Abstract
BACKGROUND:
COPD is the third most frequent cause of death globally, with much of this burden attributable to household biomass smoke exposure in developing countries. As biomass smoke exposure is also associated with cardiovascular disease, lower respiratory infection, lung cancer, and cataracts, it presents an important target for public health intervention.
METHODS:
Lung function in Guatemalan women exposed to wood smoke from open fires was measured throughout the Randomized Exposure Study of Pollution Indoors and Respiratory Effects (RESPIRE) stove intervention trial and continued during the Chronic Respiratory Effects of Early Childhood Exposure to Respirable Particulate Matter (CRECER) cohort study. In RESPIRE, early stove households received a chimney woodstove at the beginning of the 18-month trial, and delayed stove households received a stove at trial completion. Personal exposure to wood smoke was assessed with exhaled breath carbon monoxide (CO) and personal CO tubes. Change in lung function between intervention groups and as a function of wood smoke exposure was assessed using random effects models.
RESULTS:
Of 306 women participating in both studies, acceptable spirometry was collected in 129 early stove and 136 delayed stove households (n = 265), with a mean follow-up of 5.6 years. Despite reduced wood smoke exposures in early stove households, there were no significant differences in any of the measured spirometric variables during the study period (FEV1, FVC, FEV1/FVC ratio, and annual change) after adjustment for confounding.
CONCLUSIONS:
In these young Guatemalan women, there was no association between lung function and early randomization to a chimney stove or personal wood smoke exposure. Future stove intervention trials should incorporate cleaner stoves, longer follow-up, or potentially susceptible groups to identify meaningful differences in lung function.
Household air pollution from cooking with solid fuels is estimated to be the third most important global risk factor for disability-adjusted life years lost and the most important environmental health risk factor.1 It has been associated with low infant birth weight and acute lower respiratory tract infections in children, as well as cardiovascular disease, lung cancer, cataracts, and COPD in adults. Household air pollution is ubiquitous, with > 3 billion people worldwide continuing to use solid fuel for cooking or heating leading to dramatic indoor air pollution, with concentrations of particulate matter that are often 30 times higher than the air quality guidelines of the World Health Organization.2,3
Although abundant epidemiologic evidence suggests that chronic biomass smoke exposure is associated with COPD,4‐7 results from stove trials have been less consistent. Although an intention-to-treat (ITT) analysis from the Randomized Exposure Study of Pollution Indoors and Respiratory Effects (RESPIRE) in Guatemala did not identify a difference in lung function decline in young adult women receiving a chimney stove compared with open fire users over 12 to 18 months of follow-up,8 an exposure-response analysis showed that higher exhaled breath carbon monoxide (EBco) was associated with lower FEV1.9 Although the stove reduced exposures to wood smoke by > 60% based on personal carbon monoxide (CO) measurements, exposure to smoke remained high, with significant overlap in exposure distributions in the control and intervention groups.10
In this same study, receiving a chimney stove reduced respiratory symptoms during follow-up, and this reduction was significant for wheeze (relative risk, 0.42; 95% CI, 0.25-0.70).8 Additionally, increases in proxy measures of wood smoke exposure (EBco and personal CO) were associated with increased respiratory symptoms, including cough, phlegm, wheeze, and chest tightness.9 In a subgroup of these women, higher expression of genes associated with COPD pathogenesis was identified among continued open fire users (matrix metalloproteinase [MMP]-9) and among those with higher personal wood smoke exposure (MMP-9, MMP-12, IL-8, and tumor necrosis factor-α), which may suggest future COPD risk.11
In a randomized controlled trial of a different chimney stove intervention in Mexico, an ITT analysis again showed no difference in lung function between intervention and control groups of women. However, women who reported regularly using the stove had a slower decline in FEV1 over a 10-month follow-up period.12 Both the Mexican and Guatemalan stove intervention studies were limited by short follow-up and by following a group of young women, who may require many years of cumulative exposure before the accelerated lung function decline that precedes COPD becomes apparent. More recently, in a prospective cohort of adults in southern China, switching to cleaner fuels (biogas) and/or improving ventilation of biomass smoke with the addition of a chimney or exhaust fan was associated with a slower decline in FEV1 and fewer new cases of COPD over a 9-year period.13 These results are encouraging but need to be replicated in a study with an experimental design.
We attempted to address this research gap by taking advantage of > 5 years of spirometry follow-up in women participating in RESPIRE and the Chronic Respiratory Effects of Early Childhood Exposure to Respirable Particulate Matter (CRECER) cohort study. During the CRECER study, spirometry and exposure assessments were collected on nonsmoking adult women participants 4 to 5 years after the end of RESPIRE.
We hypothesized that decrements in lung function from the beginning of RESPIRE to the end of the CRECER study follow-up would be smaller in the group that received a chimney stove earlier in RESPIRE because of reduced cumulative exposure to wood smoke.
Materials and Methods
Study Design and Participants
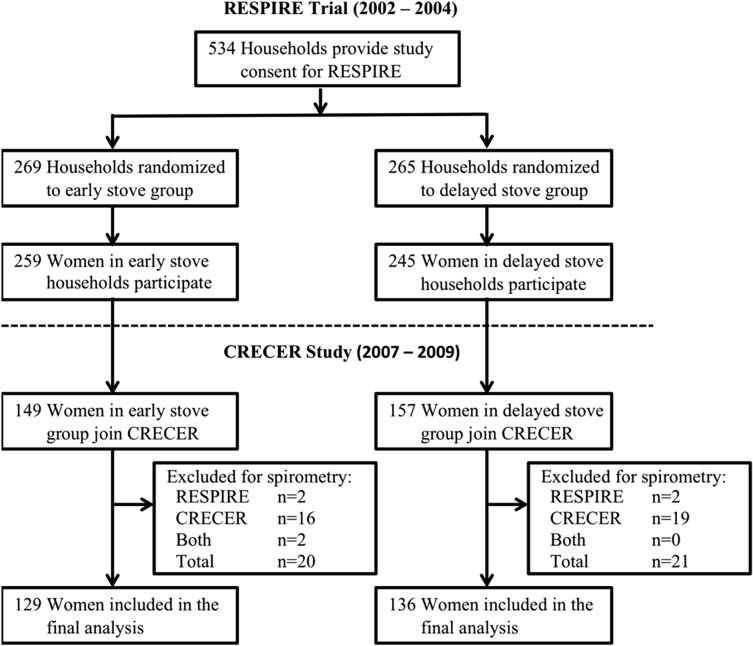
RESPIRE was a randomized controlled trial that included 534 households using traditional open wood fires for cooking located in the rural highlands of northwestern Guatemala (Fig 1). Five hundred four women from these households were recruited between October 2002 to May 2003 to participate in the adult component of RESPIRE. Households were randomized in permuted blocks of 10 to receive a chimney woodstove (plancha) or to continue cooking over an open fire between October 2002 and December 2004, after which all households received the stove. Adult women in the household were followed for 12 months to 18 months with health and exposure questionnaires, biomass smoke exposure assessments, and spirometry at baseline and every 6 months thereafter. At the end of RESPIRE, all control households (the delayed stove group) then received the chimney woodstove. Full details of RESPIRE have been published elsewhere.8,14,15
Figure 1 –
Participant profile. CRECER = Chronic Respiratory Effects of Early Childhood Exposure to Respirable Particulate Matter; RESPIRE = Randomized Exposure Study of Pollution Indoors and Respiratory Effects.
Following the end of RESPIRE, 306 women were recruited to participate in the CRECER cohort study, during which health and exposure questionnaires, spirometry, and exposure assessments were conducted between November 2008 and March 2009. As all CRECER participants now had a chimney stove, we examined the effects of differential exposure during the 12- to 18-month RESPIRE trial period on lung function over the duration of RESPIRE and the CRECER study.
Oral informed consent was obtained by local trained fieldworkers fluent in each participant’s primary language. RESPIRE was approved by the research ethics committees (Table 1) of the University of Bergen (Bergen, Norway), University of Liverpool (Liverpool, England), University of California, Berkeley (Berkeley, California), and Universidad Del Valle Guatemala (Guatemala City, Guatemala). The CRECER study was approved by the research ethics committees of University of California, Berkeley, the University of California, San Francisco (San Francisco, California ), the University of Liverpool, and Universidad Del Valle Guatemala. These studies were conducted in accordance with the amended Declaration of Helsinki.
TABLE 1 ] .
Ethics Committee Approval Numbers
| Institution | RESPIRE No. | CRECER Study No. |
| Universidad del Valle, Guatemala | 001-10-2007 | 002-12-2007 |
| University of Bergen | REK Vest nr. 122.02 | … |
| University of California, Berkeley | 2010-11-2469 | 2010-05-1553 |
| University of California, San Francisco | 14-14738 | |
| University of Liverpool | UoL # 00008210 | UoL # 00008210 |
CRECER = Chronic Respiratory Effects of Early Childhood Exposure to Respirable Particulate Matter; RESPIRE = Randomized Exposure Study of Pollution Indoors and Respiratory Effects.
Spirometry
Using a portable spirometer, we measured FEV1 and FVC without a bronchodilator. Spirometry was conducted at enrollment and every 6 months thereafter during RESPIRE. Spirometry was repeated one to two times during the CRECER study. As a result, spirometry over 4 to 6 years of follow-up with four to six sessions per participant was available for this cohort of women. Only measurements that were acceptable and reproducible by 1994 American Thoracic Society criteria were included.16,17 Airflow obstruction was defined as having FEV1/FVC ≤ 0.7; new airflow obstruction was defined as having airflow obstruction at the final spirometry session but a normal ratio at baseline. Reference values were calculated from the National Health and Nutrition Examination Survey (NHANES) III-derived equation for Mexican Americans.16 A MicroLoop (MDSpiro) portable spirometer was used for all RESPIRE spirometry, and an EasyOne (ndd Medical Technologies, Inc) portable spirometer was used for all CRECER spirometry.
To account for differences in spirometers, we conducted a validation study to estimate a correction factor. Spirometry was collected on 24 CRECER participants on the same day using both spirometers; 14 sessions were performed on the MicroLoop first and 10 sessions were performed on the EasyOne first. The paired spirometry data were regressed against each other to create a correction factor for EasyOne results. The EasyOne produced consistently lower values for FEV1 (mean correction, + 76 mL) and FVC (mean correction, + 131 mL) than the MicroLoop and were corrected according to the following equations:
Wood Smoke Exposure Assessment
In all RESPIRE participants, personal exposures to products of incomplete combustion were measured in two ways: EBco and personal CO tube measurements. Personal CO tubes (GASTEC Corporation) passively measure personal exposure to CO (in parts per million [ppm]) over a 48-h period, which correlates well with personal exposure to fine particulate matter in this population.18,19 Personal CO tubes were collected 4 to 5 times during RESPIRE, noncoincident with spirometry visits. EBco (CareFusion Corporation) is an indirect measurement of serum carboxyhemoglobin; measurements were collected following spirometry at every 6-month visit during RESPIRE and after each spirometry session in the CRECER study. EBco measurements were repeated three times at each session, and the mean of the two highest measurements was recorded. EBco monitors were calibrated against a standard CO span gas every 2 to 4 weeks during the study periods.14,20 Personal CO measurement calibration and quality control were maintained according to previously described protocols.2
Statistical Analysis
Univariate comparisons between the two treatment groups were performed using unpaired t tests, Wilcoxon rank sum, χ2, and Fisher exact tests as appropriate. Pearson correlations and Spearman correlations were used to assess correlations.
The primary end point was the difference in FEV1 during RESPIRE and the CRECER study between the early and delayed stove intervention groups. Additionally, difference in FVC and FEV1/FVC ratio was assessed between trial arms. Exposure-response analyses were conducted using visit-specific EBco measurements and mean postintervention personal CO tube measurements for the participants. Random effects models were used to assess changes in repeated measures of spirometry between intervention groups and in exposure-response analyses. All 95% CIs were based on a robust estimator.
The baseline covariates included age (median-centered) and age squared; height; weight; baseline FEV1, FVC, and FEV1/FVC ratio; asset index; cooking structure; secondhand smoke exposure; altitude; and frequency of temazcal use. Additionally, time-varying covariates included time since first spirometry visit and season. The asset index, used to assess socioeconomic status, is a number from 0 to 6 based on self-reported possession of the following items (each adding 1 point): radio, television, refrigerator, bicycle, motorcycle, or automobile.21 Cooking structure location was dichotomized based on whether the kitchen was located within the main living structure or as a separate cooking structure. A temazcal is an indigenous wood-fueled sauna that has the potential to expose women to extremely high concentrations of biomass smoke. Temazcal use was categorized as none, one to four times per month, or more than four times per month for all analyses. Secondhand tobacco smoke was considered present if anyone in the household was a current smoker. Doctor-diagnosed asthma and active tobacco use among study participants were excluded from analysis given their rarity in this population (n = 1 among all participants for both variables).
All models included exposure to secondhand tobacco smoke, age, and height. A backward elimination process was used for inclusion of other potential confounders in the final model if their inclusion resulted in a change in the β coefficient of ≥ 5% in the association between the main exposure (intervention group in RESPIRE) with the primary outcome (FEV1). We also stratified by age in subanalyses. All statistical analysis was performed using Stata software version 12.1 (StataCorp LP).
Results
Among 306 women enrolled in the CRECER study, 265 women had at least one acceptable spirometry session during both RESPIRE and the CRECER study and were included in the final analysis, with an average of 5.6 years (SD, 0.27) of follow-up and 4.1 spirometry sessions (SD, 0.84) per participant. Forty-one women were excluded for lacking at least one session of acceptable spirometry during RESPIRE (n = 4), the CRECER study (n = 35), or both (n = 2). During RESPIRE, 92% of spirometry sessions were acceptable (799 of 872 sessions among 306 participants). During CRECER, 87.3% of sessions were acceptable (283 of 324 sessions).
The early and delayed stove groups were similar at baseline with respect to sociodemographic characteristics (Table 2). Baseline values of FEV1, FVC, and FEV1/FVC ratio also were similar between the two groups. One individual in each stove group met criteria for airflow obstruction at baseline. The 41 excluded participants were similar to included participants with respect to mean baseline FEV1 % predicted (excluded 105% vs included 104%), FVC (106% vs 104%), age (29.0 years vs 28.2 years), height (143.5 cm vs 144.5 cm), and weight (48.2 kg vs 50.4 kg).
TABLE 2 ] .
Baseline Characteristics of Guatemalan Women Participants in RESPIRE and the CRECER Study From the Early Stove (Intervention) and Delayed Stove (Control) Groups
| Characteristics | Early Stove (n = 129) | Delayed Stove (n = 136) |
| Age, y | 27.9 (7.0) | 28.5 (7.0) |
| Height, cm | 144.5 (4.9) | 144.5 (4.3) |
| Weight, kg | 50.3 (5.5) | 50.4 (7.3) |
| Cooking location, No. (%) | ||
| Main house, separated by a wall | 3 (2.3) | 2 (1.5) |
| Main house, same area as bedroom | 18 (14) | 15 (11) |
| Separate enclosed cooking structure | 99 (77) | 106 (78) |
| Separate open structure (≥ 1 wall missing) | 9 (7) | 13 (10) |
| Temazcal use category, No. (%) | ||
| None | 16 (12) | 12 (8.8) |
| 1-4 times/mo | 62 (48) | 63 (46) |
| > 4 times/mo | 51 (40) | 61 (45) |
| Smoker present in home, No. (%) | 26 (20) | 37 (27) |
| Asset indexa | 1.26 (0.73) | 1.18 (0.76) |
| Baseline spirometry | ||
| FEV1, L | 2.67 (0.3) | 2.68 (0.4) |
| FEV1 % predicted | 104 (11.8) | 104 (9.9) |
| FVC, L | 3.09 (0.4) | 3.12 (0.4) |
| FVC % predicted | 103 (10.6) | 104 (12.3) |
| FEV1/FVC % | 86.2 (9.4) | 85.8 (5.6) |
| Airflow obstruction (FEV1/FVC < 0.7), No. (%) | 1 (0.8) | 1 (0.7) |
Data are presented as mean (SD) unless otherwise noted. See Table 1 legend for expansion of abbreviations.
Asset index is a number from 0 to 6 calculated based on possession of the following items (each adding 1 point): radio, television, refrigerator, bicycle, motorcycle, or automobile.
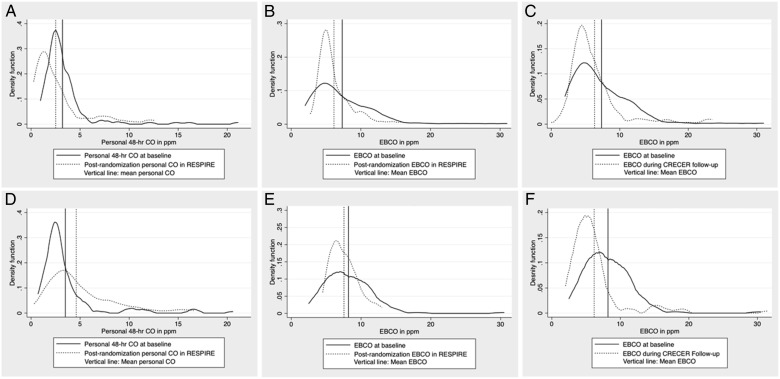
There was a significant decline in EBco in both groups during the RESPIRE follow-up period, with a mean decrease of 1.21 ppm in the early stove and 0.67 ppm in the delayed stove group (Fig 2, Table 3). There was a significant decline in EBco in both groups comparing baseline measurements collected during RESPIRE to measurements during the CRECER study, with a mean decrease of 0.94 ppm in the early stove and 2.11 ppm in the delayed stove groups. Personal CO tube measurements were similar at baseline for the early stove and delayed stove groups, at 3.2 ppm (SD, 2.4) and 3.5 ppm (SD, 3.0), respectively (P = .39). There was a mean decline in tube CO concentrations in the early stove group of 0.89 ppm (SD, 3.1) during the intervention period but an increase in the delayed stove group of 1.11 ppm (SD, 3.5) during this same period. As the distributions for EBco and personal CO tube data were positively skewed, the natural log transformations of these values were used for all regression analyses. Spearman correlation for mean postrandomization values of EBco and personal CO tube measurements was 0.30 (P < .001).
Figure 2 –
Kernel density plots of personal CO and exhaled breath CO at baseline and follow-up in the early stove and delayed stove groups. A, Personal CO, early stove group. B, EBCO in RESPIRE, early stove group. C, EBCO in CRECER, early stove group. D, Personal CO, delayed stove group. E, EBCO in RESPIRE, delayed stove group. F, EBCO in CRECER, delayed stove group. CO = carbon monoxide; EBCO = exhaled breath carbon monoxide. See Figure 1 legend for expansion of other abbreviations.
TABLE 3 ] .
Exposure Assessments During RESPIRE and the CRECER Study, EBco, and Personal 48-h CO Tubes
| Exposure Metric | Early Stove (Intervention), Mean (SD) | Delayed Stove (Control), Mean (SD) | Mean Difference (95% CI) |
| EBco (in 1 ppm) | |||
| EBco at baseline visit (preintervention) | 7.33 (4.6) | 8.20 (3.6) | 0.87 (−0.12 to 1.85) |
| EBco, RESPIRE follow-upa | 6.14 (2.5)b | 7.55 (1.9) | 1.42 (0.88 to 1.96)c |
| No. of measurements per individual | 4.19 (0.8)b | 4.13 (0.9) | … |
| EBco, CRECER study | 6.32 (4.2)b | 6.18 (4.0)b | −0.14 (−1.17 to 0.89) |
| Change in EBco, baseline to RESPIRE follow-up | −1.21 (5.0)c | −0.66 (3.5)c | −0.67 (−1.18 to −0.14)c |
| Change in EBco, baseline to CRECER study follow-up | −0.94 (5.7) | −2.11 (5.0)c | 1.15 (−0.20 to 2.50) |
| Personal 48-h CO tubes (in 1 ppm) | |||
| CO tube at baseline visit | 3.20 (2.4) | 3.49 (3.0) | 0.30 (−0.37 to 0.96) |
| CO tube at follow-upd | 2.31 (1.9)b | 4.60 (2.5) | 2.28 (1.74 to 2.83)c |
| No. of measurements per individual | 2.51 (0.6)b | 2.46 (0.6) | … |
| Change in CO tubes, baseline to RESPIRE follow-up | −0.89 (3.1)c | +1.11 (3.5)c | 1.99 (1.19 to 2.80)c |
CO = carbon monoxide; EBco = exhaled breath carbon monoxide; ppm = parts per million. See Table 1 legend for expansion of other abbreviations.
Mean of 6-mo, 12-mo, and 18-mo EBco measurements during RESPIRE.
Post stove installation exposure measurements.
Statistically significant differences (P < .05).
Mean of three to four rounds of CO tube measurements during RESPIRE.
The final regression model used for all analyses included the following covariates: age, height, frequency of temazcal use, secondhand smoke exposure, time since first spirometry, and baseline lung function. Season, age squared, altitude, weight, asset index, and cookstove location were excluded from the final model based on the model selection criteria outlined previously. A restricted F test confirmed that the restricted model fits as well as the full model.
There were no significant differences between stove groups for any of the measured spirometric variables before or after adjustment for covariates (Table 4). Likewise, exposure-response analyses did not identify any significant associations between either EBco or personal tube CO concentrations and changes in lung function. FEV1 decreased by 11.1 mL/y (95% CI, −15.5 to −6.8) overall, with similar declines of 13.0 mL/y and 9.5 mL/y in the early stove and delayed stove groups, respectively (Table 5). FVC increased by 15.9 mL/y (95% CI, 10.7-21.1) overall. There was one new case of airflow obstruction in the early stove group (0.8%) and three new cases of airflow obstruction in the delayed stove group (2.2%), respectively.
TABLE 4 ] .
Changes in Spirometry Associated With Stove Group Assignment, EBco, and Personal 48-h CO Tube Measurements
| Early vs Delayed Stove Group (n = 265) | Log EBco (n = 265) | Log CO Tubes (n = 260) | ||||
| Model | β-Coefficient | 95% CI | β-Coefficient | 95% CI | β-Coefficient | 95% CI |
| FEV1 (unadjusted), mL | −10.6 | −94.4 to 73.2 | 6.6 | −16.0 to 29.2 | 1.1 | −49.9 to 52.2 |
| FEV1 (adjusted),a mL | −13.0 | −41.1 to 15.4 | −0.46 | −20.8 to 19.9 | 13.2 | −5.4 to 31.8 |
| Final FVC (unadjusted), mL | −41.6 | −138.2 to 55.0 | −22.3 | −54.1 to 9.5 | 9.8 | −48.8 to 68.3 |
| Final FVC (adjusted), mL | −9.4 | −48.2 to 29.3 | −5.5 | −24.3 to 35.5 | 6.5 | −17.0 to 30.0 |
| FEV1/FVC (unadjusted), % | 0.84 | −0.22 to 1.89 | 0.60 | 0.011 to 1.20 | −0.21 | −0.80 to 0.38 |
| FEV1/FVC (adjusted), % | −0.058 | −0.74 to 0.62 | −0.182 | −0.78 to 0.42 | 0.22 | −0.21 to 0.65 |
β-Coefficients represent the change in lung function for each 1-unit increase in ln-transformed CO (1 ppm) from a random effects model. See Table 3 legend for expansion of abbreviations.
Final model adjusted for baseline age, height, baseline spirometry, time since baseline spirometry, secondhand smoke, and frequency of temazcal use.
TABLE 5 ] .
Change in Spirometry From the Beginning of RESPIRE to the End of the CRECER Study
| Spirometry | Early Stove (Intervention) (n = 129) | Delayed Stove (Control) (n = 136) |
| Follow-up, y | 5.59 (0.2) | 5.58 (0.2) |
| Annual change in FEV1, mL/y | −13.0 (3.1) | −9.5 (3.1) |
| Annual change in FVC, mL/y | 13.6 (3.8) | 18.0 (3.7) |
| Annual change in ratio, %/y | −0.82 (0.065) | −0.80 (0.069) |
| Airflow obstruction at baseline, FEV1/FVC < 0.7, No. (%) | 1 (0.8) | 1 (0.7) |
| New cases of airflow obstruction, No. (%) | 1 (0.8) | 3 (2.2) |
Data are presented as mean (SE) unless otherwise noted. See Table 1 legend for expansion of abbreviations.
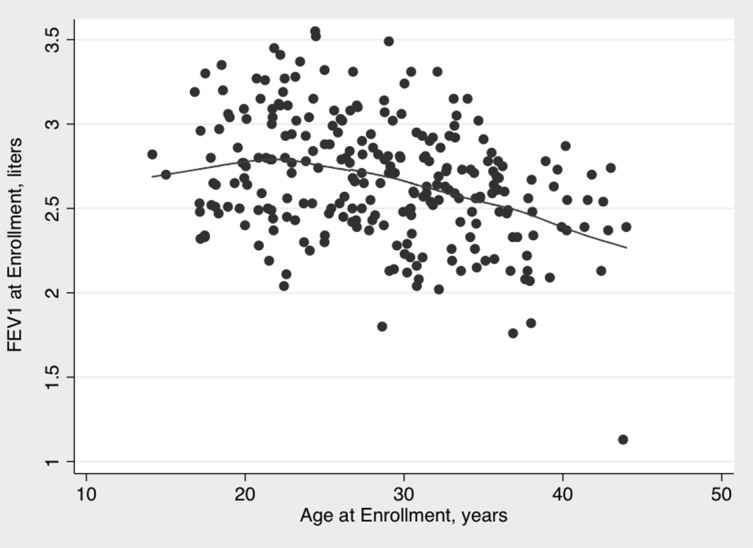
As lung function typically peaks around age 20 to 25 years in women, we performed a sensitivity analysis among participants who were ≥ 25 years old (n = 170) at baseline (Fig 3). There was no association between FEV1 and intervention group (β = −1.1, P = .95), ln-EBco (β = 8.6, P = .49), or ln-CO tubes (β = 5.6, P = .62) on adjusted analysis. The rate of decline of lung function was similar to that seen in the full analysis when limited to this age group, with a decline of 9.8 mL/y (95% CI, −14.3 to −5.3) for FEV1 and increase of 17.0 mL/y (95% CI, 10.9-23.0) for FVC. Finally, in a post hoc power analysis assuming a standard deviation of 0.35 L for FEV1 and assuming a conservative intraclass correlation of 0.7 for repeated measures of FEV1, we had 89% power to detect a 5% difference in FEV1 with this sample.
Figure 3 –
Baseline FEV1 and participant age at baseline, with locally weighted scatterplot smoothing fit line.
Discussion
In this study of 265 healthy, wood smoke-exposed women in rural Guatemala, there was no difference in lung function between the early stove (intervention) and delayed stove (control) groups with 12 to 18 months of differential exposure to a chimney stove with an average of > 5 years of follow-up. Additionally, no associations between personal wood smoke exposure and lung function changes were identified. These results are novel in that they are from, to our knowledge, the first randomized controlled stove intervention trial to measure personal exposure and lung function on a large cohort of wood smoke-exposed women with > 1,400 person-years of follow-up.
If an association between wood smoke exposure and lung function exists in this population, there are notable study limitations that may explain these negative findings. Perhaps foremost, the women differed in their exposure to wood smoke only during the 12- to 18-month RESPIRE, when the delayed stove group continued to cook over an open fire; at the end of this trial all women received a chimney stove. Any difference in lung function that may have existed at the end of this short period of differential smoke exposure may have diminished over the ensuing 3 to 4 years before spirometry was performed in the CRECER study. Moreover, both groups remained heavily exposed after stove installation; in the early stove group exposure decreased by only 30% following stove installation based on personal CO monitoring (Table 3). Many households may not have switched fully to using the stove, and use may have diminished further over years of follow-up. Additionally, personal exposure monitoring does not account for dramatic exposures these women experience while using the temazcal, with average CO exposures that are 10 times higher than the 15-min World Health Organization guideline for indoor air quality; additional research into the impact of this unique exposure on lung health is warranted.22,23 For these reasons, the overall impact of the stove on participants’ cumulative lifetime exposure during these two studies was likely small. Finally, because the exposure-response relationship between particulate matter and lung function may be nonlinear (with smaller effects at higher exposure levels), more substantial reductions in exposure may be necessary to impact lung function.10
Little is known about whether early spirometric changes that precede tobacco-associated COPD would also be present in these young individuals with biomass exposure. There is evidence to suggest that the age at onset and natural history of biomass-associated COPD is different than that observed with tobacco-associated COPD. In a recent cohort study in Mexico, the rate of lung function decline was greater in women with tobacco-associated COPD compared with biomass-associated COPD (42 mL/y vs 23 mL/y), and those with biomass-associated COPD were older on average and with less severe COPD.24 It is also interesting that these chronically biomass-exposed women had, on average, > 100% predicted values for FEV1 and FVC. Although this may suggest that lung function decline is a late finding in biomass smoke-exposed individuals, this could also be due to our use of the NHANES equation for Mexican-Americans for these Guatemalan women, who generally self-identify as being of indigenous Mayan extraction. Similar overestimations in percent predicted values for lung function have previously been observed in children from Mexico City using the NHANES prediction.25
The use of different spirometers during RESPIRE and the CRECER study complicates study interpretation. Although a correction factor for spirometer type was used, this could contribute to measurement error. As the spirometer type was consistent within each of the RESPIRE and CRECER studies, thereby affecting the early and delayed stove groups equally, this likely led to nondifferential misclassification and bias toward the null when assessing differences due to treatment group. However, this does not completely allay concerns about the impact of different spirometers on the stove intervention effect estimate. Additionally, the evidence of a systematic difference in lung function measurements between spirometers limits conclusions that can be drawn about longitudinal changes in lung function that may not be fully addressed by the correction factor.
As COPD is a disease that typically takes decades of exposure to develop, it may not be surprising that large differences in lung function were not identified between the early and delayed stove groups in our study, given a difference of only 12 to 18 months of partial reduction in smoke exposure. Because of the protracted natural history of the disease, better short-term biomarkers of airway inflammation and injury that may herald COPD risk from biomass smoke exposure need to be identified, and promising candidate markers should be evaluated in future intervention studies.
Conclusions
In rural Guatemalan women followed with spirometry over an average of 5 years, differential exposure to wood smoke during 12 to 18 months at the beginning of this period was not associated with change in lung function. Given the recognized association of biomass smoke exposure with COPD, our results suggest that future studies may need to incorporate cleaner stoves or cleaner fuels, longer follow-up, greater exposure heterogeneity, or inclusion of more susceptible individuals to further explore this association. Such studies are vital given the tremendous burden of disease associated with COPD from biomass smoke exposure in the developing world.
Acknowledgments
Author contributions: J. R. B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. E. D., D. P., K. R. S., T. S.-S., N. G. B., and J. R. B. contributed to planning of the work and M. G., E. D., D. P., E. A. E., J. M., K. R. S., T. S.-S., N. G. B., and J. R. B. contributed to the study and the drafting, review, and approval of the manuscript.
Conflict of interest: None declared.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank Alisa Jenny, MPH, for project management; Carolina Romero, BS, for organizing the fieldwork; and Rudinio Acevedo, BS, for data management. Most important, this study would not have been possible without the hard work and cooperation of the field team and study participants.
ABBREVIATIONS
- CO
carbon monoxide
- CRECER
Chronic Respiratory Effects of Early Childhood Exposure to Respirable Particulate Matter
- EBco
exhaled breath carbon monoxide
- ITT
intention to treat
- MMP
matrix metalloproteinase
- NHANES
National Health and Nutrition Examination Survey
- ppm
parts per million
- RESPIRE
Randomized Exposure Study of Pollution Indoors and Respiratory Effects
Footnotes
FUNDING/SUPPORT: Supported by the Norwegian Research Council [Grant 153987/310] and National Institute of Environmental Health Sciences [Grant NIEHS R01ES010178].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Northcross A, Chowdhury Z, McCracken J, Canuz E, Smith KR. Estimating personal PM2.5 exposures using CO measurements in Guatemalan households cooking with wood fuel. J Environ Monit. 2010;12(4):873-878. [DOI] [PubMed] [Google Scholar]
- 3.Balakrishnan K, Ghosh S, Ganguli B, et al. State and national household concentrations of PM2.5 from solid cookfuel use: results from measurements and modeling in India for estimation of the global burden of disease. Environ Health. 2013;12(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu G, Zhou Y, Tian J, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138(1):20-31. [DOI] [PubMed] [Google Scholar]
- 5.Kurmi OP, Semple S, Simkhada P, Smith WC, Ayres JG. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax. 2010;65(3):221-228. [DOI] [PubMed] [Google Scholar]
- 6.Smith KR, Bruce N, Balakrishnan K, et al. ; HAP CRA Risk Expert Group. Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185-206. [DOI] [PubMed] [Google Scholar]
- 7.Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66(3):232-239. [DOI] [PubMed] [Google Scholar]
- 8.Smith-Sivertsen T, Díaz E, Pope D, et al. Effect of reducing indoor air pollution on women’s respiratory symptoms and lung function: the RESPIRE Randomized Trial, Guatemala. Am J Epidemiol. 2009;170(2):211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pope D, Diaz E, Smith-Sivertsen T, Lie RT, Bakke P, et al. Exposure to household air pollution from wood combustion and association with respiratory symptoms and lung function in nonsmoking women: results from the RESPIRE trial, Guatemala. Environ Health Perspect. 2015;123(4):285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnett RT, Pope CA, III, Ezzati M, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014;122(4):397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarnieri MJ, Diaz JV, Basu C, et al. Effects of woodsmoke exposure on airway inflammation in rural Guatemalan women. PLoS ONE. 2014;9(3):e88455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romieu I, Riojas-Rodríguez H, Marrón-Mares AT, Schilmann A, Perez-Padilla R, Masera O. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med. 2009;180(7):649-656. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Zou Y, Li X, et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med. 2014;11(3):e1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz E, Bruce N, Pope D, et al. Lung function and symptoms among indigenous Mayan women exposed to high levels of indoor air pollution. Int J Tuberc Lung Dis. 2007;11(12):1372-1379. [PubMed] [Google Scholar]
- 15.Smith KR, McCracken JP, Weber MW, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378(9804):1717-1726. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Standardization of spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152(3):1107-1136. [DOI] [PubMed] [Google Scholar]
- 18.Naeher LP, Smith KR, Leaderer BP, Neufeld L, Mage DT. Carbon monoxide as a tracer for assessing exposures to particulate matter in wood and gas cookstove households of highland Guatemala. Environ Sci Technol. 2001;35(3):575-581. [DOI] [PubMed] [Google Scholar]
- 19.McCracken JP, Schwartz J, Diaz A, Bruce N, Smith KR. Longitudinal relationship between personal CO and personal PM2.5 among women cooking with woodfired cookstoves in Guatemala. PLoS ONE. 2013;8(2):e55670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith KR, McCracken JP, Thompson L, et al. Personal child and mother carbon monoxide exposures and kitchen levels: methods and results from a randomized trial of woodfired chimney cookstoves in Guatemala (RESPIRE). J Expo Sci Environ Epidemiol. 2010;20(5):406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCracken JP, Schwartz J, Bruce N, Mittleman M, Ryan LM, Smith KR. Combining individual- and group-level exposure information: child carbon monoxide in the Guatemala woodstove randomized control trial. Epidemiology. 2009;20(1):127-136. [DOI] [PubMed] [Google Scholar]
- 22.Thompson LM, Clark M, Cadman B, Canúz E, Smith KR. Exposures to high levels of carbon monoxide from wood-fired temazcal (steam bath) use in highland Guatemala. Int J Occup Environ Health. 2011;17(2):103-112. [DOI] [PubMed] [Google Scholar]
- 23.Lam N, Nicas M, Ruiz-Mercado I, Thompson LM, Romero C, Smith KR. Non-invasive measurement of carbon monoxide burden in Guatemalan children and adults following wood-fired temazcal (sauna-bath) use. J Environ Monit. 2011;13(8):2172-2181. [DOI] [PubMed] [Google Scholar]
- 24.Ramírez-Venegas A, Sansores RH, Quintana-Carrillo RH, et al. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am J Respir Crit Care Med. 2014;190(9):996-1002. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Briseño D, Fernández-Plata R, Gochicoa-Rangel L, et al. Longitudinal lung function growth of Mexican children compared with international studies. PLoS ONE. 2013;8(10):e77403. [DOI] [PMC free article] [PubMed] [Google Scholar]