Abstract
AIM
To describe a series of Italian patients with orbital metastasis focusing on the outcomes in relation to the different primary site of malignancy.
METHODS
Retrospective chart review of 93 patients with orbital metastasis collected in a tertiary referral centre in a period of 38y and review of literature.
RESULTS
Out of 93 patients, 52 were females and 41 were males. Median age at diagnosis was 51y (range 1 to 88y). The patients have been divided into four groups on the basis of the year of diagnosis. The frequency of recorded cases had decreased significantly (P<0.05) during the last 9.5y. Primary tumor site was breast in 36 cases (39%), kidney in 10 (11%), lung in 8 (9%), skin in 6 (6%); other sites were less frequent. In 16 case (17%) the primary tumor remained unknown. The most frequent clinical findings were proptosis (73%), limited ocular motility (55%), blepharoptosis (46%) and blurred vision (43%). The diagnosis were established by history, ocular and systemic evaluation, orbital imaging studies and open biopsy or fine needle aspiration biopsy (FNAB). Treatment included surgical excision, irradiation, chemotherapy, hormone therapy, or observation. Ninety-one percent of patients died of metastasis with an overall mean survival time (OMST) after the orbital diagnosis of 13.5mo.
CONCLUSION
Breast, kidney and lung are the most frequent primary sites of cancer leading to an orbital metastasis. When the primary site is unknown, gastrointestinal tract should be carefully investigated. In the last decade a decrease in the frequency of orbital metastasis has been observed. Surgery provides a local palliation. Prognosis remains poor with a OMST of 13.5mo ranging from the 3mo in the lung cancer to 24mo in the kidney tumor.
Keywords: orbital metastasis, primary tumor, survival time
INTRODUCTION
Metastases to the soft tissue of the orbit are relatively uncommon, with incidence ranging from 1% to 13% among reported series of orbital tumors[1]–[4]. Malignant neoplasms generally metastasize by haematogenous route to the orbit. Breast and lung carcinomas comprise the largest group of metastatic cancer with occult primary tumor forming a significant proportion[1],[4]–[7]. Several investigators have pointed out limitations of extensive medical evaluations in patients with an unknown primary site[5],[8],[9].
The purpose of this retrospective study is to report demographic and clinical features of orbital metastasis and to evaluate changes in distribution with time and outcomes in Italy.
SUBJECTS AND METHODS
Subjects
The records of 93 patients diagnosed with orbital metastasis at the Orbital Centre of the University of Naples Federico II in a 38-year period (1976-2013) were retrospectively reviewed.
General data included the patient's age, sex, race, history of cancer and interval between diagnosis of the primary cancer and orbital metastasis. Tumor data included location and type of primary neoplasm, laterality, location in the orbit. Data concerning orbital echography computed tomography (CT), magnetic resonance imaging (MRI), intraoperative observations, orbital open biopsy and fine needle aspiration biopsy (FNAB) were reviewed. Treatment modalities and outcomes were evaluated. A computerized flow sheet to record and tabulate data was used; statistical analyses on contingent tables were conducted using commercially available software packages (SPSS 14.0, SPSS Inc., Chicago, IL; Systat 11.0, Point Richmond, CA, USA). In order to evaluate changes in frequency, statistical analysis was performed using Student's t-test and regression analysis with a considered significant level of probability of P ≤0.05. Kaplan-Meier actuarial survival analysis was applied. The study was performed in accordance with the ethical standard of Declaration of Helsinki.
Only patients with evidence of metastatic cancer to the orbit by haematogenous route were included.
RESULTS
Orbital metastases represent about 3% of orbital lesions[10]. Among 93 consecutive patients, 41 were males and 52 were females, all Caucasian. Primary site, type of tumor, and patient sex are shown in Table 1. The relation between the site of primary tumor and patient age at the time of diagnosis of orbital metastasis is shown in Table 2. The patients' age ranged from 1 to 88 (median 51)y.
Table 1. Primary site, type of metastatic tumor to the orbit and sex of the 93 patients.
| Primary site | Tumor type | No. of patients | %a | M | F |
| Breast | Carcinoma | 36 | 39 | 36 | |
| Undetermined | 16 | 17 | 10 | 6 | |
| Kidney | Carcinoma | 10 | 11 | 9 | 1 |
| Lung | Carcinoma (6) Carcinoid (2) | 8 | 9 | 7 | 1 |
| Skin | Melanoma(3) Squamocell carcinoma (3) | 6 | 7 | 4 | 2 |
| Rinopharyngeal tract | Carcinoma | 4 | 4 | 2 | 2 |
| Parotid gland | Carcinoma | 3 | 3 | 3 | |
| Gastrointestinal tract | Carcinoma | 2 | 2 | 2 | |
| Thyroid | Carcinoma | 1 | 1 | 1 | |
| Prostate gland | Carcinoma | 1 | 1 | 1 | |
| Adrenal | Neuroblastoma | 1 | 1 | 1 | |
| Soft tissues | Sarcoma | 1 | 1 | 1 | |
| Penis | Carcinoma | 1 | 1 | 1 | |
| Bladder | Carcinoma | 1 | 1 | 1 | |
| Salivary gland | Carcinoma | 1 | 1 | 1 | |
| Liver | HCC | 1 | 1 | 1 | |
| Total | 93 | 100 | 41 | 52 |
aValues are rounded. HCC: Hepatocellular carcinoma.
Table 2. Relation between patient age and location of primary neoplasm.
| Primary site | 0-19y | 20-39y | 40-59y | 60-79y | ≥80y | Total |
| Breast | 4 | 16 | 11 | 5 | 36 | |
| Undetermined | 8 | 6 | 2 | 16 | ||
| Kidney | 1 | 4 | 5 | 10 | ||
| Lung | 4 | 2 | 2 | 8 | ||
| Skin | 2 | 4 | 6 | |||
| Rinopharyngeal tract | 4 | 4 | ||||
| Parotid gland | 2 | 1 | 3 | |||
| Gastrointestinal tract | 1 | 1 | 2 | |||
| Thyroid | 1 | 1 | ||||
| Prostate gland | 1 | 1 | ||||
| Adrenal | 1 | 1 | ||||
| Soft tissues | 1 | 1 | ||||
| Penis | 1 | 1 | ||||
| Bladder | 1 | 1 | ||||
| Salivary gland | 1 | 1 | ||||
| Liver | 1 | 1 | ||||
| Total | 2 | 8 | 40 | 33 | 10 | 93 |
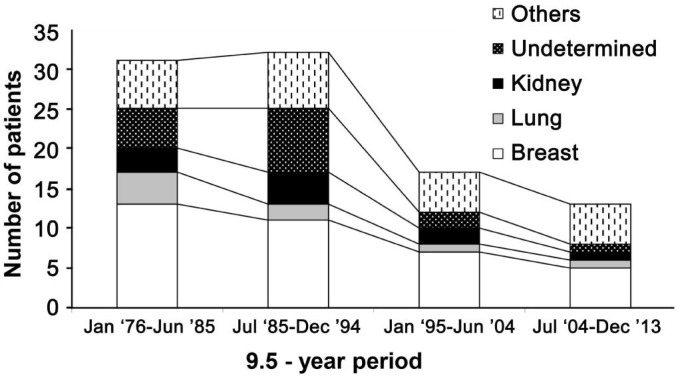
The patients have been divided into four groups on the basis of the year of diagnosis in order to evaluate the different distribution with time. The frequency of recorded cases had decreased significantly (P<0.05) during the last ten years (Figure 1).
Figure 1. Patients with orbital metastasis recorded during 1976-2013 divided into four groups on the basis of the year of diagnosis; primary site of tumor is shown.
Others includes tumors of skin, thyroid, prostate, gastrointestinal tract, parotid gland, rinopharyngeal tract, adrenal, soft tissues, penis, bladder, salivary gland and liver.
In 63 patients (68%) with a known primary neoplasm, orbital metastasis became evident on average 45mo after primary cancer diagnosis (median: 40mo; range from 2 to 166mo). Orbital metastasis of the breast cancer had a mean time of presentation of 52mo (range from 13 to 155mo), lung cancer metastasis of 6mo (range from 2 to 10mo), kidney cancer metastasis of 47mo (range from 9 to 93mo), thyroid neoplasm metastasis of 41mo (range from 26 to 70mo), melanoma metastasis of 18mo (range from 14 to 22mo). In women with less than 40y breast cancer metastasis had a mean time of presentation of 17mo (range from 13 to 21mo).
Out of 30 patients (32%) with no history of previous cancer, 27 had an orbital metastasis at the onset of the disease, 3 cases showed the orbital involvement after evidence of metastasis in other sites. Primary tumor was subsequently located in 14 cases as follows: 6 in breast, 3 in lung, 1 respectively in kidney, prostate gland, adrenal gland, gastrointestinal tract and liver. In 16 cases (17%) the primary tumor remained unknown. In these cases the histology of the orbital mass indicated a poorly differentiated carcinoma.
The right orbit was involved in 51 cases and the left in 42 cases. No patient showed bilateral involvement. Metastasis were mainly localized in the anterior orbit in 62 cases (67 %). In 17 cases extra-ocular muscles were involved, in 10 cases eyelids, in 6 lachrymal gland, in 10 temporal fossa, in 3 anterior cranial fossa, in 3 middle cranial fossa. In those cases differential diagnosis may be difficult with the following other conditions such as Graves' orbitopathy[11],[12], epithelial tumor of the eyelid[13], lachrymal gland neoplasm[14],[15], and orbital meningioma[16] respectively. Most of the orbital metastasis appeared as unique mass, only in 4 patients there was a disseminated spreading of the metastasis in the orbit. Disseminated orbital metastasis entered into differential diagnosis with lymphoma[17]. Presenting symptoms and signs are shown in Table 3.
Table 3. Presenting symptoms and signs in 93 patients with orbital metastasis.
| aSymptom or sign | No. | % |
| Proptosis | 68 | 73 |
| Limited ocular motility | 51 | 55 |
| Blepharoptosis | 43 | 46 |
| Blurred vision | 40 | 43 |
| Diplopia | 30 | 32 |
| Visible lump | 23 | 25 |
| bInflammatory signs | 20 | 22 |
| Palpable mass | 14 | 15 |
| Pain | 11 | 12 |
| Pupillar defects | 11 | 12 |
| > IOP | 11 | 12 |
| Enophthalmos | 4 | 4 |
| Conjunctival infiltration | 1 | 1 |
| Corneal invasion | 1 | 1 |
aPatients had more than one symptom or sign, explaining why numbers and percentages add to more than the number of patients; bLachrymation, ocular burn, conjunctival chemosis.
Diagnosis was made by history, ocular examination, general physical assessment, orbital CT or MRI or ECO and orbital open biopsy or FNAB. Imaging studies showed a not well circumscribed mass in 56 cases (78% of breast and 100% of prostate cancers), while it was well circumscribed in 37 (57% of lung, 83% of kidney, 100% of skin, 100% of thyroid and 100% of gastrointestinal tract cancers). Bone erosion was noted in 17 cases (18%): in the one case of prostate gland cancer (100%), in 6 cases of breast (17%), 7 of kidney (70%), 2 of lung (25%) and 1 of thyroid cancer (100%). Metastatic neuroblastoma showed extensive orbital bone involvement with ethmoidal, temporal, and sphenoid wing erosion and anterior cranial fossa invasion. Diagnosis was obtained in 24 cases by using FNAB (26%), in 27 cases by incisional biopsy (29%) and in 37 by excisional biopsy (40%). In the remaining 5 cases (5%) diagnosis was made by patient's history, clinical evidence and imaging study. Surgical approaches to the orbit for open biopsy were: superior orbitotomy in 24 cases (38%), inferior orbitotomy in 15 (23%), lateral osteoplastic orbitotomy in 13 (20%), exenteratio orbitae in 5 (8%), medial orbitotomy in 6 (9%) and hemicoronal orbitotomy in 1 case (2%).
Fifty-seven patients were treated before the onset of the orbital metastasis with chemotherapy in combination or not with radiotherapy depending on the type of primary tumor. After the diagnosis of the orbital metastasis the treatment was as follow: 24 patients received external beam irradiation (30-60 Gy); 17 were treated with additional chemotherapy with variable schemes, 5 patients (8%) were exenterated. In 11 cases (12%) the orbital metastasis was managed by observation only.
The follow-up data about the systemic status of the 93 patients were: no patient alive with no active metastasis (0%), 4 patients alive with active metastasis (4.3%), 4 deceased from other causes (4.5%), 72 deceased from metastasis and 13 unknown but presumed dead from metastasis. The mean survival time after the diagnosis of orbital metastasis was 13.5mo; the 1y survival rate was 46%, while the 2y survival rate was 14% (Figure 2). Patients with metastatic renal cell carcinoma had the longest mean survival (24mo) with a 1y survival rate of 66% and a 2y survival rate of 33%, and patients with breast cancer had the second longest mean survival (16mo) with 1y survival rate of 44% and 2y survival rate of 22%. Patients with lung cancer had the shortest mean survival time (3mo; Figure 3). There was no significant difference in survival between patients with or without known primary tumor (P=0.5).
Figure 2. Kaplan-Meier curve of survival showing 5y cumulative survival for the present series of patients with cancer metastatic to the orbit.
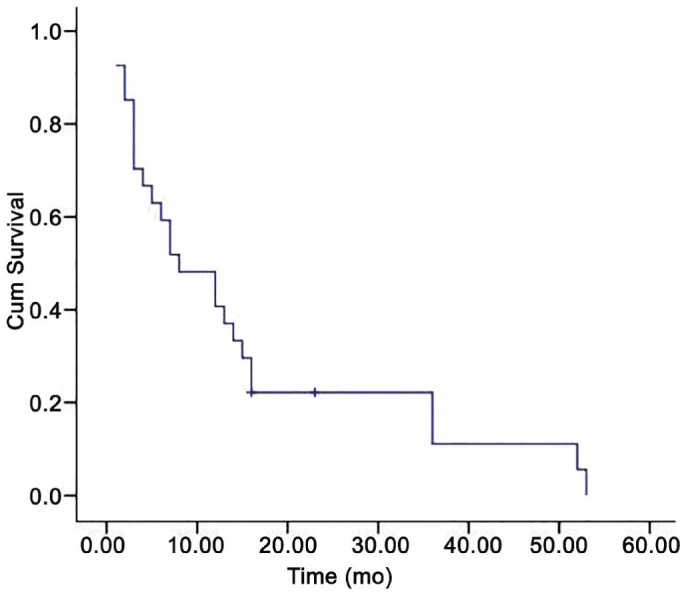
The mean survival time after the orbital diagnosis was 13.5mo.
Figure 3. Kaplan-Meier curve of survival showing 5y cumulative survival for three groups of patients with breast, lung and kidney cancer metastatic to the orbit.
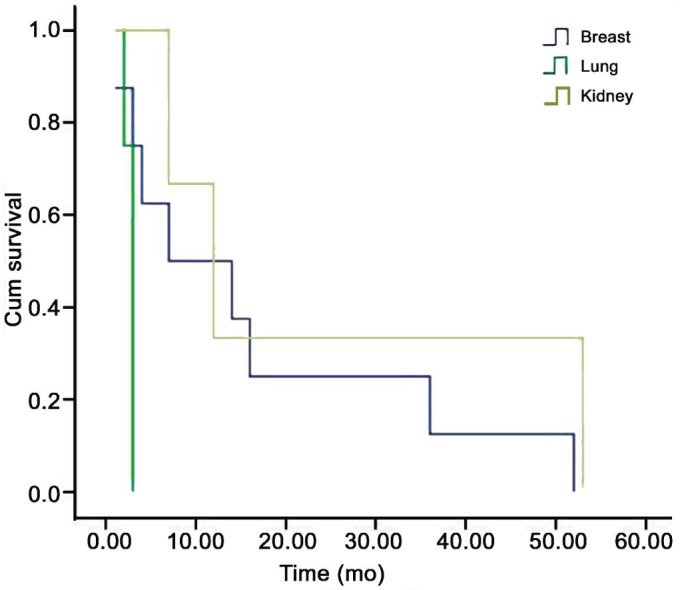
The mean survival time after the orbital diagnosis was respectively 16, 24 and 3mo.
DISCUSSION
The orbit is an unusual site for metastatic cancer. Orbital metastases ranged from 1% to 13% among reported series of all orbital tumors[1]–[4] and from 2% to 5% among patients with systemic cancer[5]. Several authors have reported their experience[3]–[4],[7]–[9],[18]. The largest series were reported by Shields et al[18] in 2000 with 100 consecutive cases referred to the Wills Eye Hospital over a 24y period and by Valenzuela et al[3] in 2009 with 80 consecutive cases treated in four tertiary orbital centres in Australia. Our series of 93 cases represent one of the largest single referral centre experience with orbital metastasis[3],[18],[19].
Breast cancer accounts for the majority of cases followed by lung cancer, as other authors have reported[3],[6],[7]. In literature prostate cancer is the third most frequent primary tumor; in our series this tumor accounts for only 1% of all orbital metastasis being the eighth for frequency. This may reflect differences in composition of background population or referral bias. Primary tumor arising from gastrointestinal tract, kidney, and skin melanoma occurs rarely. The prevalence of colorectal cancer is similar to breast and lung cancer but only one case of orbital metastasis from this site was registered; it could be due to a different metastatic pathway. There is an unusual primary site localization never described before: penis cancer (Table 1). Other unusual cases are an adrenal gland and an Ewing's sarcoma metastatic to the orbit in two children (Table 1).
Most of orbital metastases are carcinomas; sarcomas and melanomas are considerably less common. In our series, 96% were carcinomas or carcinoid tumors; this finding is similar with the already reported series[3],[18],[19].
There are not significant differences between women and men in terms of prevalence. All cases of breast cancer metastatic to the orbit occurred in women. Nine of 10 orbital metastasis from renal cell carcinoma and all prostate gland carcinoma occurred in men. Of the 8 orbital metastases from lung cancer, only one occurred in woman. Orbital metastases generally occur in adulthood and are uncommon in childhood (in 89% occurred in patients with an age of 40y or more). The adrenal neuroblastoma represents the most common orbital metastasis in children. Since in our series we report a single case of metastasis from neuroblastoma, the value of this data is probably biased by the references of children frequently treated in paediatric cancer centres.
During the study period, a decrease in number of orbital metastasis was observed although the overall incidence of cancers increased over the same period[20]. Similar findings are described by Fahmy et al[19]. Possible explanations are a decrease in the actual incidence of ophthalmic metastasis or a referral biases. Regardless of the general prognosis, the actual decrease of orbital metastasis may result by the impact of the early diagnostic methods and the improved treatment of the primary tumor[21]. Recently furthermore few patients with advanced cancer are referred to ophthalmologists and this may explain the decrease of cases with time too.
In our study 68% of patients had a known primary neoplasm with a mean time of presentation of the metastasis after the diagnosis of primary cancer of 52mo for breast cancer and of 6mo for lung cancer. Similar findings are showed in Shields et al' [18] survey. One third of cases of our series have not had a previous diagnosis of primary malignancy. This figure is similar to the one reported in Ng and Ilsen[8] series. Among our 30 patients (32%) with no history of previous cancer, the primary tumor was subsequently located in 14 cases (15%) (6 breast, 3 lung, 1 prostate gland, 1 adrenal gland, 1 liver, 1 kidney, 1 colorectal tract). When the primary sites is unknown, and breast cancer in women and lung cancer in males are excluded, attention should be paid to the gastrointestinal tract, liver, kidney and prostate gland. In 16 cases (17%) the primary tumor remained unknown despite systemic evaluation and long follow-up; this feature is similar to the one reported in other series[5],[9].
In our experience all orbital metastases occur unilaterally. In other reports a small percentage of cases of bilateral metastasis was found[4],[18]. Interestingly, clinical features of the onset in the majority of cases of the present series included rapidly progressive blurred of vision, diplopia, proptosis and pain (Table 3). Therefore it could be argued that orbital metastases very rarely tend to present as slow progressive mass. Although some authors[1]–[3] have stressed that pain is a very typical feature in orbital metastases, our percentage of patients with this symptom accounts for only 12%. Peculiar feature in present series is the enophthalmos encountered in 4 cases due to breast cancer in 2 cases, lung cancer in 1 and penis cancer in 1, respectively (Table 3). Probably, desmoplasia and fibrosis associated with these tumors cause retraction of the orbit[22]. Extraocular muscle disturbance that occurs in our series in 32% can be due to direct tumor infiltration of the muscle or to mass effect or to nerve palsies; rarely it develops as part of paraneoplastic phenomenon[23].
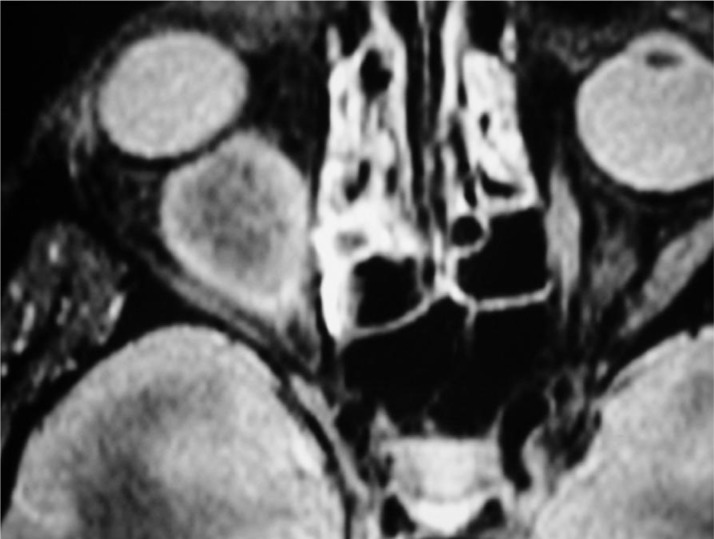
CT commonly showed a solid enhancing mass located often within the orbital fat. Bone erosion accounts for 18% in our series. Orbital metastases from breast cancer present diffuse and irregular infiltration, often growing along rectus muscles and fascial planes. Standardized B- and A-scan echography can be used to obtain a fast and an accurate differential diagnosis in case of muscle involvement or more superficial lesion. MRI may provide an accurate resolution of the mass (Figure 4).
Figure 4. T2-weighted magnetic resonance imaging scan showing an hyperintense mass involving the inferior rectus muscle in a case of orbital metastasis from an undifferentiated breast cancer.
The definitive diagnosis of orbital metastasis is made by biopsy and histopathological examination. We have reported that in 66% of cases the localization of the lesion is anterior in the orbit allowing the incisional biopsy through either transconjunctival or transcutaneous approach. If the tumor is well circumscribed, an excisional biopsy can be performed. Fine needle aspiration biopsy is an useful procedure when systemic malignancy has been previously diagnosed or when the mass is located deep within the orbit and patient is debilitated[24].
Management of the orbital metastasis depends on the primary tumor and on the staging of the systemic disease. The use of radiotherapy combined or not with chemotherapy is designed on the basis of the protocol therapy[25]. In three cases of our series the pathological examination of the orbital mass played a key role in choosing the oncological protocol treatment since the orbit was the only site of metastasis.
Potential threat to visual function and general health condition of the patient should be considered for management of orbital metastasis. In patients with progressive worsening of vision due to a mass compression, either surgical removal or debulking of lesion could be treatment option. Exenteratio orbitae has been the recommended treatment in the past (before 1980). In our case series this procedure has been used with palliative purpose in 5 cases (8%) for failure of other kind of treatments or if massive orbital infiltration was present. Observation was chosen as treatment in 11 (12%) patients because they refused further treatment.
Orbital metastases are associated with poorly prognosis. The most of reported series are small and data on prognosis are limited or absent. We were able to obtain adequate follow-up on 80 out of 93 patients. The mean survival time after the diagnosis of orbital metastasis is less than 1y, and only 14% of patients have a 2y survival period. In literature few cases with longer survival were described[4]. In our survey, patients with metastatic kidney tumor and breast cancer had the longest survival, with a mean time of 24mo and 16mo, respectively. Patients with orbital metastasis derived from lung cancer had the least favourable prognosis, with a mean survival time of 3mo. Also our 2 patients with lung carcinoid had poorly prognosis, in contrast with that reported by other authors[26]. In our study there was no difference in survival between patients with a known primary tumor and those with an unknown origin. A survival of metastatic breast cancer shorter than those reported in other series was also observed[2]. This data could be explained by the presence of breast cancers in young women (<40y) with more aggressive biological behaviour, and by the presence of familiar cases with worse course, according to their histopathological subtypes, estrogen recaptor and progesterone receptor status and positivity for human epidermal growth factor receptor 2 (HER-2 neu) receptor.
In conclusion, we have reported our experience with 93 consecutive patients with orbital metastases. Breast, lung and kidney cancers are the most frequent primary sites of orbital metastases, but other tumors can metastasize to the orbit. In the last decade a decrease in the frequency of orbital metastases has been observed. Orbital metastasis is predominantly a condition of adulthood and is usually unilateral; however it can occur in childhood too. The diagnosis can be suggested by history, ocular and systemic evaluation, orbital imaging studies and confirmed by open biopsy or FNAB. The management consists in the treatment of associated systemic malignancy with chemotherapy or hormone therapy, surgical removal or debulking eventually associated with orbital irradiation. Orbital therapies are usually effective for local palliation and disease control, but the systemic prognosis is generally poor.
Acknowledgments
We would like to thank Guido Carlomagno, MD for his help in statistical analysis.
Conflicts of Interest: Magliozzi P, None; Strianese D, None; Bonavolontà P, None; Ferrara M, None; Ruggiero P, None; Carandente R, None; Bonavolontà G, None; Tranfa F, None.
REFERENCES
- 1.Civit T, Colnat-Coulbois S, Freppel S. Orbital metastasis. Neurochirurgie. 2010;56(2–3):148–151. doi: 10.1016/j.neuchi.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part I. Ophthalmology. 2004;111(5):997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Valenzuela AA, Archibald CW, Fleming B, Ong L, O'Donnell B, Crompton JJ, Selva D, McNab AA, Sullivan TJ. Orbital metastasis: clinical features, management and outcome. Orbit. 2009;28(2–3):153–159. doi: 10.1080/01676830902897470. [DOI] [PubMed] [Google Scholar]
- 4.Chong VF. The orbits in cancer imaging. Cancer Imaging. 2006;6:S27–31. doi: 10.1102/1470-7330.2006.9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matlach J, Nowak J, Göbel W. Papilledema of unknown cause. Ophthalmologe. 2013;110(6):543–545. doi: 10.1007/s00347-012-2731-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Yang XJ, Li YY, Hei Y, Xiao LH. Diagnosis and management of orbital metastatic tumors. Zhonghua Yan Ke Za Zhi. 2008;44(8):687–690. [PubMed] [Google Scholar]
- 7.Shikishima K, Kawai K, Kitahara K. Pathological evaluation of orbital tumours in Japan: analysis of a large case series and 1379 cases reported in the Japanese literature. Clin Experiment Ophthalmol. 2006;34(3):239–244. doi: 10.1111/j.1442-9071.2006.01192.x. [DOI] [PubMed] [Google Scholar]
- 8.Ng E, Ilsen PF. Orbital metastases. Optometry. 2010;81(12):647–657. doi: 10.1016/j.optm.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Yan J, Gao S. Metastatic orbital tumors in southern China during an 18-year period. Graefes Arch Clin Exp Ophthalmol. 2011;249(9):1387–1393. doi: 10.1007/s00417-011-1660-6. [DOI] [PubMed] [Google Scholar]
- 10.Bonavolontà G, Strianese D, Grassi P, Comune C, Tranfa F, Uccello G, Iuliano A. An analysis of 2,480 space-occupying lesions of the orbit from 1976 to 2011. Ophthal Plast Reconstr Surg. 2013;29(2):79–86. doi: 10.1097/IOP.0b013e31827a7622. [DOI] [PubMed] [Google Scholar]
- 11.Comerci M, Elefante A, Strianese D, Senese R, Bonavolontà P, Alfano B, Bonavolontà B, Brunetti A. Semiautomatic regional segmentation to measure orbital fat volumes in thyroid-associated ophthalmopathy. A validation study. Neuroradiol J. 2013;26(4):373–379. doi: 10.1177/197140091302600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strianese D, Piscopo R, Elefante A, et al. Unilateral proptosis in thyroid eye disease with subsequent contralateral involvement: retrospective follow-up study. BMC Ophthalmol. 2013;13:21. doi: 10.1186/1471-2415-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iuliano A, Strianese D, Uccello G, Diplomatico A, Tebaldi S, Bonavolontà G. Risk factors for orbital exenteration in periocular Basal cell carcinoma. Am J Ophthalmol. 2012;153(2):238–241. doi: 10.1016/j.ajo.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Strianese D, Baldi G, Staibano S, Baldi A, De Rosa G, Tranfa F, Bonavolontà G. Expression of apoptosis-related markers in malignant epithelial tumours of the lacrimal gland and their relation to clinical outcome. Br J Ophthalmol. 2007;91(9):1239–1243. doi: 10.1136/bjo.2007.118661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weis E, Rootman J, Joly TJ, et al. Epithelial lacrimal gland tumors: pathologic classification and current understanding. Arch Ophthalmol. 2009;127(8):1016–1028. doi: 10.1001/archophthalmol.2009.209. [DOI] [PubMed] [Google Scholar]
- 16.Mariniello G, Maiuri F, Strianese D, Donzelli R, Iuliano A, Tranfa F, de Divitiis E, Bonavolontà G. Spheno-orbital meningiomas: surgical approaches and outcome according to the intraorbital tumor extent. Zentralbl Neurochir. 2008;69(4):175–181. doi: 10.1055/s-2008-1077077. [DOI] [PubMed] [Google Scholar]
- 17.Strianese D, Tranfa F, Finelli M, De Renzo A, Staibano S, Schiemer R, Cardone D, Pacelli R, Perna F, Mascolo M, De Rosa G, Bonavolontà G. Hepatitis C virus infection in ocular adnexal lymphomas. Arch Ophthalmol. 2010;128(10):1295–1299. doi: 10.1001/archophthalmol.2010.233. [DOI] [PubMed] [Google Scholar]
- 18.Shields JA, Shields CL, Brotman HK, Carvalho C, Perez N, Eagle RC., Jr Cancer metastatic to the orbit. The 200 Robert M. Curts Lecture. Ophthalmic Plast Reconstr Surg. 2001;17(5):346–354. doi: 10.1097/00002341-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Fahmy P, Heegaard S, Jensen OA, Prause JU. Metastasis in the ophthalmic region in Denmark 1969-98. A histopathological study. Acta Ophthalmol Scand. 2003;81(1):47–50. doi: 10.1034/j.1600-0420.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 20.AIRT Working Group Italian cancer figures-report 2006: I. Incidence, mortality and estimates. Epidemiol Prev. 2006;30(1 Suppl 2) [PubMed] [Google Scholar]
- 21.Tessari A, Palmieri D, Di Cosimo S. Overview of diagnostic/targeted treatment combinations in personalized medicine for breast cancer patients. Pharmgenomics Pers Med. 2013;7:1–19. doi: 10.2147/PGPM.S53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shields CL, Stopyra GA, Marr BP, Moster ML, Shields JA. Enophthalmos as initial manifestation of occult, mammogram-negative carcinoma of the breast. Ophthalmic Surg Lasers Imaging. 2004;35(1):56–57. [PubMed] [Google Scholar]
- 23.Iwanami M, Odaka M, Nakamura T, Hirata K. Paraneoplastic cerebellar degeneration and Lambert-Eaton myasthenic syndrome associated with anti P/Q-type voltage-gated calcium channel antibody in a patient with primary double lung cancer. Brain Nerve. 2009;61(9):1083–1087. [PubMed] [Google Scholar]
- 24.Bagchi K, Giri A, Sarkar R, Das S, Nag S. Fine needle aspiration biopsy in the diagnosis of intraocular and extra-ocular mass lesions. J Indian Med Assoc. 2010;108(7):457–459. [PubMed] [Google Scholar]
- 25.Marwaha G, Macklis R, Singh AD. Radiation therapy: orbital tumors. Dev Ophthalmol. 2013;52:94–101. doi: 10.1159/000351084. [DOI] [PubMed] [Google Scholar]
- 26.Mehta JS, Abou-Rayyah Y, Rose GE. Orbital carcinoid metastases. Ophthalmology. 2006;113(3):466–472. doi: 10.1016/j.ophtha.2005.10.051. [DOI] [PubMed] [Google Scholar]