utilization is central to the pneumococcal lifestyle
Streptococcus pneumoniae is an opportunistic respiratory pathogen that typically occupies the human airway asymptomatically but can progress beyond to normally sterile sites to cause disease. As free carbohydrates are scarce in the airway, the pneumococcus likely acquires carbon through modification and import of complex glycans [1–3]. Thus carbohydrate acquisition is essential for both colonization and pathogenesis. Accordingly, the pneumococcus devotes greater than 30% of all transport mechanisms to carbohydrate uptake though many of the annotated transporters remain unstudied in detail [4]. It is predicted that there are 21 phosphotransferase systems (PTS) and up to eight ATP binding cassette (ABC) transporters that import at least 32 distinct carbohydrates [5]. This bias towards carbohydrate uptake highlights the essentiality of carbon acquisition in the complex niche of the human airway and is unmatched by other resident airway bacteria [6–8]. Several transporters have been implicated in pathogenesis, reinforcing the need for carbon acquisition on mucosal surfaces (Table 1). There remains a need for further studies into the diversity of substrates and function of carbohydrate transporters if we are to appreciate the relationship between carbohydrate import and pathogenesis of S. pneumoniae.
Table 1.
Carbohydrate transporters in Streptococcus pneumoniae
| TIGR4 opening reading frame |
Genes | Substrate | Core genomea |
in vivo phenotypeb |
Refs. |
|---|---|---|---|---|---|
| PTS | |||||
| SP0061–4 | Core | ||||
| SP0248–50 | Core | ||||
| SP0282–4 | manLMN | Glucose, mannose, galactose, N-acetylglucosamine, glucosamine |
Core | I [43] | [5] |
| SP0305,8,10 | celBCD | Cellobiose, gentiobiose, arbutin, amygdalin |
[5, 19, 21] | ||
| SP0321,3–5 | Hyaluronic acid | Core | C,P [26, 44] | [26] | |
| SP0394–6 | mtlAF | Mannitol | [5] | ||
| SP0474,6,8 | Mannose | [5] | |||
| SP0577 | bgiP | 1-O-Methyl-β-glucoside | Core | [5] | |
| SP0645–7 | gatABC | Galactose | Core | [45] | |
| SP0758 | malt | Maltose, maltotriose, maltodextrin, glycogen |
Core | P [43] | [5] |
| SP0877 | fruA | Fructose | Core | [5] | |
| SP1185–6 | lacEF1F2 | Lactulose, lactose | Corec | [5] | |
| SP1617–9 | Id[46] | ||||
| SP1684 | Glucosamine | [5] | |||
| SP1722 | scrT | Sucrose | C [11] | [11] | |
| SP1884 | Trehalose | Core | [5] | ||
| SP2022–4 | Amygdalin | Core | [5] | ||
| SP2036–8 | Ascorbate | Core | [5] | ||
| SP2129–30 | Core | ||||
| SP2161–4 | C, Id[46, 47] | [16] | |||
| sph1925–7,9e | C,P,I [48] | [48] | |||
| ABC | |||||
| SP0090–2 | Galactose, mannose, N-acetylmannosamine |
Core | C, P, I [47, 49] | [5] | |
| SP0845–8 | Core | I [43] | |||
| SP1580 | msmK | Core | C, P, I [28, 29, 49, 50] | [28, 29] | |
| SP1681–3 | satABC | N-acetylneuraminic acid, N-acetylmannosamine |
Core | C [17] | [5, 17] |
| SP1688–90 | N-acetylneuraminic acid, N-acetylmannosamine |
Core | [5] | ||
| SP1796–8 | susT1T2X | Sucrose | P [11] | [11] | |
| SP1895–7 | rafEFG | Raffinose | Core | P [49] | [20] |
| SP2108–10 | malXCD | Maltooligosaccharides | Core | P [43, 49] | [24, 51] |
| SPD1583–5f | Sucrose | [5] | |||
| CGSSp3BS71_10433,28,23g | [10] | ||||
| Other | |||||
| SP1328 | N-acetylneuraminic acid, N-acetylmannosamine |
P [49] | [5] | ||
| SP2184 | Glycerol | Core | [5] |
As annotated by Obert et al. [9].
Capital letter denotes model of infection: C, colonization; P, pneumonia; I, invasive disease.
Only SP1186 is in the core genome.
Mutation of open reading frames SP1612-22 or SP2158-66, respectively showed the described phenotype. These regions included mutation of multiple open reading frames which included predicted transporter components.
As annotated in strain Hungary 19-A6, GenBank accession CP000936.1.
As annotated in D39 genome [52].
As annotated in strain CGSSp3-BS71 [53], GenBank accession AAZZ00000000.
Does maintenance of so many carbohydrate transporters benefit S. pneumoniae colonization and pathogenesis?
Genes encoding components for 20 carbohydrate transporters are maintained as a part of the proposed core pneumococcal genome [9]. At least two additional loci appear to always encode a transporter, although the specific genes can vary at the position [10, 11]. Based on the most recent experimental data, S. pneumoniae is capable of fermenting greater than 30 carbohydrates in vitro [5]. Rohmer and colleagues recently postulated that evolutionary adaptations to animal hosts by many bacteria was driven by nutritional diversity [12]. Therefore, gain or loss of metabolic pathways is likely greatly influenced by the host niche. One must assume that each of these transporters provides a unique advantage to S. pneumoniae for nasopharyngeal colonization, transmission or pathogenesis. In support of this, mutations in components of carbohydrate transporters have shown attenuation in vivo in both colonization and pathogenesis (Table 1).
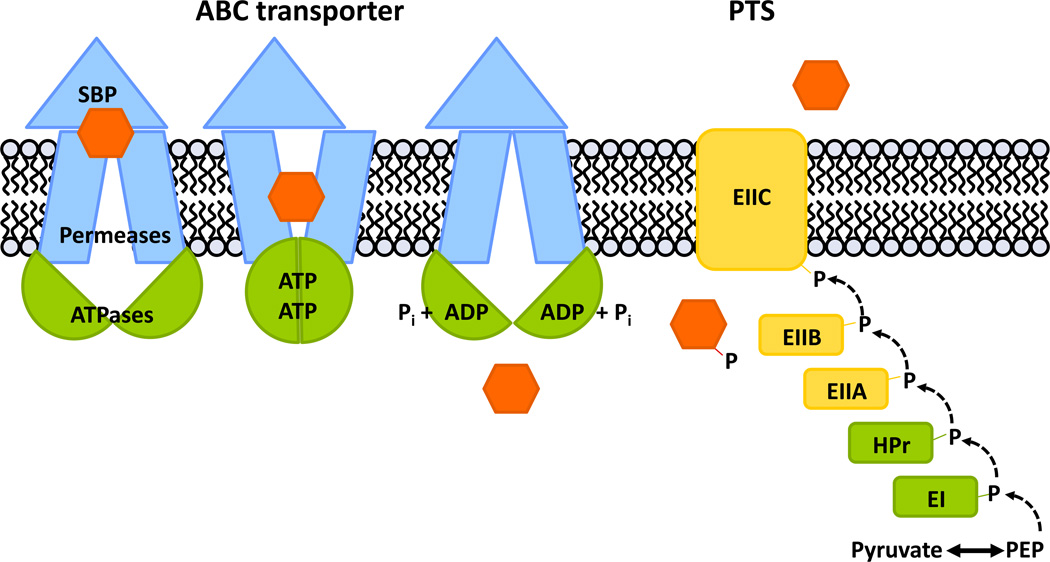
The majority of pneumococcal carbohydrate transporters are of two types: phosphotransferase systems (PTS) and ATP binding cassette (ABC) transporters (Figure 1). Import through a PTS results in the phosphorylation of the carbohydrate substrate upon transport. Strictly speaking, the carbohydrates transported through ABC transporters are not modified and as such, the term allocrite is more accurate than substrate [13]. For simplicity and continuity, substrate will be used when discussing the collective carbohydrates imported by both PTS and ABC transporters. Utilization of ABC transporter carbohydrates requires more energy than for PTS, both to energize the transport and to modify carbohydrates once intracellular. Furthermore, PTS transport intermediates exert regulatory control over ABC transporters via CcpA, and CcpA was recently show to directly and indirectly regulate up to 19% of pneumococcal genes, including carbohydrate ABC importer genes [14]. Despite the increased energy cost, ABC transporters must be advantageous or they would be lost. Although it has not been studied, it is possible that PTS substrates are size-restricted, requiring the maintenance of ABC transporters to import longer and more diverse carbohydrate linkages.
Figure 1. ABC transporters and PTS are the main carbohydrate import mechanisms in Streptococcus pneumonia.
Movement of a carbohydrate (orange) across the membrane is depicted for an ATP-binding cassette (ABC) importer and a phosphoenolpyruvate- dependent phosphotransferase system (PTS). Components that are common to transport systems are depicted in green. (left) The ABC importer is composed of two energy- generating ATPases which are shared between CUT1 ABC importers (green), two membrane-spanning permeases and a substrate binding lipoprotein (SBP) (blue). In S. pneumoniae, ATPases of the carbohydrate ABC importers are predicted to be homodimers, and the permeases are predicted to be heterodimers. (right) The PTS is composed of two general proteins enzyme I (EI) and the histidine protein (HPr) (green). Enzyme IIA (EIIA) and enzyme IIB (EIIB) are cytoplasmic phosphocarriers and the enzyme IIC (EIIC) creates the membrane channel (yellow). Mannose-type PTSs have an additional transmembrane enzyme IID (not shown). Enzymes EIIABC(D) are specific for a given substrate and while most EII are encoded as single genes, several EII fusions exist. Abbreviations: PEP, phosphoenolpyruvate.
The vast ability of S. pneumoniae to utilize carbohydrates is in stark contrast to most other nasopharyngeal bacteria and is instead more similar to oral and gastrointestinal organisms whose interaction with host and dietary carbohydrates is well studied [15]. Many other common respiratory colonizers including Haemophilus influenzae, Neisseria meningitidis, and Moraxella catarrhalis are far more restrictive in carbohydrate fermentation ability, notably with M. catarrhalis apparently unable to use carbohydrates as a carbon source [6–8]. The reason for the restricted carbohydrate utilization capabilities is uncertain but it is important to note that among those noted above, S. pneumoniae is the only species strictly reliant on carbohydrates as a carbon source. Still, this decisive difference in carbon utilization likely provides a competitive advantage to S. pneumoniae in the nasopharynx.
The possibility of non-nutritional roles for carbohydrate importers must also be considered. Presence of a fucose utilization locus in S. pneumoniae coupled with the inability to grow on fucose as a carbon source may indicate alternative roles for carbohydrate import [16]. It remains unclear whether fucose is imported and degraded but not used as a carbohydrate source or whether a fucose-containing glycan is instead imported [10, 16]. Furthermore, although S. pneumoniae can utilize sialic acid as a sole carbohydrate source in vitro, it was also suggested that sialic acid contributes to cell signaling [17, 18]. Thus, even though appreciable growth on many substrates can be detected, additional or primary roles of carbohydrate importers may have been overlooked and future work may reveal novel functions for carbohydrate transport.
Why do pneumococci ferment such diverse carbohydrates?
With humans serving as the primary host for S. pneumoniae, one assumes that all of the carbohydrate metabolic capabilities are refined for use of human-derived carbohydrates, but several transporter substrates appear to be plant derived [5, 19–22]. Adaptation of nearly all other lactic acid bacteria reveal fine tuning of the metabolic abilities to optimize life in their specific niche so we would expect to see no remaining traces for utilization of environmental carbohydrates in any of the human-adapted species [23]. This is not the case. In fact, at the turn of the last century, discrimination of pneumococci amongst streptococci was based upon the ability to ferment inulin [22]. Inulin, however, is not produced by the human body and is instead a main component of dietary fiber. Why pneumococci evolved and maintained the ability to utilize inulin is unclear but supposes that S. pneumoniae encounters inulin at some point during colonization or transmission. It is also possible that although pneumococci can ferment inulin in vitro, the transporter for inulin may have a different function in vivo.
Recently, Abbott et al. revealed that the MalXCD importer transports α1–4 linked glucose molecules of glycogen as a sole carbon source after cleavage of α1–6 branches by SpuA [24, 25]. Glycogen is structurally similar to plant starch which also contains α1–4 and α1–6 glucose repeats. Thus both maltooligosaccharide and inulin import may reflect plant carbohydrate transporters that have adapted to use structurally similar host carbohydrates. Despite this, there is compelling experimental evidence that pneumococci can ferment plant-based carbohydrates [5, 19–22].
The transporter substrates described above coupled with the overall usable carbohydrate profile call in to question the view that S. pneumoniae strictly derives carbohydrates from host glycans. Conceivably, carbohydrates of the host diet ought to be considered as another source of carbohydrates. This would reconcile the discrepancy between what S. pneumoniae has been shown to ferment and what it reasonably encounters in the host with high enough frequency to maintain selective pressure [5, 20, 22, 24]. Given the ability of S. pneumoniae to colonize the oropharynx, it is worth considering that pneumococci may modify and import dietary carbohydrates.
Perhaps underappreciated sources of carbohydrates in the airway available to S. pneumoniae are surface carbohydrates of other resident or pathogenic microbes. Indeed, in vitro studies have shown that S. pneumoniae can alter the capsule structures of other airway bacteria and utilize purified capsule as a sole carbon source [26, 27]. Modification of airway microbes would serve the dual purpose of exposing the competitor to host immune clearance as well as providing a nutrient source. Therefore, further studies of the ability of S. pneumoniae to modify and import microbe-derived carbohydrates as well as dietary carbohydrates have the potential to expand our understanding of usable carbohydrates by S. pneumoniae.
Are multiple transporters examples of redundancy?
The abundance of carbohydrate transport mechanisms begs the question whether these are redundancies. It can be argued that redundancy refers only to instances where multiple transporters import the same substrate. Indeed this has been suggested for the SusT1T2X and ScrT transporters, which are both proposed to import sucrose [11]. More recently, a survey of all predicted carbohydrate transporters indicated that transporter substrate overlap may be more widespread and growth on eight carbohydrates in vitro was affected by mutation of more than one transporter [5]. This seems counter to the evidence that each transporter is highly conserved. Although there is transporter substrate overlap in vitro, each transporter must be maintained for a distinct function. Differential regulation in vivo may reveal that transporters are important at different times during infection or in different anatomical niches, as has been suggested for the sucrose importers [11]. It is also possible additional experiments will reveal novel functions or substrates of the carbohydrate importers and that some identified substrate overlap are artifacts of in vitro systems that utilize high carbohydrate concentrations which may permit low affinity transport [5, 11]. Especially in the case of oligosaccharide importers, it is likely that substrate identification is confounded by growth phenotypes observed on constituent carbohydrates.
An alternate view of redundancy is based on the hypothesis that the need for carbohydrates as a carbon source is general to survival of the bacterium. Therefore encoding a large number of transporters may help ensure that genetic loss of one mechanism will not result in death of that bacterium. However, because each of these transporters is presumably expressed under different conditions, it seems that instead of being a functional redundancy, this mechanism is a well-evolved system to adapt to the changing carbohydrate availability encountered by S. pneumoniae. If transporters were redundant, conservation amongst the gamut of import mechanisms would be low, as loss of a single mechanism would not be detrimental. In disagreement with this, mutation of individual transporters is often associated with decreased colonization in vivo (Table 1) [9, 11, 17, 26]. Also challenging this assumption is the fact that at two loci, even when transporter genes vary, the ability to import the same substrate is apparently conserved [10, 11]. The data at present collectively argue against transporter redundancy.
Why do some ABC importers share energy generating components?
Depending on the strain, there are either six or seven predicted carbohydrate uptake transporter family 1 (CUT1) ABC importers in each pneumococcal genome [4, 5, 10]. Each locus encoding a CUT1 family carbohydrate ABC transporter is lacking a predicted ATPase which is absolutely required for import [28]. The ATPase, MsmK, was recently characterized and shown to be shared between multiple carbohydrate ABC transporters [28, 29]. It may be that the ATPase is not shared between all CUT1 ABC importers; however, no other candidate gene has been suggested or identified [5, 28]. While this is the first example of a shared ATPase in a pathogenic bacterium, other reports have demonstrated the sharing of an ATPase among carbohydrate ABC transporters in both Gram-positive and Gram-negative bacteria [30–32]. This may parallel the sharing of phosphorelay components EI and HPr of the PTS (Figure 1). Sharing of the ATPase is possibly an example of genome conservation with the potential to reduce the need for at least six ATPases to only one. This model may not explain why this phenomenon is seemingly exclusive to carbohydrate transporters; although, the argument can be made that in contrast to the transport substrates of other ABC transporters, the need for carbon as a nutrient source is general. Thus, sharing of a component for a general cellular nutrient requirement may allow for diminution of the genome through loss of extra genes encoding ATPases.
In addition, sharing an ATPase among transporters could exert preference based on the abundance of the core ABC transporter components and differences in affinity of the ATPase for different permeases where the transport substrate associated with the greatest number of functionally expressed core transporters would be favored. Although the strength of interaction between MsmK and the permease components remains unknown, this could further influence a transporter preference. For MsmK to exert a preference it must be limiting. While it is known that msmK expression increases 10–20 fold in bacteria grown in a sugar transported by an ABC-transporter compared to bacteria grown in a sugar transported by a PTS in vitro, whether MsmK it is limiting in vivo remains unknown [28].
It is well known that the HPr protein shared among PTS has extensive regulatory roles in Gram-positive bacteria [33]. Although not yet studied, a shared ATPase component may allow for an additional level of regulation over the ABC transporters. Tyx et al. showed that MsmK directly interacts with dihydrolipoamide dehydrogenase (DLDH) to alter expression of the raffinose uptake (raf) locus [29]. Whether DLDH has any regulatory capacity over the other ABC transporters and the identity of other MsmK binding partners remain unknown [29]. Furthermore, MsmK contains a C-terminal domain similar to the model ATPase MalK in Escherichia coli that has been shown to contribute to carbohydrate preference through sequestration of the activator MalT [34]. There is a great possibility that the discrete location, regulatory domain, and sharing of MsmK may serve a regulatory role over ABC transporters (or other genes) and this should be explored. An ABC transporter-wide mechanism of regulation is unprecedented and would greatly add to our understanding of carbohydrate utilization and regulation in bacteria.
Could carbon acquisition be a target for vaccine or therapy development?
As the links between cellular metabolism and pathogenesis are becoming evident, the question arises whether this knowledge can be used to help prevent pneumococcal disease [12]. ABC importers and PTS are especially attractive targets given that both mechanisms are exclusive to prokaryotes. It has been suggested that PTS and ABC importers could each be used as novel drug delivery systems [35, 36]. Engineering antimicrobials to be recognized and bound by transporters would provide an efficient and bacteria-specific route of drug delivery. An alternate approach is disrupting transport machinery directly. To the best of our knowledge, neither of these approaches is being actively pursued; however, these could represent novel therapeutic approaches for targeting S. pneumoniae or other bacterial pathogens.
There is precedence for the use of transporter components in protein-based vaccines for S. pneumoniae and the protective properties of ABC transporter substrate binding proteins have been demonstrated [37–39]. Although it has yet to be tested, the substrate binding protein of the maltooligosaccharide transporter MalX has been identified in genome-wide screens for antigenic proteins and is being considered for protein-based vaccines [40, 41]. Vaccination may provide a strong selective pressure to yield strains lacking specific importers. Furthermore, given the relatedness between commensal streptococci and S. pneumoniae, it is unlikely that vaccine targets against carbohydrate transport proteins would be specific in targeting only pneumococci [42].
Concluding remarks
Although the first genome sequence for S. pneumoniae was published in 2001, it has taken a decade for researchers to pursue large scale studies of carbohydrate uptake and metabolism [4, 5, 14]. It is clear that to understand and appreciate pneumococcal disease one must have a full understanding of metabolic processes. Continuing to study the abundance and complexity of carbohydrate importers in S. pneumoniae may reveal novel processes for modification and utilization of carbohydrates. It remains to be seen why S. pneumoniae amongst other respiratory colonizers has so many carbohydrate substrates and why several of these substrates appear to be non-host derived, why some ABC importers share an energy generating component, and whether this information can be harnessed to develop superior therapeutics and vaccines. These and other key questions are summarized in Box 1. It is hopeful then that as experimental ground work continues to be laid, the questions posed here can eventually be answered. A better understanding of the unique carbohydrate metabolic capabilities will likely shed light on the very nature of pneumococcal survival.
Box 1. Outstanding questions.
How do multiple regulatory mechanisms control pneumococcal carbohydrate utilization?
Are there alternative roles for carbohydrate transporters beyond providing a carbon source?
What are the in vivo substrates of each transporter and is there truly redundancy?
Which carbohydrates are utilized during different stages of human infection?
Does the sharing of an ATPase between CUT1 family transporters have a function beyond conservation of genome size?
Can carbohydrate transporters be utilized as novel drug delivery systems or as components of successful vaccines?
Acknowledgements
CMB is supported by the American Heart Association Predoctoral Fellowship10PRE3490014 and SJK is supported by the National Institute of Allergy and Infectious Diseases grant 1R01AI076341.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burnaugh AM, Frantz LJ, King SJ. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J Bacteriol. 2008;190:221–230. doi: 10.1128/JB.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King SJ, Hippe KR, Weiser JN. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol Microbiol. 2006;59(3):961–974. doi: 10.1111/j.1365-2958.2005.04984.x. [DOI] [PubMed] [Google Scholar]
- 3.Marion C, et al. Identification of a pneumococcal glycosidase that modifies O-linked glycans. Infect Immun. 2009;77:1389–1396. doi: 10.1128/IAI.01215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tettelin H, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293(5529):498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 5.Bidossi A, et al. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoS ONE. 2012;7(3):e33320. doi: 10.1371/journal.pone.0033320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries SP, et al. Genome analysis of Moraxella catarrhalis strain RH4, a human respiratory tract pathogen. J Bacteriol. 2010;192(14):3574–3583. doi: 10.1128/JB.00121-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leighton MP, et al. An NMR and enzyme study of the carbon metabolism of Neisseria meningitidis. Microbiology. 2001;147(Pt 6):1473–1482. doi: 10.1099/00221287-147-6-1473. [DOI] [PubMed] [Google Scholar]
- 8.Macfadyen LP, et al. Regulation of competence development and sugar utilization in Haemophilus influenzae Rd by a phosphoenolpyruvate:fructose phosphotransferase system. Mol Microbiol. 1996;21(5):941–952. doi: 10.1046/j.1365-2958.1996.441420.x. [DOI] [PubMed] [Google Scholar]
- 9.Obert C, et al. Identification of a candidate Streptococcus pneumoniae core genome and regions of diversity correlated with invasive pneumococcal disease. Infect Immun. 2006;74(8):4766–4777. doi: 10.1128/IAI.00316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins MA, et al. Blood group antigen recognition by a solute-binding protein from a serotype 3 strain of Streptococcus pneumoniae. J Mol Biol. 2009;388(2):299–309. doi: 10.1016/j.jmb.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer R, Camilli A. Sucrose metabolism contributes to in vivo fitness of Streptococcus pneumoniae. Mol Microbiol. 2007;66(1):1–13. doi: 10.1111/j.1365-2958.2007.05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohmer L, Hocquet D, Miller SI. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 2011;19(7):341–348. doi: 10.1016/j.tim.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blight MA, Holland IB. Structure and function of haemolysin B,P-glycoprotein and other members of a novel family of membrane translocators. Mol Microbiol. 1990;4(6):873–80. doi: 10.1111/j.1365-2958.1990.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho SM, et al. CcpA ensures optimal metabolic fitness of Streptococcus pneumoniae. PLoS ONE. 2011;6(10):e26707. doi: 10.1371/journal.pone.0026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95(1):50–60. doi: 10.5740/jaoacint.sge_macfarlane. [DOI] [PubMed] [Google Scholar]
- 16.Chan PF, et al. Characterization of a novel fucose-regulated promoter (PfcsK) suitable for gene essentiality and antibacterial mode-of-action studies in Streptococcus pneumoniae. J Bacteriol. 2003;185(6):2051–2058. doi: 10.1128/JB.185.6.2051-2058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marion C, et al. Sialic acid transport contributes to pneumococcal colonization. Infect Immun. 2011;79(3):1262–1269. doi: 10.1128/IAI.00832-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trappetti C, et al. Sialic acid: a preventable signal for pneumococcal biofilm formation, colonization, and invasion of the host. J Infect Dis. 2009;199(10):1497–1505. doi: 10.1086/598483. [DOI] [PubMed] [Google Scholar]
- 19.McKessar SJ, Hakenbeck R. The two-component regulatory system TCS08 is involved in cellobiose metabolism of Streptococcus pneumoniae R6. J Bacteriol. 2007;189(4):1342–1350. doi: 10.1128/JB.01170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenow C, Maniar M, Trias J. Regulation of the alpha-galactosidase activity in Streptococcus pneumoniae: characterization of the raffinose utilization system. Genome Res. 1999;9(12):1189–1197. doi: 10.1101/gr.9.12.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafeeq S, Kloosterman TG, Kuipers OP. CelR-mediated activation of the cellobiose-utilization gene cluster in Streptococcus pneumoniae . Microbiology. 2011;157(Pt 10):2854–2861. doi: 10.1099/mic.0.051359-0. [DOI] [PubMed] [Google Scholar]
- 22.Hiss PH. A contribution to the physiological differentiation of Pneumococcus and Streptococcus and to methods of staining capsules. J Exp Med. 1905;6(4–6):317–345. doi: 10.1084/jem.6.4-6.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price CE, et al. From meadows to milk to mucosa - adaptation of Streptococcus and Lactococcus species to their nutritional environments. FEMS Microbiol Rev. 2011 doi: 10.1111/j.1574-6976.2011.00323.x. [DOI] [PubMed] [Google Scholar]
- 24.Abbott DW, et al. The molecular basis of glycogen breakdown and transport in Streptococcus pneumoniae . Mol Microbiol. 2010;77(1):183–199. doi: 10.1111/j.1365-2958.2010.07199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Bueren AL, et al. Identification and structural basis of binding to host lung glycogen by streptococcal virulence factors. Nat Struct Mol Biol. 2007;14(1):76–84. doi: 10.1038/nsmb1187. [DOI] [PubMed] [Google Scholar]
- 26.Marion C, et al. Streptococcus pneumoniae can utilize multiple sources of hyaluronic acid for growth. Infect Immun. 2012;80(4):1390–1398. doi: 10.1128/IAI.05756-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shakhnovich EA, King SJ, Weiser JN. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect Immun. 2002;70(12):7161–7164. doi: 10.1128/IAI.70.12.7161-7164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marion C, et al. Identification of an ATPase, MsmK, which energizes multiple carbohydrate ABC transporters in Streptococcus pneumoniae. Infect Immun. 2011;79(10):4193–4200. doi: 10.1128/IAI.05290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyx RE, Roche-Hakansson H, Hakansson AP. Role of dihydrolipoamide dehydrogenase in regulation of raffinose transport in Streptococcus pneumoniae. J Bacteriol. 2011;193(14):3512–3524. doi: 10.1128/JB.01410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira MJ, Sa-Nogueira I. A multitask ATPase serving different ABC-type sugar importers in Bacillus subtilis. J Bacteriol. 2010;192(20):5312–5318. doi: 10.1128/JB.00832-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlosser A, Kampers T, Schrempf H. The Streptomyces ATP-binding component MsiK assists in cellobiose and maltose transport. J Bacteriol. 1997;179(6):2092–2095. doi: 10.1128/jb.179.6.2092-2095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva Z, et al. The high-affinity maltose/trehalose ABC transporter in the extremely thermophilic bacterium Thermus thermophilus HB27 also recognizes sucrose and palatinose. J Bacteriol. 2005;187(4):1210–1218. doi: 10.1128/JB.187.4.1210-1218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorke B, Stulke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6(8):613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 34.Bordignon E, Grote M, Schneider E. The maltose ATP-binding cassette transporter in the 21st century--towards a structural dynamic perspective on its mode of action. Mol Microbiol. 2010;77(6):1354–1366. doi: 10.1111/j.1365-2958.2010.07319.x. [DOI] [PubMed] [Google Scholar]
- 35.Garmory HS, Titball RW. ATP-binding cassette transporters are targets for the development of antibacterial vaccines and therapies. Infect Immun. 2004;72(12):6757–6763. doi: 10.1128/IAI.72.12.6757-6763.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parr TR, Jr, Saier MH., Jr The bacterial phosphotransferase system as a potential vehicle for the entry of novel antibiotics. Res Microbiol. 1992;143(5):443–447. doi: 10.1016/0923-2508(92)90089-7. [DOI] [PubMed] [Google Scholar]
- 37.Briles DE, et al. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae . Infect Immun. 2000;68(2):796–800. doi: 10.1128/iai.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briles DE, et al. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000;182(6):1694–1701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 39.Brown JS, et al. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect Immun. 2001;69(11):6702–6706. doi: 10.1128/IAI.69.11.6702-6706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giefing C, et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med. 2008;205(1):117–131. doi: 10.1084/jem.20071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moffitt KL, et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9(2):158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donati C, et al. Structure and dynamics of the pan-genome of Streptococcus pneumoniae and closely related species. Genome Biol. 2010;11(10):R107. doi: 10.1186/gb-2010-11-10-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogunniyi AD, et al. Identification of genes that contribute to pathogenesis of invasive pneumococcal disease by in vivo transcriptomic analysis. Infect Immun. 2012 doi: 10.1128/IAI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau GW, et al. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol Microbiol. 2001;40(3):555–571. doi: 10.1046/j.1365-2958.2001.02335.x. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman GE, Yother J. CcpA-dependent and -independent control of beta- galactosidase expression in Streptococcus pneumoniae occurs via regulation of an upstream phosphotransferase system-encoding operon. J. Bacteriol. 2007;189(14):5183–5192. doi: 10.1128/JB.00449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Embry A, Hinojosa E, Orihuela CJ. Regions of Diversity 8, 9 and 13 contribute to Streptococcus pneumoniae virulence. BMC Microbiol. 2007;7:80. doi: 10.1186/1471-2180-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orihuela CJ, et al. Microarray analysis of pneumococcal gene expression during invasive disease. Infect Immun. 2004;72(10):5582–5596. doi: 10.1128/IAI.72.10.5582-5596.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAllister LJ, et al. Contribution of a genomic accessory region encoding a putative cellobiose phosphotransferase system to virulence of Streptococcus pneumoniae. PLoS ONE. 2012;7(2):e32385. doi: 10.1371/journal.pone.0032385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol. 2002;45(5):1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 50.Polissi A, et al. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66(12):5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puyet A, Espinosa M. Structure of the maltodextrin-uptake locus of Streptococcus pneumoniae. Correlation to the Escherichia coli maltose regulon. J Mol Biol. 1993;230(3):800–811. doi: 10.1006/jmbi.1993.1202. [DOI] [PubMed] [Google Scholar]
- 52.Lanie JA, et al. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol. 2007;189(1):38–51. doi: 10.1128/JB.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiller NL, et al. Comparative genomic analyses of seventeen Streptococcus pneumoniae strains: insights into the pneumococcal supragenome. J Bacteriol. 2007;189(22):8186–8195. doi: 10.1128/JB.00690-07. [DOI] [PMC free article] [PubMed] [Google Scholar]