Abstract
BACKGROUND:
Although the benefits of early tracheostomy in patients dependent on ventilators are well established, the reasons for variation in time from intubation to tracheostomy remain unclear. We identified clinical and demographic disparities in time to tracheostomy.
METHODS:
We performed a level 3 retrospective prognostic study by querying the University HealthSystem Consortium (2007-2010) for adult patients receiving a tracheostomy after initial intubation. Time to tracheostomy was designated early (< 7 days) or late (> 10 days). Cohorts were stratified by time to tracheostomy and compared using univariate tests of association and multivariable adjusted models.
RESULTS:
A total of 49,191 patients underwent tracheostomy after initial intubation: 42% early (n = 21,029) and 58% late (n = 28,162). On both univariate and multivariable analyses, women, blacks, Hispanics, and patients receiving Medicaid were less likely to receive an early tracheostomy. Patients in the early group also experienced lower rates of mortality (OR, 0.84; 95% CI, 0.79-0.88).
CONCLUSIONS:
Early tracheostomy was associated with increased survival. Yet, there were still significant disparities in time to tracheostomy according to sex, race, and type of insurance. Application of evidence-based algorithms for tracheostomy may reduce unequal treatment and improve overall mortality rates. Additional research into this apparent bias in referral/rendering of tracheostomy is needed.
Tracheostomy, for many, is a life-saving intervention that is routinely offered to critically ill patients who require prolonged mechanical ventilation. The reasons for ventilator dependence can be quite variable, with myriad presenting illnesses, ranging from traumatic injury to exacerbated medical conditions. Often, a tracheostomy is the only way for patients to regain their respiratory independence. Regardless of the underlying pathology, patients requiring prolonged mechanical ventilation are alike in resource use and contribute greatly to the overall burden of critical illness on our health-care system, which is estimated to consume more than one-fourth of the United States’ annual $2 trillion health-care expenditures.1 In the last 2 decades, there has been an approximate 150% increase in the number of tracheostomies being performed, from 427,100 in 1993 to 643,575 in 2010.2 Presently, it is one of the most common procedures performed on patients in the ICU.3
Like other common surgical procedures, tracheostomy is not without risks. However, its benefits, such as expedited weaning from the ventilator, increased patient comfort, reduced risk of ventilator-associated pneumonia (VAP), sepsis, and potentially mortality, as well as shorter ICU and hospital stays,4‐9 typically outweigh these risks. Furthermore, fewer ventilator days, shorter ICU lengths of stay (LOSs), and shorter overall hospital LOS translate to a reduction in hospital operating costs as well as an increase in the availability of otherwise limited critical care resources.10
These benefits have, in part, fueled contentious debates surrounding tracheostomy timing for patients who are mechanically ventilated. Specifically, how many days after a patient is intubated and placed on a ventilator should the team, consisting of family members, clinicians, and ancillary staff, wait before pursuing a tracheostomy? To date, there has yet to be a consensus regarding the definition of an “early” tracheostomy across multiple specialties. In previous literature, the definition early tracheostomy ranges from 3 days to < 21 days, depending on author, specialty, and patient population.6,7,9,11,12 However, although the definition of early remains to be agreed upon, there is an increasing amount of literature that suggests > 21 days is too late.13
Given the various reasons for prolonged mechanical ventilation, some variation in timing of tracheostomy is to be expected. However, although there have been numerous studies spanning multiple specialties demonstrating sex-, racial-, age-, and insurance-based disparities in referral, as well as receipt and timeliness of care in other areas, it is unclear if such disparities exist in the receipt of a tracheostomy.14‐16 We undertook this study to validate that an early compared with a late tracheostomy does impart some benefit across diagnosis groups. Additionally, we sought to identify the specific patient and system factors that predict timing of tracheostomy and to determine whether any demographic disparities exist in the receipt of a timely tracheostomy.
Materials and Methods
Database
The University HealthSystem Consortium (UHC) is an alliance of the nation’s leading nonprofit academic medical centers composed of 116 academic medical centers and 261 affiliated hospital members. UHC’s membership includes > 90% of academic medical centers in the United States. Patient, hospital, and economic outcomes can be compared across different centers. The data housed in UHC’s Clinical Database Resource Manager (CDB/RM) is primarily acquired from submitted UB-04 billing forms that use standard International Classification of Diseases, Ninth Revision (ICD-9) codes to report both diagnoses and procedures.
The CDB/RM provides the following information: synthetic hospital and surgeon identifiers, unique patient visit identifiers, patient demographics, hospital financials, and procedural and diagnostic information. Morbidity is defined using the UHC morbidity profiler (e-Table 1 (72.9KB, pdf) ). Both cost and charge information are reported in UHC. Charges are reported by each center, and costs are then calculated using institution-specific cost-to-charge ratios obtained from the department-level Medicare cost reports. Federally reported area wage indexes are used to account for regional and center-specific variation.
For risk stratification, UHC has developed a severity of illness (SOI) score for both risk adjustment and predicted resource allocation. This method has been both verified and validated by the Agency for Healthcare Research and Quality.17 SOI takes into account a number of patient variables and weights them in the context of their illness, including other comorbid conditions, age, and diagnoses.
Cohort
Time to tracheostomy was designated as early, intermediate, or late based on a priori categories established after an extensive multispecialty literature review. Patients receiving their tracheostomy within 6 days of intubation were designated early. Patients receiving their tracheostomy on days 7 through 10 of intubation were designated intermediate. Patients receiving their tracheostomy > 10 days after intubation were designated as late.
We queried the CDB/RM from 2007 to 2010 for adult patients (≥ 18 years of age) whose initial date of endotracheal tube insertion (ICD-9 96.04) and date of tracheostomy (ICD-9 31.1, 31.2, 32.21, and 31.29) were captured. We excluded anyone who was intubated multiple times, readmitted to the ICU after an initial discharge, or did not receive a tracheostomy. Furthermore, as our primary purpose was to ascertain the benefits of early tracheostomy and determine disparities in receipt of early tracheostomy, we excluded patients who underwent tracheostomy on days 7 through 10 after initial intubation (intermediate).
Analysis
For univariate analyses, we compared patient characteristics, resource use, and outcomes between the Early and Late cohorts using Pearson χ2 for categorical variables and Kruskal-Wallis nonparametric tests for continuous variables. For multivariable analyses, a number of adjusted linear and logistic regression models were created. These models included one for each outcome of interest (in-hospital mortality, VAP, ICU LOS, hospital LOS, total charges, total direct cost) where early vs late tracheostomy was the primary predictor variable and an additional model for timing of tracheostomy to determine factors predicting early vs late tracheostomy. At each iteration, variables with univariate P values ≤ .25 entered the model, whereas those with adjusted P values ≤ .10 were retained. The final models used generalized estimating equations and robust variance calculations to account for center-specific and SOI clustering where appropriate. Covariates for these models included age groups (18-24, 25-44, 45-64, 65-83, and > 83 years of age), sex, race (white, black, Hispanic, Asian, and other), payer status (private, Medicare, Medicaid, uninsured, and other), grouped admitting diagnoses (trauma, cardiovascular, pulmonary, surgical, neurologic, sepsis, and other), SOI (minor, moderate, major, and extreme), and admission status (elective and emergent). Grouped admitting diagnoses were derived using both ICD-9 diagnosis codes and Clinical Classification Software provided by Healthcare Cost and Utilization Project.18 All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc) and JMP 10 Pro (SAS Institute Inc).
This study was reviewed by the University of Massachusetts Medical School institutional review board and was deemed appropriate for exemption from institutional review board oversight, as all data were deidentified. Statistical significance was defined as any P value < .05.
Results
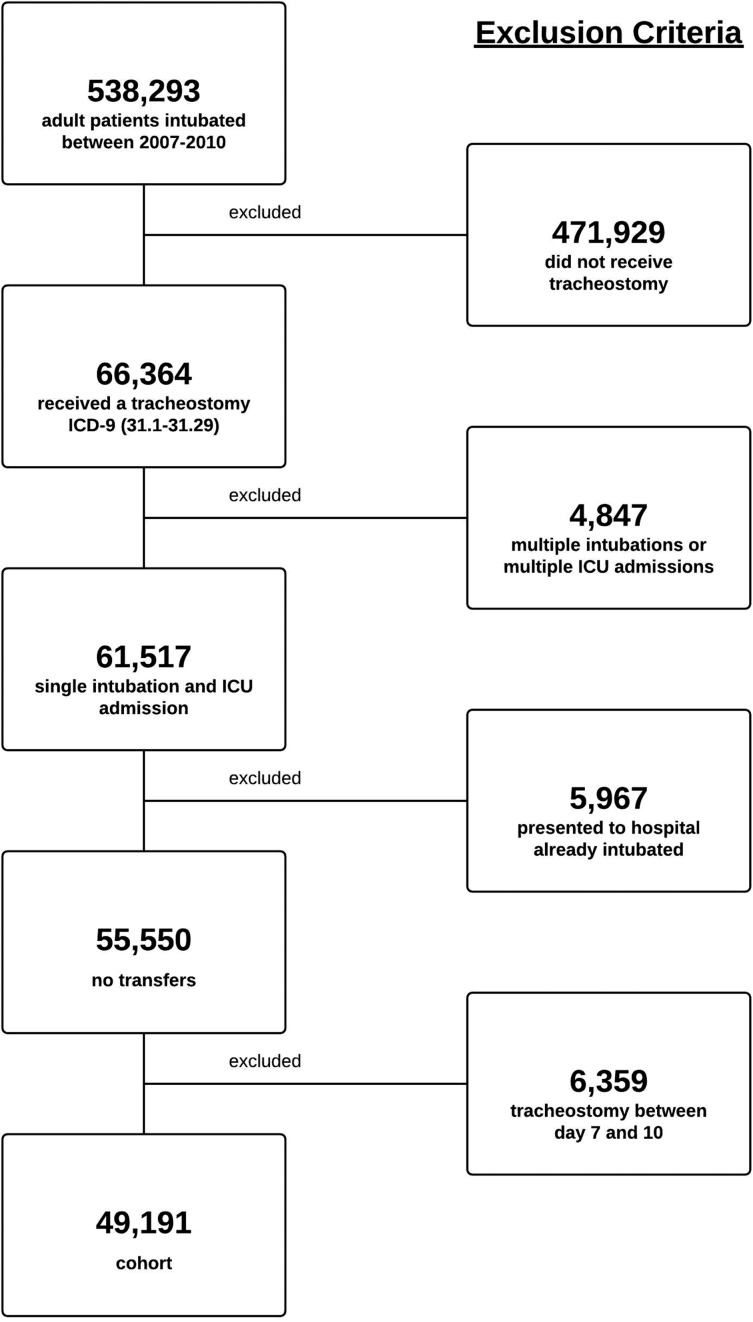
Our initial query identified 538,293 adult patients who were intubated between 2007 and 2010. A total of 471,929 patients did not receive or require a tracheostomy and were excluded. In addition, 4,847 patients were excluded because of either multiple intubations or multiple ICU admissions, 5,967 patients were excluded because they were transferred from another hospital on ventilator support, and 6,359 patients were excluded because they underwent tracheostomy on days 7 through 10 after intubation. The final population consisted of 49,191 patients from 185 different medical centers in which 7,585 different clinicians had performed a tracheostomy (Fig 1).
Figure 1 –
Exclusion criteria. ICD-9 = International Classification of Diseases, Ninth Revision.
There were 21,029 patients in the early cohort and 28,162 patients in the late cohort. Table 1 summarizes differences in sociodemographics and clinical characteristics between these two cohorts (P < .0001 for all patient characteristics). The early cohort is slightly younger (58 years vs 60 years); more likely to be men (63% vs 57%), white (63% vs 55%), and privately insured (35% vs 26%); and more likely to have a grouped admitting diagnosis related to either trauma (17% vs 10%) or an acute neurologic event (19% vs 11%).
TABLE 1 ] .
Comparison of Characteristics of Critically Ill Patients Undergoing Early vs Late Tracheostomy at 185 Academic Medical Centers/Affiliates
| Characteristic | Early Tracheostomy, < 7 d(n = 21,029) | Late Tracheostomy, > 10 d(n = 28,162) | P Valuea |
| Median age, y | 58 | 60 | < .0001 |
| Male sex, % | 63 | 57 | < .0001 |
| Race, % | < .0001 | ||
| White | 63 | 55 | |
| Black | 22 | 24 | |
| Hispanic | 5 | 7 | |
| Asian | 2 | 5 | |
| Other | 8 | 9 | |
| Payer, % | < .0001 | ||
| Private | 35 | 26 | |
| Medicaid | 17 | 19 | |
| Medicare | 42 | 47 | |
| Free care/self-pay | 3 | 4 | |
| Other | 3 | 4 | |
| Primary diagnosis category, % | < .0001 | ||
| Cardiovascular | 20 | 23 | |
| Pulmonary | 11 | 12 | |
| Surgical | 3 | 3 | |
| Trauma | 17 | 10 | |
| Acute neurologic | 19 | 11 | |
| Sepsis | 8 | 16 | |
| Other | 22 | 25 | |
| UHC severity of illness score, % | < .0001 | ||
| Minor | < 1 | < 1 | |
| Moderate | 2 | < 1 | |
| Major | 16 | 7 | |
| Extreme | 81 | 92 | |
| Admission status, % | < .0001 | ||
| Emergent | 85 | 87 | |
| Elective | 15 | 13 |
Only patients with a single intubation prior to tracheostomy where early indicates < 7 d and late indicates > 10 d. UHC = University HealthSystem Consortium.
χ2 test for categorical variables, Wilcoxon rank sum test for age.
Univariate outcomes, as depicted by Table 2, show that the incidence of VAP after tracheostomy was significantly lower for the early cohort compared with the late cohort (12% vs 15%, P value < .0001), as was mortality (14% vs 21%, P value < .0001). Patients in the early cohort had both a shorter ICU LOS and hospital LOS compared with those in the late cohort (16 days vs 27 days, P < .0001 and 25 days vs 38 days, P < .0001, respectively). Patients in the early cohort also incurred lower total direct costs and total charges compared with the late cohort ($61,163 vs $97,292, P value < .0001 and $251,333 vs $400,061, P value < .0001, respectively).
TABLE 2 ] .
Comparison of Outcomes Between Early and Late Tracheostomy in Critically Ill Patients With a Single Intubation at 185 Academic Medical Centers/Affiliates
| Outcome | Early Tracheostomy, < 7 d (n = 21,029) | Late Tracheostomy, > 10 d (n = 28,162) | P Valuea |
| Median total LOS, d | 25 | 38 | < .0001 |
| Median ICU LOS, d | 16 | 27 | < .0001 |
| Median post-tracheostomy LOS, d | 14 | 18 | < .0001 |
| Median total charges, × $1,000b | 251 | 400 | < .0001 |
| Median total direct hospital cost, × $1,000b | 61 | 97 | < .0001 |
| VAP, % | 12 | 15 | < .0001 |
| In-hospital mortality, % | 14 | 21 | < .0001 |
LOS = length of stay; VAP = ventilator-associated pneumonia.
χ2 test for categorical variables, Kruskal-Wallis test for continuous variables.
Charges are reported by each center and costs are then calculated using institution-specific cost-to-charge ratios obtained from the department-level Medicare cost reports.
In multivariable models, as shown in Tables 3 and 4, receipt of an early tracheostomy was found to independently predict mortality, ICU LOS, overall hospital LOS, total charges, and total direct costs. However, early tracheostomy did not independently predict incidence of VAP. Patients who received a late tracheostomy were 12% less likely to survive than those who received an early tracheostomy (OR, 0.88; 95% CI, 0.84-0.9). Total hospital LOS and ICU LOS were on average 11.8 (95% CI, 11.7-12.4) and 5.3 (95% CI, 5.1-5.5) days longer, respectively, for patients who underwent late tracheostomy. Total charges and hospital direct costs were on average $76,700 (95% CI, $72,800-$80,700) and $18,200 (95% CI, $17,300-$19,199) more, respectively, for patients who underwent late tracheostomy.
TABLE 3 ] .
Effect of Early Tracheostomy Compared With Late Tracheostomy on Outcomes in Linear Models
| Linear Models | Parameter Estimate | 95% CI |
| Total Hospital LOS, d | −11.8 | −12.4 to −11.7 |
| ICU LOS, d | −5.3 | −5.5 to −5.1 |
| Post-tracheostomy LOS, d | −3.7 | −6.8 to −2.3 |
| Total charges, × $1,000 | −76.7 | −80.7 to −72.8 |
| Total direct hospital cost, × $1,000 | −18.2 | −19.1 to −17.3 |
Covariates in each model included age groups (18-24, 25-44, 45-64, 65-83, and > 83 y), sex, race (white, black, Hispanic, Asian, and other), payer status (private, Medicare, Medicaid, uninsured/self-pay, and other), grouped admitting diagnoses (trauma, cardiovascular, pulmonary, surgical, neurologic, sepsis, and other), severity of illness score (minor, moderate, major, and extreme), and admission status (elective and emergent). See Table 2 legend for expansion of abbreviation.
TABLE 4 ] .
Effect of Early Tracheostomy Compared With Late Tracheostomy on Outcomes in Logistic Models
| Logistic Models | Hazards Ratio | 95% CI |
| VAP | 0.92 | 0.83-1.09 |
| In-hospital mortality | 0.88 | 0.84-0.92 |
Covariates in each model included age groups (18-24, 25-44, 45-64, 65-83, and > 83 y), sex, race (white, black, Hispanic, Asian, and other), payer status (private, Medicare, Medicaid, uninsured/self-pay, and other), grouped admitting diagnoses (trauma, cardiovascular, pulmonary, surgical, neurologic, sepsis, and other), severity of illness score (minor, moderate, major, and extreme), and admission status (elective and emergent). See Table 2 legend for expansion of abbreviation.
The multivariable model of tracheostomy timing identified male sex, younger age, white race, payer status, SOI, grouped admitting diagnosis, and elective admission as independent predictors of early tracheostomy (Table 5). Women were 16% less likely to receive early tracheostomy compared with men (OR, 0.84; 95% CI, 0.81-0.87). Blacks and Hispanics were also less likely to get an early tracheostomy compared with whites, (OR, 0.85; 95% CI, 0.81-0.90 and OR, 0.85; 95% CI, 0.78-092, respectively). Those with Medicaid and without insurance were less likely to get an early tracheostomy compared with those with private insurance (OR, 0.95; 95% CI, 0.89-0.99 and OR, 0.86; 95% CI, 0.83-0.90, respectively). Compared with those admitted with a trauma diagnosis, all other groups were less likely to get an early tracheostomy. As patients’ SOI increased from minor to extreme, they were less likely to get an early tracheostomy. Patients who had been admitted electively were more likely to receive an early tracheostomy, (OR, 1.28; 95% CI, 1.21-1.35).
TABLE 5 ] .
Logistic Regression Model of Early Tracheostomy vs Late Tracheostomy (N = 49,191)
| Covariates | Referent | OR | 95% CI |
| Sex | |||
| Male | Referent | … | … |
| Female | … | 0.84 | 0.81-0.87 |
| Race | |||
| White | Referent | … | … |
| Black | … | 0.85 | 0.81-0.90 |
| Hispanic | … | 0.85 | 0.78-0.92 |
| Asian | … | 0.90 | 0.79-1.03 |
| Other | … | 0.95 | 0.88-1.21 |
| Age groups, y | |||
| 18-24 | Referent | … | … |
| 25-44 | … | 0.88 | 0.80-0.97 |
| 45-64 | … | 0.85 | 0.77-0.93 |
| 65-83 | … | 0.86 | 0.78-0.91 |
| > 84 | … | 0.88 | 0.76-0.95 |
| Payer status | |||
| Private | Referent | … | … |
| Medicare | … | 1.01 | 0.95-1.07 |
| Medicaid | … | 0.95 | 0.89-0.99 |
| Uninsured | … | 0.86 | 0.83-0.90 |
| Other | … | 1.19 | 1.11-1.31 |
| Diagnosis | |||
| Trauma | Referent | … | … |
| Cardiovascular | … | 0.66 | 0.61-0.70 |
| Pulmonary | … | 0.86 | 0.80-0.93 |
| Surgical | … | 0.63 | 0.56-0.71 |
| Neurologic | … | 0.96 | 0.90-1.03 |
| Sepsis | … | 0.46 | 0.42-0.50 |
| Other | … | 0.69 | 0.64-0.74 |
| Severity of illness | |||
| Minor | Referent | … | … |
| Moderate | … | 0.66 | 0.44-0.94 |
| Major | … | 0.40 | 0.28-0.56 |
| Extreme | … | 0.12 | 0.08-0.16 |
| Admission status | |||
| Emergent | Referent | … | … |
| Elective | … | 1.28 | 1.21-1.35 |
Early tracheostomy timing was defined as < 7 d after index intubation; late tracheostomy was defined as > 10 d after index intubation.
Discussion
This study examined the timing of a tracheostomy at nearly 200 academic medical centers and their affiliates across the United States. Our findings that receipt of early tracheostomy decreased ICU LOS, hospital LOS, hospital costs, and overall mortality are consistent with prior studies that have found significant benefits for both the patient and hospital in the receipt of an early tracheostomy.13,19‐21
There are a number of potential explanations as to why these benefits exist. Decreased ICU LOS and hospital LOS are attributable to improved ventilator weaning that is seen in patients who have undergone a tracheostomy.11,19 The sooner this procedure happens, the sooner the weaning process can begin. A patient who can be weaned from a ventilator is also moving closer to potential ICU discharge and eventual hospital discharge, which translates into a potentially shorter LOS.19,22
Usually, patients on ventilators can only be housed in intensive care settings. Ventilator support and ICU room and board, along with all support services that they necessitate, are among the most expensive billing items in a hospital for both the patient and the hospital.23 For example, an ICU will use almost three times the nurse hours per patient day as a general floor would.24 Patients off a ventilator, tracheostomy or no tracheostomy, can usually go to less financially expensive and resource-intense floors in the hospital or could even potentially be transferred to less costly long-term care or rehabilitation facilities.25
Although others have shown that early tracheostomy reduces VAP rates,26 we did not find this to be true when controlling for other patient factors. Still, even with presumably even distribution of VAP between early and late tracheostomy groups, we found that early tracheostomy decreased ICU LOS, hospital LOS, hospital costs, and overall mortality.
Despite all the current advances in medicine, prognostication in critical illness remains an imprecise art. Yet, some ability to predict survival and quality of life must play a role in clinician decisions to offer tracheostomy to critically ill, ventilator-dependent patients. Our findings in this study of patients who all eventually undergo tracheostomy after a single intubation suggest that patients who may be assumed to have higher mortality or worse outcomes at the outset are less likely to undergo early tracheostomy. Factors such as SOI, age, and diagnosis have all been associated with worse outcome in the critically ill population.27 Furthermore, clinician prediction of high mortality has been shown to correlate with decreased use of advanced life support.28 Compared with trauma and neurologic diagnoses, which are often entirely unexpected but may result in return to acceptable quality of life with aggressive rehabilitation, patients with underlying chronic diseases or acute conditions with less-favorable long-term outcomes, such as COPD requiring intubation, MI severe enough to require intubation, or cancer surgery requiring intubation, similarly may not be considered for early tracheostomy. Thus, it is not surprising that more severely ill patients, older patients, and patients with cardiovascular, pulmonary, or surgical diagnoses are less likely to receive early tracheostomy in our study.
There is an increasing body of evidence showing sociodemographic disparities in surgical care.29‐31 It has been reported that unequal access to care is responsible for suboptimal outcome observed in certain minority and indigent groups undergoing surgery.32 However, receipt of tracheostomy for ventilator dependence is a unique surgical intervention when considering the possibility of demographic bias. The need for tracheostomy is only identified after hospital admission. Tracheostomy can be indicated for patients with various underlying diseases or reasons for admission and can be recommended and performed by a number of providers across specialties and subspecialties. Thus, the selection bias at play for other treatments may not be the same as with tracheostomy. In our study, access to care was essentially standardized by including only patients who were ventilated. Yet, we still observed sociodemographic disparities in receipt of early tracheostomy. One possibility is that of unconscious bias, wherein clinicians may be unaware that they are preferentially offering early tracheostomy to some groups while delaying it in others. Such unconscious bias may play a role in a number of observed sociodemographic disparities in surgical patients.14
Sociodemographic disparities have also been shown among critically ill patients.33 Women have been shown to require mechanical ventilation longer than men.34 It is possible that perceived aesthetic preferences by both providers and proxies may have resulted in fewer women undergoing tracheostomy. However, like the findings of Valentin et al35 in an Austrian cohort and Fowler et al36 in a Canadian cohort that critically ill men received more intensive therapy and more invasive interventions than women, we found that US men were more likely to undergo early tracheostomy. Again, physician bias may be playing a role. But, it is also possible that women and men have different cultural preferences on intensity of care in the presence of critical illness, resulting in fewer women (or their proxies) opting for early tracheostomy even if it is offered early after intubation.
We found that blacks and Hispanics are less likely than whites to undergo early tracheostomy. Nonwhite race has been shown to be associated with fewer advance directives, more discord among family members regarding goals of care, and increased deaths with full life support.37 Thus, although unconscious bias may be playing a role, inability to arrive at consensus regarding tracheostomy early after intubation may explain our finding that nonwhites were less likely to undergo early tracheostomy. Structural factors, however, may also be at play, as suggested by the findings of Mayr et al,38 Hasnain-Wynia et al,39 and others who have shown that facilities that care for higher proportions of minority patients are less likely to achieve quality benchmarks.
Lack of insurance has been shown to be associated with both higher ICU mortality and fewer invasive procedures and delays in discharge compared with insured patients.40 Our study supports these previous findings, as uninsured patients were less likely to receive early tracheostomy compared with insured patients.
There are a number of important limitations to our study. This was a retrospective study without random assignment for which we ascribed a priori categories for tracheostomy timing. Thus, we cannot account for all of the complex factors that clinicians consider for tracheostomy timing. However, given the difficulties in arranging a randomized controlled trial across this large of a cohort, disparate diagnoses, and different critical care settings, our dataset allowed us to control for a number of potential clinical confounders. The dataset, however, like all administrative data, is subject to coding errors on the original billing forms. The sample size of > 49,000 should mitigate the effect of misclassification due to coding errors. Unfortunately, we were not able to differentiate between open and percutaneous tracheostomies in our analyses. It is possible that ecologic trends for one technique over another may have been an unmeasured confounding factor in our multiyear study. It is possible that there are unmeasured differences in technological capabilities at the hospital level, in particular since certain sociodemographic groups cluster at certain hospitals; others have proposed that this may contribute to disparities in outcomes after injury, cardiac surgery, and colorectal surgery, among other interventions.41‐45 The UHC database is from academic medical centers and their affiliates whose leadership have made a commitment to quality improvement and cost control as evident by their participation in the consortium. Thus, our findings may not be generalizable to community hospitals across the United States or academic centers that do not participate in the UHC. Still, > 90% of academic medical centers along with their affiliates do participate. Given the fact that academic medical centers serve as tertiary referral centers for some of our country’s most critically ill patients, the implications of our findings are still meaningful. Despite these potential limitations, this is one of largest studies focusing on tracheostomy timing in the literature and the first, to our knowledge, to identify the benefits of early tracheostomy across multiple categories of disease and specialties.
Conclusions
Tracheostomy is a potentially life-saving intervention for ventilator-dependent patients. The benefits of tracheostomy, including shorter ICU and hospital LOS, lower costs, and improved in-hospital mortality, are greatest when tracheostomy is performed early in the course of mechanical ventilation. Yet, there are notable sociodemographic disparities in receipt of early tracheostomy even after controlling for a number of clinical factors. Further research is needed to determine why women, certain minorities, and those with Medicaid are less likely to receive an early tracheostomy. Standardized protocols to determine the timing of tracheostomy for patients who are mechanically ventilated may help to correct these disparities.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: H. P. S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. J. J. S. contributed to writing, editing, design, and statistical analysis and is first author and H. P. S. contributed to writing, editing, design, and statistical analysis, and is principal investigator of the study.
Conflict of interest: None declared.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Agency for Healthcare Research Quality, or the Patient-Centered Outcomes Research Institute.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- CDB/RM
Clinical Database Resource Manager
- ICD-9
International Classification of Diseases, Ninth Revision
- LOS
length of stay
- SOI
severity of illness
- UHC
University HealthSystem Consortium
- VAP
ventilator-associated pneumonia
Footnotes
Portions of this paper were previously published in abstract form at the American Association for the Surgery of Trauma Annual Meeting, September 12, 2012, Lihue, HI.
FUNDING/SUPPORT: This research was supported by the Agency for Healthcare Research Quality [Grant R01HS22694], the Patient Centered Outcomes Research Institute [Grant ME-1310-07682], and the University of Massachusetts Clinical Scholars Award [UL1RR031982, 1KL2RR031981-01, and 8KL2TR000160-03].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Curtis JR, Engelberg RA, Bensink ME, Ramsey SD. End-of-life care in the ICU: can we simultaneously increase quality and reduce costs? Am J Respir Crit Care Med. 2012;186(7):587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Healthcare Cost and Utilization Project (HCUP). 2006-2009. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov/. Accessed January 31, 2013. [PubMed]
- 3.Walts PA, Murthy SC, DeCamp MM. Techniques of surgical tracheostomy. Clin Chest Med. 2003;24(3):413-422. [DOI] [PubMed] [Google Scholar]
- 4.Wang HK, et al. The impact of tracheostomy timing in patients with severe head injury: an observational cohort study. Injury. 2012;43(9):1432-1436. [DOI] [PubMed] [Google Scholar]
- 5.Van Bommel J, Van der Hoven B. Tracheostomy in the ICU: is timing everything? Minerva Anestesiol. 2010;76(3):171-172. [PubMed] [Google Scholar]
- 6.Romero J, Vari A, Gambarrutta C, Oliviero A. Tracheostomy timing in traumatic spinal cord injury. Eur Spine J. 2009;18(10):1452-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizk EB, Patel AS, Stetter CM, Chinchilli VM, Cockroft KM. Impact of tracheostomy timing on outcome after severe head injury. Neurocrit Care. 2011;15(3):481-489. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI, Suarez JI, Parekh PD, Bhardwaj A. Prediction and timing of tracheostomy in patients with infratentorial lesions requiring mechanical ventilatory support. Crit Care Med. 2000;28(5):1383-1387. [DOI] [PubMed] [Google Scholar]
- 9.Quintel M, Bräuer A. Timing of tracheostomy. Minerva Anestesiol. 2009;75(6):375-383. [PubMed] [Google Scholar]
- 10.Arabi Y, Haddad S, Shirawi N, Al Shimemeri A. Early tracheostomy in intensive care trauma patients improves resource utilization: a cohort study and literature review. Crit Care. 2004;8(5):R347-R352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holevar M, Dunham JC, Brautigan R, et al. Practice management guidelines for timing of tracheostomy: the EAST Practice Management Guidelines Work Group. J Trauma. 2009;67(4):870-874. [DOI] [PubMed] [Google Scholar]
- 12.Phelan BA, Cooper DA, Sangkachand P. Prolonged mechanical ventilation and tracheostomy in the elderly. AACN Clin Issues. 2002;13(1):84-93. [DOI] [PubMed] [Google Scholar]
- 13.Gomes Silva BN, Andriolo RB, Saconato H, Atallah AN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2012;3:CD007271. [DOI] [PubMed] [Google Scholar]
- 14.Santry HP, Wren SM. The role of unconscious bias in surgical safety and outcomes.Surg Clin North Am. 2012;92(1):137-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artinyan A, Mailey B, Sanchez-Luege N, et al. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116(5):1367-1377. [DOI] [PubMed] [Google Scholar]
- 16.Chang RK, Chen AY, Klitzner TS. Female sex as a risk factor for in-hospital mortality among children undergoing cardiac surgery. Circulation. 2002;106(12):1514-1522. [DOI] [PubMed] [Google Scholar]
- 17.Mortality measurement: mortality risk adjustment methodology for University Health System Consortium. March 2009. Agency for Healthcare Research and Quality website. http://archive.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/mortality/Meurer.html. Accessed January 26, 2014.
- 18.Healthcare Cost and Utilization Project. Clinical classifications software (CCS) for ICD-9-CM. Agency for Healthcare Research and Quality website. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Published 2012. Accessed January 26, 2014. [PubMed]
- 19.Bickenbach J, Fries M, Offermanns V, et al. Impact of early vs. late tracheostomy on weaning: a retrospective analysis. Minerva Anestesiol. 2011;77(12):1176-1183. [PubMed] [Google Scholar]
- 20.BouAkl I, Bou-Khalil P, Kanazi G, Ayoub C, El-Khatib M. Weaning from mechanical ventilation. Curr Opin Anaesthesiol. 2012;25(1):42-47. [DOI] [PubMed] [Google Scholar]
- 21.Ganuza JR, Garcia Forcada A, Gambarrutta C, et al. Effect of technique and timing of tracheostomy in patients with acute traumatic spinal cord injury undergoing mechanical ventilation. J Spinal Cord Med. 2011;34(1):76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durbin CG., Jr Tracheostomy: why, when, and how? Respir Care. 2010;55(8):1056-1068. [PubMed] [Google Scholar]
- 23.Freeman BD, Morris PE. Tracheostomy practice in adults with acute respiratory failure. Crit Care Med. 2012;40(10):2890-2896. [DOI] [PubMed] [Google Scholar]
- 24.Russell LB. Intensive care. In: Technology in Hospitals: Medical Advances and Their Diffusion. Washington, DC: The Brookings Institute; 1979:42-49. [Google Scholar]
- 25.Baumann HJ, Kemei C, Kluge S. Tracheostomy in the intensive care unit [in German]. Pneumologie. 2010;64(12):769-776. [DOI] [PubMed] [Google Scholar]
- 26.Craven DE. Preventing ventilator-associated pneumonia in adults: sowing seeds of change. Chest. 2006;130(1):251-260. [DOI] [PubMed] [Google Scholar]
- 27.Carson SS, Bach PB. Predicting mortality in patients suffering from prolonged critical illness: an assessment of four severity-of-illness measures. Chest. 2001;120(3):928-933. [DOI] [PubMed] [Google Scholar]
- 28.Cook DJ, Montori VM, McMullin JP, Finfer SR, Rocker GM. Improving patients’ safety locally: changing clinician behaviour. Lancet. 2004;363(9416):1224-1230. [DOI] [PubMed] [Google Scholar]
- 29.Ayanian JZ. Determinants of racial and ethnic disparities in surgical care. World J Surg. 2008;32(4):509-515. [DOI] [PubMed] [Google Scholar]
- 30.Radowsky JS, et al. Racial disparities in voice outcomes after thyroid and parathyroid surgery. Surgery. 2013;153(1):103-110. [DOI] [PubMed] [Google Scholar]
- 31.Schoenfeld AJ, Lurie JD, Zhao W, Bono CM. The effect of race on outcomes of surgical or nonsurgical treatment of patients in the Spine Patient Outcomes Research Trial (SPORT). Spine. 2012;37(17):1505-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieracci FM, Eachempati SR, Christos PJ, Barie PS, Mushlin AI. Explaining insurance-related and racial disparities in the surgical management of patients with acute appendicitis. Am J Surg. 2007;194(1):57-62. [DOI] [PubMed] [Google Scholar]
- 33.Cooke CR, Kahn JM. Deconstructing racial and ethnic disparities in critical care. Crit Care Med. 2010;38(3):978-980. [DOI] [PubMed] [Google Scholar]
- 34.Kollef MH, O’Brien JD, Silver P. The impact of gender on outcome from mechanical ventilation. Chest. 1997;111(2):434-441. [DOI] [PubMed] [Google Scholar]
- 35.Valentin A, Jordan B, Lang T, Hiesmayr M, Metnitz PG. Gender-related differences in intensive care: a multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med. 2003;31(7):1901-1907. [DOI] [PubMed] [Google Scholar]
- 36.Fowler RA, Sabur N, Li P, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ. 2007;177(12):1513-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erickson SE, Vasilevskis EE, Kuzniewicz MW, et al. The effect of race and ethnicity on outcomes among patients in the intensive care unit: a comprehensive study involving socioeconomic status and resuscitation preferences. Crit Care Med. 2011;39(3):429-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayr FB, Yende S, D’Angelo G, et al. Do hospitals provide lower quality of care to black patients for pneumonia? Crit Care Med. 2010;38(3):759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Arch Intern Med. 2007;167(12):1233-1239. [DOI] [PubMed] [Google Scholar]
- 40.Lyon SM, Benson NM, Cooke CR, Iwashyna TJ, Ratcliffe SJ, Kahn JM. The effect of insurance status on mortality and procedural use in critically ill patients. Am J Respir Crit Care Med. 2011;184(7):809-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rangrass G, Ghaferi AA, Dimick JB. Explaining racial disparities in outcomes after cardiac surgery: the role of hospital quality. JAMA Surg. 2014;149(3):223-227. [DOI] [PubMed] [Google Scholar]
- 42.Huang LC, Tran TB, Ma Y, Ngo JV, Rhoads KF. Factors that influence minority use of high-volume hospitals for colorectal cancer care. Dis Colon Rectum. 2015;58(5):526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glance LG, Osler TM, Mukamel DB, et al. Trends in racial disparities for injured patients admitted to trauma centers. Health Serv Res. 2013;48(5):1684-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Localio AR, Hamory BH, Fisher AC, TenHave TR. The public release of hospital and physician mortality data in Pennsylvania: a case study. Med Care. 1997;35(3):272-286. [DOI] [PubMed] [Google Scholar]
- 45.Khera R, Vaughan-Sarrazin M, Rosenthal GE, Girotra S. Racial disparities in outcomes after cardiac surgery: the role of hospital quality. Curr Cardiol Rep. 2015;17(5):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement