Abstract
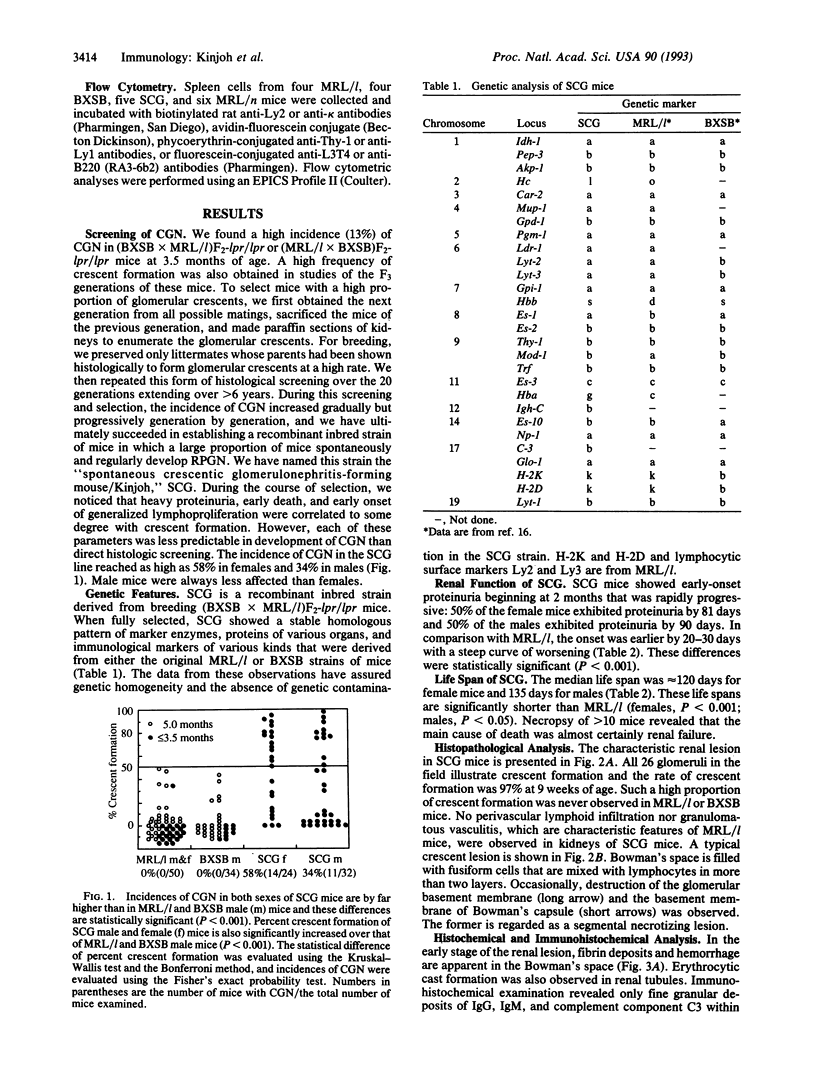
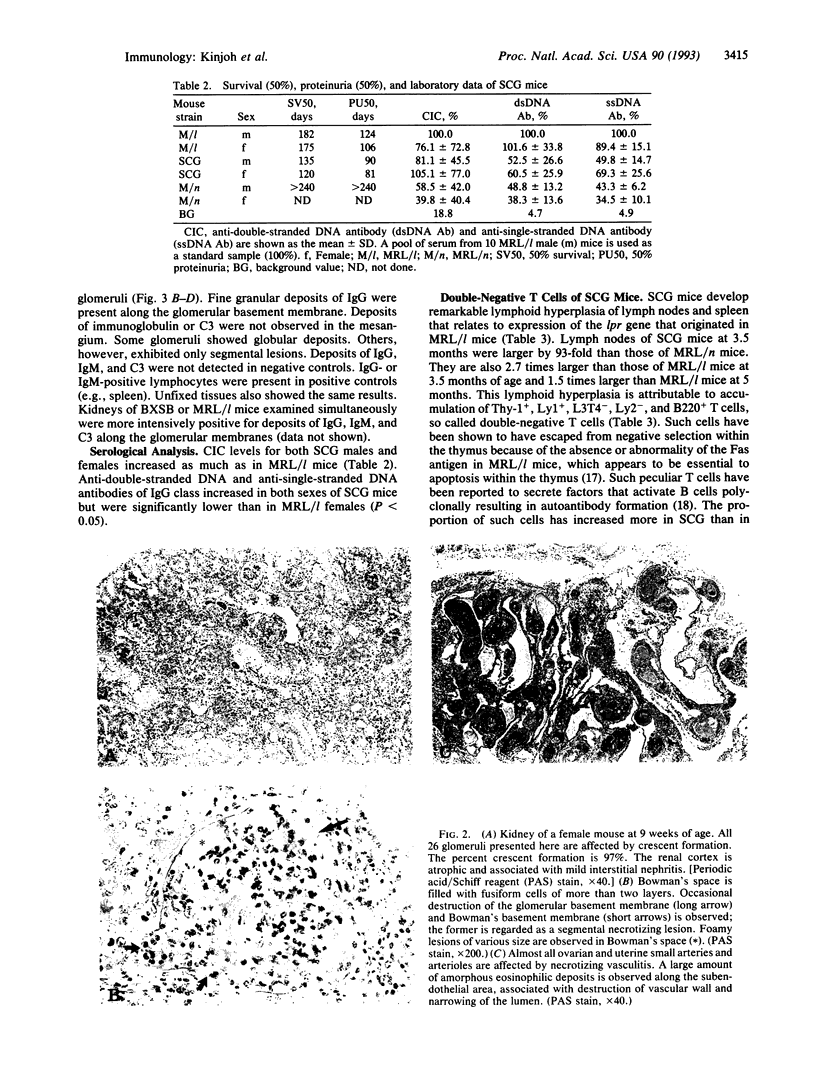
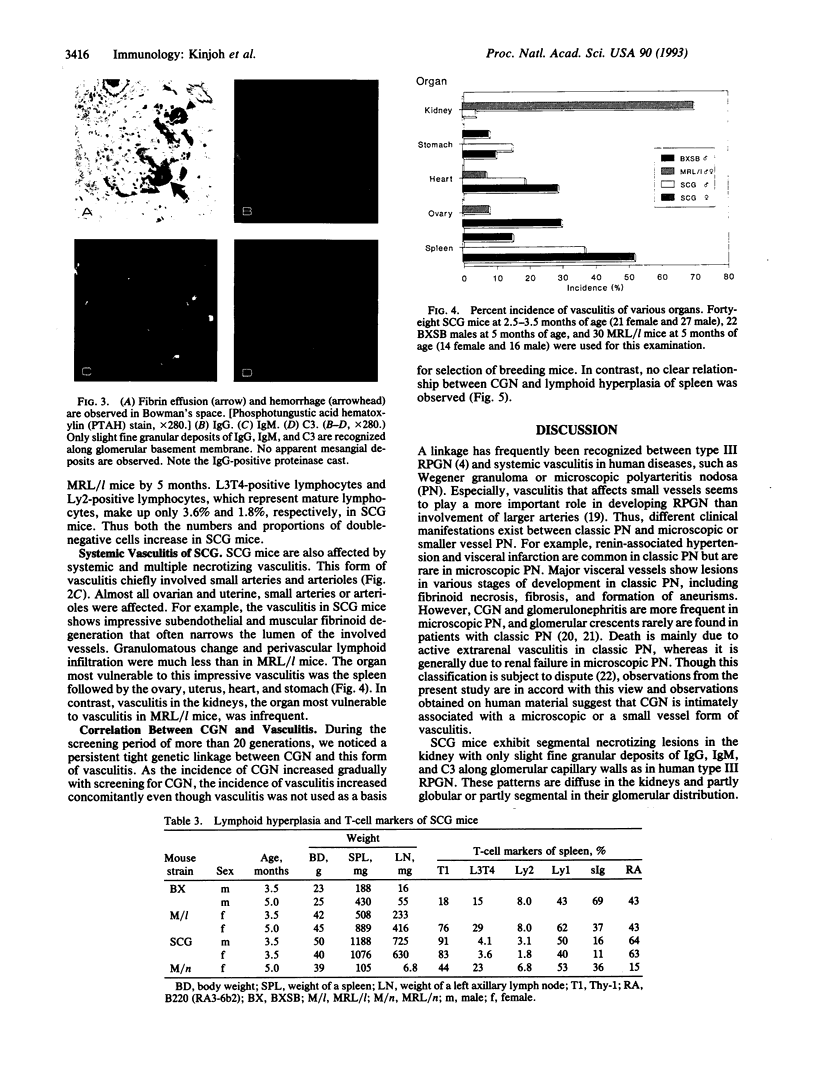
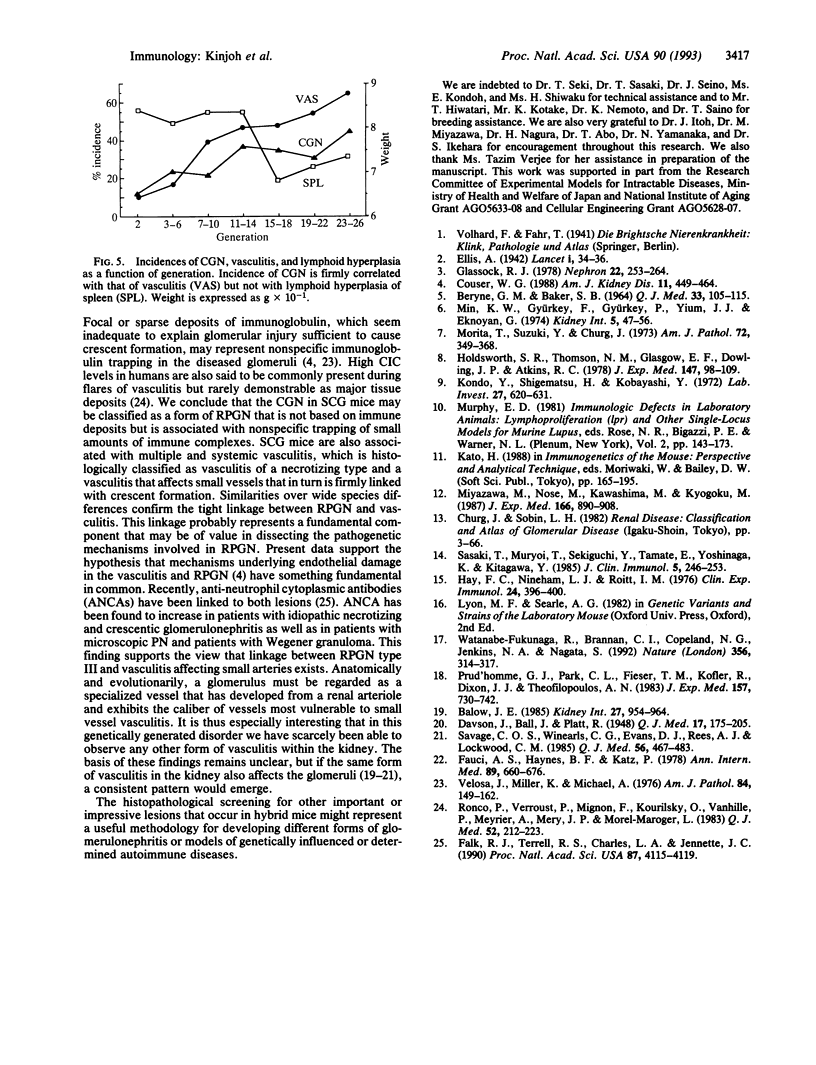
We have established a recombinant inbred strain of mouse named spontaneous crescentic glomerulonephritis-forming mouse/Kinjoh or SCG/Kj. Mice of this strain spontaneously develop rapidly progressive glomerulonephritis. This strain of mice was derived from (BXSB/Mp x MRL/Mp-lpr/lpr)F1 hybrid mice by brother x sister mating coupled with repeated histopathologic selection for breeding of mice whose parents had the highest frequency of crescent formation in the kidneys. In this strain of mice, nephritis appears earlier and is more rapidly progressive than in any other murine model of systemic lupus erythematosus. Histopathologically, the characteristic renal lesions in the mice of this strain express a most dramatic form of crescentic glomerulonephritis. The lesions in the kidneys show only slight fine granular immune deposits along the glomerular basement membrane associated with remarkable extraglomerular proliferation and hemorrhage in Bowman's space. Although selection was not based on vasculitis, mice of this strain also exhibit a high incidence of necrotizing vasculitis. These vascular lesions involve primarily small arteries and arterioles and many organs and tissues but spare the kidneys. Thus this form of vasculitis has been found to be correlated with the crescentic form of glomerulonephritis but not with lymphoid hyperplasia of the spleen. We conclude that, in this strain of mouse, the rapidly progressive glomerulonephritis is genetically restricted and that this genetic restriction is firmly linked to that responsible for the vasculitis.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLYNE G. M., DE BAKER S. B. ACUTE ANURIC GLOMERULONEPHRITIS. Q J Med. 1964 Jan;33:105–115. [PubMed] [Google Scholar]
- Balow J. E. Renal vasculitis. Kidney Int. 1985 Jun;27(6):954–964. doi: 10.1038/ki.1985.104. [DOI] [PubMed] [Google Scholar]
- Couser W. G. Rapidly progressive glomerulonephritis: classification, pathogenetic mechanisms, and therapy. Am J Kidney Dis. 1988 Jun;11(6):449–464. doi: 10.1016/s0272-6386(88)80079-9. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Terrell R. S., Charles L. A., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Haynes B., Katz P. The spectrum of vasculitis: clinical, pathologic, immunologic and therapeutic considerations. Ann Intern Med. 1978 Nov;89(5 Pt 1):660–676. doi: 10.7326/0003-4819-89-5-660. [DOI] [PubMed] [Google Scholar]
- Glassock R. J. A clinical and immunopathologic dissection of rapidly progressive glomerulonephritis. Nephron. 1978;22(1-3):253–264. doi: 10.1159/000181457. [DOI] [PubMed] [Google Scholar]
- Hay F. C., Nineham L. J., Roitt I. M. Routine assay for the detection of immune complexes of known immunoglobulin class using solid phase C1q. Clin Exp Immunol. 1976 Jun;24(3):396–400. [PMC free article] [PubMed] [Google Scholar]
- Holdsworth S. R., Thomson N. M., Glasgow E. F., Dowling J. P., Atkins R. C. Tissue culture of isolated glomeruli in experimental crescentic glomerulonephritis. J Exp Med. 1978 Jan 1;147(1):98–109. doi: 10.1084/jem.147.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y., Shigematsu H., Kobayashi Y. Cellular aspects of rabbit Masugi nephritis. II. Progressive glomerular injuries with crescent formation. Lab Invest. 1972 Dec;27(6):620–631. [PubMed] [Google Scholar]
- Min K. W., Györkey F., Györkey P., Yium J. J., Eknoyan G. The morphogenesis of glomerular crescents in rapidly progressive glomerulonephritis. Kidney Int. 1974 Jan;5(1):47–56. doi: 10.1038/ki.1974.6. [DOI] [PubMed] [Google Scholar]
- Miyazawa M., Nose M., Kawashima M., Kyogoku M. Pathogenesis of arteritis of SL/Ni mice. Possible lytic effect of anti-gp70 antibodies on vascular smooth muscle cells. J Exp Med. 1987 Oct 1;166(4):890–908. doi: 10.1084/jem.166.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Suzuki Y., Churg J. Structure and development of the glomerular crescent. Am J Pathol. 1973 Sep;72(3):349–368. [PMC free article] [PubMed] [Google Scholar]
- Prud'Homme G. J., Park C. L., Fieser T. M., Kofler R., Dixon F. J., Theofilopoulos A. N. Identification of a B cell differentiation factor(s) spontaneously produced by proliferating T cells in murine lupus strains of the lpr/lpr genotype. J Exp Med. 1983 Feb 1;157(2):730–742. doi: 10.1084/jem.157.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco P., Verroust P., Mignon F., Kourilsky O., Vanhille P., Meyrier A., Mery J. P., Morel-Maroger L. Immunopathological studies of polyarteritis nodosa and Wegener's granulomatosis: a report of 43 patients with 51 renal biopsies. Q J Med. 1983 Spring;52(206):212–223. [PubMed] [Google Scholar]
- Sasaki T., Muryoi T., Sekiguchi Y., Tamate E., Yoshinaga K., Kitagawa Y. Monoclonal human anti-DNA antibodies from EB virus-transformed lymphocytes of systemic lupus erythematosus (SLE) patients. J Clin Immunol. 1985 Jul;5(4):246–253. doi: 10.1007/BF00929459. [DOI] [PubMed] [Google Scholar]
- Savage C. O., Winearls C. G., Evans D. J., Rees A. J., Lockwood C. M. Microscopic polyarteritis: presentation, pathology and prognosis. Q J Med. 1985 Aug;56(220):467–483. [PubMed] [Google Scholar]
- Velosa J., Miller K., Michael A. F. Immunopathology of the end-stage kidney. Immunoglobulin and complement component deposition in nonimmune disease. Am J Pathol. 1976 Jul;84(1):149–162. [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]






