Abstract
Inductively coupled plasma emission spectrometry (ICP-OES) is the most common method for determination of soil Cd, yet spectral and matrix interferences affect measurements at the available analytical wavelengths for this metal. This study evaluated the severity of the interference over a range of total soil Cd by comparing ICP-OES and ICP-MS measurements of Cd in acid digests. ICP-OES using the emission at 226.5 nm generally unable to quantify soil Cd at low (near-background) levels, and gave unreliable values compared to ICP-MS. Using the line at 228.nm, a marked positive bias in Cd measurement (relative to the 226.5 nm measurement) was attributable to As interference even at soil As concentrations below 10 mg/kg. This spectral interference in ICP-OES was severe in As-contaminated orchard soils, giving a false value for soil total Cd near 2 mg kg−1 when soil As was 100–150 mg kg−1. In attempting to avoid these ICP emission-specific interferences, we evaluated a method to estimate total soil Cd using 1 M HNO3 extraction followed by determination of Cd by flame atomic absorption (FAA), either with or without pre-concentration of Cd using an Aliquat-heptanone extractant. The 1 M HNO3 extracted an average of 82% of total soil Cd. The FAA method had no significant interferences, and estimated the total Cd concentrations in all soils tested with acceptable accuracy. For Cd-contaminated soils, the Aliquat-heptanone pre-concentration step was not necessary, as FAA sensitivity was adequate for quantification of extractable soil Cd and reliable estimation of total soil Cd.
Introduction
Analytical error and bias in determination of trace elements at low levels in soils and plants is a significant but frequently unrecognized limitation in quantifying human exposure to toxic metals (McBride, 1998). In fact, soils and sediments are the most difficult of environmental samples for trace metal analysis by spectrometric methods such as ICP-OES (Daskalova and Boevski,1999). ICP-OES has the highest rate of false positives and negatives among commonly used spectrometric methods when measuring trace elements in acid digests of soils (Kimbrough and Wakakuwa,1994). It has been demonstrated that ICP-OES can not determine Cd in soil digests and extracts accurately because of spectral interferences (Anderson et al., 1998), and may overestimate Cd relative to more sensitive methods such as graphite furnace atomic absorption (GFAAS) by large factors, particularly in soil extracts containing arsenic (Waterlot and Douay, 2009). However, because of the routine usage of ICP-OES spectrometry for analysis of soil and plant digests for Cd, erroneously high estimates of total soil and plant Cd are likely to have been reported frequently. Numerous instances of both overestimation and non-detection of Cd in soils by ICP-OES can be found in the published literature (McBride,1998; Caridad-Cancela et al., 2005; Woodard et al., 2007). For example, Franklin et al. (2003) reported that Cd concentrations measured in agricultural soils of South Carolina were unusually high, with geometric means of total Cd equal to 4.1 ± 1.3, 4.7 ± 1.7 and 3.2 ± 1.8 mg kg−1 in the Blue Ridge, Piedmont and Sandhills regions, respectively. These are much higher Cd levels than reported by other studies for ultisols of the Southeastern United States, including those of Florida (0.27 mg kg−1), Georgia (0.037 mg kg−1), North Carolina (0.068 mg kg−1) and Alabama (0.037 mg kg−1) (Ma et al.,1997; Holmgren et al.,1993). In a survey of rural soils of New York State, the NYDEC and DOH reported a range of <0.05–4.15 mg kg−1 total Cd, with a median of 0.4 and a 95th percentile of 2.4 for presumed uncontaminated (source-distant) sampling sites (NY DEC and NYDOH, 2006). It is worth noting, however, that the individual Cd measurements in this case were very frequently reported by the laboratory conducting these tests to be below ICP-emission detection or quantitation limits. It is therefore likely that the DEC-reported calculated median Cd concentration for uncontaminated soils is an overestimate. The USEPA reported a median background Cd concentration in eastern U.S. soils of 0.23 mg kg−1 (USEPA, 2005) and Holmgren et al. (1993) measured an even lower geometric mean of 0.17 mg kg−1 Cd for New York State soils. In fact, when data for soil analyses are selected based on appropriately sensitive analytical techniques, Cd in the range of 0.1–0.3 mg kg−1 appears to be typical for uncontaminated mineral soils in glaciated areas of the Northeastern USA and Canada. Nevertheless, Jennings and Petersen (2006), in summarizing reported background levels of Cd in largely rural and agricultural surface soils of States and Provinces in the USA and Canada, revealed a large range from 0.04 to 3.0 mg kg−1 with numerous Cd background concentrations reported to be 1.0 or higher.
In order to understand the discrepancy between the low background Cd concentrations reported for most agricultural soils in the nation-wide survey of Holmgren et al.(1993) and other reports of much higher background Cd, it is important to note that the low soil Cd concentrations reported by Holmgren et al.(1993) were based on analysis by graphite furnace atomic absorption (GFAAS), a method with greater inherent sensitivity than ICP-OES or flame atomic absorption (FAA) spectrometry. The soil Cd results of Franklin et al.(2003) were, conversely, determined by the less sensitive and more interference-prone ICP-OES spectrometry (Anderson et al., 1998; Ure, 1990).
In a recent survey of trace metal levels, including Cd, in several hundred urban garden soils using EPA Method 3051A–6010C, we found relatively few soils to exceed 1 mg kg−1 Cd based on ICP-OES. However, reanalyzing a subset of these soils by the more sensitive ICP-MS method revealed that ICP-OES was not reliably quantifying total soil Cd, with cases of both serious overestimation and underestimation (unpublished data). The measured MDL for the line at 228.8 nm for the ICP-OES instrument used was 0.004 mg L−1 in the acid matrix of the soil digest, and predicted to be about 0.4 mg kg−1 Cd in the soil itself. However, very high concentrations of interfering elements, particularly Al and Fe, always present in soil digest, degrade the MDL severely. EPA defines the MDL as the concentration at which the analytical procedure is capable of determining with 99% certainty that the reading is above background, typically based on a mean of 7–10 separate readings on a detectable concentration of the analyte (EPA, 40CFR, 2003). However, because the standard EPA protocol for MDL does not call for spiking Cd into the sample matrices (e.g., acid soil digest) that will actually be analyzed for Cd, but rather recommends the use of pure solutions, the “operational” MDL for soil digests is not likely to be as low as reported by analytical chemists.
It might be argued that laboratory-reported soil Cd values roughly an order of magnitude higher than the true soil “background” Cd (commonly 0.1 – 0.3 mg kg−1 in rural agricultural areas) is hardly a matter for concern, but this analytical bias has potential significance for human health and ecological risk assessment. For example, the EPA has reported the soil ecological screening level (Eco-SSL) for Cd to be 0.36 mg kg−1 for mammalian wildlife (USEPA, 2005), not very much higher than natural soil background in the Northeastern USA.
The present study was undertaken to first, evaluate analytical interference factors routinely encountered in Cd analysis of soil digests that may lead to error or bias in measurement of total Cd at low (background) to moderate contamination levels, and secondly, to look for simple alternative methods of quantifying or estimating total soil Cd at these commonly encountered levels. The analytical results reported here were obtained on several high-resolution simultaneous axial-view ICP spectrometers that have been in common usage over the past decade, employing standard methods of interference minimization (matrix matching, background subtraction, internal standardization, availability of alternate emission lines). The latest generation ICP spectrometer designs may be able to reduce the severity of interferences described in this study, such as by use of radial-view for those elements with more severe spectral and matrix-induced interferences. Nevertheless, the complex matrices of soil digests and extracts still pose a serious analytical challenge for some elements because of matrix and spectral interferences that are difficult or impossible to correct.
Methods
Spectral interference with Cd determination by ICP-OES
Soils (n=24) were collected from an old orchard site where elevated levels of As and Pb were known to be present. The soils were digested in 10% HNO3 by microwave digestion (EPA Method 3051A, CEM MarsXpress). Cd and As in the digests were determined by axial-view ICP-OES at 228.8 nm and 189.0 nm, respectively.
To further test the possible effect of elemental interferences from As, Fe and Al on Cd measurement in acid digests of representative field soils from agricultural areas of New York State, 126 agricultural soils were randomly selected from soil samples submitted by growers from Western and Central New York to the Cornell soil analytical lab. These were microwave digested in 20% nitric acid (EPA Method 3051A). The digests were analyzed for Cd by axial-view ICP-OES (Thermo Jarrell Ash IRIS Advantage) at both 226.5 and 228.8 nm. In addition, As at 189.0 nm and Fe and Al at 309.2 and 271.4 nm, respectively, were measured simultaneously in the digests for the purpose of determining the possible interfering effect of these elements on Cd measurement. The NIST San Joaquin Certified Soil Standard (SRM 2709), with a Cd content of 0.38 mg kg−1 mg kg−1 was used as a standard for verifying measurement precision.
Comparison of Cd determination by ICP-OES with ICP-MS
A set of 20 soils expected to represent a range of total soil Cd was selected, including 14 urban soils from gardens and yards, 4 soils from sewage-sludge contaminated field sites, and 2 uncontaminated rural soils. These were homogenized by light grinding with mortar and pestle. Subsamples of these were digested (EPA Method 3051A), with the same digests analyzed for Cd by both ICP-OES (Thermo Jarrell Ash Trace 61E at 226.5 nm) and ICP-MS (Thermo Fisher X-7 Series) by a commercial ELAP-certified analytical lab (H2M Labs, Melville, NY). Cd recoveries for standard reference soil materials averaged 85–90% and 97% for ICP-OES and ICP-MS, respectively. This comparison allowed a determination of the degree to which discrepancies in measured soil Cd are due to errors inherent in the analytical methods as opposed to heterogeneity within samples.
Evaluation of a FAA Screening Test for Soil Cd
Subsamples of the same soils described in the methods section above were extracted for Cd by adding 50 ml of 1.0 M HNO3 to 5 g oven-dry soil (pulverized by mortar & pestle) in a 125 ml Erlenmeyer flask, agitating on a rotary shaker at 150 rpm for 1.0 hr and filtering through Whatman #42 paper. Direct determination of Cd on the filtered extracts was done by FAA at 228.8 nm. Extractable Cd (mg kg−1) by this method was compared to total soil Cd as determined by ICP-MS (see methods above).
In addition, the method of concentrating Cd from acid extracts into an iodide-containing organic phase was modified from Li et al. (1994) and tested on the extracts obtained. This method is designed to improve the detection limit of FAA measurement of Cd in solution, and is particularly suited to soils with low (near-background) concentrations of Cd. Briefly, 30 ml of each of the 1.0 M HNO3 extracts from the soils were pipetted into glass separation funnels, 3 ml of 20% (w/w) aqueous KI solution were added, and the funnels were stoppered and briefly mixed. Then 3 ml. of a solution containing Aliquat-336 (Sigma-Aldrich Co., St. Louis, MO) dissolved in 2-heptanone (1:9 ratio by volume) were added, the funnels were again stoppered and thoroughly shaken for 3 minutes. After the organic phase was allowed to separate at the surface, the lower aqueous phase was drained off and the supernatant Aliquat-heptanone solution was collected into small glass test tubes. These heptanone solutions were analysed for Cd by FAA at 228.8 nm using a lean blue flame. Each sample set included, besides the acid extracts, a reagent blank (1 M HNO3) and Cd standards of 0.010, 0.020, 0.050, 0.100, and 0.200 mg L1 in 1 M HNO3, all subjected to the same method of concentration into the Aliquat-heptanone phase. Standard curves of measured absorbance as a function of Cd concentration in 1 M HNO3 were used to calculate the Cd concentrations in the soil extracts. Two NIST standard reference soil materials (SRM-2702 and SRM-2709) were included in the set of samples analyzed by this method to determine efficiency of the 1.0 M HNO3 extraction and transfer from the aqueous to heptanone phase. (Although ascorbic acid has frequently been used in this test to prevent oxidation of the iodide to elemental iodine by the large amounts of ferric iron in acid soil digests, we did not find this reagent to be needed for the much lower levels of Fe(III) in 1 M HNO3 extracts).
The 1.0 M HNO3-extracted Cd as determined by this method was again compared to total Cd determined by ICP-MS (EPA Method 3051A-6020.
Results
Spectral interference with Cd determination by ICP-OES
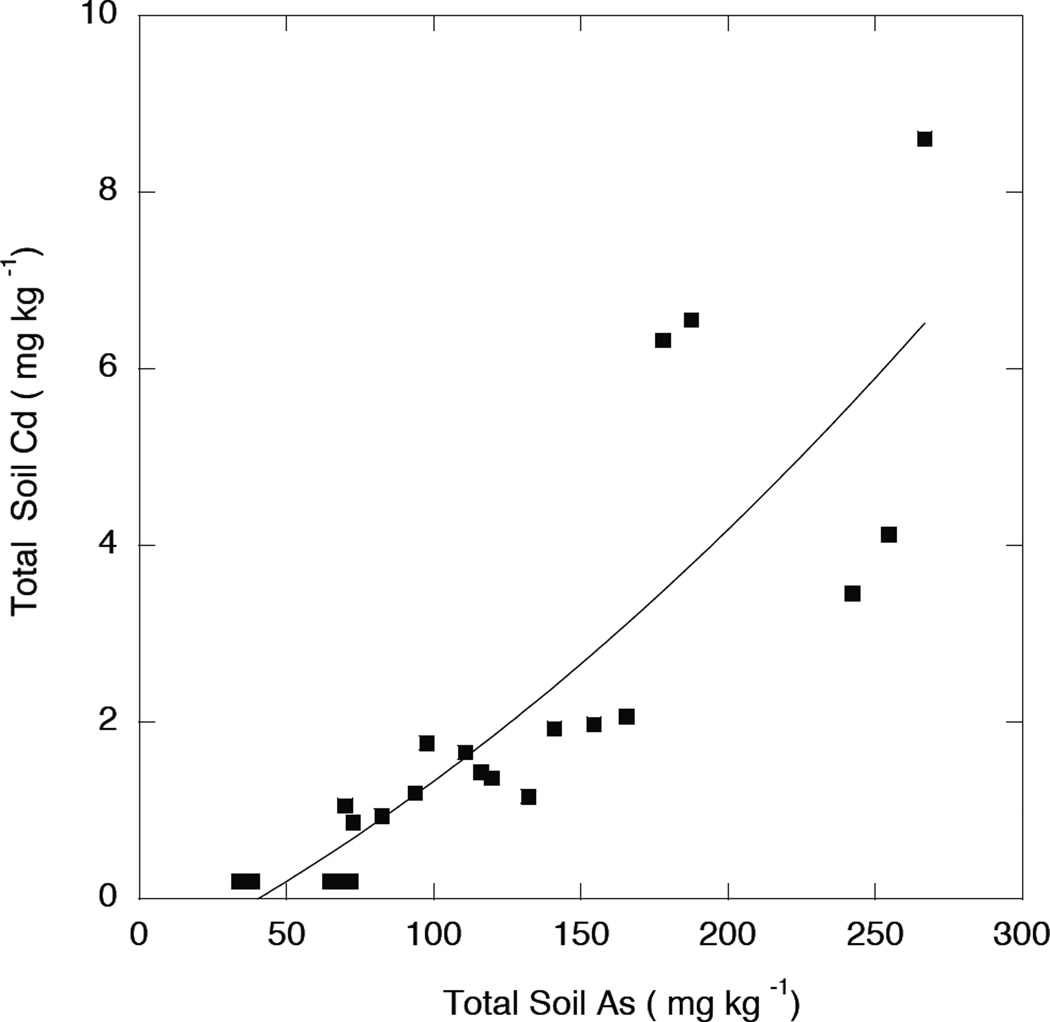
A strong correlation was observed between apparent Cd concentrations measured at 228.8 nm and As, as shown in Figure 1. As we had independent evidence that these orchard soils were not significantly contaminated by Cd based on ICP-MS analysis, these apparent high Cd measurements were spurious and demonstrate that, if soil As concentrations are higher than 50 mg kg−1, Cd determination at 228.8 nm by emission has an unacceptably high positive error.
Figure 1.
Determination of soil Cd at 228.8 nm by ICP-OES in orchard soils containing high arsenic concentration.
This result led us to conduct a more detailed study of the potential interfering effect of soil As on Cd measurement at 228.8 nm by ICP-OES in order to determine the threshold level of soil As that could be tolerated without causing a significant error in Cd determination. For this purpose, randomly selected agricultural soils (n=126) of New York State were analyzed for total trace elements by ICP-OES (EPA Method 3051A-6010C), with Cd determined at both 228.8 nm and 226.5 nm, the two emission lines most commonly used. Although the measurements of total soil Cd (mg/kg) at the 228.8 and 226.5 lines were correlated:
there was substantial scatter in the individual data points, with a number of soils plotting well off the best-fit line. Also, the intercept of 0.200 shows that the emission at 228.8 estimated soil Cd on average by 0.20 mg kg−1 units higher than the 226.5 line.
For these agricultural soils, the arithmetic mean Cd was 0.11 ± 0.14 mg kg−1 at 226.5 nm and 0.30 ± 0.21 mg kg−1 at 228.8. Based on earlier recovery tests for Cd in standard reference soils, we found that the line at 226.5 nm could estimate soil Cd reasonably well (McBride and Cherney, 2004).
The measurements of soil Cd in the agricultural soils using 228.8 nm and 226.5 nm showed no correlation to either soil Al or Fe, indicating that neither of these emission intensity measurements was being markedly affected by the high concentrations of Fe and Al in the acid soil digests. The Fe and Al concentrations in the digests were, however, highly correlated to one another (r =0.896), a possible consequence of the fact that clay and silt particles are the main source of these elements in soil digests, so that acid digests of more fine-textured soils tend to be higher in both.
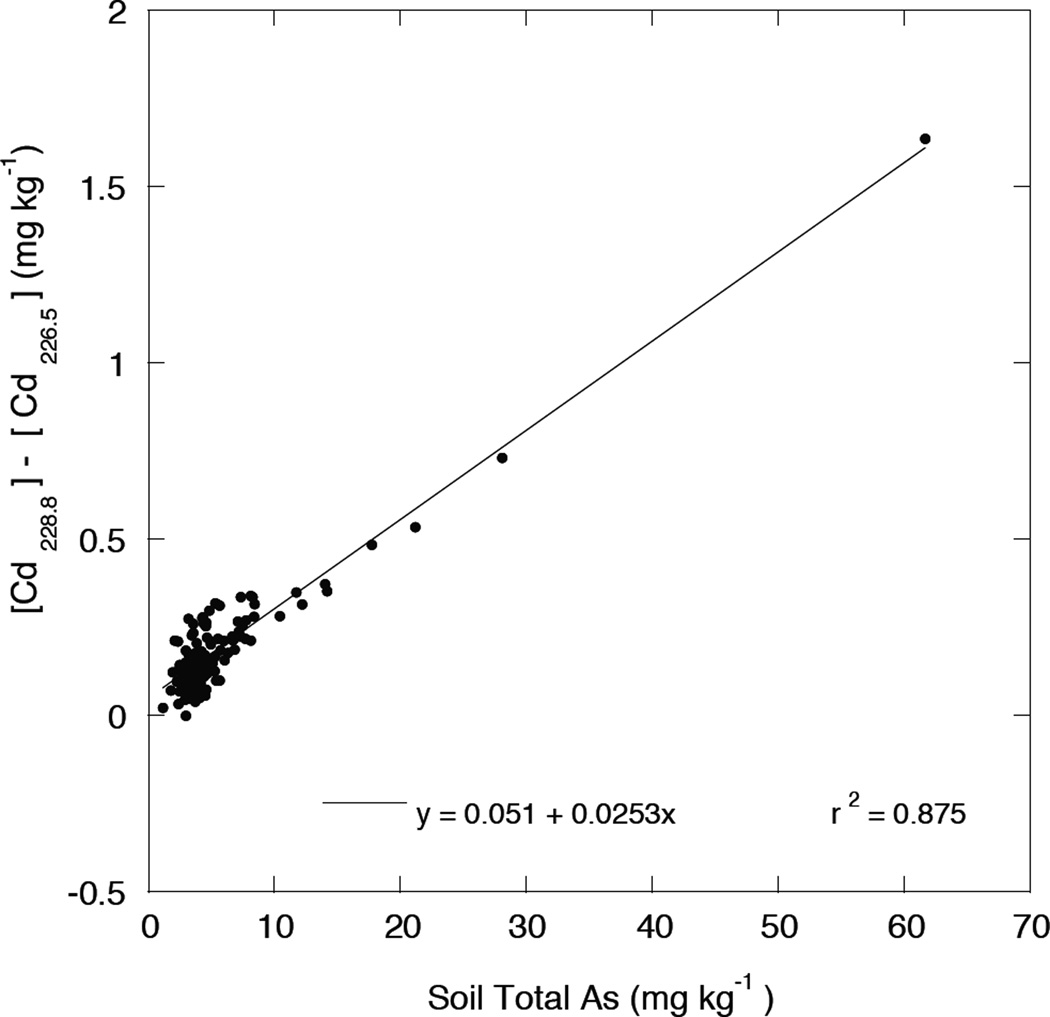
When [Cd228.8] and [Cd226.5] were tested for correlation to soil total As determined at 189.0 nm, [Cd226.5] had no tendency to correlate, whereas [Cd228.8] showed a strong relationship to soil total As (r= 0.737). Thus, the reported interference by As on soil Cd determination by ICP-OES (Waterlot and Douay,2009; Lambkin and Alloway, 2000) was confirmed to be significant for a representative sampling of agricultural soils with near-background concentrations of As. The dependence of the magnitude of this interference error on soil total As was estimated by plotting the difference between soil Cd measured at 228.8 nm and 226.5 nm, [Cd228.8] - [Cd226.5], against soil total As. This relationship is shown in Figure 2, confirming that even relatively low soil As concentrations (less than 10 mg kg−1) is associated with a significant positive error in the quantification of soil As at 228.8 nm.
Figure 2.
Difference between the soil total Cd measured by ICP-OES in acid digests at 228.8 and 226.5 nm Cd emission line as a function of soil total As.
Lambkin and Alloway (2000) indicated that As would become a serious interference for emission only when As in the soil digest exceeded 50 µg L−1 (or about 5 mg kg−1 As in the soil). Our results as indicated by the relationship in Figure 2 are in reasonable agreement with that conclusion.
This analysis assumes that Cd and As emission intensities at 226.5 and 189.0 nm, respectively, provide reliable measures of these elements in acid soil digests. In fact, the emission line used for As determination (189.0 nm) is remarkably free of interferences from other elements, including Fe and Al and Ca (Beccaloni et al., 2002; Velitchkova et al., 2004). However, the Cd emission at 226.5 nm is subject to a broad-spectrum interference by Fe and is less sensitive than the emission at 228.8 nm (Lambkin and Alloway, 2000; Waterlot and Douay, 2009). The remaining potential analytical line at 214.4 nm has a broad-spectrum Al interference. Lambkin and Alloway (2000) have suggested that flame atomic absorption (FAA), which does not show evidence of As interference, is a preferred alternative to ICP-OES measurement of Cd for soils with elevated concentrations of As.
Comparison of Cd determination by ICP-emission with ICP-MS
The results for total Cd determination by both ICP-OES (at 226.5 nm) and ICP-MS (Cd 111 isotope) in 20 soils selected to represent a range from low background to moderate contamination are listed in Table 1. Although total Cd measured by these two methods was correlated:
there was substantial scatter in the correlation despite the fact that all but three of the soils analyses carried out by the two methods were done on the same soil digests, thereby avoiding error created by possible variability in Cd from subsample heterogeneity. The three soils digested and analysed by the two methods from separate subsamples did not deviate from the correlation line, so that any discrepancies in quantifying Cd by ICP-OES and ICP-MS must be attributed to inherent errors or interferences of the spectrometric methods themselves. It is worth noting that soil Cd concentrations below 0.25 mg kg−1 are reported as below detection by both methods (Table 1). Four garden soils were reported as below detection by ICP-OES; none of these were below detection by the more sensitive ICP-MS method. However, both rural soils with no known significant sources of Cd contamination were below instrument detection limits by both methods.
Table 1.
Soils analyzed for total Cd by ICP-OES and ICP-MS and for 1 M HNO3-extractable Cd by flame atomic absorption (FAA).
| Soil No. | Land use | Soil Cd (mg kg−1) | ||
|---|---|---|---|---|
| ICP-MS* | ICP-OES* | FAA** | ||
| GT-58 | Urban garden | 1.1 | 0.62 | 0.79 |
| GT-84 | Urban garden | 0.89 | 0.34 | 0.49 |
| GT-125 | Urban garden | 0.54 | <0.25 | 0.34 |
| GT-130 | Urban garden | 1.3 | 0.68 | 1.00 |
| GT-136 | Urban garden | 1.0 | 1.04 | 0.63 |
| GT-177 | Urban garden | 1.4 | 0.95 | 1.12 |
| GT-193 | Urban garden | 0.44 | <0.25 | 0.30 |
| GT-346 | Urban garden | 0.49 | <0.25 | 0.28 |
| GT-385 | Urban garden | 1.2 | 0.62 | 1.01 |
| GT-398 | Urban garden | 0.68 | <0.25 | 0.45 |
| GT-418 | Urban garden | 4.4 | 2.72 | 3.10 |
| GT-449 | Urban garden | 4.7 | 2.98 | 3.32 |
| AS1 | Urban yard | 1.0 | 1.00 | 1.12 |
| AS7 | Urban yard | 1.1 | 0.91 | 0.90 |
| E308 | Sludged field | 2.4 | 2.10 | 2.19 |
| E316 | Sludged field | 3.6 | 3.25 | 3.90 |
| C308 | Sludged field | 1.4 | 1.17 | 1.39 |
| C316 | Sludged field | 2.8 | 2.87 | 3.12 |
| McGowan | Forest soil | <0.25 | <0.25 | 0.206 |
| Ontario | agricultural | <0.25 | <0.25 | 0.103 |
| SRM2702 | NIST reference | na | na | 0.60 |
| SRM2709 | NIST reference | na | na | 0.21 |
Same digestate analyzed by ICP-MS and ICP-OES, with exception of GT-136, GT-177 and GT-449.
Extracted by 1 M HNO3 and concentrated in Aliquat-336 in 2-heptanone
The slope of the correlation equation indicates that a significant bias existed between these ICP-MS and ICP-OES measurements of Cd in soil digests, with ICP-OES reporting lower soil Cd compared to ICP-MS.
In summary, the comparison of these two most commonly used spectroscopic methods of Cd determination in soils suggests that there is both random error (possibly due to matrix and elemental interferences) and systematic bias. Based on comparison with a third method of Cd determination using FAA (to be discussed in the next section), the evidence points to ICP-OES as being the primary source of bias in this study.
Evaluation of a FAA Screening Test for Soil Cd
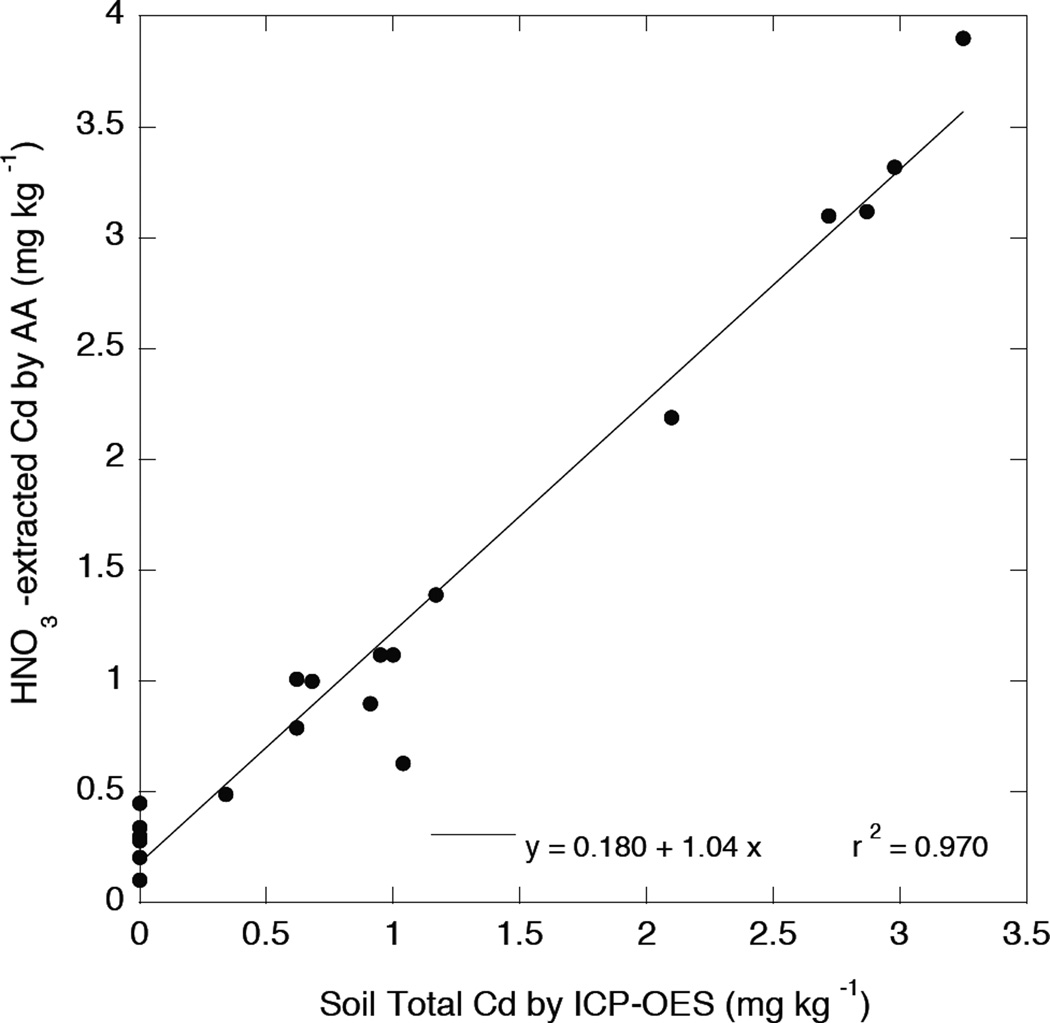
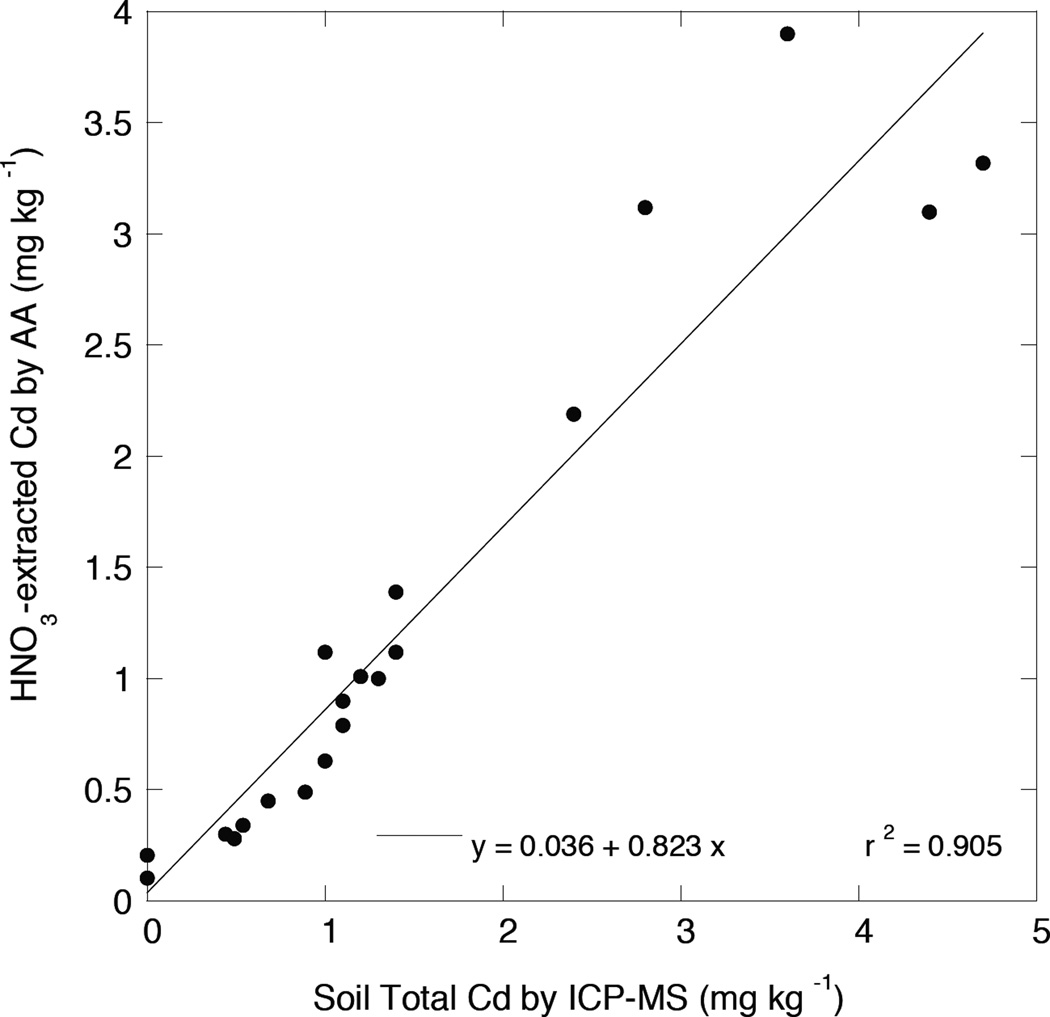
The 1.0 M HNO3–extractable Cd concentrations, measured by FAA on Aliquat-heptanone extracts as described in the methods, are reported in Table 1 for the 20 soils analyzed as described in the above section. Preliminary studies had shown that a large fraction of total Cd in most soils was readily extracted by 1.0 M HNO3, although the values in the last column of Table 1 should not be considered reliable estimates of total Cd. In fact, the Cd acid-extracted from the NIST reference soils was only 55% and 73% of total Cd for SRM2709 and SRM2702, respectively. However, as shown by the close correlation between FAA-determined 1.0 M HNO3 –extractable Cd and total soil Cd measured by both ICP-OES and ICP-MS (Figures 3 and 4), it appears that the HNO3 extraction is an excellent “screening” test for soil total Cd. The different slopes of the linear relationships between HNO3 extracted Cd and total Cd by ICP-OES (1.04) and ICP-MS (0.82) again suggest that total Cd determined by ICP-emission tends to give a low estimate, since the slope of 1.04 would indicate the unlikely result of complete dissolution and extraction of total Cd by 1 M HNO3. In fact, the 1 M HNO3 extraction efficiency at low soil Cd may be as low as 50–75% of total as indicated by the results from the SRMs. Nevertheless, overall extraction efficiency by 1 M HNO3 for the more contaminated soils was found to be about 82% (based upon total Cd as measured by ICP-MS determination of acid soil digests).
Figure 3.
Relationship between 1 M HNO3 –extracted Cd and total soil Cd as determined by acid digestion and ICP-OES.
Figure 4.
Relationship between 1 M HNO3–extracted Cd as measured by FAA and total soil Cd as determined by acid digestion and ICP-MS.
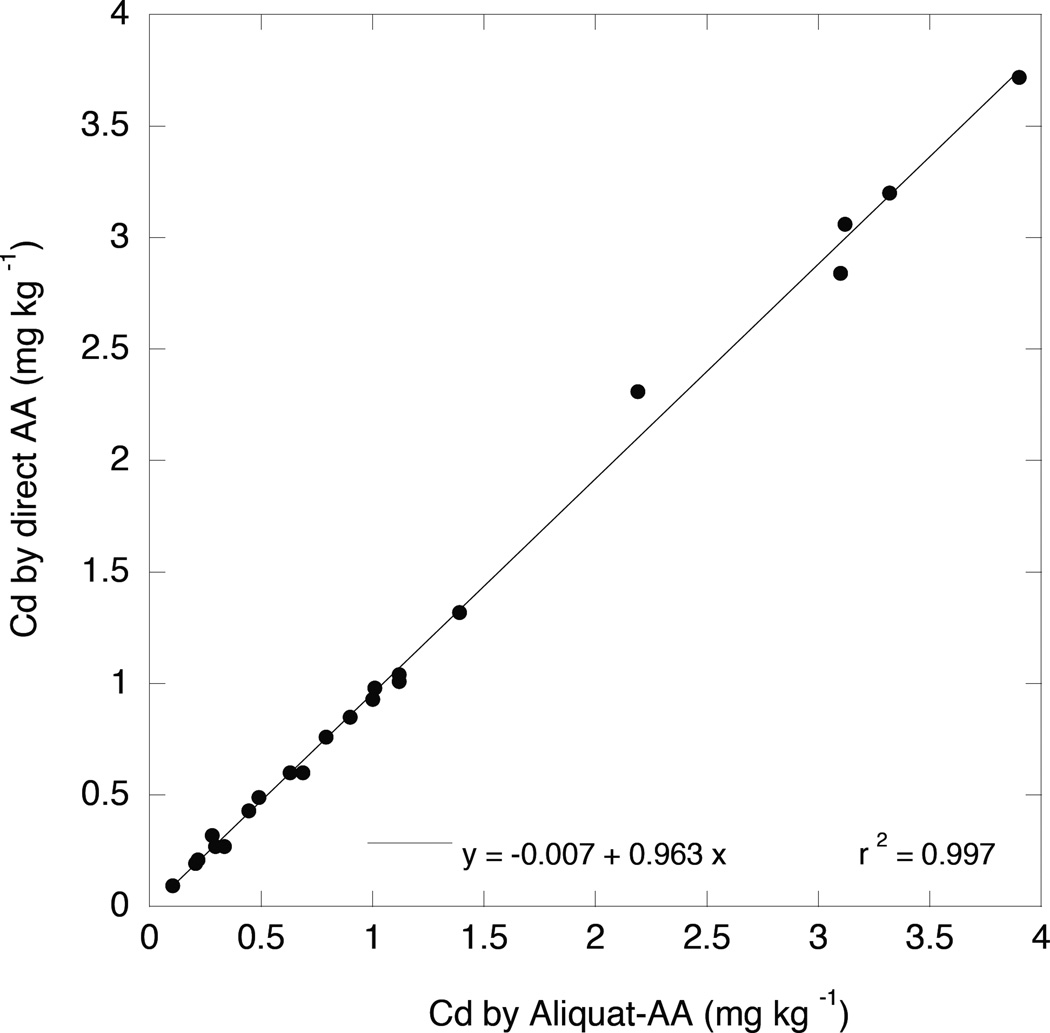
In an attempt to further simplify the 1 M HNO3 extraction test for Cd, a comparison was made between the FAA analysis of Cd in the 1 M HNO3 extracts with and without Cd preconcentration into the Aliquat-heptanone phase. In effect, this experiment was a test of whether FAA was sensitive enough to directly quantify Cd in the extracts without the additional effort required for the preconcentration step. As shown in Figure 5, the agreement between Cd concentrations determined by these two methods was remarkably good. It is concluded that preconcentration into the Aliquat-heptanone phase, while an excellent method for quantifying Cd in soils at or close to geological background levels, is probably unnecessary for measuring Cd in moderately to strongly contaminated soils.
Figure 5.
Relationship of 1 M HNO3 –extracted soil Cd measured directly by FAA (y-axis) to that measured by flame atomic absorption after concentrating the acid-extracted Cd into an Aliquat336-heptanone phase (x-axis).
Discussion and Conclusions
In recent decades, ICP-OES has become the routine method for determination of soil and plant Cd, in large part because of the instrument’s multi-element capability. The lines at 228.8 nm and 226.5 nm are, respectively, atomic and ionic emissions routinely used for soil digest analysis. Their relative intensities measured in a given sample vary depending on a number of matrix and operating conditions (Dennaud et al.,2001; Caughlin and Blades, 1984). In this study, our own comparisons of ICP-OES and ICP-MS measurements of soil total Cd indicate that ICP emission at 226.5 nm is generally unable to quantify soil Cd at near-background levels, and may provide biased values when Cd is at higher levels. It is therefore essential that researchers using ICP-OES analyze appropriate low Cd standard reference soils, and verify acceptable recovery for Cd in the concentration range of the soils being analyzed.
Our results agree with previous studies indicating that neither commonly used analytical line for Cd is completely satisfactory. The emission at 228.8 nm overlapped by an As emission (at 228.81 nm) and a nearby Ni line. Daskalova and Boevski (1999) have recommended against using this line for Cd analysis in environmental samples because of the severe As interference. Our results are consistent with this recommendation, as there is evidence of significant interference even at soil As concentrations below 10 mg kg−1. Although 226.5 nm appears to be more free of spectral interference, several Fe emission lines are nearby (Daskalova and Boevski,1999). The Fe emissions present a particularly difficult problem in soil digests because of invariably high Fe in these types of samples. In addition to spectral interferences, the high concentrations of Al, Ca and Mg in soil digests introduce potentially large background error in ICP analysis due to the high scattered light levels emitted by these elements (Ure,1990). Correction of this background error can be done by subtracting the emission intensity very near the analytical wavelength.
Although we have used ICP-MS analysis of soil Cd as our standard for comparison in this study because of its sensitivity, this is not meant to imply that ICP-MS analysis of Cd has no interference problems. In fact, spectral interferences result if oxide or hydroxide ions of Pd, Sn, In, Zr, Mo, Ru, Nb or Y are present in the sample at concentrations at least several orders of magnitude higher than the Cd concentration (May and Wiedemeyer, 1998). However, we have no reason to suspect severe interference from these trace elements in this study given that the two near-pristine soil samples that we included in the sample set were below detection for Cd by ICP-MS, an indication that the interfering elements in those soils were not high enough to produce a false positive reading for Cd.
The determination of Cd in 1 M HNO3 extracts by FAA, either with or without pre-concentration using the Aliquat-heptanone extractant, appears not to suffer from any significant interferences. It is possible to use narrower soil/solution ratios with the 1 M HNO3 extraction method (e.g., 1:5) than with soil digestion in concentrated acid (typically 1:100). The smaller dilution factor combined with relatively efficient extraction of Cd by 1 M HNO3, results in higher Cd concentrations in the final solution to be analyzed than is the case with soil digests. Furthermore, interfering elements such as Fe and Al are at many times lower concentrations in 1 M HNO3 soil extracts than soil digests. Finally, for Cd-contaminated soils, the time-consuming Aliquat-heptanone concentration step is not necessary, as FAA sensitivity is adequate for direct measurement of extracted soil Cd in 1M acid.
Acknowledgement
This project was supported in part by Award Number R21ES017921 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Abbreviations Used
- EPA
Environmental Protection Agency
- ICP-OES
Inductively coupled plasma optical emission spectrometry
- ICP- MS
Inductively coupled plasma mass spectrometry
- FAA
Flame atomic absorption spectrometry
- MDL
Method Detection Limit
- NIST
National Institute of Standards and Technology
- SRM
Standard Reference Material
References
- Anderson P, Davidson CM, Littlejohn D, Ure AM, Garden LM, Marshall J. Comparison of techniques for the analysis of industrial soils by atomic spectrometry. Int. J. Environmental Analytical Chemistry. 1998;71:19–40. [Google Scholar]
- Beccaloni E, Musmeci L, Stacul E. Determination of As in environmental solid matrix. Anal Bioanal Chem. 2002;374:1230–1236. doi: 10.1007/s00216-002-1620-4. [DOI] [PubMed] [Google Scholar]
- Caridad-Cancela R, Paz-Gonzalez A, Aparecida de Abreu C. Total and extractable nickel and cadmium contents in natural soils. Communications in Soil Science and Plant Analysis. 2005;36:241–252. [Google Scholar]
- Caughlin BL, Blades MW. An evaluation of ion-atom emission intensity ratios and local thermodynamic equilibrium in an argon inductively coupled plasma. Spectrochimica Acta. 1984;39B:1583–1602. [Google Scholar]
- Daskalova N, Boevski Iv. Spectral interferences in the determination of trace elements in environmental materials by inductively coupled plasma atomic emission spectrometry. Spectrochimica Acta Part B. 1999;54:1099–1122. [Google Scholar]
- Dennaud J, Howes A, Poussel E, Mermet J-M. Study of ionic-to-atomic line intensity ratios for two axial viewing-based inductively coupled plasma atomic emission spectrometers. Spectrochimica Acta Part B. 2001;56:101–112. [Google Scholar]
- Franklin RE, Duis L, Smith BR, Brown R, Toler JE. Elemental concentrations in soils of South Carolina. Soil Science. 2003;168:280–291. [Google Scholar]
- Holmgren GGS, Meyer MW, Chaney RL, Daniels RB. Cadmium, lead, zinc, copper and nickel in agricultural soils of the United States of America. Journal of Environmental Quality. 1993;22:335–348. [Google Scholar]
- Jennings AA, Petersen EJ. Variability of North American regulatory guidance for heavy metal contamination of residential soil. J. Environ. Eng. Sci. 2006;5:485–508. [Google Scholar]
- Kimbrough DE, Wakakuwa J. Interlaboratory comparison of instruments used for the determination of elements in acid digestates of solids. Analyst. 1994;119:383–388. [Google Scholar]
- Lambkin DC, Alloway BJ. The problem of arsenic interference in the analysis of soils for cadmium by inductively coupled plasma-optical emission spectrometry. Science of the Total Environment. 2000;256:77–81. doi: 10.1016/s0048-9697(00)00475-7. [DOI] [PubMed] [Google Scholar]
- Li Y-M, Stanislavova L, Chaney RL. Determination of total cadmium in calcareous soils by extraction using Aliquat-336 and 3-heptanone after aqua regia digestion. Communications in Soil Science and Plant Analysis. 1994;25:2029–2045. [Google Scholar]
- Ma LQ, Tan F, Harris WG. Concentrations and distributions of eleven metals in Florida soils. Journal of Environmental Quality. 1997;26:769–775. [Google Scholar]
- May TW, Wiedemeyer RH. A table of polatomic interferences in ICP-MS. Atomic Spectroscopy. 1998;19:150–155. [Google Scholar]
- McBride MB. Growing food crops on sludge-amended soils: Problems with the US Environmental Protection Agency method of estimating toxic metal transfer. Environmental Toxicology and Chemistry. 1998;17:2274–2281. [Google Scholar]
- McBride MB, Cherney J. Molybdenum, sulfur and other trace metals in farm soils and forages after sewage sludge application. Communications in Soil Science and Plant Analysis. 2004;35:517–534. [Google Scholar]
- NY DEC and DOH. New York State Brownfield Cleanup Program. Development of Soil Cleanup Objectives. Technical Support Document 2006 [Google Scholar]
- Ure AM. Methods of analysis for heavy metals in soils. In: Alloway BJ, editor. Heavy Metals in Soils. Glasgow: Blackie; 1990. pp. 40–80. [Google Scholar]
- USEPA. OSWER Directive 92857-65. Washington, D.C.: USEPA office of Solid Waste and Emergency Response; 2005. Ecological Soil Screening Levels for Cadmium. Interim Final. 20460. [Google Scholar]
- USEPA. “ Test Methods for Evaluating Soil Waste, Physical/Chemical Methods” (SW 846) http://www.epa.gov/epawaste/hazard/testsmethods/SW846/
- USEPA. 40 CFR Part 136 – Guidelines establishing test procedures for the analysis of pollutants. [July 1. 2003]; Appendix D. [Google Scholar]
- Velitchkova N, Pentcheva EN, Daskalova N. Determination of arsenic, mercury, selenium, thallium, tin and bismuth in environmental materials by inductively coupled plasma emission spectrometry. Spectrochimica Acta Part B : Atomic Spectroscopy. 2004;59:871–882. [Google Scholar]
- Waterlot C, Douay F. The problem of arsenic interference in the analysis of Cd to evaluate its extractability in soils contaminated by arsenic. Talanta. 2009;80:716–722. doi: 10.1016/j.talanta.2009.07.053. [DOI] [PubMed] [Google Scholar]
- Woodard TL, Amarasiriwardena D, Shrout K, Xing B. Roadside accumulation of heavy metals in soils in Franklin County, Massachusetts, and surrounding towns. Communications in Soil Science and Plant Analysis. 2007;38:1087–1104. [Google Scholar]