Abstract
AIM
To determine the long-term effects of selective laser trabeculoplasty (SLT) on intraocular pressure (IOP) and the number of medications used up to 5y following treatment in glaucoma patients receiving maximally tolerated medical therapy (MTMT).
METHODS
The Wills Eye Hospital Glaucoma Research Center retrospectively reviewed the charts of glaucoma patients who underwent SLT after receiving MTMT. Eyes that did not achieve their target pressure within 3mo following SLT were excluded from the study. Changes in mean IOP and number of glaucoma medications used were analyzed at 1, 3, and 5y following SLT.
RESULTS
Seventy-five eyes of 67 patients were included in the study. Fifteen eyes that received SLT failed to achieve their target pressure within 3mo and were excluded from the study. The average follow-up time was 37.4mo (±14.4). Mean IOP was significantly reduced 1y after treatment (P=0.005). It was also reduced 3, 5y after treatment without reaching statistical significance (P=0.20 and P=0.072, respectively). There was a significant decrease in mean number of medications used 1, 3, 5y after treatment (P<0.001, P<0.001, and P=0.039, respectively). In the span of 5y, 2 eyes (2.7%) underwent repeat SLT, 7 eyes (9.3%) underwent glaucoma surgery and an additional 3 eyes (4.0%) underwent both.
CONCLUSION
SLT significantly reduced the number of glaucoma medications used 5y following treatment in glaucoma patients receiving MTMT. SLT may delay operating-room surgery.
Keywords: selective laser trabeculoplasty, long-term, intraocular pressure, number of medications used, maximally tolerated medical therapy, glaucoma
INTRODUCTION
Selective laser trabeculoplasty (SLT) was first introduced as a treatment option for glaucoma in 1995[1],[2]. It employs a frequency-doubled q-switched Nd-YAG laser to target pigmented trabecular meshwork (TM) cells without affecting neighboring non-pigmented cells[3]. Although the exact mechanism of SLT is unknown, one theory proposes that the thermal energy of the laser stimulates recruitment of macrophages in the TM and remodeling of the extracellular matrix, allowing increased aqueous outflow from the eye and lowering of intraocular pressure (IOP)[4].
Multiple studies have demonstrated the effectiveness of SLT in reducing IOP in patients with glaucoma[5]–[7]. A prospective, randomized trial of 69 patients concluded that SLT and medical therapy led to similar reductions in IOP after 1y[8]. Another study has supported the use of SLT as a first-line treatment for newly diagnosed glaucoma or ocular hypertension[9]. Despite these encouraging findings, the effects of the laser treatment seem to diminish over time[10]–[12]. Thus, SLT may not be a permanent solution for maintaining IOP.
Previous studies have also demonstrated SLT to be effective up to 18mo following treatment in patients who did not achieve IOP control after receiving maximally tolerated medical therapy (MTMT)[13]–[16]. However, few retrospective or prospective studies have investigated the long-term results of SLT in this subpopulation, including the number of glaucoma medications used[10]–[12],[17]. Therefore, our aim was to determine the long-term effects of SLT on both IOP and the number of medications used up to 5y after treatment in a population of glaucoma patients receiving MTMT.
SUBJECTS AND METHODS
Subjects
A retrospective review of Electronic Health Records (NextGen, Horsham, PA, USA) of patients seen at the Glaucoma Service of Wills Eye Hospital was conducted. The Institutional Review Board of Wills Eye Hospital approved the study protocol and the study procedures conformed to the tenets of the Declaration of Helsinki. All patients treated with SLT were given an explanation of the potential risks, benefits and alternatives to the procedure and signed an informed consent form.
Methods
This investigation studied eyes diagnosed with glaucoma or ocular hypertension in which IOP was uncontrolled despite receiving MTMT. MTMT was defined as the use of all the glaucoma medications the patient could tolerate[18]. Eyes receiving SLT treatment at the Glaucoma Service at Wills Eye Hospital, a tertiary referral center, in 2007 were included and were followed for any period up to and including 2012. Eyes that did not achieve their target IOP, as determined by the specialist, within 3mo following SLT were excluded from the study. Patients with any condition preventing accurate tonometry (e.g. corneal scarring, history of corneal surgery including laser-assisted in situ keratomileusis and photorefractive keratectomy) or having a history of previous SLT, argon laser trabeculoplasty, or glaucoma surgeries in the treated eye were also excluded. If eyes had glaucoma surgery after treatment, the clinical data following surgery were censored. Ninety eyes were initially included in the study. Of these, 15 eyes did not achieve their target pressure and were excluded from the study.
Selective laser trabeculoplasty treatment
All SLTs were performed by glaucoma specialists at Wills Eye Hospital using Selecta 2 (Lumenis, Yokneam, Israel) with a 400 µm spot size and a 3-nanosecond pulse duration, starting with a power of 0.8 mJ. The energy was increased or decreased until cavitation bubbles within the TM were just noted. One hundred eighty degrees, 270° or 360° of the TM were treated. Eyes were pretreated with topical anesthesia and an alpha-adrenergic agonist. Up to 1wk after SLT, eyes were treated with topical steroids or non-steroidal anti-inflammatory agents. An IOP spike was considered a rise in IOP≥=5 mm Hg on the same day of treatment. Eyes that experienced IOP spikes were treated with the appropriate medications. The number of glaucoma medications were increased or decreased at later follow-up visits, depending upon the specialist's suggestions, in order to maintain IOP below target level.
Data collection
The primary outcome measures were IOP and the number of glaucoma medications used at each follow-up visit. For each eye, IOP measurements were documented at baseline and any or all of 4 follow-up periods: 4-8wk, 1, 3, and 5y after treatment. The number of medications used was documented at baseline and any or all of 3 follow-up periods: 1, 3 and 5y after treatment. The most recent measurement of IOP and number of medications used before treatment were considered baseline. Complications, repeat SLT treatments, and glaucoma surgeries after the initial treatment were also noted for each eye. Following the exclusion of eyes that did not achieve their target IOP, the remaining eyes were eligible to meet the success criteria. In our study, success was defined as either at least a 20% IOP reduction or a reduction in the number of medications used, with no additional laser or surgical intervention.
Statistical Analysis
The primary outcomes were summarized using means and standard deviations. For the analysis of IOP and number of medications used, eyes were separated into 3 groups based on if they had a follow-up measurement 1, 3, 5y after treatment. In each group, mean values at 4-8wk, 1, 3, and/or 5y after SLT were then compared to baseline values. Changes in mean IOP after treatment were analyzed using a paired t-test. Changes in mean number of medications used after treatment were analyzed using Wilcoxon signed rank test and tested using GEE Poisson regression models. The distribution was assumed to be 2-tailed and a significance level of 0.05 was chosen. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Seventy-five eyes of 67 patients were included in our study. The average follow-up time was 37.4mo (±14.4). Demographic and clinical characteristics of patients who underwent SLT after receiving MTMT are summarized in Table 1. The mean age was 68.2y (±11.0). Most patients were diagnosed with primary open angle glaucoma (OAG) (82.7%). The majority of patients were either of European extraction (64.7%) or African-American (29.2%).
Table 1. Demographic and clinical characteristics of patients undergoing SLT after receiving MTMT.
| Characteristics | Value |
| Age (a) | |
| Mean (SD) | 68.2 (±11.0) |
| Gender, n (%) | |
| M | 30 (44.8) |
| F | 37 (55.2) |
| Race, n (%) | |
| Caucasian | 42 (64.7) |
| African American | 19 (29.2) |
| Asian | 2 (3.1) |
| Hispanic | 1 (1.5) |
| Other | 1 (1.5) |
| Diagnosis, n (%) | |
| Primary OAG | 62 (82.7) |
| Low-tension glaucoma | 6 (8.0) |
| Pseudoexfoliation glaucoma | 4 (5.3) |
| Pigmentary glaucoma | 2 (2.7) |
| Ocular hypertension | 1 (1.3) |
OAG: Open angle glaucoma.
Of the 67 patients, 9 had both eyes treated. SLT was performed with an average of 82 applications and an average energy of 0.96 mJ. Ten eyes experienced IOP spikes after SLT. In these eyes, IOP returned to baseline after the appropriate intervention. No other complications due to SLT occurred in any eye. In the span of 5y, 2 eyes (2.7%) underwent repeat SLT, 7 eyes (9.3%) underwent glaucoma surgery and an additional 3 eyes (4.0%) underwent both.
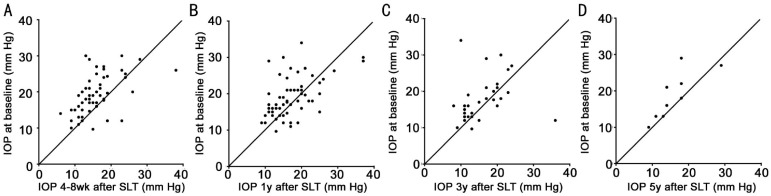
Table 2 presents mean IOP and number of glaucoma medications used. Mean IOP was significantly reduced 4-8wk and 1y after treatment (P<0.001 and P=0.005, respectively). Figure 1 illustrates IOP at baseline plotted against IOP 4-8wk, 1, 3, and 5y after treatment. At each follow-up period, the majority of eyes showed either a neutral or favorable IOP response to SLT. Success rates after 1, 3, and 5y were 62%, 50%, and 32%, respectively.
Table 2. IOP and number of glaucoma medications used in patients undergoing SLT after receiving MTMT.
| Follow-up period | Outcome | Time | n (eyes) | Mean (SD) | P1 |
| 1a | IOP (mm Hg) | Baseline | 67 | 18.7 (5.3) | - |
| 4-8wk | 56 | 15.6 (5.6) | <0.001 | ||
| 1a | 67 | 16.8 (4.1) | 0.005 | ||
| No. of medications used | Baseline | 70 | 3.4 (0.6) | - | |
| 1a | 70 | 3.0 (1.0) | <0.001 | ||
| 3a | IOP (mm Hg) | Baseline | 32 | 17.8 (6.0) | - |
| 4-8wk | 27 | 16.8 (8.0) | 0.42 | ||
| 1a | 30 | 15.7 (4.1) | 0.053 | ||
| 3a | 32 | 16.2 (5.8) | 0.20 | ||
| No. of medications used | Baseline | 32 | 3.4 (0.6) | - | |
| 1a | 31 | 2.9 (1.1) | 0.002 | ||
| 3a | 32 | 2.7 (1.2) | <0.001 | ||
| 5a | IOP (mm Hg) | Baseline | 9 | 18.8 (6.5) | - |
| 4-8wk | 9 | 13.7 (3.7) | 0.009 | ||
| 1a | 8 | 15.9 (3.6) | 0.13 | ||
| 3a | 9 | 16.8 (4.9) | 0.20 | ||
| 5a | 9 | 16.0 (5.8) | 0.072 | ||
| No. of medications used | Baseline | 9 | 3.6 (0.7) | - | |
| 1a | 9 | 3.1 (1.1) | 0.13 | ||
| 3a | 9 | 2.8 (1.0) | 0.031 | ||
| 5a | 9 | 2.1 (1.3) | 0.039 |
1Compared to baseline. Eyes were separated into three groups based on if they had a follow-up measurement 1, 3, 5y after SLT. In each group, mean values at 4-8wk, 1, 3, and/or 5y after SLT were then compared to baseline values.
Figure 1. IOP at baseline and after SLT in patients who received MTMT.
A: 4-8wk after treatment; B: 1y after treatment; C: 3y after treatment; D: 5y after treatment.
DISCUSSION
Managing glaucoma in patients who do not respond to MTMT is challenging. In order to control IOP and prevent changes in the optic disc or visual field, further intervention is needed. Invasive treatments such as surgery are generally associated with greater risks of complications. Therefore, a laser procedure such as SLT may be a safer technique to control IOP in these patients. As surgical interventions are more expensive and time-consuming than laser procedures, SLT can also provide an economic advantage. The aim of this study was to determine the long-term effects of SLT on IOP and number of glaucoma medications used. Our results indicated that SLT was mildly effective in reducing both of these outcome measures, at least temporarily.
Two prospective clinical studies have reported significant reductions in mean IOP after 4, 6y in patients who received SLT as a secondary treatment to MTMT[11],[12]. Forty-four percent and 59% of patients had at least a 20% reduction in IOP, respectively. In a retrospective study of patients with OAG, Juzych et al[10] found similar results with significant IOP-lowering effects lasting 5y following treatment. However, many of the patients enrolled in their study required additional medical or surgical intervention. The current study differs from previous reports in considering only cases in which an initial reduction of IOP was sufficient to achieve target pressure. In contrast to previous studies, the reduction in IOP after 3 and 5y was not statistically significant, possibly due to a small sample size. This discrepancy may further be explained by the magnitude of baseline IOP. Higher baseline IOP has been found to predict a greater IOP response following SLT treatment[19],[20]. The previous studies reported a higher baseline mean IOP (greater than 22 mm Hg) than our study (less than 19 mm Hg).
Although a few long-term studies have recorded the number of medications used by these patients after SLT, they did not statistically analyze their data for changes in the mean number of medications used after treatment compared to baseline. In a prospective randomized clinical study of patients with OAG, Bovell et al[17] found that the mean number of medications used decreased by 0.20, 0.30, and 0.70 medications after 1, 3, and 5y, respectively. Juzych et al[10] reported similar results in the same time frame. In our study, the mean number of medications decreased by 0.40 in eyes at 1y follow-up, 0.72 in eyes at 3y follow-up, and 1.45 in eyes at 5y follow-up. Therefore, our study found greater reductions in the mean number of medications used after treatment than previous studies. We hypothesize that this may have curtailed the reductions in IOP 3, 5y after treatment in our study. However, reducing the number of medications used may also decrease the risk of developing ocular surface disease[21].
Similar to the study by Juzych et al[10], a number of eyes (13.3%) in our study received additional IOP-lowering surgery within 5y after treatment. Interestingly, a recent study by Woo et al[22] demonstrated that the number of pre-SLT medications did not affect the IOP-lowering effectiveness of SLT; however, groups on more medications required more-pressure lowering interventions following SLT. Compared to our study, they found a slightly higher rate (22.8%) of additional IOP-lowering intervention in patients that received three or more pre-SLT glaucoma medications. Additionally, they found that most glaucoma medications, including topical prostaglandin analogues, did not impair the efficacy of SLT[22]. However, systemic or topical use of carbonic anhydrase inhibitors was associated with a higher risk of failure with primary shorter survival period following SLT treatment over 60mo[22]. Their results, in addition to ours, suggest that clinicians should strongly consider SLT in patients receiving MTMT. Since adherence rates usually decrease in patients with increased medication load, SLT also provides the added benefit of addressing this concern. A previous study has reported low adherence rates with chronic medical therapy, with rates below 50% at 1y[23].
SLT is often implemented as a second-line treatment in patients who do not respond adequately to topical medications. However, recent evidence has shown that SLT may be useful as a first-line treatment as well. A prospective clinical study by Melamed et al[6] showed that 89% of patients treated for OAG had a decrease of 5 mm Hg or more following SLT treatment, and only 7% required additional glaucoma medications after 18mo. Another prospective, randomized study by Katz et al[8] compared SLT with medical therapy as initial treatment for glaucoma. They found similar reductions in IOP between the two treatment arms at the last follow-up visit, with fewer patients in the SLT group requiring additional treatment during follow-up[8]. Also, compared with medical therapy, SLT may be less expensive as an initial treatment at a per-patient level[24].
The studies on SLT as first-line treatment often have shorter follow-up compared with those reporting the efficacy of SLT after receiving IOP-lowering medications, however, one study by Lai et al[25] had 5y follow-up period. This study randomized newly diagnosed Chinese patients with primary OAG or ocular hypertension to receive SLT in one eye and medical treatment in the fellow eye. At 5y follow-up, they concluded that with fewer medications, SLT had similar IOP reduction compared with medical therapy alone[25], supporting the use of SLT even as a first-line treatment.
Our study has several limitations. The number of eyes that were included in the 3, 5y follow-up was considerably reduced. Furthermore, as our study included a non-homogenous group of patients with various types of glaucoma, the results may not be applicable to any one subtype. A future study may compare the long-term implications of SLT on IOP in patients with different types of glaucoma. Additionally, we did not control for glaucoma severity or the extent of the angle treated by SLT. These factors may affect the magnitude of IOP reduction.
In conclusion, SLT may significantly reduce the number of medications used to control IOP 5y following treatment. It also may postpone glaucoma surgery.
Acknowledgments
Conflicts of Interest: Patel V, None; El Hawy E, None; Waisbourd M, None; Zangalli C, None; Shapiro DM, None; Gupta L, None; Hsieh M, None; Kasprenski A, None; Katz LJ, None; Spaeth GL: Research Support-Allergan, Aerie Pharmaceuticals Bausch & Lomb, Mati Therapeautics; Advisory Board-Allergan, Alcon, Glaukos, Aerie Pharmaceuticals, Bausch & Lomb, Inotek, Sensimed AG, Alimera Sciences, ForSight Vision; Honoraria-Allergan, Alcon, Merck, Lumenis; Stock-Glaukos, Mati Therapeautics, Aerie Pharmaceuticals (There are no conflicts of interest. These sources had no involvement in the study design, collection, analysis, and interpretation of data, writing of the report, and the decision to submit the report for publication).
REFERENCES
- 1.Latina MA, Tumbocon JA. Selective laser trabeculoplasty: a new treatment option for open angle glaucoma. Curr Opin Ophthalmol. 2002;13(2):94–96. doi: 10.1097/00055735-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Realini T. Selective laser trabeculoplasty: a review. J Glaucoma. 2008;17(6):497–502. doi: 10.1097/IJG.0b013e31817d2386. [DOI] [PubMed] [Google Scholar]
- 3.Latina MA, Park C. Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactions. Exp Eye Res. 1995;60(4):359–371. doi: 10.1016/s0014-4835(05)80093-4. [DOI] [PubMed] [Google Scholar]
- 4.Stein JD, Challa P. Mechanisms of action and efficacy of argon laser trabeculoplasty and selective laser trabeculoplasty. Curr Opin Ophthalmol. 2007;18(2):140–145. doi: 10.1097/ICU.0b013e328086aebf. [DOI] [PubMed] [Google Scholar]
- 5.Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: the effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol. 2009;93(4):497–501. doi: 10.1136/bjo.2008.148510. [DOI] [PubMed] [Google Scholar]
- 6.Melamed S, Ben Simon GJ, Levkovitch-Verbin H. Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol. 2003;121(7):957–960. doi: 10.1001/archopht.121.7.957. [DOI] [PubMed] [Google Scholar]
- 7.Latina MA, Sibayan SA, Shin DH, Noecker RJ, Marcellino G. Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multi-center, pilot, clinical study. Ophthalmology. 1998;105(11):2082–2088; discussion 2089–2090. doi: 10.1016/S0161-6420(98)91129-0. [DOI] [PubMed] [Google Scholar]
- 8.Katz LJ, Steinmann WC, Kabir A, Molineaux J, Wizov SS, Marcellino G, SLT/Med Study Group Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21(7):460–468. doi: 10.1097/IJG.0b013e318218287f. [DOI] [PubMed] [Google Scholar]
- 9.McIIraith I, Strasfeld M, Colev G, Hutnik CM. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma. 2006;15(2):124–130. doi: 10.1097/00061198-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Juzych MS, Chopra V, Banitt MR, Hughes BA, Kim C, Goulas MT, Shin DH. Comparison of long-term outcomes of selective laser trabeculoplasty versus argon laser trabeculoplasty in open-angle glaucoma. Ophthalmology. 2004;111(10):1853–1859. doi: 10.1016/j.ophtha.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 11.Weinand FS, Althen F. Long-term clinical results of selective laser trabeculoplasty in the treatment of primary open angle glaucoma. Eur J Ophthalmol. 2006;16(1):100–104. doi: 10.1177/112067210601600116. [DOI] [PubMed] [Google Scholar]
- 12.Gracner T, Falez M, Gracner B, Pahor D. Long-term follow-up of selective laser trabeculoplasty in primary open-angle glaucoma. Klin Monatsbl Augenheilkd. 2006;223(9):743–747. doi: 10.1055/s-2006-926725. [DOI] [PubMed] [Google Scholar]
- 13.Lanzetta P, Menchini U, Virgili G. Immediate intraocular pressure response to selective laser trabeculoplasty. Br J Ophthalmol. 1999;83(1):29–32. doi: 10.1136/bjo.83.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayin N, Alkin Z, Ozkaya A, Demir A, Yazici AT, Bozkurt E, Demirok A. Efficacy of selective laser trabeculoplasty in medically uncontrolled glaucoma. ISRN Ophthalmol. 2013;2013:975281. doi: 10.1155/2013/975281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koucheki B, Hashemi H. Selective laser trabeculoplasty in the treatment of open-angle glaucoma. J Glaucoma. 2012;21(1):65–70. doi: 10.1097/IJG.0b013e3182027596. [DOI] [PubMed] [Google Scholar]
- 16.Russo V, Barone A, Cosma A, Stella A, Delle Noci N. Selective laser trabeculoplasty versus argon laser trabeculoplasty in patients with uncontrolled open-angle glaucoma. Eur J Ophthalmol. 2009;19(3):429–434. doi: 10.1177/112067210901900317. [DOI] [PubMed] [Google Scholar]
- 17.Bovell AM, Damji KF, Hodge WG, Rock WJ, Buhrmann RR, Pan YI. Long term effects on the lowering of intraocular pressure: selective laser or argon laser trabeculoplasty? Can J Ophthalmol. 2011;46(5):408–413. doi: 10.1016/j.jcjo.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Sampaolesi R, Sampaolesi JB, Zarate J. The glaucomas: volume 2-open angle glaucoma and angle closure glaucoma. New York: Springer; 2014. Medical Therapy in Glaucoma; p. 738. [Google Scholar]
- 19.Bruen R, Lesk MR, Harasymowycz P. Baseline factors predictive of SLT response: a prospective study. J Ophthalmol. 2012;2012:642869. doi: 10.1155/2012/642869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayala M, Chen E. Predictive factors of success in selective laser trabeculoplasty (SLT) treatment. Clin Ophthalmol. 2011;5:573–576. doi: 10.2147/OPTH.S19873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi GC, Pasinetti GM, Scudeller L, Raimondi M, Lanteri S, Bianchi PE. Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur J Ophthalmol. 2013;23(3):296–302. doi: 10.5301/ejo.5000220. [DOI] [PubMed] [Google Scholar]
- 22.Woo DM, Healey PR, Graham SL, Goldberg I. Intraocular pressure-lowering medications and long-term outcomes of selective laser trabeculoplasty. Clin Experiment Ophthalmol. 2014;43(4):320–327. doi: 10.1111/ceo.12452. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl1):S57–68. doi: 10.1016/j.survophthal.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Lee R, Hutnik CM. Projected cost comparison of selective laser trabeculoplasty versus glaucoma medication in the Ontario Health Insurance Plan. Can J Ophthalmol. 2006;41(4):449–456. doi: 10.1016/S0008-4182(06)80006-2. [DOI] [PubMed] [Google Scholar]
- 25.Lai JS, Chua JK, Tham CC, Lam DS. Five-year follow up of selective laser trabeculoplasty in Chinese eyes. Clin Experiment Ophthalmol. 2004;32(4):368–372. doi: 10.1111/j.1442-9071.2004.00839.x. [DOI] [PubMed] [Google Scholar]