Abstract
Objective
Instillation of multi-walled carbon nanotubes (MWCNTs) in C57BL/6 mice results in decrements of pulmonary function specifically characterized by increases in airway resistance. In this study, we examined possible mechanisms responsible for these alterations following MWCNT exposure, including the roles of IL-33 and chronic inflammation.
Materials and Methods
To elucidate the role of IL-33, we assessed lung histology and pulmonary function in C57BL/6 and IL-33−/− mice 30 days following MWCNT instillation. In addition, the impact of MWCNT instillation on airway hyperresponsiveness (AHR) was assessed by methacholine challenges of C57BL/6 and IL-33−/− mice. To further understand the mechanisms by which MWCNTs may increase airway constriction, C57BL/6 mice were treated with aerosolized albuterol or injected with multiple doses of methylprednisolone via intra-peritoneal injections prior to the assessment of MWCNT-induced changes in pulmonary function.
Results
Total cell count, macrophages, and neutrophils were increased in the lavage fluid of C57BL/6 mice, but not in IL-33−/− mice, following MWCNT exposure. C57BL/6 mice displayed increased inflammation and fibrosis located proximal to the airways which was absent in IL-33−/− mice. Aerosolized methacholine increased parameters of airway resistance (R and Rn) in a dose-dependent manner in all groups, with MWCNT-instilled C57BL/6 mice responding more robustly compared to controls, while no differences were found in IL-33−/− mice due to MWCNT exposure. Treatment with methylprednisolone reduced both the MWCNT-induced histopathological changes and increases in R and Rn in C57BL/6 mice.
Conclusion
These findings suggest that IL-33 and chronic inflammation in general are critical in the pulmonary toxicity induced by MWCNT resulting in modified pulmonary function.
Introduction
The synthesis and utilization of nanomaterials continues to expand due to their diverse properties and applications. Carbon nanotubes (CNT) are able to undergo numerous modifications and possess a number of unique properties such as a high conductance of heat and electricity, and high strength-to-weight ratios. These properties make CNTs potentially useful as drug delivery platforms and for various biomedical and technological applications. Although humans are likely being increasingly exposed to CNTs and occupational exposure levels have been recently established, little is known regarding the human health effects (Department of Health and Human Services CDC, 2013, Erdely et al. 2013). Animal studies however have demonstrated significant cardiopulmonary effects following inhalation or instillation of CNTs into the lung. In general, these exposures have resulted in increased cardiovascular ischemia reperfusion injury, altered vascular responses, pulmonary inflammation, oxidative stress, fibrosis, altered lung function, and the exacerbation of allergen-induced airway remodeling (Cesta et al., 2010, Sayers et al., 2013, Thompson et al., 2012, Urankar et al., 2012, Wang et al., 2011a). Specifically, exposure to multi-walled carbon nanotubes (MWCNTs) has been shown in numerous studies to increase both total respiratory resistance (R), Newtonian resistance (Rn), and induce airway hyper-responsiveness (AHR) (Beamer et al., 2013, Katwa et al., 2012, Wang et al., 2011a). The mechanisms, which facilitate these MWCNT-induced changes in pulmonary function, however remain unclear.
One possible mechanism responsible for the changes in pulmonary function is the release of IL-33, a critical immune system mediator. Interleukin-33 is a member of the IL-1 family of cytokines and currently the only known ligand for the ST2 receptor (also called IL1RL1). Interleukin-33 is expressed by both non-immune cells including epithelial, endothelial, smooth muscle cells and fibroblasts, as well as, immune cells including mast cells, macrophages, and dendritic cells (Schmitz et al., 2005). Binding of IL-33 to membrane associated ST2 on various cell types, such as mast cells and Th2 cells, induces the production and release of several cytokines and chemokines, including IL-6 and IL-13, thus mediating an inflammatory response. Studies have demonstrated that IL-33 can facilitate pathological changes within the lung due to its induction of inflammatory responses. Specifically, intraperitoneal injection of IL-33 leads to development of AHR and goblet cell hyperplasia in mice (Kondo et al., 2008). Lung epithelial cells from asthmatic patients express more abundant IL-33 compared to healthy subjects, suggesting a role for IL-33 in the underlying asthmatic inflammation via the activation of alveolar macrophages (Kurowska-Stolarska et al., 2009). Inhibition of IL-33 has also been shown to reduce pulmonary inflammation following cigarette smoke exposure in mice suggesting its possible role in the development of chronic obstructive pulmonary disease (Qiu et al., 2013).
Recent studies in our laboratory and others have suggested a critical role for IL-33 in directing the inflammatory response and subsequent pathology following pulmonary exposure to MWCNT (Katwa et al., 2012, Wang et al., 2011a). We have reported that instillation of MWCNT in C57BL/6 mice results in the development of granulomas and fibrosis at the terminal bronchioles and an impairment of pulmonary function 30 days after exposure; these effects however were unseen in mice lacking the IL-33 receptor ST2. Furthermore, these changes in pulmonary pathology and function observed in C57BL/6 mice also coincided with increased bronchoalveolar lavage fluid (BALF) levels of IL-33. Recently, Beamer et al. 2012 has demonstrated increased AHR at 1 day following MWCNT exposure, which was dependent on IL-33-induced release of IL-13 and the subsequent recruitment of innate lymphoid cells into the lung (Beamer et al., 2013). These findings further highlight the importance of IL-33 and its role as an alarmin in the pulmonary toxicity induced by MWCNT exposure. It is likely that the removal or inhibition of IL-33 may lessen the effects of MWCNT exposure.
Obstructive lung diseases such as asthma and bronchitis are classified in part by increases in the pulmonary function parameters of R and Rn, airway inflammation, and increased AHR. Typical treatment to reduce the increased pulmonary resistance and ease breathing for individuals suffering from these obstructive lung diseases include β2-adrenergic receptor agonists and corticosteroids. Previous research has demonstrated that exposure to MWCNTs causes airway inflammation and increases in R and Rn (Katwa et al., 2012). It is likely that treatment with either a β2-adrenergic receptor agonist or a corticosteroid may reduce MWCNT-induced changes in pulmonary function.
A previous study has demonstrated that exposure to MWCNT induces AHR 1 day following exposure through the release of IL-33 (Beamer et al., 2013). Our work has also demonstrated that baseline pulmonary function (in the absence of a methacholine challenge) is altered 30 days following MWCNT instillation (Katwa et al., 2012). We therefore hypothesize that MWCNT exposure will induce chronic changes in pulmonary function and AHR that will persist out to 30 days and can be reduced through genetic deficiency of the alarmin IL-33. Furthermore, we will also attempt to reduce baseline resistance changes induced by MWCNT exposure via the use of pharmacologic interventions such as the β2-adrenergic receptor agonist, albuterol, and the corticosteroid methylprednisolone.
Methods
MWCNT Characterization
The multi-walled carbon nanotubes (MWCNT) utilized in this study were generously provided by NanoTech Labs, Inc. (Yadkinville, NC). For comprehensive information regarding MWCNT characterization please see our previous publications (Katwa et al., 2012, Wang et al., 2011a). Briefly, MWCNTs in the dry powder form were characterized for length, diameter and elemental composition by transmission and scanning electron microscopy while surface area was calculated by BET. MWCNTs in suspension were characterized for hydrodynamic size, by dynamic light scattering (nanosizer S90, Malvern Instruments), and zeta potential (Zeta ZS, Malvern Instruments).
Experimental Animals
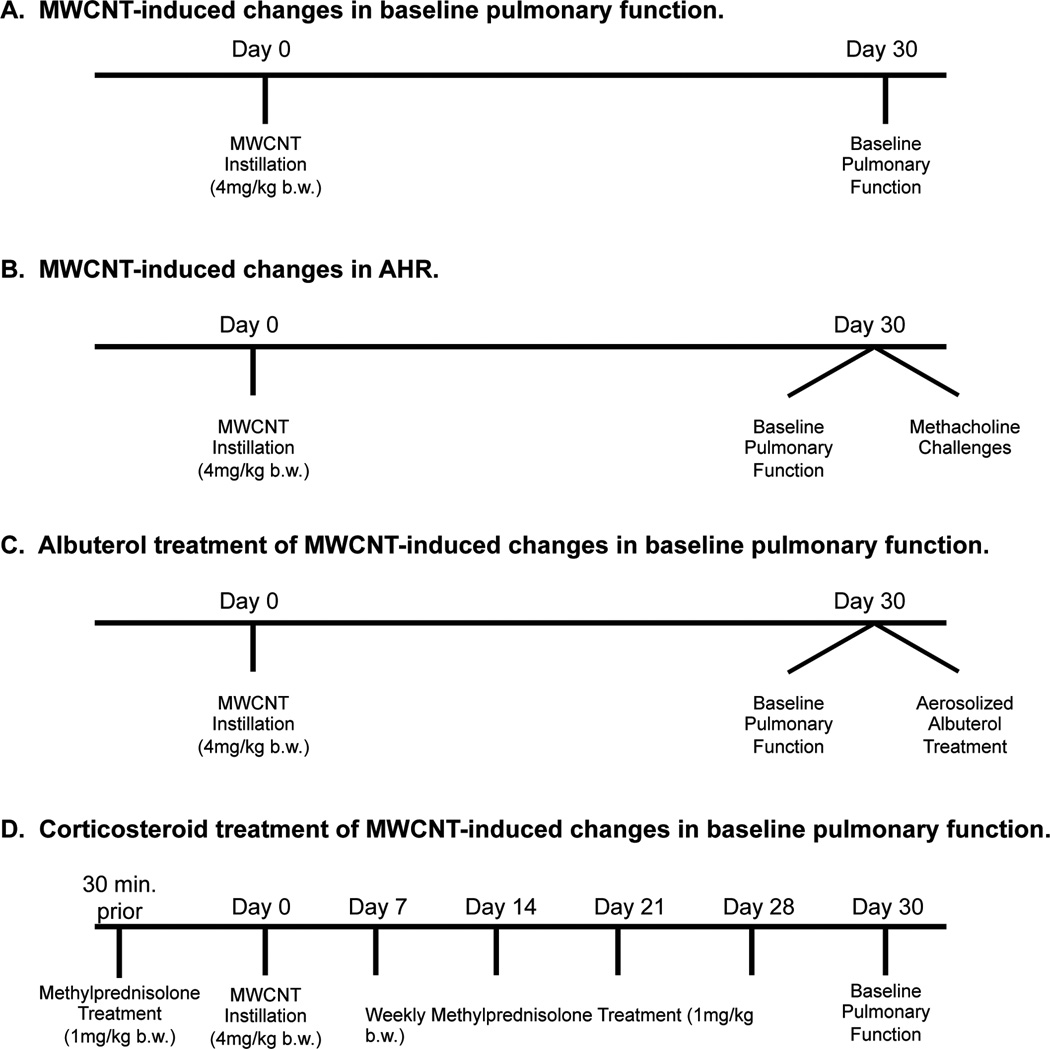
Male C57BL/6 mice were acquired from Jackson Laboratories (Bar Harbor, ME, USA) at 8 weeks of age. IL-33−/− mice were generously provided by Merck, Inc. and breeding colonies were maintained at East Carolina University. C57BL/6 and IL-33−/− mice were assigned to 2 exposure groups: vehicle (10% surfactant in saline) or MWCNTs (C57BL/6 n=18/group; IL-33−/− n=8/group), and exposed as described previously (Katwa et al., 2012, Wang et al., 2011a). Briefly, the mice were anesthetized with isofluorane and received vehicle (10% surfactant in saline) or a single dose of MWCNTs (4 mg/kg body weight) by oropharyngeal aspiration (Katwa et al., 2012, Wang et al., 2011a). The dose of MWCNTs was determined based on previously published data (Katwa et al., 2012). The dose of MWCNT used in this study, although high, allowed us to determine mechanisms responsible for the pulmonary disease caused by MWCNT exposure. Clinical grade pulmonary surfactant (Infasurf®) was kindly provided by ONY, Inc (Amherst, NY, USA). In separate cohorts of animals, baseline assessment of pulmonary function (Figure 1A), methacholine challenges (Figure 1B), and albuterol treatments (Figure 1C) were conducted 30 days post-exposure to MWCNTs. Lastly, the effectiveness of the corticosteroid treatment on MWCNT-induced pulmonary disease was assessed (Figure 1D). Following pulmonary function measurements, all animals were euthanized for collection of bronchoalveolar lavage fluid (BALF) and lung tissue. All animal procedures were conducted in accordance with the National Institutes of Health guidelines and approved by the East Carolina University Institutional Animal Care and Use Committee. All animals were treated humanely and with regard for alleviation of suffering.
Figure 1.
Outline of experimental animal groups used to assess MWCNT-induced changes in pulmonary resistance. C57BL/6 and IL-33−/− mice were instilled with MWCNTs and baseline pulmonary function was assessed 30 days post exposure (C57BL/6 n=18/group; IL-33−/− n=8/group) (A). Changes in airway hyperresponsiveness (AHR) were evaluated in a separate cohort of C57BL/6 and IL-33−/− mice via methacholine challenges 30 days following MWCNT instillation (n=6/group) (B). C57BL/6 mice underwent albuterol treatment 30 days after MWCNT instillation to determine the contribution of airway smooth muscle contraction to MWCNT-induced pulmonary resistance changes (vehicle n=4/group; MWCNT n=8/group) (C). Lastly, a separate group of C57BL/6 mice were treated with methylprednisolone, 30 min prior to and every 7 days following instillation of MWCNTs, and changes in baseline pulmonary function were determined (n=6/group) (D).
Pulmonary Function Testing
Thirty days following exposure to MWCNTs, all mice were anesthetized with tribromoethanol (TBE) (400 mg/kg), tracheostomized, and baseline pulmonary function was recorded using the FlexiVent system (SCIREQ, Montreal, QC, Canada) [6,8]. Mice were ventilated with a tidal volume of 10 ml/kg at a frequency of 150 breaths/min and a positive end expiratory pressure of 3 cm H2O to prevent alveolar collapse. In addition, mice were paralyzed with pancuronium bromide (1 mg/kg) to prevent spontaneous breathing. EKG was monitored for all mice to determine anesthetic depth and potential complications that could have arisen during testing. Total respiratory system resistance (R) and Newtonian resistance (Rn) were measured by Snapshot perturbation maneuver and Quickprime-3 perturbation maneuver, respectively, as previously described (Wang et al., 2011a). Rn is a measurement of central airway resistance; whereas R is the sum of airway, lung tissue, as well as, chest wall resistances.
Methacholine Challenge
In a separate cohort of C57BL/6 and IL-33−/− mice (n=6/group), airway responsiveness to methacholine was determined 30 days following MWCNT exposure. Animals were anesthetized, tracheostomized, and ventilated as described earlier. Mice were challenged with aerosolized saline or cumulative doses of methacholine (1.5, 3, 6, 12, and 24 mg/ml) generated by an ultrasonic nebulizer for 10 seconds (Figure 1B). The range of aerosolized methacholine doses was based on a previous publication that examined MWCNT-induced changes in pulmonary function 24 hours following exposure (Beamer et al., 2013). The degree of bronchoconstriction was expressed as enhanced R and Rn.
Albuterol and Methylprednisolone Treatments
Following the last baseline measurement, a separate cohort of C57BL/6 mice were treated with nebulized albuterol sulfate (0.083% solution in sterile saline) (Sigma-Aldrich, St. Louis, MO, USA) for 20 seconds (Wang et al., 2010). Pulmonary function was again assessed following treatment with albuterol (Figure 1C). A separate cohort of C57BL/6 mice underwent intraperitoneal injection with 1 mg/kg 6α-Methylprednisolone (Sigma-Aldrich), 30 min before instillation with vehicle or MWCNT and weekly thereafter (7, 14, 21, and 28 days after instillation) (n=6/group). Thirty days following exposure of MWCNTs, total respiratory system resistance (R) and Newtonian resistance (Rn) were record in those mice treated with methylprednisolone (Figure 1D).
BAL and Cell Differential Counts
Following completion of the pulmonary function test or methacholine challenge, the right lung of each mouse was lavaged in situ four times with a specific volume (26.25 ml/kg body weight) of ice-cold Hanks balance salt solution (HBSS). BAL fluid was centrifuged at 1000 g for 10 minutes at 4°C. Total cells from all lavages were counted and 20,000 cells were centrifuged using a Cytospin IV (Shandon Scientific Ltd., Cheshire, UK) and stained with a three-step hematology stain (Richard Allan Scientific, Kalamazoo, MI, USA). Cell differential counts were determined by morphology with evaluation of 300 cells per slide.
Lung Histopathology
Left lungs were perfused with 10% neutral buffered formalin fixative and stored for 24 h. Lungs were then processed, embedded in paraffin, sectioned at 5µm, and mounted on slides. Slides were then individually stained with hematoxylin and eosin (H&E) for identification of changes in airway morphology, Masson’s Trichrome for identification of collagen deposition, and Periodic acid-schiff (PAS) for the identification of mucus production.
Statistical Analyses
All data are expressed as mean ± SEM. Graphs and statistical analysis were performed using GraphPad Prism 5 software (GraphPad, San Diego, CA). Differences between two groups were identified by student’s t test. Methacholine responses were assessed by two-way ANOVA with differences between groups assessed using Bonferroni post –hoc test. Differences between groups were considered statistically significant at p<0.05.
Results
MWCNTs characterization
The MWCNTs used in this study have been described previously (Katwa et al., 2012, Wang et al., 2011a) and have been characterized both as a dry powder and a suspension in 10% pulmonary surfactant as the vehicle (Table 1). In summary, characterization of the dry powder form of MWCNTs was found to have limited residual Fe catalyst contamination (0.04%) and have a diameter of 22.5 ± 1.3 nm. MWCNTs were also found to disperse into a stable solution in 10% surfactant saline, based on assessment of Zeta potential and hydrodynamic size (Table 1).
Table 1.
Characterization of MWCNT in dry powder form and in suspension (10% surfactant saline).
| MWCNT Dry Powder Characteristics | MWCNT Suspension Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean Diameter by TEM (nm) |
Length Range by TEM (µm) |
Surface area by BET (m2/g) |
Spectral Content (Atomic %) |
Zeta Potential (mV) |
Hydrodynamic Size (nm) | |||
| C | Fe | Major Peak | Minor Peak | |||||
| MWCNT | 22.5 ±1.3 | 10 – 100 | 113.10 | 99.6 | 0.04 | −44.6 | 180 ± 50 | 1100 ± 200 |
MWCNT-induced changes in pathology and pulmonary function is IL-33 dependent
To assess IL-33 dependent differences in pulmonary response to MWCNT, C57BL/6 and IL-33−/− mice were instilled with MWCNTs (4 mg/kg b.w.) and evaluated for changes in pulmonary inflammation, pathology and function 30 days post-exposure (Figure 1A). Consistent with our previous findings, C57BL/6 mice demonstrated increases in total cells within the bronchoalveolar lavage fluid (BALF) at 30 days post-instillation of MWCNT. The influx of inflammatory cells following MWCNT instillation consisted of increased numbers of alveolar macrophages and neutrophils (Table 2). In contrast, IL-33−/− mice instilled with MWCNTs did not demonstrate a significant increase in the total number of cells in the BALF or any increases in alveolar macrophage or neutrophil counts. Eosinophils were slightly increased at 30 days post MWCNT instillation in C57BL/6 mice compared to controls; however, eosinophilia was diminished in IL-33−/− mice (Table 2).
Table 2.
Effect of MWCNT instillation on pulmonary cell populations in C57BL/6 and IL-33−/− mice 30 days following exposure.
| Strain | Exposure | Macrophages (×103 cells) |
Epithelial Cells (×103 cells) |
Neutrophils (×103 cells) |
Eosinophils (×103 cells) |
Lymphocytes (×103 cells) |
Total Cells (×103 cells) |
|---|---|---|---|---|---|---|---|
| C57BL/6 | Vehicle | 158.29 ± 15.13 | 2.78 ± 0.64 | 0.62 ± 0.21 | 0.00 ± 0.00 | 0.00 ± 0.00 | 161.69 ± 15.43 |
| MWCNT | 199.52 ± 17.69 | 3.75 ± 0.65 | 3.56 ± 0.65* | 0.27 ± 0.22 | 0.22 ± 0.15 | 207.33 ± 18.40 | |
| IL-33−/− | Vehicle | 109.31 ± 18.62 | 2.35 ± 0.79 | 0.33 ± 0.23 | 0.03 ± 0.03 | 0.18 ± 0.09 | 114.66 ± 20.41 |
| MWCNT | 111.31 ± 8.34# | 2.16 ± 0.59 | 1.91 ± 0.36 | 0.11 ± 0.07 | 0.18 ± 0.18 | 118.83 ± 9.51# |
Values are mean ± SEM. C57BL/6 n=18/group; IL-33−/−n=8/group.
p < 0.05 vs. C57BL/6 vehicle (10% surfactant saline),
p <0.05 vs. C57BL/6 MWCNT.
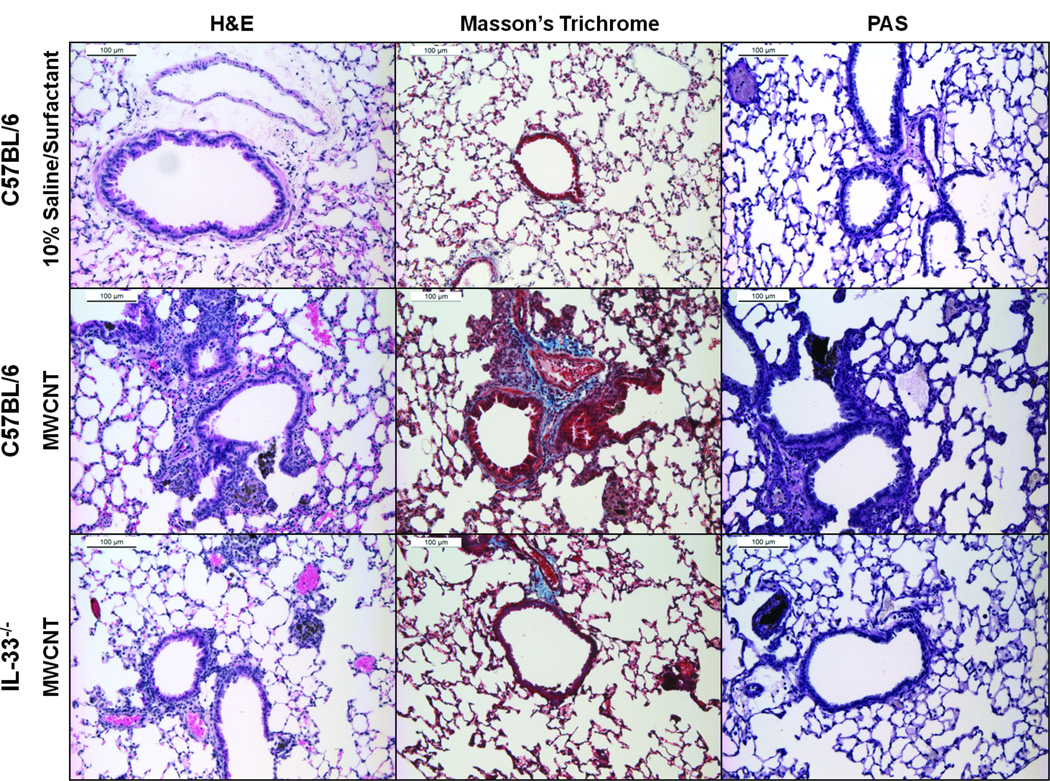
H&E staining of lungs from C57BL/6 mice demonstrated the presence of increased numbers of interstitial inflammatory cells and early granuloma formation 30 days following exposure to MWCNTs (Figure 2). This influx of interstitial inflammatory cells was less severe in IL-33−/− mice instilled with MWCNTs compared to C57BL/6 mice. As indicated by Masson’s Trichrome staining, increased collagen deposition was observed around the airways of C57BL/6 mice compared to IL-33−/− mice at 30 days following instillation of MWCNTs (Figure 2). Neither C57BL/6 mice nor IL-33−/− mice exposed to MWCNTs demonstrated increased staining for mucus production as shown with periodic acid-schiff (PAS) staining of lung tissue (Figure 2).
Figure 2.
Representative images of H&E, Masson’s trichrome, and PAS stained lung tissue obtained 30 days following instillation of vehicle (10% surfactant saline) (upper row) or MWCNTs (middle row) in C57BL/6 mice, and MWCNTs in IL-33−/− mice (lower row).
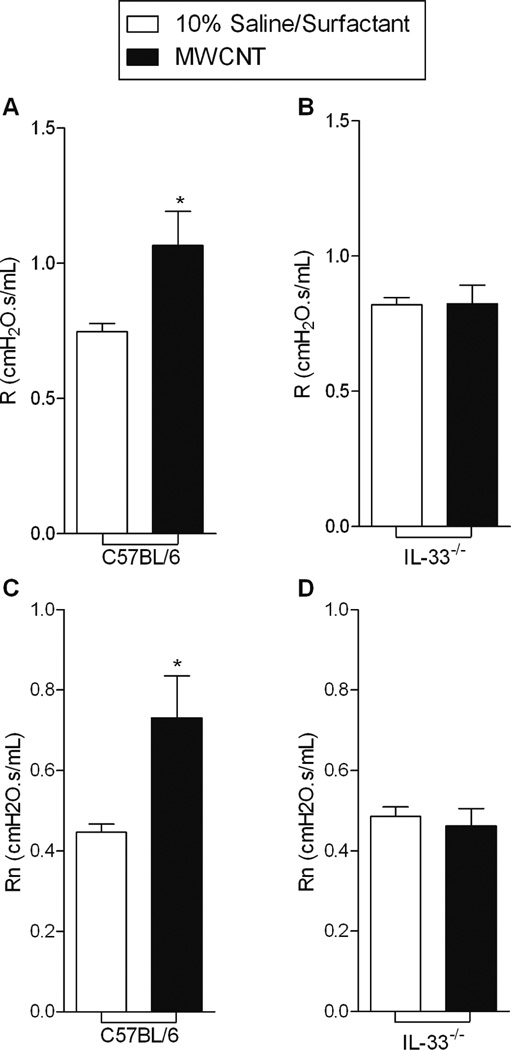
Thirty days following instillation of MWCNTs, pulmonary function was assessed in C57BL/6 and IL-33−/− mice (Figure 1A). C57BL/6 mice exposed to MWCNTs demonstrated increased pulmonary resistance (R) and Newtonian resistance (Rn) compared to vehicle (Figure 3). IL-33−/− mice instilled with MWCNTs, however, did not demonstrate alterations in either R or Rn.
Figure 3.
C57BL/6 mice instilled with MWCNTs demonstrated increases in A) pulmonary resistance (R) and C) Newtonian resistance (Rn) compared to 10% Saline/Surfactant treated animals, while no change in R and Rn occurred in IL-33−/− mice (B and D) 30 days following exposure. All values are expressed as mean ± SEM (C57BL/6 n=18/group; IL-33−/− n=8/group). *p < 0.05, compared to C57BL/6 10% surfactant saline.
Airway hyperresponsiveness induced by MWCNT exposure is IL-33 dependent
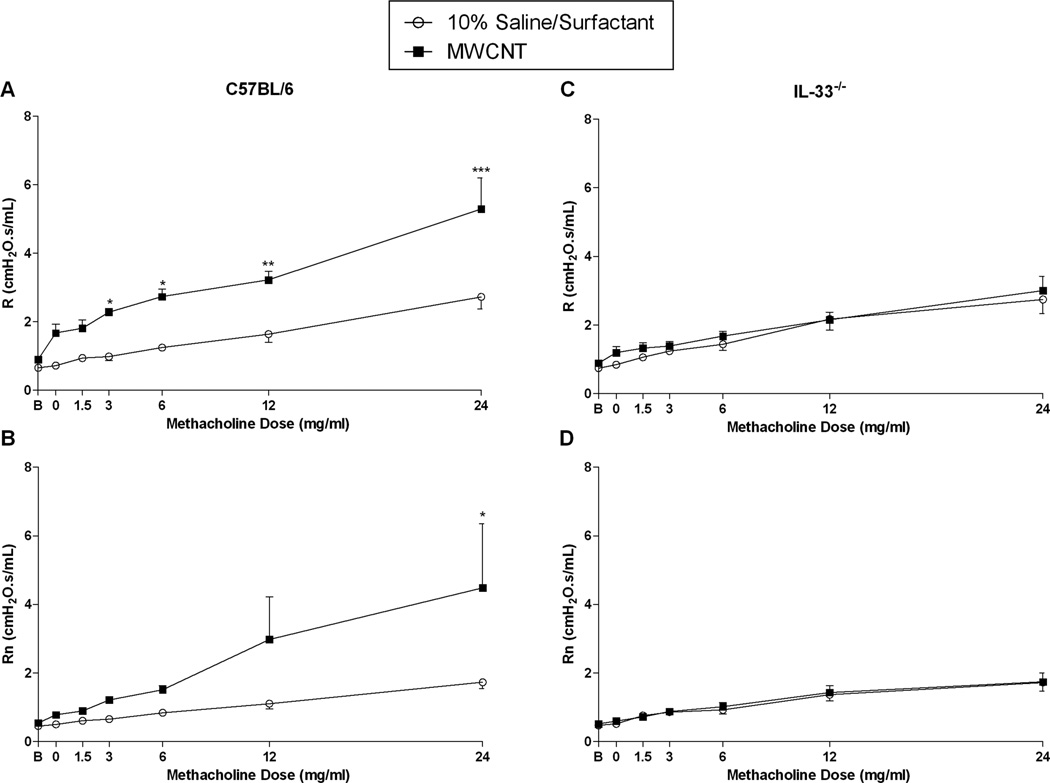
We evaluated the development of AHR in C57BL/6 and IL-33−/− mice at 30 days following MWCNT exposure to determine if these effects were chronic following a single exposure (Figure 1B). As expected, vehicle exposed C57BL/6 and IL-33−/− mice challenged with increasing doses of methacholine were found to exhibit similarly dose-dependent increases in R and Rn (Figure 4). However, when C57BL/6 mice were challenged with increasing concentrations of methacholine 30 days following instillation of MWCNT, we observed a significant increase in both R and Rn parameters at the highest dose of methacholine (24 mg/ml) compared to vehicle exposed animals indicating that MWCNTs are capable of exacerbating AHR (Figure 4A and B). In contrast, when the same experiment was performed in IL-33−/− mice, we found no difference in AHR during the methacholine challenge at 30 days following instillation of MWCNTs as compared to vehicle exposed IL33−/− mice (Figure 4C and D).
Figure 4.
Pulmonary resistance (R) and Newtonian resistance (Rn) dose response curves following challenge with increasing concentrations of methacholine in aerosolized saline (0, 1.5, 3, 6, 12, and 24 mg/ml) for C57BL/6 mice (A and B) and IL-33−/− mice (C and D) 30 days following instillation with vehicle (10% saline surfactant) or MWCNT. All data are expressed as mean ± SEM (n = 6/group). *p < 0.05, compared to vehicle (10% surfactant saline) exposed C57BL/6.
MWCNT-induced changes in pulmonary resistance is primarily inflammation dependent
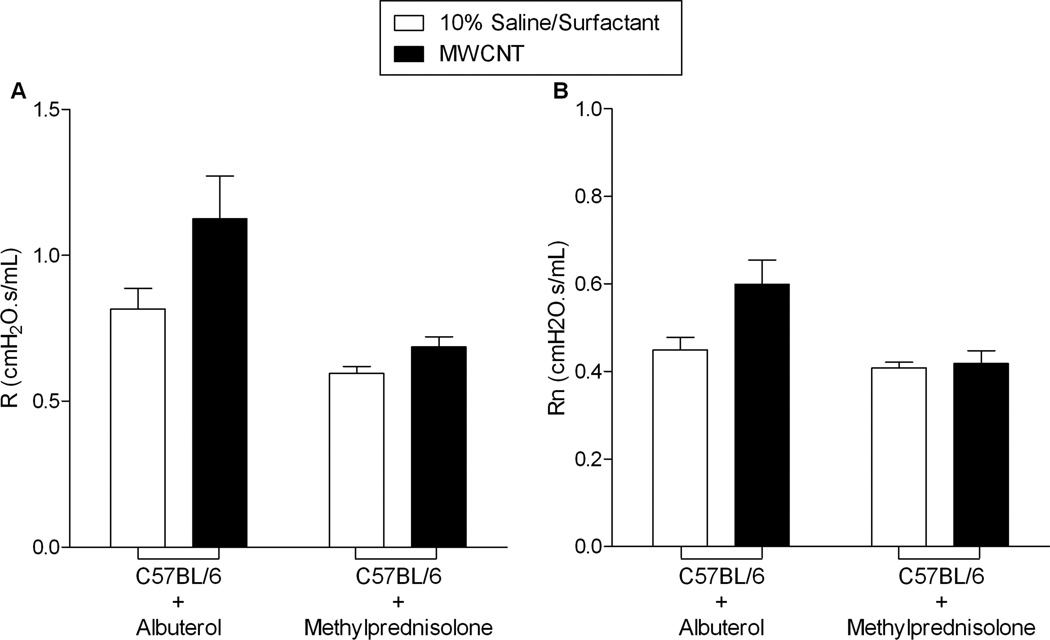
In an attempt to inhibit MWCNT-induced changes in pulmonary resistance (Figure 3) and elucidate the contribution of smooth muscle tone in the airways, C57BL/6 mice were administered albuterol, a β2-adrenergic receptor agonist. Thirty days following MWCNT exposure, C57BL/6 mice were treated with aerosolized albuterol following baseline assessment of MWCNT-induced chronic changes in pulmonary resistance parameters (Figure 1C). Treatment with albuterol did not reverse the MWCNT-induced increases in the resistance parameters, R and Rn (Figure 5).
Figure 5.
The treatment of C57BL/6 mice with albuterol or methylprednisolone was conducted as two separate experimental series. Thirty days following instillation, albuterol treatment (0.083% solution in saline) failed to reverse MWCNT-induced chronic enhancement of R and Rn (A and B). Methylprednisolone treatment (1mg/kg b.w.) 30 minutes prior to instillation of MWCNT and at 7, 14, 21, and 28 days after exposure via i.p. injection diminished MWCNT-induced chronic enhancement of R and Rn (A and B). All values are expressed as mean ± SEM (Albuterol: vehicle n=4/group; MWCNT n=8/group; Methylprednisolone: n=6/group).
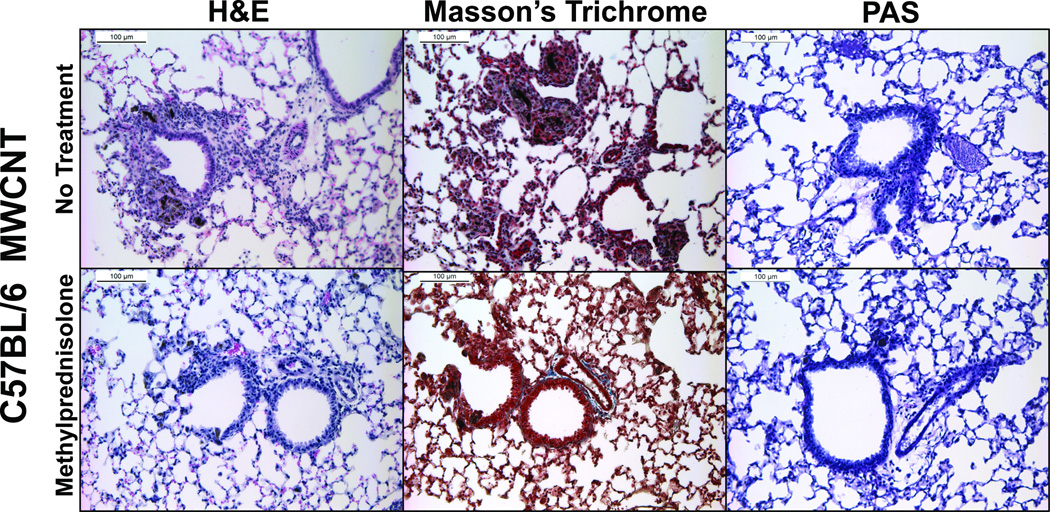
To assess the role of inflammation in MWCNT-induced pulmonary resistance, C57BL/6 mice were treated with a corticosteroid, methylprednisolone, prior to and after exposure to MWCNTs (Figure 1D). Thirty days following MWCNT instillation in C57BL/6 mice receiving the methylprednisolone regiment (intraperitoneal injection of methylprednisolone 30 min prior to MWCNT installation and at 7, 14, 21, and 28 days following MWCNT instillation), there were no increases in R or Rn compared to vehicle treated mice (Figure 5). Treatment with methylprednisolone was not found to mitigate MWCNT-induced influx of inflammatory cells in the BALF, possibly due to the route of corticosteroid administration (Table 3). However, H&E staining of lung tissue demonstrated a decrease in inflammatory cell influx and early granuloma formation near the airways of C57BL/6 mice receiving methylprednisolone treatment (Figure 6). Furthermore, MWCNT-induced collagen deposition was reduced with methylprednisolone treatment as determined by Masson’s Trichrome staining (Figure 6). Mucus production again was not altered due to exposure to MWCNTs or in conjunction with methylprednisolone treatment (Figure 6).
Table 3.
Effect of albuterol and methylprednisolone treatment on pulmonary cell populations in C57BL/6 mice 30 days following exposure to MWCNT.
| Strain | Treatment | Exposure | Macrophages (×103 cells) |
Epithelial Cells (×103 cells) |
Neutrophils (×103 cells) |
Eosinophils (×103 cells) |
Lymphocytes (×103 cells) |
Total Cells (×103 cells) |
|---|---|---|---|---|---|---|---|---|
| Albuterol | Vehicle | 105.63 ± 17.87 | 1.83 ± 0.74 | 0.094 ± 0.094 | 0.0 ± 0.0 | 0.72 ± 0.20 | 108.38 ± 18.19 | |
| MWCNT | 226.99 ± 19.02* | 4.39 ± 1.52 | 2.06 ± 0.42* | 0.37 ± 0.22 | 2.43 ± 0.96 | 236.25 ± 19.92* | ||
| C57BL/6 | Methyl- prednisolone |
Vehicle | 216.01 ± 27.79 | 1.76 ± 0.57 | 0.094 ± 0.094 | 0.55 ± 0.23 | 1.08 ± 0.49 | 219.50 ± 28.11 |
| MWCNT | 359.97 ± 44.03* | 4.18 ± 1.85 | 5.05 ± 1.26* | 0.95 ± 0.51 | 5.08 ± 1.26* | 375 ± 45.03* |
Values are mean ± SEM. Albuterol: vehicle n=4/group; MWCNT n=8/group; Methylprednisolone: n=6/group.
p < 0.05 vs. vehicle (10% surfactant saline).
Figure 6.
Representative images of H&E, Masson’s trichrome, and PAS stained lung tissue obtained 30 days following instillation of MWCNTs in C57BL/6 mice without (upper panels) or with methylprednisolone treatment (lower panels). C57BL/6 mice were treated with methylprednisolone (1 mg/kg body weight) via i.p. injection 30 minutes prior to instillation of MWCNT and 7, 14, 21, and 28 days after exposure.
Discussion
Rapid increases in the application of nanotechnology in industry have resulted in the production of numerous diverse nanomaterials with extensive applications. Currently, there is a lack of understanding regarding their potential adverse health effects from both environmental and occupational exposures. Previous studies have elucidated that pulmonary exposure of carbon nanotubes in rodents causes pulmonary inflammation and fibrosis, alteration of immune function, and exacerbation of allergic response (Wang et al., 2011a, Beamer et al., 2013, Wang et al., 2013, Ryman-Rasmussen et al., 2009, Bonner et al., 2013). In the present study, we demonstrated that pulmonary exposure of MWCNTs induces IL-33 dependent chronic deterioration of pulmonary pathology and function, as well as, an enhancement of airway hyperresponsiveness 30 days following a single exposure. This study also evaluated possible underlying mechanisms responsible for the impaired pulmonary function following MWCNT exposure, which was identified as inflammation leading to further development of pulmonary fibrosis and airway remodeling. By blocking the inflammatory response induced by MWCNT exposure using methylprednisolone treatment, we successfully eliminated the worsening of both pulmonary pathology and function; however, simply relaxing smooth muscle tone using a β2-adrenergic receptor agonist did not fully reverse the increases in both R and Rn.
It has been demonstrated that pulmonary exposure to MWCNTs causes damage to epithelial cells within the lung resulting in the release of IL-33 (Beamer et al., 2013, Katwa et al., 2012, Ronzani et al., 2013). A number of recent publications support the hypothesis that IL-33 stimulates an inflammatory response within the lung thereby significantly contributing to lung diseases such as asthma (Beamer et al., 2013, Inoue et al., 2009, Katwa et al., 2012, Wang et al., 2011a). Signaling of IL-33 through the ST2 receptor on the surface of mast cells and other immune cells has been shown to induce the release of pro-inflammatory cytokines such as IL-6 and IL-13 (Moussion et al., 2008, Sabatino et al., 2012, Wolters et al., 2005). In previous studies we have addressed the role of mast cells and the IL-33/ST2 axis by using KitW-sh mice, a mast cell deficient mouse model, and ST2−/− mice. Both mouse models were found to respond similar to IL-33−/− mice used in our current study when exposed to MWCNTs. Our results demonstrate a lack of increase in the total number of cells in the BALF in IL-33−/− mice instilled with MWCNTs, which suggests a blunted inflammatory response due to the absence of IL-33. Recent research has demonstrated the importance of IL-33 in the promotion of eosinophilia through the recruitment of lymphoid cells and release of IL-13 at 24 hours following MWCNT exposure (Beamer et al., 2013). Consistent with this previous report, small increases in eosinophils were observed at 30 days following instillation of MWCNT in C57BL/6 mice but not in IL-33−/− mice. It is likely that eosinophilia was enhanced at earlier time points as seen at 24 hours in a previous study (Beamer et al., 2013) and had nearly returned to base line by 30 days in our study. Increased deposition of collagen 30 days following instillation of MWCNT in C57BL/6 mice at the terminal airways was consistent not only with our previous studies but also the work of others (Ryman-Rasmussen et al., 2009, Wang et al., 2011b). Interleukin-33−/−, KitW-sh, and ST2−/− mice all significantly lacked development of interstitial inflammation, granuloma formation, fibrosis, and changes in pulmonary resistance as compared to C57BL/6 mice. Taken together these studies reveal the importance of the IL-33/ST2 axis and mast cells in stimulating an inflammatory response within the lung following exposure to MWCNTs. In addition, the lack of pulmonary function changes in IL-33−/− mice instilled with MWCNTs further suggests that the observed pathology near the terminal bronchioles significantly contributes to these changes in pulmonary function. The location of these sites of fibrosis and granuloma formation is likely due to the site of MWCNT deposition within the lung. Other studies have demonstrated altered pulmonary function at earlier time points, however, our current study points to the possibility of the development of a chronic disease condition following MWCNT exposure which is mediated through the release of IL-33. It is likely over time this inflammatory response will resolve, however, due to the location and persistence of these fibrotic and inflammatory lesions pulmonary function may not entirely return to baseline.
Overall, investigations concerning the pulmonary fibrotic potential of CNTs are somewhat conflicting. Whereas some studies have demonstrated the development of substantial fibrotic lesions following pulmonary exposure others have observed only inflammation with no discernable fibrosis (Cesta et al. 2010, Ryman-Rasmussen et al. 2009, Wang et al. 2013). These differences in pathology are likely due to the type of CNT being evaluated as well as the route of exposure (intratracheal vs inhalation) or suspension method. Our current study utilizing a single high concentration aspiration exposure demonstrated pulmonary fibrosis, which ultimately impacted lung function. While the concentration of MWCNTs used in this study was high compared to recent assessments of occupational exposure levels, it allowed for the examination of in vivo mechanisms, which may facilitate CNT-induced chronic disease conditions (Erdely et al. 2013).
Airway hyperresponsiveness (AHR) is a hallmark of inflamed airways. Furthermore, IL-33 has been shown to induce AHR in other models, including exposure to MWCNTs at earlier time points (Beamer et al., 2013). The similar dose-dependent response in vehicle exposed C57BL/6 and IL-33−/− demonstrate there is no difference in AHR between these two strains of mice. IL-33 release following exposure to MWCNTs has been shown to stimulate the release of IL-13 and IL-5 from innate lymphoid cells resulting in AHR and the recruitment of eosinophils (Wang et al., 2013, Beamer et al., 2013). In our current study we found that mice, which lack IL-33, do not demonstrate increased AHR or eosinophil influx when exposed to MWCNTs. Furthermore, we demonstrated that AHR and mild eosinophilia persists up to 30 days following a single exposure to MWCNTs in C57BL/6 mice. It is likely that exposure to MWCNTs may contribute to the progression or exacerbation of underlying pulmonary diseases such as asthma and COPD. Specifically, sensitization of mice to a combination of ovalbumin and MWCNT has been shown to induce greater AHR and eosinophilia than exposure to ovalbumin alone (Inoue et al., 2009, Mizutani et al., 2012). It is also possible that individuals, who already suffer from AHR and pulmonary inflammation due to diseases such as asthma, may be increasingly sensitive to these prolonged effects of MWCNT exposure. This is supported by findings in a mouse model of allergic asthma that when exposed to MWCNT has increased collagen deposition compared to non-asthmatic mice, possibly due to the presence of disease related inflammation already present within their lungs prior to exposure (Ryman-Rasmussen et al., 2009). Based on our current study, IL-33 may represent a novel therapeutic target for the treatment of MWCNT-induced lung disease.
In many obstructive airway diseases, where pulmonary resistance parameters are elevated, treatment includes a β2-adrenergic receptor agonist to relax the smooth muscle in the airways. This agonist allows for much easier airflow into the lungs by relaxing airway smooth muscle and therefore decreasing pulmonary resistance. Treatment with albuterol however was not found to significantly reduce MWCNT-induced chronic increases in resistance parameters suggesting that smooth muscle contraction is not solely responsible for the observed changes in pulmonary resistance. Furthermore, since treatment with the β2-adrenergic receptor agonist did not alleviate MWCNT-induced changes in pulmonary resistance it is likely that these alterations are not due exclusively to smooth muscle tone or smooth muscle hypertrophy. Instead the changes in pulmonary resistance are likely related to the presence of pathological lesions within the lung, as well as, airway remodeling.
Treatments with corticosteroids alleviated the prolonged increase in pulmonary resistance and therefore suggest that inhibition of the early stages of inflammation can mitigate compromised pulmonary function and pathology in response to MWCNT exposure. Treatment with corticosteroids has been shown to reduce the expression of IL-1β, TNF-α, GM-CSF, and other cytokines in vitro (Muroya et al., 2012, Newton et al., 2010). It has been specifically shown that treatment with dexamethasone fails to abolish the up-regulation of IL-33 in airway smooth muscle cells (Prefontaine et al., 2009). Treatment with methylprednisolone was found to reduce MWCNT-induced changes in pulmonary resistance but not to control levels. It is likely that treatment with methylprednisolone blunted the overall immune response to the MWCNT but did not completely inhibit the IL-33 pathway. This result is consistent with a recent report of corticosterioid resistant airway inflammation that develops via induction of the IL-33 pathway (Kabata et al., 2013). Due to other studies that have demonstrated robust inflammation and changes in pulmonary resistance at 24 hours following MWCNT exposure it is likely that methylprednisolone treatment inhibited the robust acute inflammatory response (Beamer et al., 2013, Bonner et al., 2013, Girtsman et al., 2012). This initial acute inflammatory response is likely responsible for the chronic changes we observe at 30 days. In the occupational setting, when workers are exposed to MWCNT, early treatment with methylpredisolone might alleviate the delayed pulmonary fibrosis. Therefore, corticosteroid treatment might be an effective therapeutic treatment for early pulmonary exposure of MWCNT.
Conclusion
In summary, consistent with our previous studies and those of other laboratories, we demonstrated alterations in baseline pulmonary function, pathology and AHR 30 days following MWCNT instillation in C57BL/6 mice. Alternatively, IL-33−/− mice did not demonstrate alterations in baseline pulmonary function, pathology, or AHR following exposure to MWCNT, further validating the role of IL-33 in MWCNT-induced pulmonary toxicity. In our study, treatment with albuterol was not found to alleviate the increases in pulmonary resistance in mice; while treatment with the corticosteroid methylprednisolone was found to reduce the pulmonary pathology induced by MWCNT and also partially prevent the MWCNT-directed changes in pulmonary resistance. Based on these findings, we conclude that the observed changes in chronic pulmonary resistance, pathology and AHR following MWCNT instillation are primarily due to inflammation and the IL-33 pathway.
Acknowledgments
This work was supported by NIEHS R01 ES019311.
List of Abbreviations
- AHR
Airway Hyperresponsiveness
- BALF
Bronchoalveolar Lavage Fluide
- CNT
Carbon Nanotubes
- H&E
Hematoxylin and Eosin
- IL-33
Interleukin-33
- MWCNT
Multi-walled Carbon Nanotubes
- Rn
Newtonian Resistance
- PAS
Periodic Acid-Schiff
- R
Total respiratory system resistance
Footnotes
Declaration of Interest Statements
The authors declare that they have no competing interests.
References
- Beamer CA, Girtsman TA, Seaver BP, Finsaas KJ, Migliaccio CT, Perry VK, Rottman JB, Smith DE, Holian A. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 2013;7:1070–1081. doi: 10.3109/17435390.2012.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JC, Silva RM, Taylor AJ, Brown JM, Hilderbrand SC, Castranova V, Porter D, Elder A, Oberdorster G, Harkema JR, Bramble LA, Kavanagh TJ, Botta D, Nel A, Pinkerton KE. Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ Health Perspect. 2013;121:676–682. doi: 10.1289/ehp.1205693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesta MF, Ryman-Rasmussen JP, Wallace DG, Masinde T, Hurlburt G, Taylor AJ, Bonner JC. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am J Respir Cell Mol Biol. 2010;43:142–151. doi: 10.1165/rcmb.2009-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services CDC, N. I. F. O. S. A. H. Current Intelligence Bulletin 65 - Occupational Exposure to Carbon Nanotubes and Nanofibers. NIOSH Publications and Products 2013. 2013 [Google Scholar]
- Erdely A, Dahm M, Chen B, Zeidler-Drdely P, Fernback J, Birch M, Eans D, Kashon M, Deddens J, Hulderman T, Bilgesu S, Battelli L, Schwegler-Berry D, Leonard H, Mckinney W, Frazer D, Antonini J, Porter D, Castranova V, Schubauer-Berigan M. Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology. Part. Fibre Toxicol. 2013 doi: 10.1186/1743-8977-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girtsman TA, Beamer CA, Wu N, Buford M, Holian A. IL-1R signalling is critical for regulation of multi-walled carbon nanotubes-induced acute lung inflammation in C57Bl/6 mice. Nanotoxicology. 2012 doi: 10.3109/17435390.2012.744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Koike E, Yanagisawa R, Hirano S, Nishikawa M, Takano H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol Appl Pharmacol. 2009;237:306–316. doi: 10.1016/j.taap.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, Betsuyaku T, Koyasu S, Asano K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. doi: 10.1038/ncomms3675. [DOI] [PubMed] [Google Scholar]
- Katwa P, Wang X, Urankar RN, Podila R, Hilderbrand SC, Fick RB, Rao AM, Ke PC, Wingard CJ, Brown JM. A carbon nanotube toxicity paradigm driven by mast cells and the IL-(3)(3)/ST(2) axis. Small. 2012;8:2904–2912. doi: 10.1002/smll.201200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, Hoshino T, Fujimoto J, Nakanishi K. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, Van rooijen N, Shepherd M, Mcsharry C, Mcinnes IB, Xu D, Liew FY. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- Mizutani N, Nabe T, Yoshino S. Exposure to multiwalled carbon nanotubes and allergen promotes early- and late-phase increases in airway resistance in mice. Biol Pharm Bull. 2012;35:2133–2140. doi: 10.1248/bpb.b12-00357. [DOI] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroya M, Chang K, Uchida K, Bougaki M, Yamada Y. Analysis of cytotoxicity induced by proinflammatory cytokines in the human alveolar epithelial cell line A549. Biosci Trends. 2012;6:70–80. [PubMed] [Google Scholar]
- Newton R, King EM, Gong W, Rider CF, Staples KJ, Holden NS, Bergmann MW. Glucocorticoids inhibit IL-1beta-induced GM-CSF expression at multiple levels: roles for the ERK pathway and repression by MKP-1. Biochem J. 2010;427:113–124. doi: 10.1042/BJ20091038. [DOI] [PubMed] [Google Scholar]
- Prefontaine D, Lajoie-kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- Qiu C, Li Y, Li M, Liu X, Mcsharry C, Xu D. Anti-interleukin-33 inhibits cigarette smoke-induced lung inflammation in mice. Immunology. 2013;138:76–82. doi: 10.1111/imm.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzani C, Casset A, Pons F. Exposure to multi-walled carbon nanotubes results in aggravation of airway inflammation and remodeling and in increased production of epithelium-derived innate cytokines in a mouse model of asthma. Arch Toxicol. 2013 doi: 10.1007/s00204-013-1116-3. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2009;40:349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino G, Nicoletti M, Neri G, Saggini A, Rosati M, Conti F, Cianchetti E, Toniato E, Fulcheri M, Caraffa A, Antinolfi P, Frydas S, Pandolfi F, Potalivo G, Galzio R, Conti P, Theoharides TC. Impact of IL-9 and IL-33 in mast cells. J Biol Regul Homeost Agents. 2012;26:577–586. [PubMed] [Google Scholar]
- Sayers BC, Taylor AJ, Glista-Baker EE, Shipley-Phillips JK, Dackor RT, Edin ML, Lih FB, Tomer KB, Zeldin DC, Langenbach R, Bonner JC. Role of COX-2 in Exacerbation of Allergen-Induced Airway Remodeling by Multi-Walled Carbon Nanotubes. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2013-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, Mcclanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Thompson LC, Frasier CR, Sloan RC, Mann EE, Harrison BS, Brown JM, Brown DA, Wingard CJ. Pulmonary instillation of multi-walled carbon nanotubes promotes coronary vasoconstriction and exacerbates injury in isolated hearts. Nanotoxicology. 2012 doi: 10.3109/17435390.2012.744858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urankar RN, Lust RM, Mann E, Katwa P, Wang X, Podila R, Hilderbrand SC, Harrison BS, Chen P, Ke PC, Rao AM, Brown JM, Wingard CJ. Expansion of cardiac ischemia/reperfusion injury after instillation of three forms of multi-walled carbon nanotubes. Part Fibre Toxicol. 2012;9:38. doi: 10.1186/1743-8977-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Nie X, Wang Y, Li Y, Ge C, Zhang L, Wang L, Bai R, Chen Z, Zhao Y, Chen C. Multiwall carbon nanotubes mediate macrophage activation and promote fibrosis through TGF- β/Smad signaling pathway. Small. 2013 doi: 10.1002/smll.201300607. [DOI] [PubMed] [Google Scholar]
- Wang X, Katwa P, Podila R, Chen P, Ke PC, Rao AM, Walters DM, Wingard CJ, Brown JM. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part Fibre Toxicol. 2011a;8:24. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Podila R, Shannahan JH, Rao AM, Brown JM. Intravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. Int J Nanomedicine. 2013;8:1733–1748. doi: 10.2147/IJN.S44211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Reece S, Olmstead S, Wardle RL, Van scott MR. Nocturnal thoracoabdominal asynchrony in house dust mite-sensitive nonhuman primates. J Asthma Allergy. 2010;3:75–86. doi: 10.2147/jaa.s11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xia T, Ntim SA, Ji Z, Lin S, Meng H, Chung CH, George S, Zhang H, Wang M, Li N, Yang Y, Castranova V, Mitra S, Bonner JC, Nel AE. Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung. ACS Nano. 2011b;5:9772–9787. doi: 10.1021/nn2033055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]