Abstract
BACKGROUND:
Adenotonsillectomy (AT) is commonly performed for childhood OSA syndrome (OSAS), but little is known about prognosis without treatment.
METHODS:
The Childhood Adenotonsillectomy Trial (CHAT) randomized 50% of eligible children with OSAS to a control arm (watchful waiting), with 7-month follow-up symptom inventories, physical examinations, and polysomnography. Polysomnographic and symptomatic resolution were defined respectively by an apnea/hypopnea index (AHI) <2 and obstructive apnea index (OAI) <1 and by an OSAS symptom score (Pediatric Sleep Questionnaire [PSQ]) < 0.33 with ≥ 25% improvement from baseline.
RESULTS:
After 194 children aged 5 to 9 years underwent 7 months of watchful waiting, 82 (42%) no longer met polysomnographic criteria for OSAS. Baseline predictors of resolution included lower AHI, better oxygen saturation, smaller waist circumference or percentile, higher-positioned soft palate, smaller neck circumference, and non-black race (each P < .05). Among these, the independent predictors were lower AHI and waist circumference percentile < 90%. Among 167 children with baseline PSQ scores ≥ 0.33, only 25 (15%) experienced symptomatic resolution. Baseline predictors were low PSQ and PSQ snoring subscale scores; absence of habitual snoring, loud snoring, observed apneas, or a household smoker; higher quality of life; fewer attention-deficit/hyperactivity disorder symptoms; and female sex. Only lower PSQ and snoring scores were independent predictors.
CONCLUSIONS:
Many candidates for AT no longer have OSAS on polysomnography after 7 months of watchful waiting, whereas meaningful improvement in symptoms is not common. In practice, a baseline low AHI and normal waist circumference, or low PSQ and snoring score, may help identify an opportunity to avoid AT.
TRIAL REGISTRY:
ClinicalTrials.gov; No.: NCT00560859; URL: www.clinicaltrials.gov.
More than 500,000 adenotonsillectomies (ATs) are performed annually in the United States.1 Many nonrandomized studies2‐7 and one multicenter, randomized clinical trial—the Childhood Adenotonsillectomy Trial (CHAT)8—have indicated that OSAS treatment improves behavior, sleepiness, symptoms, and quality of life (QoL). However, given the frequency of AT and the potential for serious, if uncommon, complications,9,10 surprisingly little is known about the natural history of childhood OSAS in the absence of treatment. Without surgery, habitual snoring resolves in one-half to two-thirds of affected children within 1 to 3 years.11‐13 Studies of documented OSAS, however, have been small, and focused mainly on risk that untreated OSAS might worsen over time,13,14 rather than spontaneous resolution of untreated OSAS.
The CHAT study randomized just over 200 OSAS subjects to “watchful waiting and supportive care” for 7 months: 46% showed resolution of their OSAS on repeat polysomnography (PSG),8 although a small number of participants nonetheless had surgery prior to 7-month follow-up. Resolution on follow-up PSG was predicted by milder OSAS on baseline PSG, absence of obesity, and non-black race. Further insight into predictors of spontaneous resolution in the absence of AT would be useful in practice when decisions are made about this common surgery. Therefore, we investigated whether baseline findings on histories, physical examinations, laboratory tests, or polysomnograms predicted spontaneous OSAS resolution on either follow-up PSG or symptom inventories.
Materials and Methods
Participants
The CHAT design has been described previously,8,15 and additional details relevant to the present study are available in the online supplement (e-Appendix 1 (246.8KB, pdf) ). Institutional review board approvals (e-Table 1 (246.8KB, pdf) ), consent from caregivers, and assent from children aged ≥ 7 years were obtained. Participants aged 5 to 9 years, recruited from pediatric sleep clinics and otolaryngology practices, had an obstructive apnea/hypopnea index (AHI) ≥ 2 events per hour of sleep, or an obstructive apnea index (OAI) ≥ 1. Exclusion criteria included severe OSAS (AHI > 30, OAI > 20, or oxygen saturation < 90% for ≥ 2% of total sleep time), recurrent tonsillitis, a BMI z score ≥ 3, or medication for attention-deficit/hyperactivity disorder (ADHD). Among 453 children randomized in CHAT, 194 had complete follow-up, remained untreated surgically, and provided data for the current analyses.
Outcomes
Objective resolution of OSAS was defined as an AHI < 2 and an OAI < 1 on PSG at 7-month follow-up. PSG followed standard guidelines, as did centralized scoring using pediatric criteria.8,15,16 Resolution of OSAS symptoms was defined by scores on the caregiver-completed Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder (PSQ-SRBD) scale. This well-validated17,18 symptom inventory includes 22 items on snoring; other symptoms of obstructed breathing; observed apneas; ancillary symptoms such as mouth breathing; sleepiness; and behavioral symptoms. Component scores include subscales for sleepiness, snoring, and behavior. Substantive resolution of OSAS symptoms was defined by a total PSQ-SRBD score ≥ 0.33 at baseline,17 < 0.33 at 7-month follow-up, and at least 25% below baseline at follow-up.
Explanatory Variables
Variables tested for ability to predict spontaneous resolution of OSAS included demographic, history, physical examination, and polysomnographic findings. History data included several symptoms from the PSQ-SRBD scale, the subscales described in the previous section, and the total score. Additional variables included caregiver report of allergies, nasal allergies more specifically, frequent colds or upper respiratory infections, asthma, frequent ear infections, attention-deficit disorder or ADHD, nasal steroids, montelukast, or a current smoker in the household. Global QoL was assessed by the Pediatric Quality of Life Inventory (PedsQL) caregiver version as well as the child version, with higher scores indicating better QoL.19,20 Behavior was assessed by the Conners Rating Scale Revised: Long Version ADHD and hyperactivity T scores.21
Physical examinations generated BMI z scores,8 waist circumference,22 waist circumference percentiles,23 and classification of tonsil size as < 50% vs > 50% of the oropharyngeal cross-sectional area. The tongue and palatal position were classified as Friedman class I or II vs class III or IV,24 and also as Mallampati class I or II vs III or IV.25 Neck circumference was assessed just below the thyroid prominence. Morning, resting systolic, and diastolic BPs were measured three times, and blood was taken to measure C-reactive protein (CRP) level. Polysomnographic OSAS severity was assessed by the AHI, OAI, percent of sleep time with oxygen saturation < 92%, and minimum oxygen saturation.
Analyses
Univariate associations were tested using logistic regression models. Variables predictive (P < .05) of either objective or symptom-based resolution of OSAS as the outcome were considered in two different forward stepwise logistic multiple regression models, with twofold cross-validation, to assess for independent predictive value. The entry criterion was P < .20 and explanatory variables were retained as long as they showed P < .10. The only exception to this procedure occurred in the analysis that used objective resolution of OSAS as the outcome. As AHI and OAI were not independent of each other (a child with OAI < 1 had to have an AHI > 2 to participate, and a child with AHI < 2 had to have an OAI > 1), and AHI was the stronger predictor in univariate analysis, OAI was not entered in the multiple regression models. All calculations were performed using SAS version 9.2 (SAS Institute Inc). No adjustment was made for multiple comparisons.
Results
Participants
The mean ± SD age among 194 participants was 6.5 ± 1.4 years, and 92 (47%) were girls. The mean ± SD baseline AHI was 6.7 ± 5.6 (range, 1.1-29.3) and mean ± SD minimum oxygen saturation was 88.8% ± 5.1% (range, 59%-97%). The mean ± SD baseline PSQ-SRBD score was 0.48 ± 0.18 (range, 0.05-0.90).
Polysomnographic Resolution of OSAS
Among the 194 participants, 82 (42%) experienced spontaneous resolution with AHI < 2 and OAI < 1 on PSG at 7 months. The likelihood of resolution did not vary by sex or age (Table 1). Black children in comparison with others were less likely to experience resolution (P = .029). Family income and study site showed no associations.
TABLE 1 ] .
Demographic Variables at Baseline as Predictors of Spontaneous OSA Resolution, as Assessed by PSG or Symptoms at 7-Month Follow-upa
| Polysomnographic Evidence for OSA | Symptoms Suggestive of OSA | |||||
| Characteristic | Not Resolved (n = 112) | Resolved (n = 82) | P Value | Not Resolved (n = 142) | Resolved (n = 25) | P Value |
| Sex | .80 | .03 | ||||
| Male | 58 (56.9) | 44 (43.1) | 79 (90.8) | 8 (9.2) | ||
| Female | 54 (58.7) | 38 (41.3) | 63 (78.8) | 17 (21.3) | ||
| Age, mean ± SD, y | 6.6 ± 1.4 | 6.5 ± 1.4 | .43 | 6.6 ± 1.3 | 6.4 ± 1.3 | .42 |
| Race | .03 | .92 | ||||
| Black | 67 (65.0) | 36 (35.0) | 78 (84.8) | 14 (15.2) | ||
| Non-black | 45 (49.5) | 46 (50.5) | 64 (85.3) | 11 (14.7) | ||
| Family income, USD | .47 | .43 | ||||
| < 30,000 | 43 (54.4) | 36 (45.6) | 62 (82.7) | 13 (17.3) | ||
| ≥ 30,000 | 54 (60.0) | 36 (40.0) | 62 (87.3) | 9 (12.7) | ||
| Missing | 15 (60.0) | 10 (40.0) | 18 (85.7) | 3 (14.3) | ||
| Study site | .26 | .74 | ||||
| 1 | 36 (58.1) | 26 (41.9) | 41 (82.0) | 9 (18.0) | ||
| 2 | 13 (40.6) | 19 (59.4) | 28 (90.3) | 3 (9.7) | ||
| 3 | 34 (65.4) | 18 (34.6) | 39 (83.0) | 8 (17.0) | ||
| 4 | 15 (53.6) | 13 (46.4) | 19 (82.6) | 4 (17.4) | ||
| 5 | 5 (71.4) | 2 (28.6) | 7 (87.5) | 1 (12.5) | ||
| 6 | 9 (69.2) | 4 (30.8) | 8 (100) | … | ||
Data given as No. (row %) unless otherwise indicated. PSG = polysomnography; USD = US dollars.
Tests were conducted excluding subjects with missing values.
Habitual snoring, loud snoring, or observed apneas (or lack thereof), as reported on the PSQ-SRBD scale, all failed to predict polysomnographic resolution, as did scores on the total scale and its subscales (Table 2). QoL ratings, those for ADHD symptoms and hyperactive behavior, and several historical features and comorbidities all failed to predict resolution. Absence of a current smoker in the household also showed no predictive value. At baseline, use of nasal steroids was reported for 17 children (9.3%). Usage was unchanged at follow-up, and no evidence emerged that nasal steroids improved likelihood of spontaneous polysomnographic resolution. Similarly, baseline use of montelukast did not change at follow-up and did not predict resolution, though only six children used this medication.
TABLE 2 ] .
Symptom, Questionnaire, and Historical Variables at Baseline as Predictors of Spontaneous OSA Resolution, as Assessed by PSG or Symptoms at 7-Month Follow-upa
| Polysomnographic Evidence for OSA | Symptoms Suggestive of OSA | |||||
| Baseline Variable | Not Resolved (n = 112) | Resolved (n = 82) | P Value | Not Resolved (n = 142) | Resolved (n = 25) | P Value |
| Habitual snoring | .64 | .03 | ||||
| Yes | 88 (59.9) | 59 (40.1) | 120 (87.0) | 18 (13.0) | ||
| No | 16 (55.2) | 13 (44.8) | 9 (64.3) | 5 (35.7) | ||
| Missing/not sure | 8 (44.4) | 10 (55.6) | 13 (86.7) | 2 (13.3) | ||
| Loud snoring | .61 | .04 | ||||
| Yes | 83 (60.1) | 55 (39.9) | 118 (88.1) | 16 (11.9) | ||
| No | 28 (56.0) | 22 (44.0) | 21 (72.4) | 8 (27.6) | ||
| Missing/not sure | 1 (16.7) | 5 (83.3) | 3 (75.0) | 1 (25.0) | ||
| Observed apneas | .58 | .02 | ||||
| Yes | 52 (57.8) | 38 (42.2) | 76 (90.5) | 8 (9.5) | ||
| No | 55 (61.8) | 34 (38.2) | 51 (76.1) | 16 (23.9) | ||
| Missing/not sure | 5 (33.0) | 10 (66.0) | 15 (93.8) | 1 (6.3) | ||
| PSQ-SRBD scale total score, mean ± SD | 0.48 ± 0.18 | 0.49 ± 0.17 | .95 | 0.57 ± 0.14 | 0.44 ± 0.08 | <.0001 |
| Snoring subscale | 0.76 ± 0.28 | 0.74 ± 0.30 | .63 | 0.85 ± 0.23 | 0.70 ± 0.31 | .01 |
| Sleepiness subscale | 0.43 ± 0.33 | 0.46 ± 0.31 | .53 | 0.55 ± 0.30 | 0.45 ± 0.26 | .14 |
| Behavioral subscale | 0.46 ± 0.35 | 0.48 ± 0.34 | .62 | 0.57 ± 0.32 | 0.41 ± 0.33 | .03 |
| PedsQL, parent | 76.96 ± 15.57 | 77.30 ± 14.89 | .88 | 73.65 ± 15.18 | 80.73 ± 11.50 | .03 |
| PedsQL, child | 65.40 ± 14.52 | 67.07 ± 15.08 | .44 | 65.02 ± 15.80 | 67.66 ± 13.79 | .43 |
| Conners ADHD | 52.59 ± 11.67 | 52.72 ± 9.72 | .93 | 55.40 ± 11.54 | 50.16 ± 6.59 | .03 |
| Hyperactivityb | 53.69 ± 10.84 | 54.26 ± 9.92 | .71 | 55.85 ± 11.15 | 51.84 ± 6.68 | .09 |
| Allergies | .07 | .14 | ||||
| Yes | 52 (65.8) | 27 (34.2) | 62 (89.9) | 7 (10.1) | ||
| No | 60 (52.6) | 54 (47.4) | 79 (81.4) | 18 (18.6) | ||
| Missing | … | 1 (100) | 1 (100) | … | ||
| Nasal allergies | .78 | .14 | ||||
| Yes | 27 (60.0) | 18 (40.0) | 36 (92.3) | 3 (7.7) | ||
| No | 83 (57.6) | 61 (42.4) | 102 (82.3) | 22 (17.7) | ||
| Missing | 2 (50.0) | 2 (50.0) | 4 (100) | … | ||
| Use of nasal steroids | .92 | .36 | ||||
| Yes | 10 (58.8) | 7 (41.2) | 14 (93.3) | 1 (6.7) | ||
| No | 102 (57.6) | 75 (42.4) | 128 (84.2) | 24 (15.8) | ||
| Use of montelukast | .65 | .98 | ||||
| Yes | 4 (66.7) | 2 (33.3) | 5 (100) | … | ||
| No | 108 (57.4) | 80 (42.6) | 137 (84.6) | 25 (15.4) | ||
| Frequent colds/influenza | .93 | .50 | ||||
| Yes | 54 (58.1) | 39 (41.9) | 73 (86.9) | 11 (13.1) | ||
| No | 58 (57.4) | 43 (42.6) | 69 (83.1) | 14 (16.9) | ||
| Frequent ear infections | .06 | .78 | ||||
| Yes | 9 (39.1) | 14 (60.9) | 20 (87.0) | 3 (13.0) | ||
| No | 102 (60.0) | 68 (40.0) | 122 (84.7) | 22 (15.3) | ||
| Not sure | 1 (100) | … | … | … | ||
| Asthma | .12 | .24 | ||||
| Yes | 41 (66.1) | 21 (33.9) | 50 (89.3) | 6 (10.7) | ||
| No | 70 (54.3) | 59 (45.7) | 88 (82.2) | 19 (17.8) | ||
| Missing/not sure | 1 (33.0) | 2 (66.0) | 4 (100) | … | ||
| ADD/ADHD | .40 | .99 | ||||
| Yes | 1 (33.3) | 2 (66.7) | 2 (100) | … | ||
| No | 111 (58.7) | 78 (41.3) | 137 (84.6) | 25 (15.4) | ||
| Missing | … | 2 (100) | 3 (100) | … | ||
| Current smoker in household | 0.54 ± 0.86 | 0.43 ± 0.80 | .37 | 0.58 ± 0.89 | 0.20 ± 0.50 | .05 |
| Months of entry to study | .06 | .93 | ||||
| December-July | 75 (53.6) | 65 (46.4) | 109 (85.2) | 19 (14.8) | ||
| August-November | 37 (68.5) | 17 (31.5) | 33 (84.6) | 6 (15.4) | ||
Data given as No. (row %) unless otherwise indicated. ADD = attention-deficit disorder; ADHD = attention-deficit/hyperactivity disorder; Conner ADHD = Conners Rating Scale-Revised: Long Version, Attention-Deficit/Hyperactivity Disorder subscale; PedsQL = Pediatric Quality of Life Inventory; PSQ-SRBD = Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder. See Table 1 legend for expansion of other abbreviation.
Tests were conducted excluding subjects with missing values.
Hyperactivity refers to Conners Rating Scale-Revised: Long Version, Hyperactivity subscale.
On physical examination at baseline, children destined to have spontaneous polysomnographic resolution of their OSAS had smaller waist circumference percentiles compared with others; more widely patent oropharyngeal anatomy by Friedman, if not Mallampati, position assessments; and smaller neck circumferences (Table 3). Lower BMI z scores and lower log CRP values showed P values < .10 and < .05, respectively. Tonsillar size and BPs showed no predictive value.
TABLE 3 ] .
Physical Examination and Laboratory Variables at Baseline as Predictors of Spontaneous OSA Resolution, as Assessed by PSG or Symptoms at 7-Month Follow-upa
| Baseline Variable | Polysomnographic Evidence for OSA | Symptoms Suggestive of OSA | ||||
| Not Resolved (n = 112) | Resolved (n = 82) | P Value | Not Resolved (n = 142) | Resolved (n = 25) | P Value | |
| BMI z score | 1.02 ± 1.29 | 0.68 ± 1.17 | .07 | 0.97 ± 1.17 | 0.90 ± 1.41 | .79 |
| Waist circumference, mean ± SD | 64.29 ± 13.67 | 59.36 ± 10.67 | .01 | 63.12 ± 13.23 | 63.07 ± 12.95 | .99 |
| Waist circumference, cm | .02 | .95 | ||||
| < 90th percentile | 66 (51.6) | 62 (48.4) | 90 (84.9) | 16 (15.1) | ||
| ≥ 90th percentile | 46 (69.7) | 20 (30.3) | 52 (85.3) | 9 (14.8) | ||
| Tonsillar size | .41 | .07 | ||||
| 0-50 | 33 (62.3) | 20 (37.7) | 43 (93.5) | 3 (6.5) | ||
| 50-100 | 78 (55.7) | 62 (44.3) | 98 (81.7) | 22 (18.3) | ||
| Missing | 1 (100) | … | 1 (100) | … | ||
| Friedman palate position | .02 | .93 | ||||
| I/II | 29 (45.3) | 35 (54.7) | 38 (84.4) | 7 (15.6) | ||
| III/IV | 80 (63.0) | 47 (37.0) | 102 (85.0) | 18 (15.0) | ||
| Not done | 3 (100) | … | 2 (100) | … | ||
| Mallampati position | .10 | .54 | ||||
| I/II | 58 (52.3) | 53 (47.7) | 82 (86.3) | 13 (13.7) | ||
| III/IV | 52 (64.2) | 29 (35.8) | 58 (82.9) | 12 (17.1) | ||
| Not done | 2 (100) | … | 2 (100) | … | ||
| Neck circumference, mean ± SD, cm | 28.33 ± 2.89 | 27.48 ± 2.37 | .03 | 28.16 ± 2.80 | 27.88 ± 3.14 | .65 |
| Log C-reactive protein level, mean ± SD, mg/L (n = 131) | −0.48 ± 1.82 | −1.18 ± 1.81 | .02 | −0.68 ± 1.83 | −0.72 ± 1.76 | .93 |
| Systolic BP, mean ± SD, mm Hg | 63.19 ± 8.34 | 61.43 ± 7.08 | .13 | 62.56 ± 7.94 | 62.75 ± 6.94 | .91 |
| Diastolic BP, mean ± SD, mm Hg | 98.63 ± 8.72 | 97.10 ± 8.50 | .22 | 98.03 ± 8.32 | 98.04 ± 10.14 | .99 |
Data given as No. (row %) unless otherwise indicated. See Table 1 legend for expansion of abbreviation.
Tests were conducted excluding subjects with missing values.
Polysomnographic findings at baseline included the strongest predictors of spontaneous polysomnographic resolution at follow-up (Table 4), as might be expected; the mildest OSAS at baseline only required a small change in AHI, OAI, or both to be considered resolved. Low OAI and especially low AHI at baseline were predictive; the only other effective predictors were minimum oxygen saturation and percent of sleep time with CO2 level > 50 mm Hg.
TABLE 4 ] .
Polysomnographic Variables at Baseline as Predictors of Spontaneous OSA Resolution, as Assessed by PSG or Symptoms at 7-Month Follow-upa
| Polysomnographic Evidence for OSA | Symptoms Suggestive of OSA | |||||
| Baseline Variable | Not Resolved (n = 112) | Resolved (n = 82) | P Value | Not Resolved (n = 142) | Resolved (n = 25) | P Value |
| Apnea/hypopnea index | 8.40 ± 6.19 | 4.41 ± 3.72 | < .0001 | 7.08 ± 5.90 | 7.27 ± 6.02 | .88 |
| Obstructive apnea index | 2.50 ± 3.20 | 1.48 ± 1.69 | .01 | 2.30 ± 3.09 | 1.88 ± 2.18 | .52 |
| % of sleep time with oxygen saturation < 92% | 1.12 ± 4.67 | 0.31 ± 0.76 | .07 | 0.92 ± 4.20 | 0.72 ± 1.23 | .82 |
| Minimum oxygen saturation, % | 87.88 ± 5.16 | 90.02 ± 4.87 | .01 | 88.95 ± 4.65 | 87.96 ± 6.82 | .37 |
| % of sleep time with CO2 > 50 mm Hg | 10.52 ± 19.54 | 5.31 ± 13.96 | .08 | 9.46 ± 19.04 | 8.71 ± 18.07 | .87 |
Data given as mean ± SD unless otherwise indicated. See Table 1 legend for expansion of abbreviation.
Tests were conducted excluding subjects with missing values.
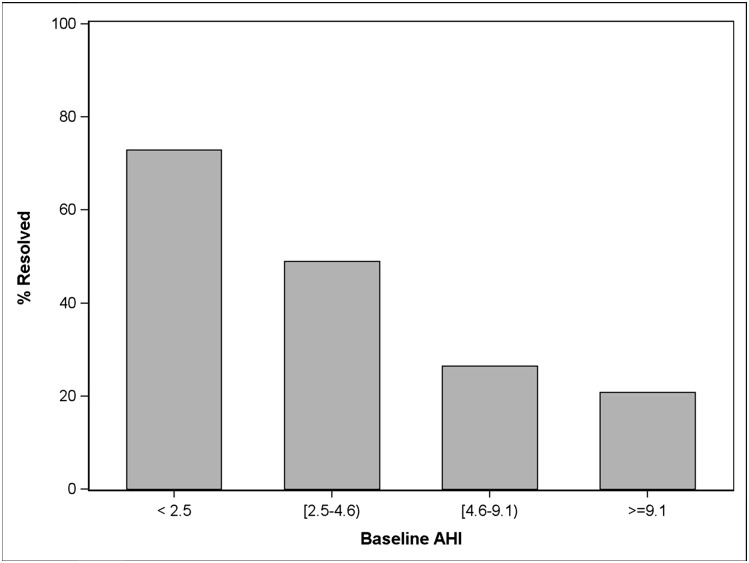
A forward stepwise multiple logistic regression of polysomnographic resolution on all baseline explanatory variables that had shown significance (P < .05) on univariate analyses yielded a final model with R2 = 0.22, but only two predictors were retained: low AHI (OR, 0.83; 95% CI, 0.77-0.90) and waist circumference < 90th percentile (OR, 2.0; 95% CI, 1.03-3.91). Figure 1 illustrates the number of participants within each baseline AHI quartile who experienced spontaneous polysomnographic resolution.
Figure 1 –
For 194 participants randomized to watchful waiting and supportive care, the percent whose OSA resolved after 7 mo, within each baseline AHI quartile, is shown. AHI = apnea/hypopnea index.
To reduce influence of small changes in AHI among children whose baseline AHI was already close to 2, the described univariate analyses were repeated after limiting the sample of 194 children to 95 children who all had AHI > 5 at baseline, 23 (24%) of whom showed spontaneous resolution. In these analyses, however, no baseline predictive variables were identified (though AHI did come close: P = .09), so no multivariate analysis was performed.
Resolution of OSAS Symptoms
Among the 194 children, 167 (86%) had baseline PSQ-SRBD scale scores > 0.33 and available follow-up PSQ-SRBD scores. Follow-up scores < 0.33 with a minimum 25% improvement from baseline (defining “symptom resolution”) were observed in only 25 (15%) of the 167 children. Girls were more than twice as likely as boys to experience symptom resolution (P = .033), but no other demographic variables were predictive (Table 1). Among the 167 children, only 20 (12%) showed both polysomnographic and symptomatic resolution at 7 months.
In univariate analyses, PSQ-SRBD symptom resolution was predicted by baseline absence of each of the three symptoms analyzed—habitual snoring, loud snoring, and observed apneas (each P < .05)—and by lower PSQ-SRBD scale scores (P < .0001), snoring subscales (P = .012), and behavioral subscales (P = .026) (Table 2). Better QoL on parent- but not child-completed measures predicted spontaneous resolution. Lower Conner scores for ADHD symptoms, though not those for hyperactive behavior per se, predicted resolution. Absence of a household smoker was associated with resolution of symptoms. Resolution of OSAS symptoms was not associated with polysomnographic findings, laboratory findings, physical examination findings, nasal allergies, or other comorbidities (Tables 2-4).
Multiple logistic regression of OSAS symptom resolution on explanatory variables that were significant in the univariate analyses showed that the only independent predictors retained in the final model were lower baseline PSQ-SRBD scale total scores (lower by 0.1 on a scale of 0.0 to 1.0, representing a change of positive to negative responses for about two of 22 symptom-items; OR, 0.38; 95% CI, 0.23-0.62) and lower PSQ-SRBD snoring subscale scores (OR, 0.18; 95% CI, 0.04-0.94).
Discussion
This sizable, multicenter study of outcomes in untreated childhood OSAS—analyses made possible by the control arm of CHAT—suggests that almost one-half (42%) of children considered to be surgical candidates by their otolaryngologists no longer have OSAS on PSG when it is repeated after 7 months of watchful waiting. In contrast, only 15% of untreated children who were significantly symptomatic at baseline experienced meaningful improvement and resolution of their OSAS symptoms. Few baseline independent predictors of spontaneous polysomnographic OSAS resolution were identified: Other than lower baseline polysomnographic measures of OSAS severity, only less central obesity, defined by a waist circumference under the 90th percentile, was found to add independent predictive value. Similarly, independent baseline predictors of spontaneous symptom resolution, other than lower total symptom scores at study entry, only included a snoring subscale. All these findings, including the absence of many tested associations, provide new insight that should be useful to clinicians and families who must make difficult decisions about whether to pursue AT for children with OSAS. These data represent some of the most specific patient-oriented evidence yet generated to assist with predictions of spontaneous improvement in objective or subjective OSAS features without surgery.
Multiple potential mechanisms could explain objective or subjective improvement in OSAS over a 7-month period without specific intervention: Reduction in tonsillar size, airway enlargement with growth, and regression to the mean may have all contributed. “Supportive care” during watchful waiting may also have helped, but nasal saline rinses often went unused, and only a small minority of subjects took antiinflammatory agents. Given plausible explanations for “spontaneous” improvement, however, the discrepancy between polysomnographic and symptomatic resolution may seem surprising. In part, the discrepancy arose because 25% improvement, in addition to a specified threshold, was required to achieve substantive symptomatic but not polysomnographic resolution. Previous studies of fewer symptoms did show frequent resolution of habitual snoring.11,12 In our cohort, 34 of 147 habitual snorers (23%) were no longer habitual snorers at follow-up (data not shown). However, within a 7-month period, overall OSAS symptom burden appears unlikely to diminish substantially without AT.
Interestingly, physical examination features appeared more promising than symptoms or comorbidities as predictors in our univariate models of spontaneous polysomnographic resolution of OSAS. Epidemic obesity has been blamed for failure of OSAS to resolve after AT, especially in samples that have included many obese subjects.8,26 Similarly, among nonoperated children, we found two measures of obesity—large waist circumference, particularly as a percentile, and neck circumference—to predict less OSAS resolution. In practice, this observation may be useful to assist in decisions to defer surgical treatment of children who have not had PSG.27,28 Surprisingly, the baseline AHI and measures of hypoxemia did not help predict spontaneous symptom resolution. However, many previous studies,2,29‐34 in addition to the CHAT study itself,8 have shown little or no association between baseline OSAS polysomnographic measures and concurrent morbidity or future outcomes.
Several limitations of the present study deserve discussion. First, the finding that AHI and PSQ-SRBD scores most strongly predicted objective and symptomatic spontaneous resolution of OSAS, respectively, is likely to arise, in part, from use of these same variables as outcome measures. The finding that children with low AHI or low PSQ-SRBD scores at baseline were most likely to cross specified thresholds for spontaneous resolution may arise in part from test-retest variability and regression to the mean. Children who crossed over from the watchful waiting arm to AT were excluded from analyses. However, the number of crossovers was small and their exclusion provided a more realistic view of outcomes without surgery. Finally, the severity of OSAS among CHAT participants was predominantly mild to moderate, as in previous studies that recruited AT candidates from otolaryngology clinics,2,35 and most likely reflects national clinical practice. Only 3.5% of CHAT candidates were excluded because of severe OSAS.8 However, spontaneous resolution of more severe OSAS may be less common, and its predictors may be different.
Nonetheless, our observations about frequent polysomnographic resolution and less frequent symptomatic improvement in nonoperated pediatric OSAS do have several implications for clinical practice. In making a decision about AT, for a patient with OSAS suspected on clinical grounds, a preoperative polysomnogram may be useful not only to confirm objective evidence for OSAS, as recommended by the American Academy of Pediatrics,36 but also to assess the likelihood of its spontaneous resolution with time. A child with OSAS but an AHI < 5 may be more likely than not to have a negative PSG after several months of watchful waiting (Fig 1). Similarly, the PSQ-SRBD scale may be useful in practice to help predict in a systematic way which children with OSAS may have substantive resolution of symptoms with watchful waiting. Symptoms often matter more to patients and families than do laboratory results. Current findings suggest that watchful waiting may be a reasonable option in children with low OSAS symptom burden and, especially, little snoring, who also have low AHIs and do not have central obesity. Although other potentially useful predictors of spontaneous resolution are identifiable in our data, they all tend to vary with symptom burden, snoring, AHI, and low waist circumference percentile, to the extent that they add little when these four key measures are available.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: R. D. C. and C. L. R. assume responsibility for the content of the manuscript. R. D. C. and C. L. R. contributed to study design, acquisition of data, and interpretation of data, drafted the manuscript, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; S. S. E. and X. H. contributed to study design, analysis of data, and interpretation of data, revised the manuscript critically for important intellectual content, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; and C. L. M., S. L. G., E. S. K., E. K. H., R. B. M., D. T. J., R. Arens, R. Amin, and S. R. contributed to study design, acquisition of data, and interpretation of data, revised the manuscript critically for important intellectual content, approved the final version, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest: R. D. C. is named in or has developed patented and copyrighted materials owned by the University of Michigan and designed to assist with assessment or treatment of sleep disorders. These materials include the Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder scale, used in the research now reported. Currently, this questionnaire is licensed online at no charge by the University of Michigan to appropriate users, and licensed (for electronic use) to Zansors LLC. R. D. C. serves on the boards of the American Academy of Sleep Medicine and the International Pediatric Sleep Society, is an editor for UpToDate, is a book editor for Cambridge University Press, has received support for research and education from Philips Respironics (Koninklijke Philips NV) and Fisher & Paykel Healthcare Ltd, and has consulted for MC3 Inc and Zansors LLC. C. L. M. received a loan of research equipment from Philips Respironics (Koninklijke Philips NV) and Ventus Medical Inc. S. R. reports that Brigham and Women’s Hospital received research grant support from ResMed Foundation and research equipment (unrelated to this study) from Philips Respironics (Koninklijke Philips NV) and ResMed Inc. C. L. R. has consulted for Natus Medical Inc, Advance Medical Inc, and Jazz Pharmaceuticals plc. The study received equipment from Philips Respironics (Koninklijke Philips NV) for use in this trial. None declared (S. S. E., X. H., S. L. G., E. S. K., E. K. H., R. B. M., D. W. J., R. Arens, R. Amin).
Role of sponsors: The sponsors played no role in data collection or analysis or manuscript preparation and had no access to study data.
Collaborators: Childhood Adenotonsillectomy Trial Investigators: Eliot Katz, MD; Janice Ware, PhD; Dwight Jones, MD; Susan Redline, MD, MPH; Rui Wang, PhD; Ron Mitchell, MD; Shalini Paruthi, MD; Karen Snyder, MS; Carole Marcus, MBBCh; Nina H. Thomas, PhD; Lisa Elden, MD; Raouf Amin, MD; Dean Beebe, PhD; Paul Willging, MD; Raanan Arens, MD; Hiren Muzumdar, MD; Shelby Harris, PsyD CBSM; Carol Rosen, MD; H. Gerry Taylor, PhD; Robert Sprecher, MD; James Arnold, MD; David Gozal, MD; Ronald Chervin, MD; Susan Garetz, MD; Bruno Giordani, PhD; Tim Hoban, MD; Susan Ellenberg, PhD; Reneé H. Moore, PhD; Kim Lacy, RN, BSN.
Other contributions: The authors are indebted to the children and families who made the effort to participate in CHAT. The authors gratefully acknowledge superb support from the CHAT research staff and the CHAT Data and Safety Monitoring Board.
Additional information: The e-Appendix and e-Table can be found in the Supplemental Materials area of the online article.
ABBREVIATIONS
- ADHD
attention-deficit/hyperactivity disorder
- AHI
apnea/hypoponea index
- AT
adenotonsillectomy
- CHAT
Childhood Adenotonsillectomy Trial
- CRP
C-reactive protein
- OAI
obstructive apnea index
- OSAS
OSA syndrome
- PedsQL
Pediatric Quality of Life Inventory
- PSG
polysomnography
- PSQ
Pediatric Sleep Questionniare
- PSQ-SRBD
Pediatric Sleep Questionnaire Sleep-Related Breathing Disorder
- QoL
quality of life
Footnotes
FOR EDITORIAL COMMENT SEE PAGE 1129
FUNDING/SUPPORT: This study was supported by the National Institutes of Health [Grants HL083075, HL083129, UL1 RR024134, and UL1 RR024989].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Contributor Information
for the Childhood Adenotonsillectomy Trial:
Eliot Katz, Janice Ware, Dwight Jones, Susan Redline, Rui Wang, Ron Mitchell, Shalini Paruthi, Karen Snyder, Carole Marcus, Nina H. Thomas, Lisa Elden, Raouf Amin, Dean Beebe, Paul Willging, Raanan Arens, Hiren Muzumdar, Shelby Harris, Carol Rosen, H. Gerry Taylor, Robert Sprecher, James Arnold, David Gozal, Ronald Chervin, Susan Garetz, Bruno Giordani, Tim Hoban, Susan Ellenberg, Reneé H. Moore, and Kim Lacy
References
- 1.Bhattacharyya N, Lin HW. Changes and consistencies in the epidemiology of pediatric adenotonsillar surgery, 1996-2006. Otolaryngol Head Neck Surg. 2010;143(5):680-684. [DOI] [PubMed] [Google Scholar]
- 2.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769-e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell RB, Kelly J. Quality of life after adenotonsillectomy for SDB in children. Otolaryngol Head Neck Surg. 2005;133(4):569-572. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell RB, Kelly J. Behavioral changes in children with mild sleep-disordered breathing or obstructive sleep apnea after adenotonsillectomy. Laryngoscope. 2007;117(9):1685-1688. [DOI] [PubMed] [Google Scholar]
- 5.Tran KD, Nguyen CD, Weedon J, Goldstein NA. Child behavior and quality of life in pediatric obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2005;131(1):52-57. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein NA, Fatima M, Campbell TF, Rosenfeld RM. Child behavior and quality of life before and after tonsillectomy and adenoidectomy. Arch Otolaryngol Head Neck Surg. 2002;128(7):770-775. [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C, Winkle R, Korobkin R, Simmons B. Children and nocturnal snoring: evaluation of the effects of sleep related respiratory resistive load and daytime functioning. Eur J Pediatr. 1982;139(3):165-171. [DOI] [PubMed] [Google Scholar]
- 8.Marcus CL, Moore RH, Rosen CL, et al. ; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coté CJ, Posner KL, Domino KB. Death or neurologic injury after tonsillectomy in children with a focus on obstructive sleep apnea: Houston, we have a problem! Anesth Analg. 2014;118(6):1276-1283. [DOI] [PubMed] [Google Scholar]
- 10.Goldman JL, Baugh RF, Davies L, et al. Mortality and major morbidity after tonsillectomy: etiologic factors and strategies for prevention. Laryngoscope. 2013;123(10):2544-2553. [DOI] [PubMed] [Google Scholar]
- 11.Ali NJ, Pitson D, Stradling JR. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch Dis Child. 1994;71(1):74-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urschitz MS, Guenther A, Eitner S, et al. Risk factors and natural history of habitual snoring. Chest. 2004;126(3):790-800. [DOI] [PubMed] [Google Scholar]
- 13.Anuntaseree W, Kuasirikul S, Suntornlohanakul S. Natural history of snoring and obstructive sleep apnea in Thai school-age children. Pediatr Pulmonol. 2005;39(5):415-420. [DOI] [PubMed] [Google Scholar]
- 14.Li AM, Au CT, Ng SK, et al. Natural history and predictors for progression of mild childhood obstructive sleep apnoea. Thorax. 2010;65(1):27-31. [DOI] [PubMed] [Google Scholar]
- 15.Redline S, Amin R, Beebe D, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34(11):1509-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson A, Quan SF, for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Vol 1 Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 17.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21-32. [DOI] [PubMed] [Google Scholar]
- 18.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133(3):216-222. [DOI] [PubMed] [Google Scholar]
- 19.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329-341. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree VM, Varni JW, Gozal D. Health-related quality of life and depressive symptoms in children with suspected sleep-disordered breathing. Sleep. 2004;27(6):1131-1138. [DOI] [PubMed] [Google Scholar]
- 21.Conners CK. Conners’ Rating Scales - Revised. North Tonawanda, NY: Multi-Health Systems Publishing; 1997. [Google Scholar]
- 22.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Ford ES, Mokdad AH, Cook S. Recent trends in waist circumference and waist-height ratio among US children and adolescents. Pediatrics. 2006;118(5):e1390-e1398. [DOI] [PubMed] [Google Scholar]
- 24.Friedman M, Tanyeri H, La Rosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109(12):1901-1907. [DOI] [PubMed] [Google Scholar]
- 25.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429-434. [DOI] [PubMed] [Google Scholar]
- 26.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149(6):803-808. [DOI] [PubMed] [Google Scholar]
- 27.Weatherly RA, Mai EF, Ruzicka DL, Chervin RD. Identification and evaluation of obstructive sleep apnea prior to adenotonsillectomy in children: a survey of practice patterns. Sleep Med. 2003;4(4):297-307. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell RB, Pereira KD, Friedman NR. Sleep-disordered breathing in children: survey of current practice. Laryngoscope. 2006;116(6):956-958. [DOI] [PubMed] [Google Scholar]
- 29.Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001;24(3):313-320. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien LM, Holbrook CR, Mervis CB, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003;111(3):554-563. [DOI] [PubMed] [Google Scholar]
- 31.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145(4):458-464. [DOI] [PubMed] [Google Scholar]
- 32.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114(3):768-775. [DOI] [PubMed] [Google Scholar]
- 33.Beebe DW, Wells CT, Jeffries J, Chini B, Kalra M, Amin R. Neuropsychological effects of pediatric obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10(7):962-975. [DOI] [PubMed] [Google Scholar]
- 34.Emancipator JL, Storfer-Isser A, Taylor HG, et al. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch Pediatr Adolesc Med. 2006;160(2):203-210. [DOI] [PubMed] [Google Scholar]
- 35.Chervin RD, Ruzicka DL, Hoban TF, et al. Esophageal pressures, polysomnography, and neurobehavioral outcomes of adenotonsillectomy in children. Chest. 2012;142(1):101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576-584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement