Abstract
The availability of Pb and As in an historically contaminated orchard soil, after amendment with compost and aging in the field, was determined by single-step chemical extraction with 1.0 M ammonium acetate at pH 4.8, sequential extraction using the modified BCR test, and a redworm bioassay in the laboratory. The efficiency of soil Pb extraction by ammonium acetate was greater at higher total soil Pb but was reduced by compost amendment. Conversely, the extraction efficiency of total soil As increased with compost amendment, but was not sensitive to total soil As. The redworm bioassay indicated Pb (but not As) bioavailability to be reduced by soil amendment with compost, a result consistent with the ammonium acetate extraction test but not reflected in modified BCR test. Electron microprobe studies of the orchard soil revealed Pb and As to be spatially associated in discrete particles along with phosphorus and iron.
Keywords: soil lead, soil arsenic, bioavailability, bioaccumulation, orchard soils, compost, remediation, sequential extraction
INTRODUCTION
Lead and arsenic are toxic metals that coexist at elevated concentrations in old orchard soils due to the historical use of lead arsenate insecticide (Veneman et al., 1983). Their solubility and bioavailability to plants, soil organisms and humans depends on the form of these metals in the solid phase of the soil. Lead activity and solubility in slightly to moderated contaminated soils are controlled by strong adsorption of the Pb2+ ion on soil minerals (particularly Fe oxides) and organic matter (Gustafsson et al., 2011; Sauve et al., 2000). Surface complexation models predict that Pb adsorption is dominated by organic matter at soil pH < 6, whereas adsorption on Fe oxides is more prevalent at pH > 6 (Gustafsson et al., 2011). Soil pH is critically important in determining the free Pb2+ activity in soil solution, as Pb adsorption and most precipitation reactions are favored by higher pH. Dissolved organic matter also has a key role in controlling Pb solubility in soils, forming organo-Pb complexes that increase Pb solubility as the pH is raised above 6.5 (Sauve et al., 1998). In fact, most of the water-soluble and potentially leachable Pb in non-acid soils occurs as Pb-DOM complexes (Ashworth and Alloway, 2008; Weng et al., 2002). In severely contaminated soils, some mineral forms of Pb are sufficiently stable to limit Pb solubility. Thus, for example, litharge (PbO) cerussite (PbCO3) and hydrocerussite (Pb3(CO3)2(OH)2) have been detected in Pb-contaminated soils (Essington et al., 2004) and pyromorphite (Pb5(PO4)3Cl) is a stable form of Pb in soils with high phosphate content (Ryan et al., 2001; Scheckel et al., 2005).
Arsenic bioavailability is complicated by the existence of two prevalent oxidation states of this metalloid in soil environments, occurring as the arsenate (AsO43−) and arsenite (As(OH)30) species. Arsenate in most situations adsorbs more strongly than arsenite, behaving much like phosphate and chemisorbing most strongly on Fe oxide and silicate minerals in the pH range of 5.0–6.5 (Manning and Goldberg, 1996a, 1996b). Consequently, phosphate amendments to soils (e.g., as fertilizers, manures or composts) tend to enhance solubility, mobility and bioavailability of As in soils (Cao et al., 2003; Wang and Mulligan,2006). Bonding of arsenate on soil organic matter is relatively weak, probably occurring indirectly via ternary complexes involving polyvalent cations such as Ca2+, Al3+ and Fe3+ (Thanabalasingam and Pickering, 1986). Unlike Pb, As solubility tends to increase at higher pH. Anoxic conditions in soils can also mobilize As due to reductive dissolution of Fe oxides, reduction of arsenate to arsenite and an increase in dissolved organic acids that compete with arsenate for mineral adsorption sites. In acidic oxidized soils, Ca and Fe arsenate minerals may control As solubility, whereas in extremely anoxic soils insoluble As(III) sulfides can limit solubility (Sadiq,1997). Scorodite (FeAsO4), formed during the weathering of arsenopyrite (Mihaljevic et al., 2010), is a stable mineral form of As in severely contaminated soils (Meunier et al., 2010).
Because the most prevalent ionic forms of Pb and As in aerated soils, Pb2+ and AsO43− (arsenate), behave very different chemically, it is expected that remediation schemes dependent on the modification of soil chemical properties may affect the solubility and bioavailability of these two elements in very different ways. Thus, remediation of soils containing both elements by removal or chemical stabilization may be difficult. Various soil amendments have been tested for their ability to reduce the solubility, leachability, and bioavailability of Pb, As and other toxic metals in contaminated soils. Materials composed primarily of natural organic matter can be expected to decrease the level of Pb extractability and bioavailability in soil due to the very strong affinity of organic complexation sites for Pb2+ cations. Thus, peats and composts have consistently shown the ability to stabilize soil Pb and reduce its bioavailability (Hashimoto et al., 2011; Karam et al., 2011; Kumpiene et al., 2007; Nwachukwu and Pulford, 2009; Zhou et al., 2012) in spite of the fact that these amendments are likely to increase dissolved organic matter (DOM) which potentially can increase Pb solubility (Sauve et al., 1998).
Experimental results for the effect of compost amendments on As behavior have, in contrast to Pb, indicated a tendency for organic-rich amendments to increase rather than decrease solubility and extractability (Hartley et al., 2010; Beesley et al., 2010; Yan et al., 2012; Cao et al., 2003; Wang et al., 2006), a result expected from the known weak interaction of arsenate with solid organic matter as well as competition between dissolved organic matter (DOM) and arsenate for adsorption sites.
Generally, bioavailability of metals in soils is estimated using chemical extraction tests or more directly determined using bioassays. One of the most common bioassays for evaluating heavy metal bioavailability uses bioaccumulation by earthworms (Maenpaa et al., 2002; Janssen et al., 1997). In this study, our objective was to assess the impact of compost addition on availability of Pb and As in an historically contaminated orchard soil by using a traditional chemical test and a soil invertebrate bioassay. A common soil extraction method (Modified Morgan solution) was used to estimate Pb and As availability by soil test, and a redworm (Eisenia foetida) bioassay was used to measure bioavailability. A widely used sequential extraction method (the modified BCR) was also applied to evaluate Pb availability in the compost-amended soils.
METHODS
Soil Pb and As extractability
The soils used in this study are silty clay loams (Hudson series), sampled from an ongoing field experiment involving six separate miniplots situated on an old apple orchard near the Cornell university campus. Lead arsenate had been applied repeatedly to this orchard in the first half of the 20th century, and topsoils accumulated highly variable concentrations of Pb and As. The miniplots represent a wide range of soil Pb and As concentration, and each one is subdivided into beds which have been supplemented with 0, 5, or 10% (on a dry weight basis) compost having low As (0.97 mg/kg) and Pb (16.1 mg/kg) content. The pH of the compost in water was 7.0, and concentrations of major elements in the compost were 4.41% (Ca), 1.62% (Mg), 1.51% (K), 0.82% (P), 0.45% (S), 0.88% (Fe) and 0.60% (Al). Concentrations of trace elements were 23.9 mg/kg (Ni), 65.4 mg/kg (Cu), 507 mg/kg (Mn), 42.7 mg/kg (Cr), and 298 mg/kg (Zn).
Miniplot construction, which included building the frames for the miniplots and incorporating the compost, was done in June 2009 for four of the soils (labeled here as A, C, D, and E). The other two miniplots (B and F) were set up in the spring of 2010. The compost added to the miniplots was taken from the Cornell composting facility and hand mixed into the beds. The beds were further cultivated during cropping in 2009 and 2010. One of the miniplots (F) was sampled in December 2010 while the five remaining miniplots (A, B, C, D, and E) were sampled in July 2011. The sampled soils were pulverized and sieved (2mm) in preparation for testing. Subsamples were analyzed for total lead and arsenic by the Cornell Nutrient Analysis Laboratory (CNAL) using ICP emission spectrometry after digestion of the soils by EPA method 3051. Total Pb and As concentrations in the miniplot soils with 0,5 and 10% compost amendments are listed in Table 1.
Table 1.
Relevant properties, Pb and As total concentrations and Modified Morgan extractable Pb and As percentages of the orchard miniplot soils.
| Miniplot | Compost | OM (%) | pH | Total Pb (mg/kg) | Extractable Pb (%) | Total As (mg/kg) | Extractable As (%) |
|---|---|---|---|---|---|---|---|
| A | 0 | 5.1 | 6.4 | 66.1 | 1.99 ± 0.17 | 28.5 | 1.54 ± 0.45 |
| 5 | 5.9 | 6.5 | 31.1 | 1.28 ± 0.08 | 13.9 | 3.30 ± 1.00 | |
| 10 | 9.1 | 6.6 | 22.3 | 1.48 ± 0.11 | 11.7 | 2.94 ± 0.34 | |
| B | 0 | 5.8 | 5.9 | 127 | 2.50 ± 0.03 | 51.7 | 1.74 ± 0.09 |
| 5 | 9.1 | 6.6 | 105 | 1.86 ± 0.00 | 50.0 | 5.06 ± 0.32 | |
| 10 | 14.9 | 6.8 | 117 | 1.06 ± 0.06 | 42.2 | 6.65 ± 0.11 | |
| C | 0 | 6.7 | 6.0 | 236 | 4.73 ± 0.07 | 61.8 | 2.61 ± 0.22 |
| 5 | 12.5 | 6.3 | 265 | 1.54 ± 0.06 | 60.7 | 5.29 ± 0.85 | |
| 10 | 18.2 | 6.7 | 240 | 0.77 ± 0.01 | 53.6 | 7.43 ± 1.36 | |
| D | 0 | 5.8 | 6.2 | 303 | 3.05 ± 0.08 | 73.1 | 1.66 ± 0.20 |
| 5 | 7.9 | 6.8 | 257 | 1.62 ± 0.01 | 61.9 | 6.66 ± 1.21 | |
| 10 | 13.3 | 6.9 | 255 | 0.96 ± 0.06 | 60 | 6.14 ± 1.04 | |
| E | 0 | 7.1 | 6.2 | 532 | 3.08 ± 0.01 | 138 | 5.39 ± 0.04 |
| 5 | 12.1 | 6.6 | 1420 | 1.77 ± 0.07 | 236 | 7.12 ± 0.05 | |
| 10 | 15.8 | 6.7 | 694 | 1.62 ± 0.12 | 122 | 9.93 ± 2.39 | |
| F | 0 | 8.7 | 5.8 | 892 | 4.33 ± 0.10 | 185 | 1.41 ± 0.06 |
| 5 | 12.3 | 6.6 | 768 | 3.18 ± 0.02 | 165 | 4.97 ± 0.26 | |
| 10 | 15.7 | 6.7 | 667 | 2.23 ± 0.02 | 151 | 6.36 ± 0.14 |
In order to measure the pH of the soils, 5 g of dry soil were measured out and mixed with 10 mL of deionized water. The mixture was allowed to settle for 30 minutes and pH was then measured using a pH meter equipped with a glass electrode. The Loss on Ignition (LOI) method was used to calculate the percentage of organic matter in the soils. This was done by measuring weight loss of 5 g soil in a crucible, pre-dried at 105°C, after heating the soil in a muffle furnace at 375°C for 24 hours (Davies, 1974). Percent organic matter and pH of the miniplot soils are given in Table 1. Free Fe and Mn oxide content of the orchard soils were determined using the standard citrate-dithionite method (Mehra and Jackson, 1960) for Miniplot A soils with 0, 5 and 10% compost amendment, and averaged 1.1 g Fe/kg and 0.12 g Mn/kg in the control (0%) soil, and slightly lower (for Fe) and higher (for Mn) in the compost-amended soils.
Extraction for Pb and As was processed using Modified Morgan’s solution (1M ammonium acetate at pH 4.8). The extraction procedure was conducted in duplicate on each soil collected from the miniplots. 5 g of each soil and 20 mL of Modified Morgan were measured into 125 ml Erlenmeyer flasks. The flasks were agitated on a rotary shaker at 150 rpm for 15 minutes, allowed to stand for 5–10 minutes, then filtered through Whatman No. 42 Paper to produce clear solutions. The extracts were analyzed for Pb by flame atomic absorption spectrometry (FAA) at a wavelength of 217 nm, and for As by ICP-emission spectroscopy at 189.0 nm. The Pb and As extraction results using Modified Morgan are presented as percentages of the total Pb and As extracted into solution (Table 1). Because the pH of the compost-amended miniplot soils were always higher than those of the unamended control soils (see Table 1), the Modified Morgan extraction was repeated on miniplot F soils after gradually adjusting samples from the 0, 5 and 10% compost treatments to pH 5.5 over 24 hours using 1 M HCl.
The modified BCR test, a 3-step sequential extraction procedure that is frequently used for the speciation of toxic metals in soils (Rauret et al., 1999) was applied in triplicate to samples of the miniplot F soils only. This procedure extracts metals sequentially using, in order : 1. Acetic acid (0.11 M) at pH 2.55 (Fraction A), 2. Hydroxylamine hydrochloride (0.5 M) at pH 1.54 (Fraction B), 3. Hydrogen peroxide (8.8 M) at pH 2.0 (Fraction C). The metal fractions extracted, although definable in operational terms only, are commonly referred to, respectively, as:
the exchangeable and weak-acid soluble (chemically labile) forms
bound to Fe and Mn oxide minerals
bound to oxidizable solids (specifically organic matter and sulfides)
The metal fraction remaining in the soil residue after these 3 steps is referred to as Fraction D, the residual form. Metal in fraction D may be occluded in insoluble minerals or otherwise bound extremely strongly on solids, but is considered not to be bioavailable.
Scanning Electron Microscopy (SEM) and Elemental Mapping
Orchard soil with high Pb and As contamination was collected near the miniplot F site, dried and pulverized to pass a 1-mm sieve. A thin layer of this soil was mounted on an aluminum holder using two-sided adhesive tape, then coated with a thin elemental carbon layer under vacuum prior to making observations by SEM (JEOL 8900 Electron Probe Microanalyzer) operating at 15.0 kV. Imaging and chemical element mapping for particles of interest was done on the scanning electron microscope (SEM) equipped with energy dispersive x-ray detector (EDS). The scanning system forms images from secondary and backscattered electrons and elemental maps from secondary X-rays. This is not a surface analysis method, as the high-energy electrons focussed on the sample penetrate at least several micrometers into the sample particles.
Redworm Bioassay
Additional soil was collected from miniplot F as well as from an uncontaminated nearby field (Caldwell Field) to serve as a soil control in March 2012. The miniplot F soils included samples supplemented with 0, 5%, or 10% compost. Relevant properties of the miniplot F soils are included in Table 1, showing Pb concentrations in the 600–900 mg/kg range depending on the level of compost amendments. The control soil was a low-organic matter silty-clay loam with a pH of 5.5 and total Pb content of 11.1 mg/kg. The soils were pulverized and sieved (2mm). For each soil type, 500 grams with three replicates were placed in plastic containers, creating a total of twelve samples. Each soil sample was saturated to a field-moist state using deionized water.
Redworms (Eisenia foetida) obtained from Carolina Biological Supply Company were rinsed with deionized water before being separated into groups of eight individuals and deposited into the 12 containers. The worms were fed oatmeal twice during a total of 27 days exposure. To stop the experiment, worms were removed from the containers, rinsed with deionized water, and weighed fresh. Each group of worms was depurated in a separate clean container with oatmeal for three days. The worms were then frozen, dried overnight at 70°C, weighed dry, and pulverized using a mortar and pestle. The tissues were digested in 30 ml glass beakers by adding 10 ml trace-metal grade concentrated nitric acid followed by 3 hours refluxing on a hot plate, with additional 1–2 ml of 30% H2O2 near the end of the refluxing period. The heat was then increased to reduce the acid digest volume to 2 ml, and the beakers were removed from the hot plate and cooled. After adding 10 ml of deionized water to each beaker, the digests were brought to near-boiling to allow solids to re-dissolve. Finally, the solution volumes were diluted to 25 ml exactly with deionized water and went through Whatman #42 filter papers. The clear solutions were analyzed by ICP emmision spectrometry for Pb and As.
RESULTS AND DISCUSSION
Pb and As Extractability by Modified Morgan’s Test
The six miniplot soils had a large range of total Pb (22–1420 mg/kg) and As (11.7–236 mg/kg) concentrations as is shown in Table 1, with the miniplots labeled A, B, C, D, E, F in the order of increasing Pb and As level. Soil Pb concentration was variable among the treated sub-plots of some miniplots, with miniplot E showing the most extreme variation (532–1420 mg/kg) due to the inherently spotty nature of the historical application of Pb arsenate insecticide (Veneman et. al., 1983). The pH of the unamended miniplot soils ranged from 5.6 to 6.4, but compost addition raised the pH into the 6.3 to 6.9 range. Data in table 1 shows that, disregarding the initial spatial variability of soil Pb and As, compost additions had the general effect of decreasing soil total Pb and As concentrations due to dilution by the low-metal compost (As = 0.97 mg/kg, Pb = 16.1 mg/kg).
The extractable Pb levels as measured using the modified Morgan (MM) soil test (Table 1), were strongly dependent on total soil Pb. The overall correlation between MM-extractable Pb (PbMM) and total Pb (PbT) was :
| (1) |
This regression equation reveals that, on average, MM extracted only about 2.8% of the total Pb in these compost-amended and unamended orchard soils. However, over all soil Pb concentrations, the extractability of Pb for the unamended soils averaged 3.28%, substantially higher than the 1.61% measured for the compost-amended soils. The average efficiency of extraction of the compost-amended soils by MM is also substantially lower than that reported by McBride et al. (2011) and Hamel et al. (2003) for Pb-contaminated soils having various sources of contamination. In fact, unamended soils collected earlier from the same orchard site used in this study had an average efficiency of Pb extraction by MM of 5.3%, somewhat higher than the 3.28% reported here for unamended orchard soil, and much higher than that measured here for the compost-amended soils..
An increase in soil organic matter due to compost addition caused a decreasing extraction efficiency of Pb by MM, as shown by the lower extraction percentages for Pb in soils amended with 5 and 10% compost (see Table 1). All of these reduced Pb extractabilities were significantly lower than those of the unamended control soils. In most miniplots, the 10% compost amendment had a significantly larger effect in lowering Pb extractability than the 5% amendment. However, the efficiency of lead extractability also tended to increase with increasing total Pb in the soils. Thus, regression analysis confirmed a significant relationship of Pb extraction efficiency to both soil organic matter content (r= 0.359) and soil total Pb (r= 0.313). Because these two factors were correlated to Pb extraction efficiency, we conducted a multiple regression analysis to confirm a statistically significant relationship between the fraction of total Pb extracted factors when both factors are considered together. The best-fit equation (2) shows a stronger relationship of Pb extraction efficiency to these soil factors when they are considered simultaneously:
| (2) |
In this equation, OM (%) is the measured organic matter content (%) of the soil and PbT is the total soil Pb concentration (mg/kg). The physical-chemical interpretation of the organic matter effect in reducing Pb extractability must consider, besides the well-known ability of organic matter to complex Pb strongly, the fact that the compost amendments raised soil pH significantly. In fact, there was a highly significant correlation between Pb extractability and soil pH for the soils sampled from all miniplots and treatments:
| (3) |
The results indicate that three factors may be involved in controlling the soil Pb extraction efficiency: total soil Pb concentration, soil organic matter content and soil pH. Because the variables of soil pH and organic matter content are highly correlated, it is not possible to discern from this initial extraction at the field soil pH if reduced Pb extractability arose solely from the pH effect on Pb adsorption or was largely a consequence of Pb bonding to the organic matter of the added compost. It is also conceivable that the high P content of the compost could have provided enough soluble phosphate to promote Pb phosphate precipitation (Bosso et al., 2008; Cao et al., 2003).
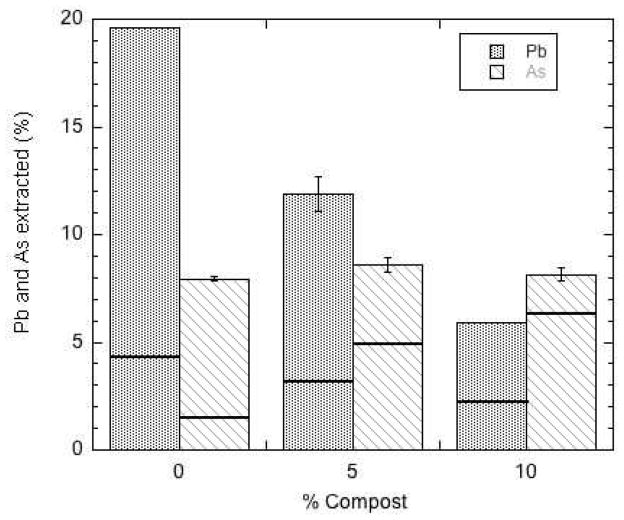
Figure 1 exemplifies, based on the data for miniplots C and F (Table 1) the pronounced effect of compost amendment in reducing the efficiency of Pb extractability. Similar trends to those in Figure 1 were apparent for miniplots B, D and E (see Table 1). The only exception to the trend of decreasing Pb extractability with increasing compost addition was seen in the low-Pb (control) miniplot A.
Figure 1.
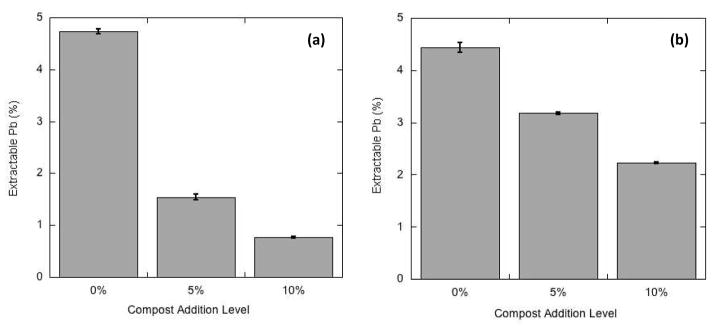
Percentage of total soil Pb extracted by Modified Morgan solution from miniplot soils with 3 levels (0, 5, 10%) of compost addition: (a) miniplot C soils containing about 240 mg/kg total Pb; (b) miniplot F soils containing about 700–900 mg/kg total Pb.
The extractable As levels, as measured using the modified Morgan (MM) soil test, were strongly dependent on total soil As. The overall correlation between MM-extractable As (AsMM) and total As (AsT) is given by equation (3):
| (4) |
The correlation of extractable to total As was slightly weaker than that observed for Pb. This regression equation reveals that, on average, MM extracted about 5.3% of the total As in these orchard soils, about twice the extraction efficiency observed for Pb. However, the fraction of total arsenic that was extractable increased with compost addition to the miniplots (see Table 1), a trend exemplified in Figure 2 for miniplots C and F. The 5% compost amendment significantly increased the efficiency of As extractability in all the miniplots (with the possible exception of miniplot A, which had the lowest As content), but the 10% compost addition did not in every case increase As extractability over that in the 5% treatment (Table 1).
Figure 2.
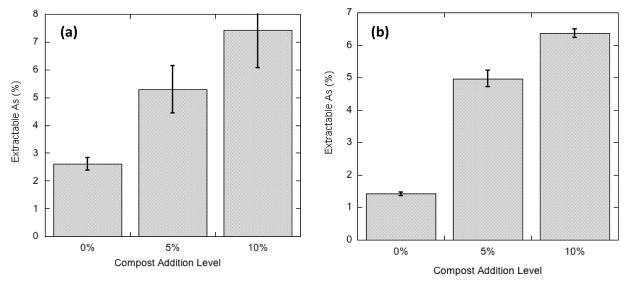
Percentage of total soil As extracted by Modified Morgan solution from miniplot soils with 3 levels (0, 5, 10%) of compost addition: (a) miniplot C soils containing about 60 mg/kg total As; (b) miniplot F soils containing about 150–180 mg/kg total As.
The results suggest a strong relationship between soil organic matter content and the ease of extractability of soil arsenic. Regression analysis confirms the consistency of this trend, with the extractable fraction of As strongly correlated to soil organic matter content over all miniplot soils as shown by equation (5) :
| (5) |
Unlike the trend observed for Pb, there is no statistically significant relationship between the extractable fraction of As and soil total As. However, as discussed earlier for the case of Pb, the fact that compost amendments raised soil pH in the field means that the specific mechanism causing As to become more extractable with compost amendment is uncertain. The efficiency of As extraction was correlated significantly to soil pH as well as organic matter content:
| (6) |
It is possible that the pH increase associated with compost amendment reduced the adsorption strength of As on soil mineral components (Manning and Goldberg, 1996a), but the added compost may also have promoted As dissolution by, for example, competing for As adsorption sites via organic acids and phosphate (the compost had a high P content of 0.82 % but only a trace level of As). Regardless of the specific mechanism, numerous other studies have reported the mobilization of arsenic upon organic matter amendment of soils (Cao and Shiralipour, Wang and Mulligan, 2006). Compost amendments to soils may also promote As reduction in the soil to arsenite, a potentially more soluble and mobile form of As (Hartley et al., 2010).
Because it was not possible to separate effects of soil pH and soil organic matter content on extractability of Pb and As from the field-collected soils, a follow-up experiment was done with adjustment of pH to 5.5 for soils of miniplot F prior to extraction using MM. The results, shown in Figure 3, reveal two important trends. First, both MM-extractable Pb and As increased in compost-amended and unamended soils with the pH decrease, indicating that acidification had the effect of making both metals more available. Second, the compost effect in reducing Pb extractability, observed for the soils before pH equalization, persisted after the pH was equalized across compost treatments. However, no effect of compost was observed for the extractability of As at pH 5.5, and the greater extractability of As at a lowered pH is in contradiction of known adsorption behavior of arsenate on soil minerals, with chemisorption being generally more favorable at lower pH (Manning and Goldberg, 1996a, 1996b). The possible presence of precipitated forms of As such as mimetite (Pb5(AsO4)3Cl) or Ca arsenate (Bothe and Brown, 1999) in these soils could account for increased extractability of both As and Pb at lower pH. Mimetite, for example, has minimum solubility near pH 7.5, with increasing As and Pb solubility as pH is lowered toward 4 (Bajda, 2010).
Figure 3.
Percentage of total soil Pb and As extracted by Modified Morgan solution from miniplot F soils with 3 levels (0, 5, 10%) of compost addition and pH adjusted to 5.5. The solid horizontal lines denote extraction percentages measured for these same soils before pH adjustment.
The strong effect of Pb immobilization by organic-rich amendments as observed here has been observed in numerous other studies (Farrell and Jones, 2010; Zhou et al., 2012; Hashimoto et al., 2011; Nwachukwu and Pulford, 2009). Free Fe and Mn oxides of these soils measured by the citrate-dithionite method were low (free Fe averaged 0.11% in the unamended soil), suggesting that adsorption on Fe oxides is unlikely to have a major role in Pb adsorption be these soils.
In summary, soils with higher As concentrations do not have a notably higher As extractability, a result in contrast to that observed for Pb. In addition, soils with higher organic matter content from compost addition clearly have more readily extractable As, quite the reverse of the case for Pb, but consistent with observations from other studies of compost-amended soils (Hartley et al., 2010).
Pb Extractability by Modified BCR Sequential Extraction
The results of the 3-step sequential extraction of compost-amended orchard soils from Miniplot F are summarized in Table 2. They show that most of the Pb is in Fraction B, the operationally defined “reducible fraction”, with only a small percentage (> 5 %) in the most labile Fraction A. This distribution of Pb by the BCR extraction is consistent with that observed for metal-contaminated soils from a Pb mining area (Anju and Banerjee, 2011). The compost amendments had no measurable effect on the percentage of Pb in the most labile or “reducible” forms. It is only Fraction C, the form of Pb dissolved by oxidation, that increased with increasing compost amendment, with a concomitant reduction of the percentage of Pb in the highly-resistant (residual) Fraction D. These results suggest that, following the amendment of the Pb-contaminated soil by compost, some Pb originally bound to older and highly stable organic matter (Fraction D) migrated into the younger and more chemically oxidizable organic matter of the added compost (Fraction C). The BCR extraction results, taken alone, suggest that the compost amendment had no substantial effect on Pb bioavailability, since the labile Fraction A is generally considered to represent bioavailable forms of soil Pb. However, the MM extraction results and the earthworm assay (see below) contradict this inference. It seems necessary to conclude that the first BCR extraction step (using acetic acid at pH 2.55) is too aggressive to adequately assess the bioavailable fraction of soil Pb.
Table 2.
Modified BCR extracted fractions of Pb (mg/kg) in Miniplot F soils treated with 0%. 5%, and 10% compost.
| Compost Level (%) | Fraction A | Fraction B | Fraction C | Fraction D* | Total Pb |
|---|---|---|---|---|---|
| 0 | 43 ± 5 | 541 ± 11 | 93 ± 0 | 215 | 892 |
| 5 | 36 ± 2 | 499 ± 6 | 111 ± 4 | 121 | 768 |
| 10 | 36 ± 2 | 406 ± 14 | 143 ± 16 | 82 | 667 |
Determined by difference between total Pb and the sum of Fractions A+B+C.
Scanning Electron Microscopy and Elemental Mapping
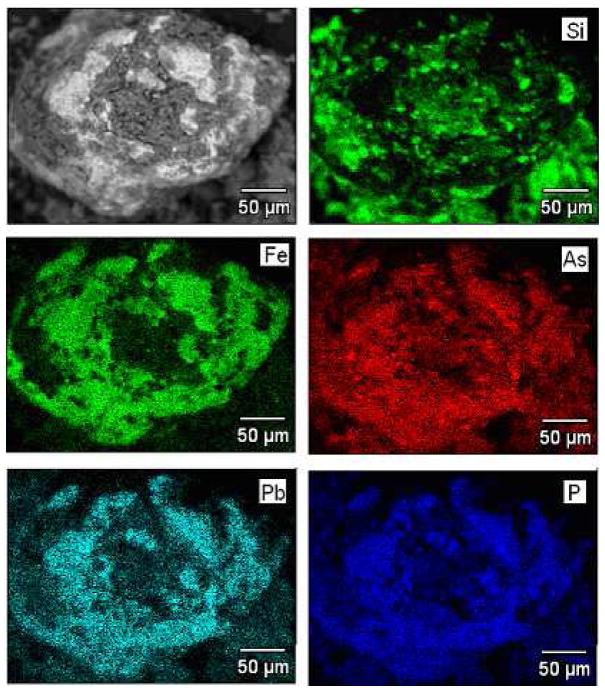
Using the electron microprobe, it was possible to identify a few isolated soil particles with high Pb and As concentrations. One of these particles is pictured in Fig. 4 along with maps of soil elements of interest. A spatial association of Pb with As, P and Fe is apparent, possibly indicating that some part of the Pb arsenate originally applied to the orchard soil has been transformed over decades to more stable mineral phases such as pyromorphite (Pb phosphate), scorodite (ferric arsenate), or mimetite (Pb arsenate) (Drahota and Filippi, 2009; Bosso et al., 2008; Magalhaes and Silva, 2003). Poorly crystalline Fe oxides could also become enriched in Pb and As through chemisorption processes. Earlier unpublished work at this orchard site for a large set of analyzed soils showed a very strong spatial correlation in the surface soil between total As (AsT) and Pb (PbT) :
| (7) |
Figure 4.
Mapping by electron microprobe of specific elements associated with a Pb-rich particle identified in the orchard soil.
Based on the reasonable assumption that “acid lead arsenate” (PbHAsO4) was the main form of insecticide used in in this region on apple orchards to control codling moth (Schooley et al., 2008), the initial As/Pb ratio in the contaminated soils should be approximately 1/1 on a mole basis or 0.36 based on weight. However, the slope of 0.218 in equation (7) is substantially less than the value of 0.36, suggesting that there has been some loss of As relative to Pb in the soil. Based on the actual measured to theoretical ratio of 0.218/0.361 = 0.604, a loss of about 40% of the As in the topsoil soil relative to Pb appears to have occurred over the time since the pesticide was applied. This inferred greater mobility of soil As in orchard soils is consistent with the greater extractability of As compared with Pb in this study using a relatively mild extractant (Modified Morgan’s solution). Greater As mobility is also implied by the presently measured aqueous solubility of As in these orchard soils, generally several times greater for As compared to Pb (unpublished data) in any given soil sample. For example, one soil from our experimental site containing 915 mg/kg Pb and 211 mg/kg As averaged 153 μg/L Pb and 745 μg/L As in soil water extracts, indicating that the partitioning coefficient (Kd) for Pb is about 20 times greater than that of As in this orchard soil. Other studies have also reported greater solubility and mobility of As compared to Pb in orchard soil profiles (Aten et al., 1980; Peryea and Creger, 1994).
In earlier work using synchrotron XRF microprobe elemental mapping of the orchard soils studied here, we found Pb and As to be highly concentrated in isolated and discrete particles with dimensions of about 50 micrometers (McBride et al., 2011). In the present study, using the electron microprobe, we again identified Pb-rich particles associated with As, but association with Fe and P was also evident. The mineralogy of these environmentally persistent and stable particles is as yet uncertain, possibly involving low-solubility Pb and arsenate mineral phases or Pb and As chemisorbed on poorly crystalline Fe oxides. A possible candidate mineral that could explain the pronounced long-term Pb-As association is mimetite, probably the most stable and low-solubility Pb-As phase in the environment (Magalhaes and Silva, 2003). In order for mimetite to form by aging of the applied PbHAsO4 insecticide powder in the soil environment, the reaction :
| (8) |
would be necessary. Thus, the As/Pb mole ratio in the solid phase would change from 1/1 to 3/5, a 40% reduction in the As relative to Pb, which is in agreement with that observed. The released arsenate may leach downward and possibly readsorb (at least in part) in the subsoil. Pb remains in the topsoil in a relatively insoluble form. However, it is possible that more complex soil geochemistry is involved with multiple or mixed insoluble phases containing Pb and As formed during aging, since Fe and P are also spatially associated with Pb and As in this soil. Possible stable minerals involving these elements include scorodite (ferric arsenate) and pyromorphite (Pb phosphate), both known to form and persist under certain soil surface conditions (Drahota and Filippi, 2009; Bosso et al., 2008). Although the association of As with Fe could also indicate that As has adsorbed on iron oxides, the electron microprobe is a “bulk” analytical method and is unlikely to be very sensitive to surface-adsorbed As. In addition, the free Fe oxide content of this particular soil is quite low (≈ 0.15% Fe2O3). Further research using methods sensitive to specific mineral structures (e.g., XRF, EXAFS) will be needed to determine which specific mineral or adsorbed forms of As and Pb are present in this soil.
Redworm Bioassay
Much higher Pb and As concentration in the whole redworm tissues were found for worms exposed to the orchard soils compared to an uncontaminated control soil (Table 3). However, worm tissue concentrations of Pb were substantially lower than soil Pb concentrations, with Pb bioaccumulation factors (BAF) of 0.40, 0.10 and 0.11 for the 0%, 5% and 10% compost- amended soils, respectively. Conversely, the tissue As concentrations were higher, comparable to the soil As concentrations, with As BAFs calculated to be 0.86, 1.07 and 1.12 for the 0%, 5% and 10% compost- amended soils, respectively. Compost amendment caused a highly significant reduction in the tissue concentrations of Pb as shown by an ANOVA test (p= 0.00250), with redworms in the 5% and 10% compost amended soils both having much lower Pb tissue concentrations than those in uncomposted soils. In contrast, neither of the compost amendments showed an ability to reduce As concentrations in the redworm tissues (p= 0.810). These earthworm bioassay results show a similar trend in the effects of compost to a related woodlice (Porcellio scaber, L.) bioassay conducted on the same orchard soils, in which the BAF for As was generally greater than that for Pb, and compost amendment decreased Pb but increased As bioavailability (Udovic and McBride, 2012).
Table 3.
Redworm tissue concentrations of Pb and As (mg/kg dry wt.)
| Treatment | Replicate number | Tissue Pb (mg/kg) | Tissue As (mg/kg) |
|---|---|---|---|
| Control (Caldwell soil) | 3 | 13.1 ± 5.9 | 8.7 ± 1.8 |
| 0% Compost | 2 | 354 ± 107 | 160 ± 13.4 |
| 5% Compost | 3 | 73.8 ± 14.8 | 177 ± 34.6 |
| 10% Compost | 3 | 70.5 ± 0.1 | 169 ± 26.5 |
In general, earthworm bioaccumulation factors for toxic metals in soils correlate well to equilibrium partitioning of the metals between soil solids and soil solution (Janssen et al., 1997). Because of the much lower partitioning coefficients of As in these orchard soils compared to Pb, the As BAFs are as expected greater than the Pb BAFs, and Pb is not bioconcentrated by the earthworms. However, BAFs for Pb and As in different soils vary by several orders of magnitude depending on soil pH, organic matter content and other factors (Vermeulen et al., 2009). Gal et al. (2007) reported earthworm As concentrations up 960 mg/kg for soils from an abandoned mining area, although their As BAFs generally did not exceed 1.0.
Despite the fact that the redworms were depurated, tissue Pb concentration was positively correlated to both tissue Al and Fe for the worms exposed to the orchard soils (data not shown), a suggestion that ingestion of mineral soil particles could account for at least part of the measured Pb in tissues. Specifically, the worms exposed to the unamended orchard soil (0% compost) contained substantially higher Fe and Al concentrations than those exposed to the 5% and 10% compost treatments. In contrast, there was no significant correlation of tissue As to tissue Al or Fe.
These bioassay results parallel the soil test data showing a strong effect of compost amendment in reducing the extractability of soil Pb, yet revealing an increased extractability of As with compost amendment. However, the reduced body burden of Pb in worms could be at least partly due to selective ingestion of organic-rich particles with low initial Pb concentration by worms exposed to the compost-treated soil. Thus, greater mineral particle, and therefore Pb, ingestion by redworms would have been likely in the 0% compost treatment, as suggested by the higher tissue Al and Fe. The fact that added compost did not reduce the redworm body burden of As as well as Pb is curious, since our research has shown that much of the Pb and As in these same orchard soils has remained closely associated in microparticles (McBride et al., 2011). It would therefore be expected that reduced Pb ingestion in the form of soil particles would necessarily lead to reduced As ingestion by the same exposure pathway. However, since the extractable As by MM is increased by increasing compost addition in soil, As availability is presumably increased by compost addition. Consequently, in the compost-amended soils, dermal exposure could become a more significant pathway for As bioaccumulation than in unamended soil. For very insoluble metals such as Pb, most absorption by earthworms occurs via the intestine, but for metals such as As with higher solubility, bioaccumulation is better explained by exposure to metals dissolved in the soil pore water (Janssen et al., 1997; Nannoni et al., 2011).
CONCLUSIONS
In this study of an orchard soil contaminated more than 50 years ago by lead arsenate pesticide, both the Modified Morgan soil test and the bioassay pointed to the effectiveness of recently added compost amendments in reducing Pb availability. Part of this effect may have been due to the fact that the compost raised soil pH significantly, but the compost effect in reducing Pb extractability persisted after equalization of soil pH across compost treatments. A commonly used sequential extraction method, the modified BCR test, did not detect a significant effect of compost on Pb availability as measured by the size of the chemically labile fraction. This insensitivity of the BCR, in contrast to that of the Modified Morgan test, can be attributed to the strongly acidic and aggressive nature of the extractants used in the BCR procedure.
In contrast to Pb, compost amendments and higher soil organic matter failed to reduce As availability as measured by Modified Morgan’s test and the worm bioassay. Although much of the Pb and As in this historically contaminated orchard soil is in the form of micrometer-sized particles, these particles nevertheless slowly release both Pb and As into soil solution. Acidification of the soils to pH 5.5 increased the fraction of extractable As, a result consistent with acid dissolution of precipitated forms of As such as mimetite, but not with mineral adsorption-desorption processes. Readsorption of Pb onto soil organic matter in high organic matter soils may limit the extractability and bioavailability of this metal, whereas As released from the particles is more mobile and bioavailable because of the low affinity of arsenate for organic matter.
Highlights.
Soil Pb and As in an old orchard were concentrated in discrete particles
compost amendment of contaminated soil reduced Pb bioavailability
compost amendment of contaminated soil did not reduce As bioavailability
ammonium acetate extraction test reflected bioavailability of soil Pb and As
Acknowledgments
This project was supported in part by Award Number R21ES017921 from the National Institute of Environmental Health Sciences.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anju M, Banerjee DK. Associations of cadmium, zinc and lead in soils from a lead and zinc mining area as studied by single and sequential extractions. Environmental Monitoring and Assessment. 2011;176:67–85. doi: 10.1007/s10661-010-1567-4. [DOI] [PubMed] [Google Scholar]
- Ashworth DJ, Alloway BJ. Influence of dissolved organic matter on the solubility of heavy metals in sewage-sludge-amended soils. Communications in Soil Science and Plant Analysis. 2008;39:538–550. [Google Scholar]
- Aten GF, Bourke JB, Martini JH, Walton JC. Arsenic and lead in an orchard environment. Bulletin of Environmental Contamination and Toxicology. 1980;24:108–115. doi: 10.1007/BF01608083. [DOI] [PubMed] [Google Scholar]
- Bajda T. Solubility of mimetite Pb5(AsO4)3Cl at 5–55°C. Environmental Chemistry. 2010;7:268–278. [Google Scholar]
- Beesley L, Moreno-Jimenez E, Gomez-Eyles JL. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environmental Pollution. 2010;158:2282–2287. doi: 10.1016/j.envpol.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Bosso ST, Enzweiler J, Angelica RS. Lead bioaccessibility in soil and mine wastes after immobilization with phosphate. Water Air and Soil Pollution. 2008;195:257–273. [Google Scholar]
- Bothe JV, Brown PW. Arsenic immobilization by calcium arsenate formation. Environmental Science and Technology. 1999;33:3806–3811. [Google Scholar]
- Cao RX, Ma LQ, Chen M, Singh SP, Harris WG. Phosphate-induced metal immobilization in a contaminated site. Environmental Pollution. 2003;122:19–28. doi: 10.1016/s0269-7491(02)00283-x. [DOI] [PubMed] [Google Scholar]
- Cao X, Ma LQ, Shiralipour A. Effects of compost and phosphate amendments on arsenic mobility in soils and arsenic uptake by the hyperaccumulator, Pteris vittata L. Environmental Pollution. 2003;126:157–167. doi: 10.1016/s0269-7491(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Davies BE. Loss-on-ignition as an estimate of soil organic matter. Soil Science Society of America Proceedings. 1974;38:150–151. [Google Scholar]
- Drahota P, Filippi M. Secondary arsenic minerals in the environment: A review. Environment International. 2009;35:1243–1255. doi: 10.1016/j.envint.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Essington ME, Foss JE, Roh Y. The soil mineralogy of lead at Horace’s Villa. Soil Science Society of America Journal. 2004;68:979–993. [Google Scholar]
- Farrell M, Jones DL. Use of composts in the remediation of heavy metal contaminated soil. Journal of Hazardous Materials. 2010;175:575–582. doi: 10.1016/j.jhazmat.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Gal J, Hursthouse A, Cuthbert S. Bioavailability of arsenic and antimony in soils from an abandoned mining area, Glendinning (SW Scotland) Journal of Environmental Science and Health. Part A. 2007;42:1263–1274. doi: 10.1080/10934520701435585. [DOI] [PubMed] [Google Scholar]
- Gustafsson JP, Tiberg C, Edkymish A, Berggren Kleja D. Modelling lead (II) sorption to ferrihydrite and soil organic matter. Environmental Chemistry. 2011;8:485–492. [Google Scholar]
- Hamel SC, Heckman JR, Shilke-Gartley KL, Hoskins B. Lead extraction using three soil fertility tests and Environmental Protection Agency Method 3050. Communications in Soil Science and Plant Analysis. 2003;34:2853–2873. [Google Scholar]
- Hartley W, Dickinson NM, Riby P, Leese E, Morton J, Lepp NW. Arsenic mobility and speciation in a contaminated urban soil are affected by different methods of green waste compost application. Environmental Pollution. 2010;158:3560–3570. doi: 10.1016/j.envpol.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Yamaguchi N, Takaoka M, Shiota K. EXAFS speciation and phytoavailability of Pb in a contaminated soil amended with compost and gypsum. Science of the Total Environment. 2011;409:1001–1007. doi: 10.1016/j.scitotenv.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Janssen RPT, Posthuma L, Baerselman R, DenHollander HA, vanVeen RPM, Peijnenburg JGM. Equilibrium partitioning of heavy metals in Dutch field soils.2. Prediction of metal accumulation in earthworms. Environmental Toxicology and Chemistry. 1997;16:2479–2488. [Google Scholar]
- Karam N, Clemente R, Moreno-Jimenez E, Lepp NW, Beesley L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. Journal of Hazardous Materials. 2011;191:41–48. doi: 10.1016/j.jhazmat.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Kumpiene J, Lagerkvist A, Maurice C. Stabilization of Pb- and Cu-contaminated soil using coal fly ash and peat. Environmental Pollution. 2007;145:365–373. doi: 10.1016/j.envpol.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Maenpaa KA, Kukkonen JVK, Lydy MJ. Remediation of heavy metal-contaminated soils using phosphorus: Evaluation of bioavailability using an earthworm bioassay. Archives of Environmental Contamination and Toxicology. 2002;43:389–398. doi: 10.1007/s00244-002-1248-6. [DOI] [PubMed] [Google Scholar]
- Magalhaes MCF, Silva MCM. Stability of lead (II) arsenates. Monatshefte fur Chemie. 2003;134:735–743. [Google Scholar]
- Manning BA, Goldberg S. Modeling arsenate competitive adsorption on kaolinite, montmorillonite and illite. Clays and Clay Minerals. 1996a;44:609–623. [Google Scholar]
- Manning BA, Goldberg S. Modeling competitive adsorption of arsenate with phosphate and molybdate on oxide minerals. Soil Science Society of America Journal. 1996b;60:121–131. [Google Scholar]
- McBride MB, Mathur RR, Baker LL. Chemical extractability of lead in field-contaminated soils: implications for estimating total lead. Communications in Soil Science and Plant Analysis. 2011;42:1581–1593. doi: 10.1080/00103624.2011.581729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra OP, Jackson ML. Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clays and Clay Minerals. 1960;7:317–327. [Google Scholar]
- Meunier L, Walker SR, Wragg J, Parsons MB, Koch I, Jamieson HE, Reimer KJ. Effects of soil composition and mineralogy on the bioaccessibility of arsenic from tailings and soil in gold mine districts of Nova Scotia. Environmental Science and Technology. 2010;44:2667–2674. doi: 10.1021/es9035682. [DOI] [PubMed] [Google Scholar]
- Mihaljevic M, Ettler V, Sebek O, Drahota P, Strnad L, Prochazka R, Zeman J, Sracek O. Alteration of arsenopyrite in soils under different vegetation covers. Science of the Total Environment. 2010;408:1286–1294. doi: 10.1016/j.scitotenv.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Nannoni F, Protano G, Riccobono F. Uptake and bioaccumulation of heavy metals by two earthworm species from a smelter contaminated area in northern Kosovo. Soil Biology and Biochemistry. 2011;43:2359–2367. [Google Scholar]
- Nwachukwu OI, Pulford ID. Soil metal immobilization and ryegrass uptake of lead, copper and zinc as affected by application of organic materials as soil amendments in a short-term greenhouse trial. Soil Use Management. 2009;25:159–167. [Google Scholar]
- Peryea FJ, Creger TL. Vertical distribution of lead and arsenic in soils contaminated with lead arsenate pesticide residues. Water Air and Soil Pollution. 1994;78:297–306. [Google Scholar]
- Rauret G, Lopez-Sanchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. Journal of Environmental Monitoring. 1999;1:57–61. doi: 10.1039/a807854h. [DOI] [PubMed] [Google Scholar]
- Ryan JA, Zhang PC, Hesterberg D, Chou J, Sayers DE. Formation of chloropyromorphite in a lead-contaminated soil amended with hydroxyapatite. Environmental Science and Technology. 2001;35:3798–3803. doi: 10.1021/es010634l. [DOI] [PubMed] [Google Scholar]
- Sadiq M. Arsenic chemistry in soils: An overview of thermodynamic predictions and field observations. Water Air and Soil Pollution. 1997;93:117–136. [Google Scholar]
- Sauve S, Martinez CE, McBride M, Hendershot W. Adsorption of free lead (Pb2+) by pedogenic oxides, ferrihydrite and leaf compost. Soil Science Society of America Journal. 2000;64:595–599. [Google Scholar]
- Sauve S, McBride M, Hendershot W. Soil solution speciation of lead(II): Effects of organic matter and pH. Soil Science Society of America Journal. 1998;62:618–621. [Google Scholar]
- Scheckel KG, Ryan JA, Allen D, Lescano NV. Determining speciation of Pb in phosphate-amended soils: method limitations. Science of the Total Environment. 2005;350:261–272. doi: 10.1016/j.scitotenv.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Schooley T, Weaver MJ, Mullins D, Eick M. The history of lead arsenate use in apple production: comparison of its impact in Virginia with other states. Journal of Pesticide Safety Education. 2008;10:22–53. [Google Scholar]
- Thanabalasingam P, Pickering WF. Arsenic sorption by humic acids. Environmental Pollution (Series B) 1986;12:233–246. [Google Scholar]
- Udovic M, McBride MB. Influence of compost addition on lead and arsenic bioavailability in reclaimed orchard soil assessed using Porcellio scaber bioaccumulation test. Journal of Hazardous Materials. 2012;205–206:144–149. doi: 10.1016/j.jhazmat.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneman PLM, Murray JR, Baker JH. Spatial distribution of pesticide residues in a former apple orchard. Journal of Environmental Quality. 1983;12:101–104. [Google Scholar]
- Vermeulen F, Van den Brink H, D’Have H, Mubiana VK, Blust R, Bervoets L, De Coen W. Habitat-based bioaccumulation and risk assessment of metal and As contamination in earthworms, beetles and woodlice. Environmental Pollution. 2009;157:3098–3105. doi: 10.1016/j.envpol.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Wang S, Mulligan CN. Effect of natural organic matter on arsenic release from soils and sediments into groundwater. Environmental Geochemistry and Health. 2006;28:197–214. doi: 10.1007/s10653-005-9032-y. [DOI] [PubMed] [Google Scholar]
- Weng LP, Temminghoff EJM, Lofts S, Tipping E, Van Riemsdijk WH. Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environmental Science and Technology. 2002;36:4804–4810. doi: 10.1021/es0200084. [DOI] [PubMed] [Google Scholar]
- Yan X, Zhang M, Liao X, Tu S. Influence of amendments on soil arsenic fractionation and phytoavailability by Pteris vittata L. 2012. Chemosphere. 2012;88:240–244. doi: 10.1016/j.chemosphere.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Zhou YF, Haynes RJ, Naidu R. Use of inorganic and organic wastes for in situ immobilization of Pb and Zn in a contaminated alkaline soil. Environmental Science and Pollution Research. 2012;19:1260–1270. doi: 10.1007/s11356-011-0648-4. [DOI] [PubMed] [Google Scholar]