Abstract
In recent years, the potentially adverse role of sleep-disordered breathing in cancer incidence and outcomes has emerged. In parallel, animal models of intermittent hypoxia (IH) and sleep fragmentation (SF) emulating the two major components of OSA have lent support to the notion that OSA may enhance the proliferative and invasive properties of solid tumors. Based on several lines of evidence, we propose that OSA-induced increases in sympathetic outflow and alterations in immune function are critically involved in modifying oncologic processes including angiogenesis. In this context, we suggest that OSA, via IH (and potentially SF), promotes changes in several signaling pathways and transcription factors that coordinate malignant transformation and expansion, disrupts host immunologic surveillance, and consequently leads to increased probability of oncogenesis, accelerated tumor proliferation, and invasion, ultimately resulting in adverse outcomes.
The last two decades have witnessed parallel research efforts in the exploration of the mechanistic roles of hypoxia in cancer biology and also how sleep duration and biologic clock perturbations may be epidemiologically associated with increased risk for either developing cancer or adversely affecting cancer outcomes. Consequently, it was not surprising that such parallel fields of investigation would ultimately spark the hypothesis that a highly prevalent sleep disorder, namely OSA, may be a major modulator of tumor biology. Here, we perform an up-to-date critical review of the evidence supporting possible associations between OSA and cancer and further explore any putative biologically relevant mechanisms.
Hypoxia can elicit divergent responses that are either adaptive or maladaptive and are contingent on different levels of stimulus severity and presentation.1‐3 Most of the existing work has thus far revolved around the characterization of how sustained or monophasic hypoxia modulates the transcriptional activity of a large group of redox-sensitive transcription factors (eg, hypoxia-inducible factors [HIFs], nuclear factor-κB/Rel family). However, it is becoming increasingly clear that intermittent hypoxia (IH) and sustained hypoxia (SH) may elicit quite different cellular responses and that the duration and cycling frequency of IH, as occurs in sleep apnea, may also elicit contradictory cellular phenomena, namely preconditioning or cell death.1‐3 In addition, SH, and more prominently IH, will promote the formation of excessive reactive oxygen species and inflammatory products that damage or alter function of proteins, lipids, and DNA.4
The occurrence of cellular hypoxia in tumors emanates from the high proliferative rates of malignant cells that are not accompanied by parallel and commensurate angiogenesis to match the bioenergetics needs of the tissue. It is, therefore, likely that different regions of solid tumors will undergo both SH and IH, which can then elicit discrepant activation of transcription factors such as HIF-1 and HIF-2.5,6 In the absence of diseases associated with IH, such as OSA, the time constants of intratumoral oxygen tension are markedly lengthy. However, patients with OSA are characteristically sustaining IH episodes that are concurrent with the repetitive obstructions of the upper airway during sleep. The oscillatory nature of tissue Po2 in these patients imposes similar repercussions on tissue Po2 with a much shorter time constant7 and could be a predisposing factor for increased incidence of malignancies. This is particularly relevant when considering that oxidative stress is a major risk factor in oncogenesis and that increased oxidative stress enhances the probabilistic mutational rate of rapidly replicating cells.8,9 Second, mechanisms enhancing tumor growth, invasion, and regional and distant metastatic potential are enhanced by IH.6,10‐12 However, studies examining how IH may affect cancer progression have revolved around the replication of the putative changes in tissue oxygenation that develop during the fast tumor growth and the concomitant and uneven process of vascularization (ie, processes with oscillatory frequencies in the range of hours to even days).10 These hypoxia/reoxygenation cycles are at least one order of magnitude longer and certainly vastly different from those experienced by patients with moderate to severe OSA. Since some of the molecular pathways triggered by IH are stringently dependent on the frequency and cumulative number of IH events,2,13 it was important to explore the effects of IH in the frequency and severity range occurring in OSA in cancer.
In comparison with the possible role of IH, much less is currently known on the potential impact of sleep fragmentation (SF) on tumorigenesis. There is growing evidence, however, suggesting that poor sleep quality or sleep discontinuity (collectively termed SF here) may in fact have a carcinogenic role. In a review by Haus and Smolensky,14 the summary evidence presented conclusively indicated that lifestyle patterns that disrupt the stability and homeostasis of sleep and circadian rhythms (eg, shift work) are associated with epigenetic modifications of several key circadian genes, which in turn modify transcriptional regulation and affect the expression of cancer-related susceptibility genes, while also disrupting gene networks that coordinate cell division and DNA repair.
In the following paragraphs, we summarize initially some of the bench-based evidence linking IH and SF to solid tumor biology and follow these with the currently available epidemiologic literature suggesting a link between OSA and cancer in human populations. Finally, we attempt to present a holistic mechanistic view of potential pathways that may account for the observations reported herein that merit future research in both human- and animal-based models.
Animal Models of IH, SF, and Cancer
Does Fast Hypoxia/Reoxygenation Cycling Mimicking OSA Facilitate Malignant Transformation in Otherwise Normal Cells?
Karoor et al15 injected chemical carcinogenic agents in mice and observed the appearance of lung tumors when the animals were under normoxia, continuous hypoxia (SH), and IH and found that although SH increased tumor formation, no changes occurred in IH (Fig 1). More recently, Zhang et al16 reported that alteration in brain-derived neurotrophic factor (which prevents neuronal damage by oxidative stress and is linked to changes in leptin production and β-adrenergic receptors) and miR (a target of P53 promoting cell cycle arrest and tumor suppression) occurs with IH exposures. These findings may reflect indirect evidence of increased propensity for tumorigenesis.15‐17
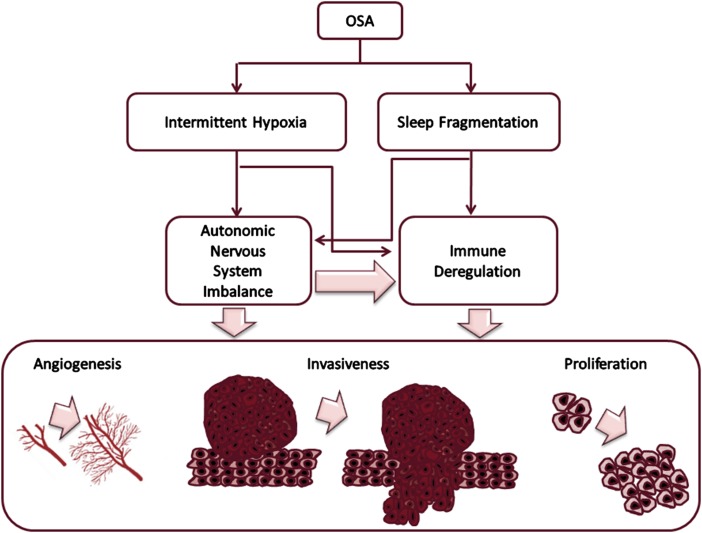
Figure 1 –
Theoretical framework of possible mechanisms by which sleep fragmentation or intermittent hypoxia in the context of OSA may not only lead to maladaptive transcriptional regulation, potentially affecting malignant transformation potential, but also disrupt sympathetic outflow and alter multiple immunoregulatory cellular targets that in turn will impose changes in the oncogenic properties of the tumor (eg, vessel formation, invasiveness, proliferation).
Does IH Affect Tumor Growth?
Rofstad et al18 induced intradermal A-07 human melanoma xenografts in mice and subjected the animals to 4 h of IH, after which tumors had increased blood perfusion, microvascular density, and vascular endothelial growth factor expression. Almendros et al19 subjected mice to a realistic IH paradigm mimicking oxygenation patterns in patients with OSA during the light hours (sleep hours in rodents). This IH pattern was applied to both obese and lean mice that were injected into the flank with murine melanoma cells, a model whereby a local tumor forms and progressively increases in size over 2 to 4 weeks, with subsequent metastases typically occurring in the lungs. The mice subjected to IH exhibited faster tumor growth rate with more necrotic regions, a signature feature of tumor aggressiveness.19,20 Interestingly, using a different cancer model that uses murine lung epithelial tumor cells in mice subjected to a nearly identical IH protocol has resulted in very similar findings.21
Does IH Mimicking OSA Promote Cancer Metastasis?
In 2001, Cairns et al22 showed that IH is more effective than SH in enhancing metastasis in vivo, even if the cycling frequency of IH was low. Rofstad et al18 also reported that mice exposed to cyclic hypoxia showed increased incidence of pulmonary metastases (approximately threefold vs normoxic mice), an effect that was ascribed to increased expression of hypoxia-vascular endothelial growth factor-A in the primary tumors, resulting in accelerated angiogenesis and blood perfusion and, therefore, facilitating tumor cell intravasation and hematogenous dissemination. Concordant with such findings, Almendros et al23 showed a sixfold increase in the incidence of micrometastases to the lung using a murine melanoma model. These findings have been replicated and further demonstrated that IH favors HIF-1α over HIF-2α transcriptional regulatory changes that seem to be conducive to higher metastatic rates by differentially regulating metalloproteinases.24 We have also expanded these observations to a lung tumor model in mice, whereby local invasion was increased under IH conditions. Of note, hyperoxia, rather than hypoxia, appears to have a beneficial effect on tumors by curtailing their growth and enabling host antitumor immunity to operate more favorably.25 The latter findings should bode well for the potential benefits of CPAP treatment in patients with OSA with concurrent malignant tumors. We should also point out that the putative effects of IH on cancer may not be always directly relevant to tumor cells alone, and the effect of IH may not only vary from one tumor cell type to another12,17‐23 but also actually necessitate the involvement of the host immune response26 as well as the autonomic nervous system (see Fig 1 and the Cancer, SF, IH, and the Sympathetic-Catecholaminergic System section).
What About SF?
To investigate the potential implications of SF on tumor proliferation and invasiveness, we exposed mice to an SF paradigm in which mice undergo periodic arousals during their sleep period but still retain their normal sleep duration and sleep state distribution.27,28 The TC-1 and 3-LLC murine cancer cell lines were injected, and accelerated tumor growth became apparent in mice exposed to SF along with clear histologic evidence of capsule invasion into adjacent tissues, with such processes appearing to be mediated, at least in part, by altered tumor-associated macrophage polarity involving innate immunity Toll-like receptor 4 pathways.29
Cancer and Immunity
Among the comprehensive array of immune effector cells, macrophages have emerged as major operators within tumors. The tumor microenvironment delivers signals that shape macrophage phenotypes into preferentially promoting tumor growth instead of attacking tumor cells through the release of growth factors, cytokines, inflammatory mediators, and proteolytic enzymes implicated in tumor growth and invasion.26 Besides the direct effect that IH and SF could directly impose on cancer cells, they can boost recruitment of macrophages and alter their polarity, thereby enhancing tumor growth, invasion, and metastasis. Interestingly, proteomic analysis revealed that IFIT1, IFIT3, TAP1, and TAP2, proteins that are involved in the interferon response and antigen processing and presentation, respectively, were markedly decreased in tumor-associated macrophages (TAMs) isolated from mice treated for IH. Also, cell surface expression of CD86 and CD40 (M1 markers) and TFRC and CD206 (M2 markers) in TAMs showed a shift toward the M2 phenotype in IH-exposed tumors. To test their different function, TAMs were isolated and cocultured with tumor cells in vitro.21 When compared with tumors of mice exposed to normoxia or normal sleep conditions, TAMs from IH- and SF-exposed mice showed a greater effect in increasing cancer cell proliferation, migration, and extravasation.
Moreover, application of IH and SF can also boost recruitment of macrophages, as suggested by histologic and flow cytometry analyses. Alterations in the tumor microenvironment can differentially activate macrophages in the adipose tissue surrounding the tumor, and, therefore, the adipose tissue becomes de facto a depot of resident macrophages that migrate toward the tumor.30 In addition, T regulatory cells and adipocyte progenitor stem cells are recruited to the tumor microenvironment in response to IH. T regulatory cells and adipocyte progenitor stem cells have been associated with increased malignancy properties of tumors, because of their ability to suppress the immune response, and by changing the stiffness of tumor stroma, respectively.30 Accordingly, host immunologic responses that are modified by the presence of OSA can become a major determinant in the potentially adverse cancer outcomes that could be anticipated in patients with OSA.
Cancer, SF, IH, and the Sympathetic-Catecholaminergic System
During arousals from sleep, surges in sympathetic outflow activity occur and are accompanied by bursts of systemic and local tissue catecholamine release, further attesting to the critical importance of the adrenergic system in arousal systems and sleep state regulation.31 We should note that increases in sympathetic outflow have been reported in humans subjected to short-term SF.32,33 Similar to SF, an extensive body of evidence indicates that IH is a powerful recruiter of sympathetic outflow both centrally and peripherally, with IH and SH eliciting very different patterns of catecholamine synthesis and release.34,35 To date, the effects of SF or IH on intratumoral levels of catecholamines or their effects on host immune cells within the tumor have yet to be explored. As will become evident, macrophages are able to synthesize and release catecholamines, which can then modulate their own activity, polarity, and fate through ligand-mediated activation of adrenergic receptors located in macrophage surface.36
The biologic effects of catecholamines result from the coordinated actions elicited by the ligation of these compounds to α1-, α2-, and β-adrenergic receptor families that are ubiquitously distributed in nearly every cell or organ.37 Notably, β-adrenergic receptor subtypes are expressed in many sites of tumor growth and metastasis, and their downstream signaling pathways regulate the function of several cellular substrates within tumors, such as epithelial cells, vascular myocytes and pericytes, adipocytes, fibroblasts, neural and glial cells, and most of the lymphoid and myeloid immune cells.38 Several studies have indicated that long-term treatment with β-blockers may reduce the prevalence and improve outcomes of several cancers in humans.39 β-Adrenergic receptor signaling can regulate multiple cellular processes involved in cancer progression, including tumor cell proliferation, extracellular matrix invasion, angiogenesis, activation of matrix metalloproteases, and inflammatory and chemotactic cytokines. Adrenergic recruitment of macrophages into the tumor leads to marked enhancements in tumor invasion and metastasis.38 In addition, macrophages and monocytes can also synthetize and release catecholamines that will in turn modulate surrounding cellular substrates within the tumor.36,38 Thus, since OSA alters sympathetic function, it may in turn alter cancer-related biologic pathways. A study has shown that the increased malignant properties triggered in tumor cells by TAMs isolated from mice exposed to IH21 can be abolished by applying inhibitors of α- and β-adrenergic receptors, thereby suggesting an important role of the sympathetic-catecholaminergic system.40
Cancer and Microvesicles/Exosomes
Exosomes are microvesicles that are actively generated and released by multiple cell types into body fluids and have been suggested as a potential mechanism of intercellular communication promoting tumor malignancy. Exosomes released from the tumor or in response to injurious stimuli in tissues include a large variety of proteins, lipids, messenger RNAs, and microRNAs. Application of hypoxia resulted in an increased release of proangiogenic extracellular exosomes41‐43 mediated by HIF.44 In addition, exosomes shed by breast cancer cells subjected to hypoxia can increase focal adhesion formation, invasion, and metastasis in nonexposed tumor cells.45 In recent work from our laboratory, plasma-derived exosomes obtained from IH and SF-exposed mice increased the malignancy of tumor cells in comparison with exosomes derived from mice in control conditions.46,47
IH, SF, and Cancer in Humans
As discussed in a previous review,48 the topic of the present article represents a clear instance where the epidemiologic evidence followed (rather than preceded or prompted) the research aimed at assessing a biologic link between a potential novel risk factor (ie, OSA) and a disease (cancer in this case). Based on the emerging evidence from the laboratory and animal experiments summarized in the preceding section, data from existing cohort studies and electronic health records have been interrogated in recent years to examine whether an association between OSA and cancer incidence or cancer mortality can be observed.
Three studies, two using population-based samples in the United States49 and in Australia50 and one using a multicenter clinical cohort in Spain,51 assessed whether markers of OSA (apnea-hypopnea index [AHI] or % time below 90% oxygen saturation [hypoxemia index (HI)]) are associated with an increased risk of cancer mortality. In a 22-year prospective follow-up analysis among participants in the Wisconsin Sleep Cohort study (n = 1,522), hazard ratios (HRs) revealed a highly statistically significant dose-response relationship for both AHI and HI; the association was stronger for the latter, however, with participants with a severe sleep breathing disorder (defined by an HI ≥ 11.2%) about 8.6 times more likely to die of cancer than those without OSA syndrome (HI < 0.8%), even after accounting for possible confounding variables (age, sex, BMI, smoking, diabetes; P for trend = .0008).49 The Australian Busselton, albeit including a smaller number of subjects (397 patients followed for 20 years), corroborated the Wisconsin study findings, whereby the fully adjusted models showed that moderate to severe OSA (AHI ≥ 15) was significantly associated with cancer mortality, with HRs of 3.4.50 In the Spanish study, 5,427 patients referred to sleep clinics because of suspicion of OSA were followed for about 4.5 years; in this study, although an association with AHI was not observed, when compared with the lower severity category, the fully adjusted HR (95% CI) of cancer mortality for the highest tertile of HI (> 13%) was 2.06 (95% CI, 1.72-4.58).51
Studies looking at cancer incidence have produced mixed results. In both the Busselton cohort50 and the Spanish cohort,51 a significant association was also observed between OSA and overall cancer incidence, although slightly weaker than that for mortality in Busselton, and also limited to the HI, but not for the AHI in the Spanish study. Two studies from Taiwan used electronic health records to assess the association between OSA and incidence of site-specific cancers.52,53 In one of these studies, based on > 90,000 claim records and a 10-year follow-up, an AHI ≥ 5/h of total sleep time was associated with a statistically significant, yet modest, increase in the risk of CNS cancers (HR = 1.5).52 In another clinic-based prospective study in Taiwan, OSA cases (n = 846) exhibited a 2.1-higher hazard of breast cancer incidence over 5 years compared with non-OSA control subjects (n = 4,230).53
Two other studies, however, found no associations between OSA and cancer incidence.54,55 In a population-based retrospective cohort (n = 8,783) in Copenhagen, Denmark, history of snoring and daytime sleepiness were not associated with cancer incidence54; inferences from this study are limited by the lack of objective measures of OSA.56 Another clinic-based study in Toronto, Ontario, Canada, that used 9,629 clinical records and covered about 8 years of follow-up (627 incident cancer cases), polysomnogram-assessed OSA was not associated with the incidence of cancer.55
Studies in humans have also addressed mechanistic questions. For example, a recent prospective pilot study suggested a direct association between a quantitative index of IH and markers of aggressiveness of human cutaneous malignant melanoma.57 Fifty-six patients with documented data of melanoma aggressiveness (tumor mitotic rate, Breslow index, presence of ulceration, stage of disease, and growth rate of melanoma) underwent an overnight sleep study, and nocturnal oxygen desaturation indexes and measures of tumor aggressiveness were examined. In the fully adjusted multivariate analyses, oxygen desaturation indexes were independently associated with an increased melanoma growth rate along with other well-established aggressiveness factors of cutaneous malignant melanoma.57 In another study assessing the transcriptomic signatures of OSA, Gharib et al58 studied gene expression in peripheral blood leukocytes from 18 patients with severe OSA before and after 1 month of CPAP treatment. Comparisons before and after treatment using gene set enrichment analyses identified gene sets traditionally involved in neoplastic processes that were markedly upregulated in OSA before treatment.58
With respect to SF, the epidemiologic evidence regarding its association with cancer in humans is as limited as the animal evidence discussed above. In fact, we are not aware of specific assessments of SF in patient cohorts and cancer, although there is substantial indirect evidence from studies demonstrating that long-term night workers and shift workers have increased incidence of solid malignancies59,60 that appear to be attributable, at least partly, to reduced melatonin secretion.61‐63 Similarly, the cumulative evidence gathered thus far would suggest that sleep duration is an important factor in cancer incidence and outcomes. In a series of meta-analyses, shift work was strongly associated with a higher risk of breast cancer,64‐66 but most if not all the studies had major sociodemographic factors and comorbidity confounders (including obesity and metabolic disease). Similarly, although some studies suggest that short sleep duration is associated with higher risk of breast cancer,67 other studies could not find evidence of such an effect.68 In a recent large prospective study in postmenopausal women from Denmark, exposures to road traffic and railway noise (presumably associated with poor sleep quality and increases in sleep discontinuity) did not identify evidence of a strong association with breast cancer but reported modestly elevated risk of estrogen receptor-negative breast cancer.69
In summary, there is growing but still limited epidemiologic evidence suggesting that OSA and other sleep disorders may be associated with cancer outcomes. Although the results from studies that examined cancer incidence are mixed, the few studies that looked at OSA in relation to cancer mortality have shown consistently stronger associations. This is consistent with the data from laboratory studies and animal models that suggest that IH, SF, or both promote changes in the tumor microenvironment and tumor growth, which suggests that OSA (via IH, SF, or both) might influence cancer progression and decrease cancer survival, rather than affect cancer initiation.70
In addition to this critical question, many other unresolved epidemiologic questions remain unresolved. The possibility of confounders (eg, obesity, smoking) cannot be ruled out entirely, although it is not highly likely, given the strong associations observed in many of the studies reviewed above even after carefully controlling for these and other factors. Another important outstanding question is whether the putative association between OSA and cancer risk or cancer mortality is generic or specific to certain cancer types. Most studies published thus far have used incidence or mortality of all cancers combined as the outcome measure. Future studies that are powered to study specific cancer sites and types are urgently needed.
Finally, at this time, evidence regarding whether proper treatment of OSA in affected patients with cancer improves cancer survival and cancer outcomes is lacking. This is an area that merits further investigation.
Conclusions and Future Directions
The overall cumulative evidence appears to support that OSA or its two major constitutive elements, SF and IH, promote increased risk of neoplastic processes. The data from epidemiologic studies in humans suggest that IH, and possibly SF, may contribute to cancer risk in OSA, but conclusive evidence is still lacking. Recent experiments in animal models have thus far provided supportive findings, whereby both IH and SF paradigms have begun to unravel their potential effects on cancer biology and are likely contributing to increases in cancer incidence and mortality in patients with sleep breathing disorders, most likely via alterations in sympathetic outflow, angiogenesis, and immune function. More intensive research is undoubtedly needed to better understand the pathophysiologic mechanisms and the clinical implications of OSA and OSA treatment on cancer prevention, cancer management, and cancer prognosis.
Acknowledgments
Conflict of interest: None declared.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- HI
hypoxemia index
- HIF
hypoxia-inducible factor
- HR
hazard ratio
- IH
intermittent hypoxia
- SF
sleep fragmentation
- SH
sustained hypoxia
- TAM
tumor-associated macrophage
Footnotes
FUNDING/SUPPORT: Dr Gozal is supported by National Institutes of Health [Grant HL-65270] and the Herbert T. Abelson Endowed Chair in Pediatrics. Dr Farré is supported by Ministerio Español de Economia y Competitividad [Grant FIS-2014/00004] and SEPAR [2014-086]. Dr Nieto is supported in part by National Institutes of Health [Grant R01HL062252-11] and by the University of Wisconsin Helfaer Endowed Chair of Public Health.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Prabhakar NR, Fields RD, Baker T, Fletcher EC. Intermittent hypoxia: cell to system. Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L524-L528. [DOI] [PubMed] [Google Scholar]
- 2.Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. 2014;307(2):L129-L140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mateika JH, El-Chami M, Shaheen D, Ivers B. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J Appl Physiol (1985). 2015;118(5):520-532. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92(3):967-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Span PN, Bussink J. Biology of hypoxia. Semin Nucl Med. 2015;45(2):101-109. [DOI] [PubMed] [Google Scholar]
- 6.Nanduri J, Prabhakar NR. Intermittent hypoxia: mechanistic pathways influencing cancer. In: Redline S, Berger NA, eds. Impact of Sleep and Sleep Disturbances on Obesity and Cancer. New York, NY: Springer; 2014. [Google Scholar]
- 7.Almendros I, Farré R, Planas AM, et al. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34(8):1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121(11):2381-2386. [DOI] [PubMed] [Google Scholar]
- 9.Kontogianni K, Messini-Nikolaki N, Christou K, Gourgoulianis K, Tsilimigaki S, Piperakis SM. DNA damage and repair capacity in lymphocytes from obstructive sleep apnea patients. Environ Mol Mutagen. 2007;48(9):722-727. [DOI] [PubMed] [Google Scholar]
- 10.Toffoli S, Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J. 2008;275(12):2991-3002. [DOI] [PubMed] [Google Scholar]
- 11.Zhu H, Wang D, Zhang L, et al. Upregulation of autophagy by hypoxia-inducible factor-1α promotes EMT and metastatic ability of CD133+ pancreatic cancer stem-like cells during intermittent hypoxia. Oncol Rep. 2014;32(3):935-942. [DOI] [PubMed] [Google Scholar]
- 12.Miao ZF, Zhao TT, Wang ZN, et al. Influence of different hypoxia models on metastatic potential of SGC-7901 gastric cancer cells. Tumour Biol. 2014;35(7):6801-6808. [DOI] [PubMed] [Google Scholar]
- 13.Carreras A, Kayali F, Zhang J, Hirotsu C, Wang Y, Gozal D. Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period. Am J Physiol Regul Integr Comp Physiol. 2012;303(7):R700-R709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17(4):273-284. [DOI] [PubMed] [Google Scholar]
- 15.Karoor V, Le M, Merrick D, Fagan KA, Dempsey EC, Miller YE. Alveolar hypoxia promotes murine lung tumor growth through a VEGFR-2/EGFR-dependent mechanism. Cancer Prev Res (Phila). 2012;5(8):1061-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Guo X, Shi Y, Ma J, Wang G. Intermittent hypoxia with or without hypercapnia is associated with tumorigenesis by decreasing the expression of brain derived neurotrophic factor and miR-34a in rats. Chin Med J (Engl). 2014;127(1):43-47. [PubMed] [Google Scholar]
- 17.Magnon C. Role of the autonomic nervous system in tumorigenesis and metastasis. Molecular & Cellular Oncology. 2015;2(2):e975643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rofstad EK, Gaustad JV, Egeland TA, Mathiesen B, Galappathi K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int J Cancer. 2010;127(7):1535-1546. [DOI] [PubMed] [Google Scholar]
- 19.Almendros I, Montserrat JM, Ramírez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39(1):215-217. [DOI] [PubMed] [Google Scholar]
- 20.Almendros I, Montserrat JM, Torres M, et al. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012;13(10):1254-1260. [DOI] [PubMed] [Google Scholar]
- 21.Almendros I, Wang Y, Becker L, et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2014;189(5):593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cairns RA, Kalliomaki T, Hill RP. Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res. 2001;61(24):8903-8908. [PubMed] [Google Scholar]
- 23.Almendros I, Montserrat JM, Torres M, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol. 2013;186(3):303-307. [DOI] [PubMed] [Google Scholar]
- 24.Eubank T, Sherwani S, Peters S, Gross A, Evans R, Magalang UJ. Intermittent hypoxia augments melanoma tumor metastases in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2013;187:A2302. [Google Scholar]
- 25.Hatfield SM, Kjaergaard J, Lukashev et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med. 2015;7(277):277ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair D, Zhang SXL, Ramesh V, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184(11):1305-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramesh V, Nair D, Zhang SX, et al. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J Neuroinflammation. 2012;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakim F, Wang Y, Zhang SX, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74(5):1329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almendros I, Gileles-Hillel A, Khalyfa A, et al. Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: effect of tumor microenvironment. Cancer Lett. 2015;361(2):233-239. [DOI] [PubMed] [Google Scholar]
- 31.Hunsley MS, Palmiter RD. Altered sleep latency and arousal regulation in mice lacking norepinephrine. Pharmacol Biochem Behav. 2004;78(4):765-773. [DOI] [PubMed] [Google Scholar]
- 32.Chouchou F, Pichot V, Pépin et al; PROOF Study Group. Sympathetic overactivity due to sleep fragmentation is associated with elevated diurnal systolic blood pressure in healthy elderly subjects: the PROOF-SYNAPSE study. Eur Heart J. 2013;34(28):2122-2131. [DOI] [PubMed] [Google Scholar]
- 33.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197-210. [DOI] [PubMed] [Google Scholar]
- 34.Prabhakar NR, Kumar GK, Peng YJ. Sympatho-adrenal activation by chronic intermittent hypoxia. J Appl Physiol (1985). 2012;113(8):1304-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hakim F, Gozal D, Kheirandish-Gozal L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front Neurol. 2012;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480(7375):104-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly CJ, McGrath JC. Previously unsuspected widespread cellular and tissue distribution of β-adrenoceptors and its relevance to drug action. Trends Pharmacol Sci. 2011;32(4):219-226. [DOI] [PubMed] [Google Scholar]
- 38.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18(5):1201-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29(19):2635-2644. [DOI] [PubMed] [Google Scholar]
- 40.Almendros I, Khalyfa A, Gileles-Hillel A, Qiao Z, Farre R, Gozal D. Intermittent hypoxia-induced adrenergic alterations modulate tumor proliferation in a mouse model of obstructive sleep apnea. Am J Respir Crit Care Med. 2015;191:A2699. [Google Scholar]
- 41.Park JE, Tan HS, Datta A, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9(6):1085-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson KJ, Kucharzewska P, Christianson HC, et al. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci U S A. 2011;108(32):13147-13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kucharzewska P, Christianson HC, Welch JE, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang T, Gilkes DM, Takano N, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111(31):E3234-E3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almendros I, Khalyfa A, Gileles-Hillel A, Farre R, Gozal D. Tumor cell malignant properties are enhanced by circulating exosomes in a mouse model of intermittent hypoxia mimicking sleep apnea. Am J Respir Crit Care Med. 2015;191:A5154. [Google Scholar]
- 47.Khalyfa A, Almendros I, Gileles-Hillel A, Gozal D. Exosomes released into the circulation under chronic sleep fragmentation potentiate tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2015;191:A2693. [Google Scholar]
- 48.Nieto FJ, Farré R. Association of sleep apnea and cancer: from animal studies to human epidemiologic data. In: Berger N, Redline S, eds. Impact of Sleep and Sleep Disturbances on Obesity and Cancer. New York, NY: Springer; 2014:121-136. [Google Scholar]
- 49.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186(2):190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. ; Spanish Sleep Network. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99-105. [DOI] [PubMed] [Google Scholar]
- 52.Chen JC, Hwang JH. Sleep apnea increased incidence of primary central nervous system cancers: a nationwide cohort study. Sleep Med. 2014;15(7):749-754. [DOI] [PubMed] [Google Scholar]
- 53.Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, Lin YW, Tsai SJ. Sleep apnea and the subsequent risk of breast cancer in women: a nationwide population-based cohort study. Sleep Med. 2014;15(9):1016-1020. [DOI] [PubMed] [Google Scholar]
- 54.Christensen AS, Clark A, Salo P, et al. Symptoms of sleep disordered breathing and risk of cancer: a prospective cohort study. Sleep. 2013;36(10):1429-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kendzerska T, Leung RS, Hawker G, Tomlinson G, Gershon AS. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ. 2014;186(13):985-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peppard PE, Nieto FJ. Here come the sleep apnea-cancer studies. Sleep. 2013;36(10):1409-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martínez-García MA, Martorell-Calatayud A, Nagore E, et al. Association between sleep disordered breathing and aggressiveness markers of malignant cutaneous melanoma. Eur Respir J. 2014;43(6):1661-1668. [DOI] [PubMed] [Google Scholar]
- 58.Gharib SA, Seiger AN, Hayes AL, Mehra R, Patel SR. Treatment of obstructive sleep apnea alters cancer-associated transcriptional signatures in circulating leukocytes. Sleep. 2014;37(4):709-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176(9):751-759. [DOI] [PubMed] [Google Scholar]
- 60.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18(1):182-183. [DOI] [PubMed] [Google Scholar]
- 61.Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, Reynolds P. Light at night and breast cancer risk among California teachers. Epidemiology. 2014;25(5):697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blask DE, Dauchy RT, Dauchy EM, et al. Light exposure at night disrupts host/cancer circadian regulatory dynamics: impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS ONE. 2014;9(8):e102776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dauchy RT, Xiang S, Mao L, et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014;74(15):4099-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jia Y, Lu Y, Wu K, et al. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013;37(3):197-206. [DOI] [PubMed] [Google Scholar]
- 65.Ijaz S, Verbeek J, Seidler A, et al. Night-shift work and breast cancer—a systematic review and meta-analysis. Scand J Work Environ Health. 2013;39(5):431-447. [DOI] [PubMed] [Google Scholar]
- 66.Wang F, Yeung KL, Chan WC, et al. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann Oncol. 2013;24(11):2724-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kakizaki M, Kuriyama S, Sone T, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99(9):1502-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res. 2006;15(3):241-249. [DOI] [PubMed] [Google Scholar]
- 69.Sørensen M, Ketzel M, Overvad K, Tjønneland A, Raaschou-Nielsen O. Exposure to road traffic and railway noise and postmenopausal breast cancer: a cohort study. Int J Cancer. 2014;134(11):2691-2698. [DOI] [PubMed] [Google Scholar]
- 70.Nieto FJ. Cancer: an epidemiological perspective. In: Barbe F, Pepin JL, eds. Obstructive Sleep Apnea. ERS Monograph 2015;67:205-220. [Google Scholar]