Abstract
AIM
To compare the efficacy of intravitreal ranibizumab (IVR) alone or in combination with photodynamic therapy (PDT) vs PDT in patients with symptomatic polypoidal choroidal vasculopathy (PCV).
METHODS
A systematic search of a wide range of databases (including PubMed, EMBASE, Cochrane Library and Web of Science) was searched to identify relevant studies. Both randomized controlled trials (RCTs) and non-RCT studies were included. Methodological quality of included literatures was evaluated according to the Newcastle-Ottawa Scale. RevMan 5.2.7 software was used to do the Meta-analysis.
RESULTS
Three RCTs and 6 retrospective studies were included. The results showed that PDT monotherapy had a significantly higher proportion in patients who achieved complete regression of polyps than IVR monotherapy at months 3, 6, and 12 (All P≤0.01), respectively. However, IVR had a tendency to be more effective in improving vision on the basis of RCTs. The proportion of patients who gained complete regression of polyps revealed that there was no significant difference between the combination treatment and PDT monotherapy. The mean change of best-corrected visual acuity (BCVA) from baseline showed that the combination treatment had significant superiority in improving vision vs PDT monotherapy at months 3, 6 and 24 (All P<0.05), respectively. In the mean time, this comparison result was also significant at month 12 (P<0.01) after removal of a heterogeneous study.
CONCLUSION
IVR has non-inferiority compare with PDT either in stabilizing or in improving vision, although it can hardly promote the regression of polyps. The combination treatment of PDT and IVR can exert a synergistic effect on regressing polyps and on maintaining or improving visual acuity. Thus, it can be the first-line therapy for PCV.
Keywords: photodynamic therapy, ranibizumab, combination treatment, monotherapy, polypoidal choroidal vasculopathy, Meta-analysis, systematic review
INTRODUCTION
Polypoidal choroidal vasculopathy (PCV) is characterized by the polyp-like aneurysmal dilations of choroidal vascular networks and the presence of typical hyper-fluorescent nodules in the early phase of indocyanine green angiography (ICGA)[1]. Subretinal hemorrhage or fluid accumulation, persistent leakage, serous pigment epithelial detachment or neurosensory detachment, retinal pigment epithelium (RPE) atrophy and subretinal fibrosis are the clinical manifestations of PCV, and can cause serious and permanent vision loss[2]. The prevalence of PCV can reach up to 54% in Asian patients with undiagnosed exudative age-related macular degeneration (AMD) while it is relatively lower in white patients. However, with the development of diagnostic techniques, PCV has been more frequently diagnosed in all patient populations[3].
Among currently available therapy methods for PCV, verteporfin photodynamic therapy (PDT) has always been considered the most promising modality with complete regression of polyps in a high proportion of patients and stable or improvable visual acuity (VA)[4]–[6]. Verteporfin binds to low-density lipoprotein receptors and preferentially bound by choroidal neovascular tissue. Irradiation of the neovascular lesion by the laser creates toxic reactive oxygen species that induces thrombosis and the closure of choroidal neovascularisation (CNV)[7]. After PDT, the polyps can be occluded, and the exudative lesions are resolved [8]. Nevertheless, PDT cannot complete occlude the branching vascular network (BVN). The ulterior recurrence of PCV is inevitable as new polyps form from the residual BVN[9]. In some cases, a persistent BVN may result in leakage, and PDT can also induce the evolution of BVN into CNV[9],[10]. Several long-term studies have revealed that after PDT monotherapy, a high proportion of patients developed recurrence of PCV after 12mo[5],[11],[12]. Meanwhile, studies with more than two years follow-up have showed that the effect of vision improvement of PDT declines after the initial year[5],[11],[13]. Furthermore, increased levels of pro-angiogenesis growth factors like vascular endothelial growth factor (VEGF) have also been observed in patients after PDT and these may potentially increase the risk of recurrence.
The elevated expression of VEGF in aqueous humor[14] and vascular endothelium[15] of eyes with PCV provides a biological fundamental for the treatment with anti-VEGF agent like ranibizumab, an antigen-binding fragment (Fab) of a recombinant and humanized monoclonal antibody that targets VEGF-A and has been approved by the U.S. Food and Drug Administration (FDA) for the special application to ocular neovascular diseases[7],[16]. Recent reports have showed that the monotherapy using ranibizumab led to reduction of leakage, resolution of subretinal hemorrhage, and improvement of vision in PCV. However, it might be ineffective for complete regression of polyps [17]–[19]. To achieve complete regression of the polypoidal lesions and to maximize the VA outcome, combination treatment using PDT and ranibizumab may be helpful and provide a synergistic effect of angio-occlusion and antiangiogenesis.
In order to explore the best therapeutic schedule for PCV, we performed this Meta-analysis to compare the efficacy of PDT monotherapy vs intravitreal ranibizumab (IVR) monotherapy and PDT monotherapy vs combination treatment of PDT and IVR.
SUBJECTS AND METHODS
Search Strategy
Four databases (PubMed, EMBASE, Cochrane Library and Web of Science) were last searched on June 5, 2014. Three domains of terms were searched: 1) polypoidal choroidal vasculopathy or equivalents (e.g. PCV, choroidal vasculopathy); 2) ranibizumab or lucentis; 3) photodynamic therapy or equivalents (e.g. PDT, photodynamic treatment, photochemotherapy). The results from each domain were then combined with AND. Meanwhile, reference lists of relevant articles were also searched. There was no restriction on language or study design. Retrieved articles were imported into EndNote X6 (Thomson Reuters, New York, NY, USA) where duplicate articles were manually removed.
Inclusion and Exclusion Criteria
Published studies, regardless of study design or sample size, were included if they 1) included treatment-naive patients with PCV; 2) compared PDT vs IVR or PDT vs combination treatment of PDT and IVR; 3) used standard-fluence verteporfin (6 mg/m2) PDT (50 J/cm2) and standard-fluence IVR (0.5 mg/0.05 mL); 4) reported one or more of the following outcomes: proportion of patients in achieving complete regression of polyps, best-corrected visual acuity (BCVA), proportion of patients gain/loss ≥3 lines of BCVA, central retinal thickness (CRT), number of treatments, and ocular or systemic adverse events. Exclusion criteria were: 1) patients received previous treatment for PCV; 2) patients changed the initial therapy strategy casually. In addition, conference abstracts that had not been published were also excluded. If two or more studies were based on the same population, they would be combined as a single. The titles and abstracts were independently scanned by two reviewers for the inclusion qualification and full texts were read as necessary. Inconsistencies were resolved by discussion and consensus.
Data Extraction and Quality Assessment
For each qualified study, the following data were extracted: 1) basic data: name of first author, the year of publication and location of the study, the design and phase of the study, major inclusion and exclusion criteria, various intervention groups, number of subjects, age and gender of patients, and duration of follow-up; 2) outcomes: the primary outcomes were the number of patients in achieving complete regression of polyps and the mean change of BCVA from baseline. The secondary outcomes were number of patients with VA gain/loss ≥3 lines, mean change of CRT from baseline, recurrent number of polyps, number of treatments, and ocular or systemic adverse events.
The methodological quality of included articles was evaluated using the Newcastle-Ottawa Scale (NOS) [20]. This scale consists of three broad perspectives with a score range of 0-9: the selection of the study groups, the comparability of the groups, and the ascertainment of the exposure or outcome of interest. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21].
Data Processing and Statistical Analysis
Two authors (Tang K and Si JK) independently extracted information by reading full texts and tables and calculating raw data. We contacted authors to request missing data and captured the rest in figures using GetData Graph Digitizer 2.26 (supplied by S. Fedorov, available at http://getdata-graph-digitizer.com/index.php). Early Treatment Diabetic Retinopathy Study (ETDRS) VA letter scores of EVEREST [22] were converted into the logarithm of minimal angle of resolution (logMAR) scale with mathematical method according to the retreatment guidelines for ranibizumab in Japan[23],[24] to analysis the mean change of BCVA. Mean changes from baseline were computed by posttreatment mean minus pretreatment mean, and the unavailable standard deviation (SD) values were estimated according to Cochrane Handbook 5.1.0 (chapter 16.1, available at http://handbook.cochrane.org/). The final data round to two decimal places.
Statistical Analysis
Statistical analysis was performed with Review Manager 5.2.7 software supplied by Cochrane Collaboration. In the Meta-analysis, the effect sizes of each study were presented as mean difference with 95% confidence intervals (CI) for continuous data, and as risk ratio with 95%CI for dichotomous data. Weighted mean difference (WMD) and pooled risk ratio (RR) were calculated by fixed-effect model or random-effect model depending on the significance of heterogeneity. The clinical heterogeneity was evaluated depending on the baseline characteristics and the statistical heterogeneity was assessed by I-square (I2) statistic. The statistical heterogeneity was considered insignificant when I2<50%. Furthermore, the sensitivity analysis was performed by omitting one study and reconducting Meta-analysis with remaining studies. The pooled effect sizes were considered significant when the 95%CI of WMD did not cross zero or when the 95%CI of pooled risk ratio did not cross 1.0.
RESULTS
Selection and Categorization of Studies
A total of 144 relevant literatures were initially identified through searching databases and 63 literatures were acquired after eliminating duplicates. Forty-four studies included reviews, case reports, letters and non comparative studies were eliminated after reviewing titles and abstracts. After review of full-text, 9 studies were finally selected, including 3 randomized controlled trials (RCTs)[22],[25],[26] and 6 retrospective studies[27]–[32].
Characteristics of Included Studies
The basic characteristics of the included studies were showed in Tables 1, 2. These studies were published between 2011 and 2013. Eight of them were performed in Asia (one in Singapore, two in Korea and five in Japan) and the other one was in Greece. The sample size of the trials ranged from 20 to 93. All patients were elderly ≥50y. Every study reported balanced baseline characteristics between comparison groups. In the study by Kang and Koh [28], bevacizumab was used for the combination treatment group, so the information of this group was excluded. Thus, five studies [22],[26]–[28],[30] were involved in the comparison of PDT and IVR monotherapy, and six studies [22],[25],[29]–[32] performed the comparison of combination treatment and PDT monotherapy. Almost all of the studies used regression of polyps and BCVA indicators to evaluate the efficacy of treatment on PCV. However, clinical heterogeneity could be found in several areas such as treatment protocols or the duration of follow-up. In two RCTs [22],[25], patients in combination groups were administered ranibizumab from 1 to 24h after PDT. Whereas in other four studies[29]–[32], the treatment order varied. Patients with 3-month follow-up were received single treatment of PDT or IVR in the study by Lee et al[25]. None of these studies had a follow-up period longer than 24mo.
Table 1. Basic characteristics of the included nine studies.
| Study | Country of publication | Major inclusion criteria | M/F | Mean age (a) | No. of eyes | Intervention groups | Follow-up | NOSscore |
| Koh et al 2012 [22] | Singapore | 1) Treatment-naive patients with symptomatic PCV; 2) BCVA between 73 and 24 letters; 3) GLD<5400 µm; 4) without other fundus disease or surgery |
G1: 11/8 G2: 15/6 G3: 15/6 |
G1: 63.8±8.30 G2: 62.2±9.77 G3: 69.3±8.27 |
G1: n=19 G2: n=21 G3: n=21 |
G1: PDT (50 J/cm2, PRN)+IVR(0.5 mg, 3+PRN) G2: PDT (50 J/cm2, PRN)+sham injection G3: IVR (0.5 mg, 3+PRN)+sham PDT |
6mo | 9 |
| Lee et al 2013 [25] | Korea | 1) Treatment-naive patients with symptomatic PCV; 2) without other fundus disease or surgery; 3) no use of immunosuppressive drugs |
G1: 11/1 G2: 5/3 |
G1: 63.68±8.78 G2: 66.33±7.85 |
G1: n=12 G2: n=8 |
G: PDT (50 J/cm2)+IVR (0.5 mg) G2: PDT (50 J/cm2) |
3mo | 8 |
| Oishi et al2013 [26] | Japan | 1) Treatment-naive patients with PCV; 2) VA≤0.6 logMAR unit; 3) GLD<5400 µm; 4) Without other fundus disease or surgery |
G1: 32/15 G2: 28/18 |
G1: 75.0±8.0 G2: 75.4±6.9 |
G1: n=47 G2: n=46 |
G1: PDT (50 J/cm2, PRN) G2: IVR (0.5 mg, 3+PRN) |
24mo | 9 |
| Inoue et al 2013 [27] | Japan | 1) Treatment-naive patients with PCV; 2) BCVA≥20/400; 3) without eye diseases that couldinfluence VA |
G1: 9/14 G2: 30/14 |
G1: 73.2±7.5 G2: 71.0±7.8 |
G1: n=33 G2: n=44 |
G1: IVR (0.5 mg, 3+PRN)G2: PDT (50 J/cm2, PRN) | 24mo | 6 |
| Kang and Koh 2014 [28] | Korea | 1) Treatment-naive patients with symptomatic PCV;2) presence of BVN and polypoidallesions on ICGA;3) without other fundus disease | NR | G1: 66.21±9.0G2: 68.05±8.12 | G1: n=19 G2: n=23 | G1: PDT (50 J/cm2, PRN)G2: IVR (0.5 mg, 3+PRN) | 24mo | 5 |
| Maruko et al 2011 [29] | Japan | Treatment-naive patients with symptomatic PCV | G1: 12/4 G2: 6/5 | G1: 71.8 G2: 71.0 | G1: n=16 G2: n=11 | G1: PDT (50 J/cm2, PRN) G2: PDT (50 J/cm2, PRN)+IVR(0.5 mg, 3+PRN) |
6mo | 6 |
| Rouvas et al 2011 [30] | Greece | 1) Treatment-naive patients with PCV; 2) VA≤20/30; 3) identification of polyps and interconnecting vessels on the ICGA; 4) presence of subretinal hemorrhages or exudation |
G1: 5/6 G2: 4/6 G3: 4/5 |
G1: 62.9 G2: 66.5 G3: 64.67 |
G1: n=11 G2: n=10 G3: n=9 |
Group1: PDT (50 J/cm2, PRN) Group2: IVR (0.5 mg, 3+PRN) Group3: PDT (50 J/cm2, PRN)+IVR(0.5 mg, 3+PRN) |
12mo | 5 |
| Saito et al2013 [31] | Japan | 1) Treatment-naive patients; 2)VA≤20/40; 3) without other fundus disease or surgery |
G1: 17/8 G2: 27/5 |
G1: 74.0±8.6 G2: 75.0±6.5 |
G1: n=25 G2: n=32 |
G1: PDT (50 J/cm2, PRN)+IVR(0.5 mg, 3+PRN) G2: PDT (50 J/cm2, PRN) |
24mo | 6 |
| Sakurada and Iijima 2013 [32] | Japan | 1) Treatment-naive patients with consecutive PCV; 2) BCVA≤1.0 logMAR unit |
G1: 25/9 G2: 16/8 |
G1: 70.1±7.1 G2: 73.2±7.4 |
G1: n=34 G2: n=24 |
G1: PDT (50 J/cm2, PRN) G2: PDT (50 J/cm2, PRN)+IVR(0.5 mg, 3+PRN) |
24mo | 6 |
G1: Group 1; G2: Group 2; G3: Group 3; RCT: Randomized controlled trial; RS: Retrospective study; NR: Not reported; PCV: Polypoidal choroidal vasculopathy; BVN: Branching vascular network; BCVA: Best-corrected visual acuity; VA: Visual acuity; PRN: Pro re nata; 3+PRN: 3 monthly injections followed by pro re nata treatment; CRT: Central retinal thickness; GLD: Greatest linear dimension; PDT: Photodynamic therapy; IVR: Intravitreal ranibizumab; logMAR: Logarithm of minimal angle of resolution.
Table 2. Basic BCVA, GLD and CRT characteristics of the included nine studies.
| Study | Combination group |
PDT group |
IVR group |
||||||
| Initial logMAR BCVA | Initial GLD (µm) | Initial CRT (µm) | Initial logMAR BCVA | Initial GLD (µm) | Initial CRT (µm) | Initial logMAR BCVA | Initial GLD (µm) | Initial CRT (µm) | |
| Koh et al 2012[22] | 0.57±0.42 | <5400 | 334.7±118.9 | 0.56±0.26 | <5400 | 285.3±105.6 | 0.72±0.32 | <5400 | 268.5±97.8 |
| Lee et al 2013 [25] | 0.46±0.30 | NR | 391.13±130.25 | 0.53±0.35 | NR | 481.33±128.46 | |||
| Oishi et al 2013 [26] | 0.57±0.31 | 3051.1±1177.7 | 366.8±113.6 | 0.48±0.27 | 3347.4±1288.3 | 418.9±168.6 | |||
| Inoue et al 2013 [27] | 0.52±0.28 | 3640±2120 | NR | 0.48±0.38 | 4171±2631 | NR | |||
| Kang and Koh 2014 [28] | 0.68±0.36 | 2810.87±974.1 | 408.01±116.51 | 0.67±0.43 | 2790.05±871.5 | 404.18±118.12 | |||
| Maruko et al 2011 [29] | 0.55±0.49 | 2905±1122 | 455±198 | 0.53±0.34 | 3013±1059 | 364±114 | |||
| Rouvas et al 2011 [30] | 0.81±0.30 | NR | 289 | 0.53±0.33 | NR | 304.36 | 0.79±0.32 | NR | 310.9 |
| Saito et al 2013 [31] | 0.52 | 4074±1459 | 385±154 | 0.58 | 4867±1855 | NR | |||
| Sakurada and Iijima 2013 [32] | 0.51±0.22 | 2039±847 | NR | 0.55±0.26 | 2364±716 | NR | |||
PDT: photodynamic therapy; IVR: Intravitreal ranibizumab; logMAR: Logarithm of minimal angle of resolution; BCVA: Best-corrected visual acuity; CRT: Central retinal thickness; GLD: Greatest linear dimension of polypoidal lesion; SD: Standard deviation; RCT: Randomized controlled trial; RS: Retrospective study; NR: Not reported.
x±s
Comparison of Photodynamic Therapy and Intravitreal Ranibizumab
The number of patients achieved complete regression of polyps from five studies [22],[26]–[28],[30] was extracted. If there were RCTs and retrospective studies, the subgroup analysis would be carried out. The proportion of patients who gained complete regression of polyps in PDT group was significantly higher than that in IVR group, with a pooled RR (95% CI) of 2.09 (1.25-3.51), 2.50 (1.21-5.18), 4.22 (1.41-12.62) at months 3, 6 and 12, respectively. However, this result showed no significant difference at month 24 between the two groups. According to two studies [27],[28] that reported the polys recurrence, eyes treated with PDT achieved significantly lower recurrence than that treated with IVR in 24mo [pooled RR 0.64 (0.46-0.88), P=0.007].
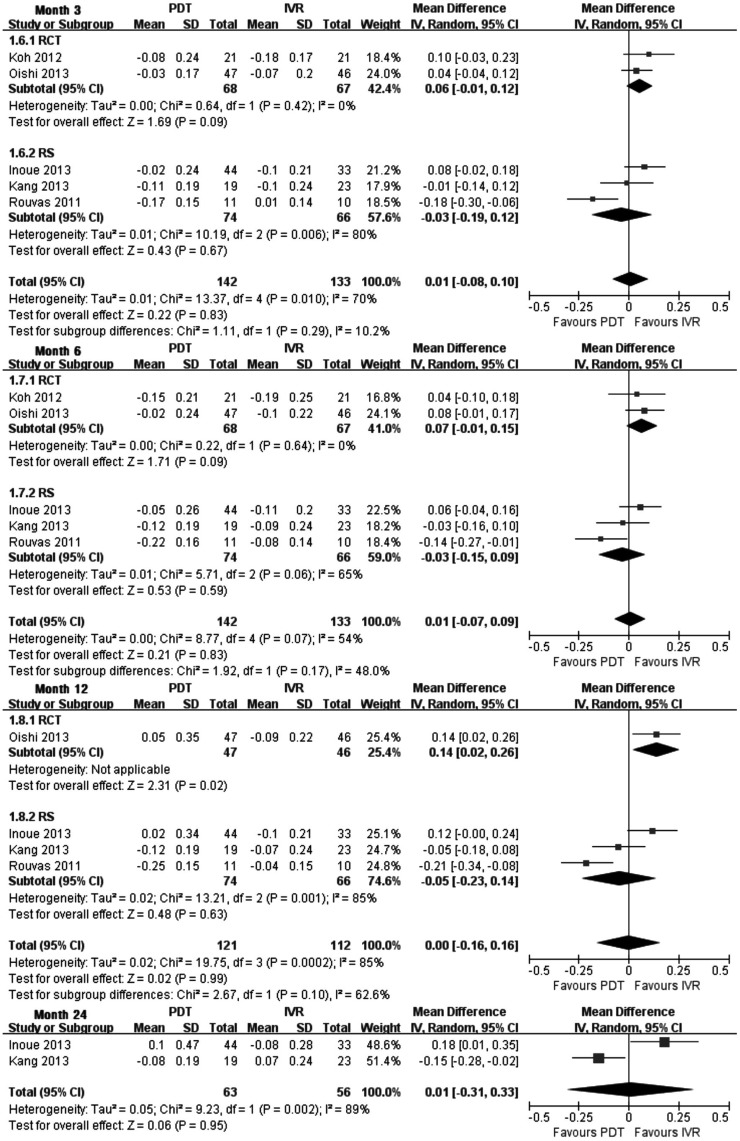
Mean change of BCVA was used to reduce the clinical heterogeneity came from baseline VA. The pooled results revealed that there was no significant difference about the change of BCVA between PDT group and IVR group at months 3, 6, 12 and 24 (Figure 1). In the meantime, according to two high-quality RCTs [22],[26], there was also no significant difference at months 3 and 6, yet significant effect of improving VA in IVR group could be found at month 12 by Oishi et al [26]. Compared with the baseline of vision, eyes gained three lines or more were considered increased VA, while lost three lines or more was decreased VA, and stable VA defined as a gain or loss of less than 3 lines. The pooled RR of increased and stable VA (loss less than 3 lines) showed there was insignificant difference between the two groups at month 6 [1.00 (0.91-1.09), P=1.0], month 12 [0.92 (0.74-1.14), P=0.46] and month 24 [0.91 (0.58-1.42), P=0.67]. The analysis of CRT could not be assessed due to the inadequate data in PDT and IVR groups.
Figure 1. Mean change of BCVA from baseline at months 3, 6, 12 and 24 PDT vs IVR.
Each square represents a study and the size is proportional to the precision of the mean treatment effect in that study. The horizontal line represents 95%CI of each study for the treatment effect. The center of the diamond is the average treatment effect across studies, and the width of diamond denotes its 95%CI. RCT: Randomized controlled trial; RS: Retrospective study.
Comparison of Combination Treatment and Photodynamic Therapy
The efficacy of PDT combined with IVR vs PDT was showed in six studies, included two RCTs [22],[25] and four retrospective studies[29]–[32]. The proportion of patients who gained complete regression of polyps revealed no significant difference among groups receiving combination treatment vs PDT monotherapy at months 3, 6, 12 and 24, respectively. And the recurrence of polys in 12mo [pooled RR 1.22 (0.09-16.92), P=0.88] and in 24mo [pooled RR 0.78 (0.50-1.22), P=0.28] also showed insignificant difference between the two therapies. Sensitivity analysis showed that no studies substantially influenced the pooled effect sizes.
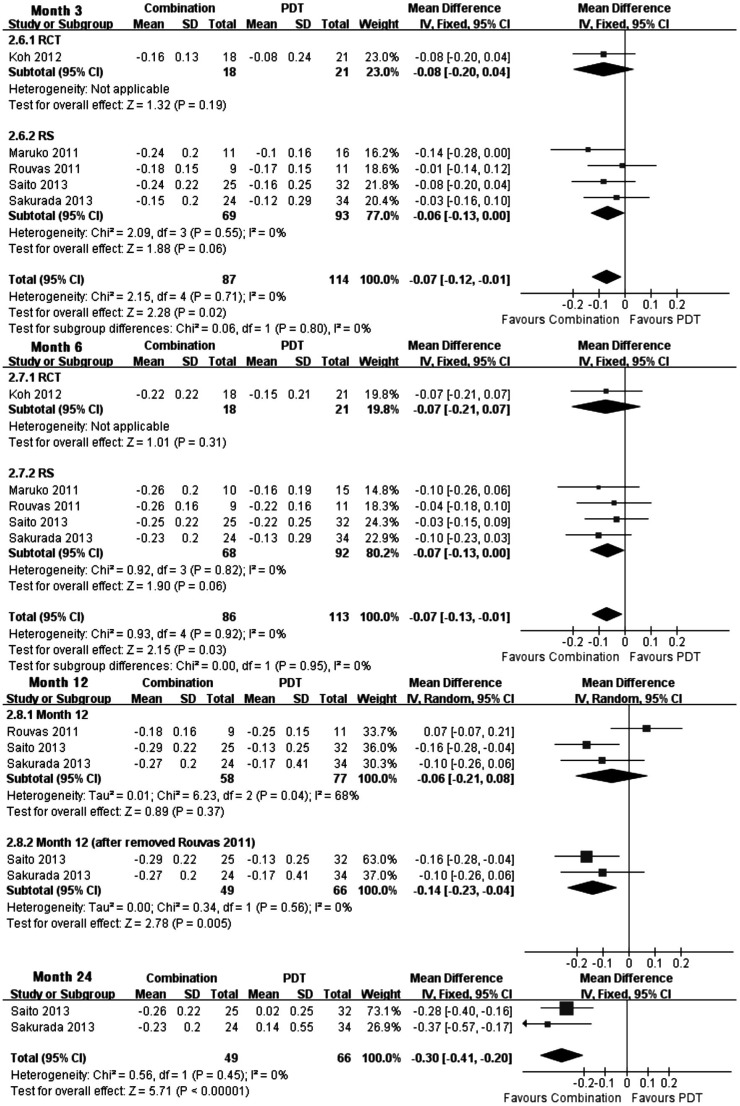
Five studies were in agreement with each other, showing significantly more effect in improving BCVA from baseline in combination group at month 3 (WMD, -0.07; 95%CI, -0.12 to -0.01; P=0.02), month 6 (WMD, -0.07; 95%CI, -0.13 to -0.01; P=0.03) and month 24 (WMD, -0.30; 95%CI, -0.41 to -0.20; P<0.00001) (Figure 2). At month 12, the pooled results of the mean BCVA change revealed insignificant difference. Nevertheless, the clinical heterogeneity could be found in the Rouvas' study, whose baseline VA of logMAR scale was 0.81±0.30 (mean±SD) in the combination group, which is much worse than in other groups. In order to exclude the heterogeneity among studies, The Rouvas' study was removed to apply sensitivity analysis and the result indicated that the mean change of BCVA in combination group had significant advantage than in PDT group [WMD, -0.14; 95%CI, -0.23 to -0.04; P=0.005]. Thus, we concluded that the clinical heterogeneity altered the statistical result. In addition, the pooled RR of increased, stable or decreased VA at months 6 and 12 were respectively analyzed, and no significant difference was found between the two therapies. However, according to the analysis result of increased and stable VA, there was significant more eyes avoid the loss of vision with combination treatment according to two retrospective studies[31], [32] at month 24 [pooled RR 1.36 (1.17 to 1.58), P<0.0001].
Figure 2. Mean change of BCVA from baseline at months 3, 6, 12 and 24.
Combination treatment vs PDT.
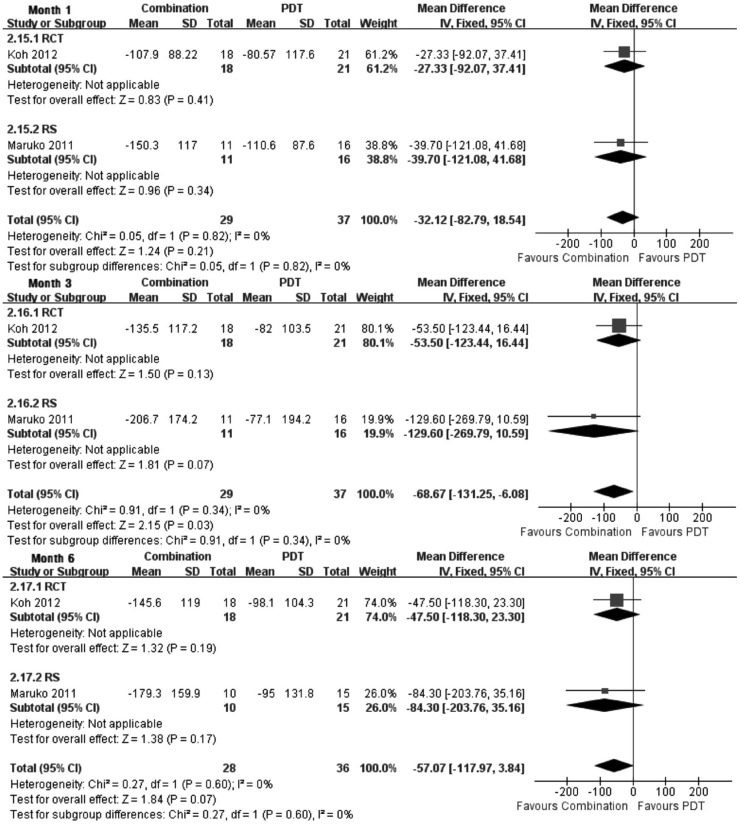
CRT is another indicator that represents the anatomic change after treatment. The superiority of combination treatment over PDT monotherapy in reducing CRT had also been observed at month 3 (Figure 3) according to an RCT[22] and a retrospective study[29] (WMD, -68.67; 95%CI, -131.25 to -6.08; P=0.03). Although no significant CRT reduction was detected at months 1 and 6, combination treatment tended to have greater reduction of CRT for PCV.
Figure 3. Mean change of CRT from baseline at months 1, 3 and 6.
Combination treatment vs PDT.
Adverse Events and Number of Treatment
Only one RCT by Koh et al [22] reported the occurrence of adverse events in detail. Although five retrospective studies mentioned adverse events, the accuracy of records can not be guaranteed. Ocular and systemic adverse events of PDT, IVR and combination treatment for PCV reported in all identified studies were summarized in Table 3 without statistical analysis.
Table 3. Main ocular adverse events and systemic adverse events.
| Adverse events | Combination group |
PDT group |
IVR group |
|||
| RCT (N=19) |
RS (N=58) |
RCT (N=21) |
RS (N=140) |
RCT (N=21) |
RS (N=66) |
|
| 6mo | 24mo | 6mo | 24mo | 6mo | 24mo | |
| Ocular AEs | ||||||
| Retinal hemorrhage a | 2 (10.5) | 0 | 3 (14.3) | 22 (15.8) | 0 | 2 (3.0) |
| RPE detachment or subretinal fluid | 0 | 1 (1.7) | 0 | 0 | 0 | 9 (13.6) |
| RPE atrophy | 0 | 0 | 2 (1.4) | 0 | 2 (3.0) | |
| Macular scar | 1 (5.3) | 0 | 1 (0.7) | 1 (4.8) | 2 (3.0) | |
| Macular edema | 1 (5.3) | 0 | 0 | |||
| Epiretinal membrane | 0 | 0 | 0 | 0 | 0 | 2 (3.0) |
| Vitreous hemorrhage | 0 | 1 (4.8) | 0 | |||
| Intraocular pressure increased | 1 (5.3) | 0 | 1 (4.8) | 0 | 1 (4.8) | 0 |
| Conjunctival hemorrhage | 0 | 0 | 1 (4.8) | |||
| Conjunctival hyperemia | 1 (5.3) | 0 | 0 | |||
| Dry eye | 1 (5.3) | 1 (4.8) | 0 | |||
| VA reduced or impairment | 1 (5.3) | 0 | 0 | 1 (4.8) | ||
| Asthenopia | 0 | 1 (4.8) | 0 | |||
| Non-ocular AEs | ||||||
| Angina pectoris | 0 | 0 | 1 (4.8) | |||
| Noncardiac chest pain | 0 | 1 (4.8) | 0 | |||
| Blood pressure increased | 1 (5.3) | 1 (4.8) | 1 (4.8) | |||
| Gastric cancer | 0 | 1 (4.8) | 0 | |||
| Vomiting | 1 (5.3) | 0 | 1 (4.8) | |||
| Abdominal pain | 0 | 0 | 1 (4.8) | |||
| Nasopharyngitis | 1 (5.3) | 4 (19.0) | 0 | |||
| Dyspnoea | 2 (10.5) | 0 | 0 | |||
| Dizziness | 0 | 0 | 1 (4.8) | |||
aInclude intraretinal, subretinal, and subretinal pigment epithelium hemorrhage; N: No. of total patients; n: No. of eyes with adverse events; %: Incidence of adverse events; AEs: Adverse events; RPE: Retinal pigment epithelium; VA: Visual acuity.
n (%)
The mean number of treatment showed a reduction of IVR treatment numbers in combination group (mean 3.90, 4.30 and 4.50 injections) compared with the IVR monotherapy group (mean 5.20, 5.72 and 8.61 injections) in 6, 12 and 24mo, respectively. According to three studies[22],[30],[31], the mean PDT treatment numbers in combination group (mean 1.40, 1.34 and 1.40 injections) is also fewer than that in PDT monotherapy group (mean 1.70, 1.91 and 2.60 injections) in 6, 12 and 24mo, respectively.
DISCUSSION
It is still unclear whether PCV is a subtype of neovascular AMD or a unique choroidal vascular abnormality. On the one hand, the results of several histopathology and optical coherence tomography (OCT) studies on PCV were found to be inconsistent in terms of pathogenic mechanisms [25]. On the other hand, according to the determination of the concentration of VEGF, VEGF levels in PCV eyes were higher than in normal eyes but lower in comparison to eyes with neovascular AMD[14],[15],[33]. Thus, VEGF might have a minor contribution to the pathogenesis of PCV compared to neovascular AMD. Furthermore, compared with CNV in AMD, the choroidal vascular changes of PCV are more mature and structured, and this possibly resulted in the limited therapeutic response to anti-VEGF agents [34].
The refractory to ranibizumab of eyes documented to have PCV on ICGA was confirmed in the significant disadvantage of promoting the regression and suppressing recurrence of polyps. A two years prospective study showed that an as need reinjection schedule after initial continuous three monthly IVR could not prevent polypoidal lesions or BVNs from recurrence[35]. The anatomic outcome of PCV is also important when considering long-term results of therapy. In studies with PDT[5] or IVR[27],[28],[35] monotherapy, the effects of both two therapies were reported to decline in the second year, which were consistent with our analysis. Despite the inevitable recurrence of polyps, PDT could remain effective for 5y, and represents a good therapeutic approach to PCV [36]. What's more, the comparison in polyps regression or recurrence between PDT and combination treatment showed that there was no difference in the follow-up of 24mo. Thus, PDT alone or in combination with ranibizumab was superior than ranibizumab monotherapy in achieving complete regression of polyps in treatment-naive patients with symptomatic PCV.
Based on the maintenance of vision improvement for at least 12mo, Mori et al[37] considered IVR for PCV was useful for eyes with logMAR BCVA of 0.22 to 0. The baseline VA of studies that we included were all worse than 0.22. And the comparison of mean BCVA change had inconsistency between PDT and IVR, three studies included two RCTs favored IVR while the other two favored PDT. The pooled results showed that there was no significant difference for the mean change of BCVA between PDT and IVR at months 3, 6, 12 and 24 (Figure 1). We also noticed that IVR had a tendency to be more effective in improving VA than PDT on the basis of RCTs [22],[26], especially at month 12. Saito et al[38],[39] reported that IVR monotherapy could maintain or improve VA and the retinal thickness in 24mo in eyes with PCV with recurrent or residual exudation from BVN after previous PDT monotherapy. Moreover, in the PDT group of LAPTOP study[26], patients who drop-out or switched treatment strategy due to poor efficacy, deterioration or their will showed more loss of vision than in IVR monotherapy group. So, we speculate that compare with PDT, IVR has non-inferiority in terms of stabilizing and improving vision.
Most retrospective clinical studies reported that combined PDT with IVR for PCV could maintain or improve VA and reduce the exudation [40],[41]. In long-term (two patients lasted for 36 and 58mo) results of IVR combined with PDT for the treatment of PCV, Fernández et al[4] showed combination therapy is an effective treatment for symptomatic PCV. Nevertheless, several studies with 2-year follow-up suggested that the benefit of combined therapy seems to decrease with time. Three studies[28],[42],[43] reported similar results that VA significantly improved during the first year of treatment, yet the benefit diminished in the second year with insignificant difference compared with baseline. From our results, the statistical result showed that combined therapy is superior to PDT monotherapy at months 3, 6 and 24 after initial injection according to five corresponding studies (Figure 2). The lack of significance at month 12 in three articles could be attributed to the unbalanced baseline and small sample size of Rouvas' article [30]. After removal of this study, the data of Saito et al[31] and Sakurada and Iijima[32] showed the combination therapy of PDT and IVR still kept a favourable position compared with PDT monotherapy during the second year.
Both studies reported by Kon et al[22] and Rouvas et al[30] indicate that there was no statistically significant difference in CRT decrease for combination treatment and PDT monotherapy, whereas the patients in IVR monotherapy group had a significantly inferior mean CRT change compared with those in other two groups. Furthermore, our Meta-analysis found that CRT in combination treatment group tended to have a greater reduction for PCV than in PDT group at month 6.
Some eyes with PCV might develop severe complications like recurrent lesions and massive subretinal hemorrhage (SRH) due to the up-regulation of VEGF after PDT monotherapy[44]. Intravitreal injection of anti-VEGF agent could therefore block the adverse effects induced by the increased VEGF expression, which might account for the limited visual loss observed in the combined PDT group[32]. Hatz and Prünte[45] found that after initiation of combination therapy, eyes with PCV received obvious lesser ranibizumab injections/year and had prolonged stabilization of VA and regression of polyps. Our statistical results also indicated that the mean number of both PDT and IVR in combination group was less than in monotherapy group, thus we speculate that the relevant adverse events might reduce in combination treatment with the decreased number of PDT or IVR.
Standard-fluence verteporfin PDT may result in extensive choroidal nonperfusion and inflammation. To reduce the possible complications, efforts have been made and have also achieved quite promising results using reduced-fluence PDT combined with IVR for the treatment of PCV[46]–[48]. The deficiency is the lack of comparison of efficacy with standard-fluence PDT. In addition, bevacizumab might be a more attractive alternative than ranibizumab as its lower cost to reduce the economic burden. However, it has not received FDA approval for eye diseases. The efficacy of intravitreal bevacizumab combined with PDT and the incidence rate of adverse events compared between ranibizumab and bevacizumab also need to be analyzed.
In conclusion, current evidences confirmed that the first-line therapy for PCV might be PDT combine with ranibizumab, which could efficiently promote the regression of polyps and significantly improve VA with comparative safety. Given the high incidence of PCV in Asian populations, three RCTs with slightly different interventions and measuring indicators were small samples at the present time, we think first of all to formulate unified criteria of diagnosis, observation and prognosis evaluation, and future large-scale, multicenter, double-blind, prospective, long-term RCTs are warranted to confirm our preliminary results of PDT combine with ranibizumab in the treatment of PCV.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81373826, No.81100658); Development Project of Science and Technology of Traditional Chinese Medicine of Shandong Province (No.2013ZDZK-083); Development Project of Medicine and Health Science Technology of Shandong Province (No.2013WS0251).
Conflicts of Interest: Tang K, None; Si JK, None; Guo DD, None; Cui Y, None; Du YX, None; Pan XM, None; Bi HS, None.
REFERENCES
- 1.Imasawa M, Sakurada Y, Iijima H. Classic choroidal neovascularization developing after photodynamic therapy in eyes with polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2011;55(3):241–247. doi: 10.1007/s10384-011-0006-6. [DOI] [PubMed] [Google Scholar]
- 2.Yoon JS, Lee J, Lee SC, Koh HJ, Kim SS, Kwon OW. Polypoidal choroidal vasculopathy in Korean patients with large submacular hemorrhage. Yonsei Med J. 2007;48(2):225–232. doi: 10.3349/ymj.2007.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA. Polypoidal choroidal vasculopathy: a review. Surv Ophthalmol. 2010;55(6):501–515. doi: 10.1016/j.survophthal.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Fernández M, Gil M, Gomez-Ulla F, Charlón P. Verteporfin photodynamic therapy combined with intravitreal ranibizumab for polypoidal choroidal vasculopathy controversy concerning long-term followup. Case Rep Med. 2012;2012:897097. doi: 10.1155/2012/897097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuchiya D, Yamamoto T, Kawasaki R, Yamashita H. Two-year visual outcomes after photodynamic therapy in age-related macular degeneration patients with or without polypoidal choroidal vasculopathy lesions. Retina. 2009;29(7):960–965. doi: 10.1097/IAE.0b013e3181a3b7c5. [DOI] [PubMed] [Google Scholar]
- 6.Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, Ikuno Y, Tano Y. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008;115(1):141–146. doi: 10.1016/j.ophtha.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Si JK, Tang K, Bi HS, Guo DD, Guo JG, Du YX, Cui Y, Pan XM, Wen Y, Wang XR. Combination of ranibizumab with photodynamic therapy vs ranibizumab monotherapy in the treatment of age-related macular degeneration: a systematic review and meta-analysis of randomized controlled trials. Int J Ophthalmol. 2014;7(3):541–549. doi: 10.3980/j.issn.2222-3959.2014.03.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YH, Lee EK, Shin KS, Lee KM, Kim JY. Intravitreal ranibizumab combined with verteporfin photodynamic therapy for treating polypoidal choroidal vasculopathy. Retina. 2011;31(7):1287–1293. doi: 10.1097/IAE.0b013e3182003ccd. [DOI] [PubMed] [Google Scholar]
- 9.Akaza E, Yuzawa M, Matsumoto Y, Kashiwakura S, Fujita K, Mori R. Role of photodynamic therapy in polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2007;51(4):270–277. doi: 10.1007/s10384-007-0452-3. [DOI] [PubMed] [Google Scholar]
- 10.Quaranta M, Mauget-Faÿsse M, Coscas G. Exudative idiopathic polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Am J Ophthalmol. 2002;134(2):277–280. doi: 10.1016/s0002-9394(02)01516-7. [DOI] [PubMed] [Google Scholar]
- 11.Akaza E, Mori R, Yuzawa M. Long-term results of photodynamic therapy of polypoidal choroidal vasculopathy. Retina. 2008;28(5):717–722. doi: 10.1097/IAE.0b013e31816577cb. [DOI] [PubMed] [Google Scholar]
- 12.Lai TY, Lam CP, Luk FO, Chan RP, Chan WM, Liu DT, Lam DS. Photodynamic therapy with or without intravitreal triamcinolone acetonide for symptomatic polypoidal choroidal vasculopathy. J Ocul Pharmacol Ther. 2010;26(1):91–95. doi: 10.1089/jop.2009.0073. [DOI] [PubMed] [Google Scholar]
- 13.Kurashige Y, Otani A, Sasahara M, Yodoi Y, Tamura H, Tsujikawa A, Yoshimura N. Two-year results of photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2008;146(4):513–519. doi: 10.1016/j.ajo.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, Lam DS. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141(3):456–462. doi: 10.1016/j.ajo.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004;88(6):809–815. doi: 10.1136/bjo.2003.032466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lantry LE. Ranibizumab, a mAb against VEGF-A for the potential treatment of age-related macular degeneration and other ocular complications. Curr Opin Mol Ther. 2007;9(6):592–602. [PubMed] [Google Scholar]
- 17.Cho HJ, Kim JW, Lee DW, Cho SW, Kim CG. Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye (Lond) 2012;26(3):426–433. doi: 10.1038/eye.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br J Ophthalmol. 2010;94(3):297–301. doi: 10.1136/bjo.2008.150029. [DOI] [PubMed] [Google Scholar]
- 19.Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, Ohtsuka H, Ariga H. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am J Ophthalmol. 2012;154(1):117–124. doi: 10.1016/j.ajo.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S, Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32(8):1453–1464. doi: 10.1097/IAE.0b013e31824f91e8. [DOI] [PubMed] [Google Scholar]
- 23.Tano Y, Ohji M, Ishibashi T, Shiraga F, Tokoro T, Yuzawa M, Yoshimura N. Re-treatment guideline of ranibizumab (genetical recombination) in the maintenance phase. Nihon Ganka Gakkai Zasshi. 2009;113(11):1098–1103. [PubMed] [Google Scholar]
- 24.Saito M, Iida T, Kano M. Intravitreal ranibizumab for exudative age-related macular degeneration with good baseline visual acuity. Retina. 2012;32(7):1250–1259. doi: 10.1097/IAE.0b013e318236e503. [DOI] [PubMed] [Google Scholar]
- 25.Lee MY, Lee WK, Baek J, Kwon OW, Lee JH. Photodynamic therapy versus combination therapy in polypoidal choroidal vasculopathy: changes of aqueous vascular endothelial growth factor. Am J Ophthalmol. 2013;156(2):343–348. doi: 10.1016/j.ajo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Oishi A, Kojima H, Mandai M, Honda S, Matsuoka T, Oh H, Kita M, Nagai T, Fujihara M, Bessho N, Uenishi M, Kurimoto Y, Negi A. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol. 2013;156(4):644–651. doi: 10.1016/j.ajo.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Inoue M, Arakawa A, Yamane S, Kadonosono K. Long-term outcome of intravitreal ranibizumab treatment, compared with photodynamic therapy, in patients with polypoidal choroidal vasculopathy. Eye (Lond) 2013;27(9):1013–1020; quiz 1021. doi: 10.1038/eye.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang HM, Koh HJ. Two-year outcome after combination therapy for polypoidal choroidal vasculopathy: comparison with photodynamic monotherapy and anti-vascular endothelial growth factor monotherapy. Ophthalmologica. 2014;231(2):86–93. doi: 10.1159/000354546. [DOI] [PubMed] [Google Scholar]
- 29.Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T. Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2011;151(4):594–603. doi: 10.1016/j.ajo.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 30.Rouvas AA, Papakostas TD, Ntouraki A, Douvali M, Vergados I, Ladas ID. Photodynamic therapy, ranibizumab, and ranibizumab with photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Retina. 2011;31(3):464–474. doi: 10.1097/IAE.0b013e3181f274ec. [DOI] [PubMed] [Google Scholar]
- 31.Saito M, Iida T, Kano M, Itagaki K. Two-year results of combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251(9):2099–2110. doi: 10.1007/s00417-013-2323-6. [DOI] [PubMed] [Google Scholar]
- 32.Sakurada Y, Iijima H. Two-year results of photodynamic therapy with or without intravitreal ranibizumab for polypoidal choroidal vasculopathy. J Ocul Pharmacol Ther. 2013;29(9):832–836. doi: 10.1089/jop.2013.0044. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki S, Miyazaki D, Miyake K, Terasaka Y, Kaneda S, Ikeda Y, Funakoshi T, Baba T, Yamasaki A, Inoue Y. Associations of IL-23 with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2012;53(7):3424–3430. doi: 10.1167/iovs.11-7913. [DOI] [PubMed] [Google Scholar]
- 34.Kim KS, Lee WK. Bevacizumab for serous changes originating from a persistent branching vascular network following photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2011;55(4):370–377. doi: 10.1007/s10384-011-0045-z. [DOI] [PubMed] [Google Scholar]
- 35.Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, Ohtsuka H, Kitamei H, Shioya S. Results of 2 years of treatment with as-needed ranibizumab reinjection for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2013;97(5):617–621. doi: 10.1136/bjophthalmol-2012-302652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang HM, Kim YM, Koh HJ. Five-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;155(3):438–447. doi: 10.1016/j.ajo.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Mori R, Yuzawa M, Akaza E, Haruyama M. Treatment results at 1 year of ranibizumab therapy for polypoidal choroidal vasculopathy in eyes with good visual acuity. Jpn J Ophthalmol. 2013;57(4):365–371. doi: 10.1007/s10384-013-0245-9. [DOI] [PubMed] [Google Scholar]
- 38.Saito M, Iida T, Kano M. Intravitreal ranibizumab for polypoidal choroidal vasculopathy with recurrent or residual exudation. Retina. 2011;31(8):1589–1597. doi: 10.1097/IAE.0b013e31820f4b21. [DOI] [PubMed] [Google Scholar]
- 39.Saito M, Iida T, Kano M, Itagaki K. Two-year results of intravitreal ranibizumab for polypoidal choroidal vasculopathy with recurrent or residual exudation. Eye (Lond) 2013;27(8):931–939. doi: 10.1038/eye.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito M, Iida T, Kano M. Combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2012;32(7):1272–1279. doi: 10.1097/IAE.0b013e318236e624. [DOI] [PubMed] [Google Scholar]
- 41.Lai TY, Lee GK, Luk FO, Lam DS. Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina. 2011;31(8):1581–1588. doi: 10.1097/IAE.0b013e31820d3f3f. [DOI] [PubMed] [Google Scholar]
- 42.Kim M, Kim K, Kim do G, Yu SY, Kwak HW. Two-year results of photodynamic therapy combined with intravitreal anti-vascular endothelial growth factor for polypoidal choroidal vasculopathy. Ophthalmologica. 2011;226(4):205–213. doi: 10.1159/000330793. [DOI] [PubMed] [Google Scholar]
- 43.Nemoto R, Miura M, Iwasaki T, Goto H. Two-year follow-up of ranibizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Clin Ophthalmol. 2012;6(1):1633–1638. doi: 10.2147/OPTH.S37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, Mandai M, Yoshimura N. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27(3):335–341. doi: 10.1097/01.iae.0000233647.78726.46. [DOI] [PubMed] [Google Scholar]
- 45.Hatz K, Prünte C. Polypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor ranibizumab response. Br J Ophthalmol. 2014;98(2):188–194. doi: 10.1136/bjophthalmol-2013-303444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakurai M, Baba T, Kitahashi M, Yokouchi H, Kubota-Taniai M, Bikbova G, Oshitari T, Yamamoto S. One-year results of intravitreal ranibizumab combined with reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathy. Clin Ophthalmol. 2014;28(8):235–241. doi: 10.2147/OPTH.S54578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricci F, Calabrese A, Regine F, Missiroli F, Ciardella AP. Combined reduced fluence photodynamic therapy and intravitreal ranibizumab for polypoidal choroidal vasculopathy. Retina. 2012;32(7):1280–1288. doi: 10.1097/IAE.0b013e318236e835. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida Y, Kohno T, Yamamoto M, Yoneda T, Iwami H, Shiraki K. Two-year results of reduced-fluence photodynamic therapy combined with intravitreal ranibizumab for typical age-related macular degeneration and polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2013;57(3):283–293. doi: 10.1007/s10384-013-0234-z. [DOI] [PubMed] [Google Scholar]