Abstract
Although significant effort is concentrated toward gait retraining during stroke rehabilitation1, thirty-three percent of community-dwelling individuals following stroke continue to demonstrate gait asymmetries following participation in conventional rehabilitation2. Recent studies utilizing the split-belt treadmill indicate that subjects post stroke retain the ability to learn a novel locomotor pattern. Through the use of error-augmentation, this novel locomotor pattern can provide a temporary improvement in symmetry which can be exploited through repetitive task specific locomotor training. Here, we review findings from this experimental paradigm in chronic stroke survivors and discuss the future questions that must be addressed in order to provide optimal rehabilitation interventions.
Keywords: stroke, motor learning, locomotion, split belt, adaptation
INTRODUCTION
Stroke is the leading cause of long term disability in the United States with approximately 795,000 people experiencing a new or recurrent stroke each year3. A primary concern of individuals experiencing a stroke is the ability to regain ambulatory function4. Moreover, improved ambulatory function post-stroke is linked to increased community participation, improved cardiovascular fitness and decreased risk of stroke recurrence3. As such, gait retraining is a major component of rehabilitation1.
Gait post-stroke is characterized by pronounced asymmetry5. Following stroke, individuals increase reliance on the non-paretic lower extremity in static standing as well as during ambulation. This results in a shortened non-paretic swing phase and increased stance phase on the non-paretic lower extremity. The resulting spatiotemporal asymmetries (stance time, swing time and step length asymmetries) are well documented in individuals post-stroke2,5. Step length asymmetry, in particular, has been shown to influence other gait deviations. By taking a shorter non-paretic step the propulsive force of the paretic limb is decreased thereby limiting forward propulsion of the body 6. Step length asymmetry, and its associated gait deviations, have been linked to decreased walking speed6,7 and efficiency8 as well as decreased dynamic balance9 thereby limiting safe functional ambulation. Various novel rehabilitation interventions have attempted to target these asymmetries to improve safe locomotion. In particular, several studies have utilized principles of motor learning to target specific gait deviations utilizing a split-belt treadmill 10–13.
The split-belt treadmill has two independent belts, one under each leg, so that subjects can walk with belts moving at the same speed, “tied”, or with the belts moving at different speeds “split-belt”. By splitting the treadmill belt speeds in a 2:1 ratio, the paradigm requires both neurologically intact14 and subjects post-stroke15 to alter their coordination while walking. Initially, both spatial and temporal characteristics of step symmetry are altered, however over a period of ten to fifteen minutes this asymmetry will be reduced with the use of trial and error practice 10,11,14. When returning the treadmill to a normal walking condition or a “tied belt” configuration, both neurologically intact and subjects post stroke demonstrate “after-effects” with a reversal of the initial asymmetry induced by the split-belt treadmill configuration. The presence of this “after-effect” indicates that the nervous system has learned and stored a new locomotor pattern14–16. The use of trial and error practice, or adaptation, to a perturbing environment provides important insight into the ability of the post-stroke central nervous system (CNS) to temporarily store and recall a motor memory.
Thus, the split-belt treadmill paradigm allows exploration of various aspects of motor learning including adaptation and retention of a novel locomotor pattern, but also allows exploration of the capacity of the nervous system for error recognition and correction. Recent evidence suggests exaggeration of post stroke gait asymmetry using the split belt treadmill can lead to after-effects resulting in a more symmetric pattern of walking on the treadmill as well as over ground11,15. With repeated exposure to split-belt treadmill walking subjects post stroke demonstrate longer-term improvements in step length symmetry12,17. Consequently, the split belt treadmill can be utilized to facilitate improvements in asymmetric gait post stroke, or can be utilized as a specific probe of motor learning. Within this review we discuss the current role of the split belt treadmill in the examination of locomotor learning as well as a potential therapeutic tool for intervention in individuals post-stroke.
ADAPTATION
Research employing principles of motor learning, specifically adaptation, have recently gained interest due to the ability to target specific gait abnormalities in individuals post-stroke11,12,15,18. Within these studies, adaptation may be defined as the process of modifying or adjusting an already well-learned movement or motor skill based on error feedback16. This process of adaptation occurs over a period of trial-and-error practice in response to novel task demands16,19. Given this definition, motor adaptation can be considered as one specific component of motor skill learning. Once fully adapted, storage of a new motor pattern within the CNS is reflected through “after-effects”. Upon removal of the stimulus, the subject is not able to retrieve the previous motor behavior20,21. The subject must de-adapt, during a period of continued practice without the perturbation, in order to return to their previous baseline motor performance21,22.
Given that motor adaptation involves relearning an already well known movement pattern, the process strongly reflects the re-learning process of those post stroke early within a therapeutic intervention. As a short term learning process, adaptation has gained interest within motor learning research, particularly locomotor learning. Locomotor adaptation affords the ability to learn and unlearn a given locomotor pattern rapidly depending on the environment, allowing for flexibility and efficiency18. The capacity of the nervous system to adapt to and store a new locomotor pattern that approximates an already stored walking pattern may provide insight into the ability of the damaged nervous system to regain a more normal walking pattern10,21,23.
When subjects are asked to walk on a split belt treadmill, participants with chronic stroke and neurologically intact participants demonstrate an immediate reaction in which the leg on the slow belt will immediately spend more time in stance and the fast leg will spend less time in stance to accommodate the difference in belt speeds14,15. When the belts are returned to normal conditions, “tied belts”, subjects immediately return stance times to the baseline walking pattern. Additional intralimb characteristics, including swing time, stride length, and intralimb joint kinematics appear to demonstrate a similar pattern of reactive change to the split belt treadmill condition with absence of adaptation and after-effects14,15. These reactive changes require utilization of peripheral feedback and do not appear to rely on adaptive processes as indicated by lack of after-effects14,15. In contrast, interlimb gait parameters of step length, double support time, and center of oscillation have been found to change slowly during locomotor adaptation and demonstrate appreciable after-effects14. Specifically, step length asymmetry exaggerated by the split-belt paradigm requires a period of trial and error practice in order to reduce this asymmetry toward baseline. During the split-belt condition an adaptive response occurs via feedforward changes in interlimb coordination in order to allow a return to ‘normal’ step symmetry, despite the treadmill belts going two different speeds10,14. When the belts are returned to ‘tied’, neurologically intact subjects demonstrate step asymmetry in the opposite direction, while chronic stroke subjects may demonstrate an improved symmetry relative to baseline.
In those post stroke, improved step length symmetry is only achieved through exaggeration of the subjects’ initial step length asymmetry15. Specifically, an individual with stroke who ambulates with a longer paretic step length relative to the non-paretic will walk on the split-belt treadmill with the paretic leg on the slow belt. When the treadmill is set to a 2:1 speed ratio, “split belt”, the subject will initially walk with an even longer paretic step than demonstrated with baseline “tied belt” walking, thus exaggerating their step length asymmetry or ‘error’. Over a period of 10–15 minutes individuals post stroke are able to reduce this asymmetry. When the belts are returned to the “tied belt” condition, after-effects are evident with subjects maintaining a more symmetric walking pattern10. The improved gait pattern also transfers to overground walking11. As in a true adaptation paradigm however, the improved symmetry is short-lived and subjects return to baseline asymmetry within several minutes. Despite the brevity of improved symmetry, the current findings demonstrate that individuals post-stroke maintain the ability to ambulate with a more symmetric walking pattern 10,11.
The studies presented above demonstrate that individuals post-stroke retain the basic capacity to adapt their walking pattern to novel environmental conditions. Recent locomotor adaptation studies also indicate however, that the rate of adaptation is slowed after stroke 13,24. Rate deficits appear to be specific to spatial rather than temporal gait parameters in those post-stroke13. Differences in adaptation rates of temporal versus spatial characteristics of gait have been previously demonstrated in neurologically intact subjects25. Specifically, Malone and Bastian (2010) demonstrated that neurologically intact subjects walking on a split-belt treadmill were able to adapt limb phasing at a rate twice that of step length25. In addition, temporal characteristic of gait appear to be much more resistant to manipulations of practice structure25 as well as developmental stage26. Young children demonstrate rates of adaption of center of oscillation and step length symmetry which improve with age, while rates of limb phase adaptation are similar regardless of age26. These differences in temporal versus spatial gait characteristics have been postulated to be due to differing sites of neural control 22,27 with temporal characteristics thought to be under subcortical control.
Recent exploration of locomotor adaptation in neurologically intact subjects utilizing the split belt treadmill highlight additional constructs that may be utilized to enhance the rate of adaptation of spatial parameters after stroke. Providing visual feedback in order to allow conscious correction of step symmetry during split-belt adaptation in neurologically intact subjects resulted in an increased rate of adaptation, however also increased the rate of de-adaptation, thereby limiting the potential for increased retention of the split-belt pattern25. Performance of a secondary task while walking on the split-belt treadmill, however, resulted in a decreased rate of adaptation and de-adaptation, thereby allowing enhanced retention of the split-belt pattern. It is currently unknown whether this paradigm could be utilized in those post-stroke given that individuals post-stroke demonstrate difficulties with interference between cognitive tasks and motor control activities including gait 28. Further research identifying specific task parameters that may improve or limit adaptation and retention of a novel locomotor pattern are required in order to develop targeted and effective interventions in those post-stroke.
While individuals with stroke retain the basic ability to adapt to the split-belt treadmill, recent evidence indicates that damage to the cerebellum, whether due to stroke or other causes, interferes with this adaptive capacity23. When exposed to the split-belt walking condition, patients with cerebellar lesions are able to make reactive, feedback driven changes to intralimb characteristics including stride length, stance and swing time. Subjects with cerebellar damage, however, are unable to utilize trial and error practice to adapt characteristics of interlimb coordination that require feedforward, predictive changes in gait23. Similar to previously cited studies, Morton and Bastian (2006) indicate differing levels of locomotor adaptability, feedforward versus reactive, may be under separate neural control. Given that the control of motor learning is suggested to occur in multiple brain areas that may be affected by stroke, it is plausible that specific deficits in locomotor adaptation may be a result of deficits in acquisition of a motor skill within particular damaged cortical areas.
RETENTION
Motor learning can be defined as a set of processes associated with practice or experience leading to relatively permanent changes in skilled behavior (Schmidt, 1988). To learn a motor skill requires increased practice over longer time periods and may be influenced by offline-learning, consolidation and long term storage processes along with various cognitive processes including attention and decision making18. Restoration of movement function post-stroke is thought to be a function of motor learning or relearning29. However, the optimal characteristics of learning which promote functional recovery of walking are not well defined for the post-stroke population.
Few studies have examined the motor learning capability of individuals post stroke11,13,24,30–32 with most evidence confined to the upper extremity 30–37. Winstein et al. 1999 demonstrated that those with chronic stroke retain the ability to utilize augmented feedback in a manner similar to neurologically intact controls to learn a novel upper extremity task, however demonstrate greater errors and increased variability in their movements compared to controls31. Platz and colleagues32 also demonstrated that those post-stroke retain the ability to learn both simple and complex upper extremity motor tasks compared to neurologically intact controls. Subjects post-stroke however, demonstrated increased variability in their movements, increased errors, and required increased time for performance during skill acquisition of a more complex maze coordination task and peg board task32. These studies highlight the capability of those post-stroke to perform and retain a novel motor task with use of the upper extremity. They also, however, call attention to an increase in variability and error during motor performance, in comparison to neurologically intact controls. It is plausible that this increased error and slowed performance may be increasingly detrimental in more complex functional tasks, such as locomotion, which may require increased practice to achieve learning and retention of the motor skill.
The capacity to learn a novel walking pattern has been explored in both neurologically intact14,22,38 and individuals following stroke 10,11 utilizing the split-belt treadmill during single sessions of adaptation. The split belt treadmill has also recently been used to characterize longer-term motor learning 13,27. To assess longer-term motor learning, participants are asked to walk on the split belt treadmill over the course of days. If participants have learned something about how to walk on the split belt treadmill, with each subsequent day of exposure the participant will be less perturbed by the split-belt condition and will therefore demonstrate less initial asymmetry 27. In addition, it would be expected that with each day, the time required to reduce the asymmetry and achieve a more stable pattern of locomotion would be reduced, indicating a faster rate of re-adaptation. Through evaluation of the rate and magnitude of learning one can determine whether subjects can successfully learn a novel walking pattern specific to the split-belt treadmill.
Tyrell and colleagues (2014) had subjects with chronic stroke and age and sex-matched neurologically intact controls participate in 15 minutes of split belt walking at 2:1 speed ratio for five consecutive days. Retention of the newly learned walking pattern was assessed each day and with a final 15 minute split belt treadmill session after 2 days without exposure to the split belt treadmill. Similar to previously cited studies10,11, Tyrell et al. (2014) found that those with chronic stroke retain the ability to acquire a novel locomotor pattern through adaptation to an exaggeration of step length asymmetry. Subjects post-stroke also demonstrated similar retention of the split-belt locomotor pattern on the sixth day of split-belt practice. However, compared to neurologically intact controls, subjects post stroke demonstrated a slowed rate of adaptation to the split belt paradigm on the initial day of practice as well as a slowed rate of re-adaptation and reduction in initial step asymmetry over the course of 5 days13. These results are similar to previously evidenced results in the upper extremity31,32 as well as a recent locomotor adaptation paradigm39. Savin et al. (2013) required subjects with hemiparesis as well as neurologically intact controls to overcome a novel swing phase resistance during treadmill walking39. They found that both neurologically intact and chronic stroke subjects were able to adapt both temporal and spatial parameters of gait. Those with chronic stroke however, differed in the rate of adaptation, requiring increased repetition compared to controls. This study did not examine the rate of learning over subsequent days.
Currently, the study by Tyrell and colleagues (2014) is the only study to demonstrate that learning over multiple days, in addition to acquisition in a single session, of a novel locomotor pattern is slowed in those post-stroke. The study, however, also indicates that although slowed, if given sufficient practice, individuals with chronic stroke can achieve gains through locomotor learning similar to neurologically intact individuals. In the animal model the amount of practice needed to directly influence task dependent neuroplastic changes and demonstrate significant improvements in stepping quality is greater than 1,000 steps per session40. Corroborating this effect, Moore and colleagues (2010) previously demonstrated a dose- response relationship between the amount of stepping practice and improved community ambulation in those post stroke41. Despite the apparent dose-response relationship, patients often receive a limited amount of locomotor practice within a physical therapy session41. This inconsistency highlights a crucial role for empirical evaluation of optimal motor learning strategies that may be utilized for efficient and effective locomotor rehabilitation.
TRAINING
Although preserved, a more symmetric, and possibly more efficient8, safe9 and speedy6,7 gait pattern is not easily achieved in those post-stroke. Within the split-belt treadmill paradigm, after-effects resulting in improved symmetry are only achieved when the initial step length asymmetry is exaggerated and returns to baseline asymmetry. Previous use of error augmentation has been shown to be effective at targeting specific movement deficits following stroke10,11,39,42. The exaggeration of asymmetry, or “error”, is critical because it provides the nervous system with a cue to correct the gait deviation induced by the split- belt paradigm10,11. Error augmentation may be particularly useful for those with chronic stroke whose gait deviations are now perceived as “normal” by the nervous system18. By providing an exaggeration of the locomotor asymmetry, the split-belt paradigm draws attention to the “error” in order to correct the gait asymmetry.
Recent studies have assessed whether short-term improvements in step length asymmetry could be capitalized upon with repetitive split-belt treadmill training in order to produce longer term improvements in gait post-stroke12,17 Utilizing an error-augmentation strategy, a single participant trained 3 days per week for 4 weeks with each training session consisting of six 5-minute bouts of split belt treadmill walking17. The belt configuration during split-belt walking was set so that the participant’s baseline step length asymmetry would be exaggerated. Utilizing the ‘after effect’ of improved step length symmetry, the participant practiced walking overground for 5 minutes following each split-belt walking session. Improved step length symmetry during overground walking was reinforced with verbal cuing from the physical therapist. Following 4 weeks of treadmill training, the participant demonstrated an increase in self-selected and fastest walking speed which was maintained at 1 month follow up. Gait analysis comparison from pre to post treadmill training revealed that step length asymmetry improved from 21% asymmetry at baseline to 9% post training. Decrements in step length asymmetry continued at 1 month follow up to 7% asymmetry17. Expanding upon these results, Reisman and colleagues (2013) trained 12 subjects with chronic stroke with the identical paradigm as described above (Fig. 1). Across the twelve subjects step length asymmetry improved significantly from baseline to post-testing (Fig. 2A). In addition, subjects demonstrated a difference between step length asymmetry at baseline and 1 and 3 month follow-up evaluations that approached significance. In order to identify responders to the intervention, a double baseline evaluation was employed to assess day-to-day differences in step-length asymmetry. Seven participants demonstrated a change in step length asymmetry greater than day to day variability (responders) from pre-training to post-training while 5 did not (non-responders) (Fig. 2C and 2D respectively). The improvements in step-length symmetry occurred as a result of an increased step length bilaterally, with a relatively larger change on the extremity with the shorter step at baseline 12(Fig. 2B). A decreased step length on the extremity with a longer step would have also provided an improved step-length bilaterally, however the improvements achieved through increased step length provides increased functionality, given the association between increased step length and increased walking speed in post-stroke walking interventions43. It is important to note, that the only gait parameter demonstrating long term change, i.e. step length asymmetry, was targeted specifically by the split belt intervention through exaggeration of baseline asymmetry. Longer-term changes were not noted for double-support time, percent stance time, or over-ground gait speed12.
Figure 1.

Split-belt training paradigm.
Figure 2.
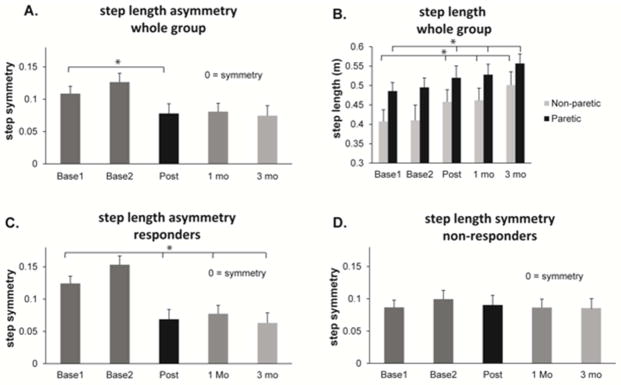
Step length during over ground walking measured at each baseline session (Base1, Base2), at post-training (Post) and at 1 and 3 months after training (1 mo and 3 mo respectively). (A) Step length asymmetry for the entire group (n=12). (B) Step length in meters for the non-paretic (grey bars) and paretic (black bars) leg for the entire group (n=12). (C) Step length asymmetry in the group identified as responders based on their pre to post-training change in asymmetry (n=7). (D) Step length asymmetry in the group identified as non-responders based on their pre to post-training change in asymmetry (n=5). * indicates p <0.05.
The above studies demonstrate that repetitive short-term adaptations observed in previous single session split belt studies can be capitalized on through repetitive practice and can lead to longer term improvements in gait deficits after stroke. The ability to maintain an improvement in step length symmetry at 1 and 3 month follow-up suggests that the effects of training are sustainable, which is particularly important given that gait asymmetries post stroke, namely step length asymmetry, are particularly resistant to rehabilitative training2. These results suggest a potential therapeutic role for the split-belt treadmill in gait rehabilitation. Further empirical evaluation of post-stroke motor learning strategies and an understanding of “for whom” this intervention is effective are required.
SUMMARY
Current evidence indicates that the split-belt treadmill may be utilized as a probe of motor learning after stroke, or as a therapeutic intervention in order to promote a more symmetric gait pattern in individuals post-stroke. Existing evidence indicates that the split belt paradigm may influence some aspects of gait to a greater degree than others. Location of neural injury, type of feedback, and task constructs, all may impact the adaptability of specific gait parameters with exposure to the split-belt paradigm. Further study is required to fully characterize the adaptive capacity of the post-stroke nervous system in order to develop rational methods for improved locomotor rehabilitation.
Key points.
The split-belt paradigm can be used to examine motor learning or potentially as a rehabilitation intervention after stroke.
Persons post-stroke retain the ability to adapt their walking pattern to new constraints.
Locomotor adaptation and learning may be slowed after stroke
Exaggeration of spatial gait asymmetries using the split-belt treadmill results in improved spatial gait symmetry.
Acknowledgments
Funding from NIH grant 1R01HD078330-01A
Footnotes
Disclosures: The authors declare no material financial interests that relate to this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jette DU, Latham NK, Smout RJ, Slavin MD, Horn SD. Physical Therapy Interventions for Patients With Stroke in Inpatient. Phys Ther. 2005;(85):238–248. [PubMed] [Google Scholar]
- 2.Patterson KK, Parafianowicz I, Danells CJ, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89(2):304–310. doi: 10.1016/j.apmr.2007.08.142. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics--2014 Update: A Report from the American Heart Association. 2014:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohannon RW, Andrews AWSM. Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res. 1998;(11):181–183. [Google Scholar]
- 5.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31(2):241–246. doi: 10.1016/j.gaitpost.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanian CK, Bowden MG, Neptune RR, Kautz Sa. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. [Accessed February 6, 2015];Phys Ther. 1994 74(9):872–885. doi: 10.1093/ptj/74.9.872. http://www.ncbi.nlm.nih.gov/pubmed/8066114. [DOI] [PubMed] [Google Scholar]
- 8.Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking Speed and Step Length Asymmetry Modify the Energy Cost of Walking After Stroke. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314552528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewek MD, Bradley CE, Wutzke CJ, Zinder SM. The relationship between spatiotemporal gait asymmetry and balance in individuals with chronic stroke. J Appl Biomech. 2014;30(1):31–36. doi: 10.1123/jab.2012-0208. [DOI] [PubMed] [Google Scholar]
- 10.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130(7):1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23(7):735–744. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair. 2013;27(5):460–468. doi: 10.1177/1545968312474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyrell CM, Helm E, Reisman DS. LEARNING THE SPATIAL FEATURES OF A LOCOMOTOR TASK IS SLOWED AFTER STROKE. J Neurophysiol. 2014;112(2):480–489. doi: 10.1152/jn.00486.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94(4):2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- 15.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130(Pt 7):1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms: II. Specificity and storage of multiple gaze--throw calibrations. Brain. 1996;119(4):1199–1211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- 17.Reisman DS, McLean H, Bastian AJ. Split-belt treadmill training poststroke: a case study. J Neurol Phys Ther. 2010;34(4):202–207. doi: 10.1097/NPT.0b013e3181fd5eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisman DS, Bastian AJ, Morton SM. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Phys Ther. 2010;90(2):187–195. doi: 10.2522/ptj.20090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng Y-W, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98(1):54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- 20.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. [Accessed July 19, 2014];J Neurosci. 1994 14(5 Pt 2):3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. http://www.ncbi.nlm.nih.gov/pubmed/8182467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21(6):628–633. doi: 10.1097/WCO.0b013e328315a293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres-Oviedo G, Vasudevan E, Malone L, Bastian AJ. Locomotor adaptation. Prog Brain Res. 2011;191:65–74. doi: 10.1016/B978-0-444-53752-2.00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26(36):9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savin DN, Tseng S-C, Whitall J, Morton SM. Poststroke hemiparesis impairs the rate but not magnitude of adaptation of spatial and temporal locomotor features. Neurorehabil Neural Repair. 2013;27(1):24–34. doi: 10.1177/1545968311434552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103(4):1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasudevan EVL, Torres-Oviedo G, Morton SM, Yang JF, Bastian AJ. Younger is not always better: development of locomotor adaptation from childhood to adulthood. J Neurosci. 2011;31(8):3055–3065. doi: 10.1523/JNEUROSCI.5781-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malone LA, Vasudevan EVL, Bastian AJ. Motor adaptation training for faster relearning. J Neurosci. 2011;31(42):15136–15143. doi: 10.1523/JNEUROSCI.1367-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haggard P, Cockburn J, Cock J, Fordham C, Wade D. Interference between gait and cognitive tasks in a rehabilitating neurological population. [Accessed January 27, 2015];J Neurol Neurosurg Psychiatry. 2000 69(4):479–486. doi: 10.1136/jnnp.69.4.479. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1737140&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. [Accessed February 17, 2015];J Rehabil Med. 2003 (41 Suppl):7–10. doi: 10.1080/16501960310010070. http://www.ncbi.nlm.nih.gov/pubmed/12817650. [DOI] [PubMed]
- 30.Hanlon RE. Motor Learning. 1996 Aug;77 doi: 10.1016/s0003-9993(96)90262-2. [DOI] [PubMed] [Google Scholar]
- 31.Winstein CJ, Merians AS, Sullivan KJ. Motor learning after unilateral brain damage. [Accessed August 25, 2014];Neuropsychologia. 1999 37(8):975–987. doi: 10.1016/s0028-3932(98)00145-6. http://www.ncbi.nlm.nih.gov/pubmed/10426521. [DOI] [PubMed] [Google Scholar]
- 32.Platz T, Denzler P, Kaden B, Mauritz KH. Motor learning after recovery from hemiparesis. Neuropsychologia. 1994;32(10):1209–1223. doi: 10.1016/0028-3932(94)90103-1. http://www.ncbi.nlm.nih.gov/pubmed/7845561. [DOI] [PubMed] [Google Scholar]
- 33.Pohl PS, McDowd JM, Filion DL, Richards LG, Stiers W. Implicit learning of a perceptual-motor skill after stroke. [Accessed August 25, 2014];Phys Ther. 2001 81(11):1780–1789. http://www.ncbi.nlm.nih.gov/pubmed/11694171. [PubMed] [Google Scholar]
- 34.Boyd LA, Vidoni ED, Wessel BD. Motor learning after stroke: is skill acquisition a prerequisite for contralesional neuroplastic change? Neurosci Lett. 2010;482(1):21–25. doi: 10.1016/j.neulet.2010.06.082. [DOI] [PubMed] [Google Scholar]
- 35.Vidoni ED, Boyd LA. Preserved motor learning after stroke is related to the degree of proprioceptive deficit. Behav Brain Funct. 2009;5:36. doi: 10.1186/1744-9081-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meehan SK, Randhawa B, Wessel B, Boyd LA. Implicit sequence-specific motor learning after subcortical stroke is associated with increased prefrontal brain activations: an fMRI study. Hum Brain Mapp. 2011;32(2):290–303. doi: 10.1002/hbm.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyd LA, Winstein CJ. Implicit motor-sequence learning in humans following unilateral stroke: the impact of practice and explicit knowledge. Neurosci Lett. 2001;298(1):65–69. doi: 10.1016/S0304-3940(00)01734-1. [DOI] [PubMed] [Google Scholar]
- 38.Savin DN, Tseng S-C, Morton SM. Bilateral adaptation during locomotion following a unilaterally applied resistance to swing in nondisabled adults. J Neurophysiol. 2010;104(6):3600–3611. doi: 10.1152/jn.00633.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savin DN, Tseng S-C, Whitall J, Morton SM. Poststroke hemiparesis impairs the rate but not magnitude of adaptation of spatial and temporal locomotor features. Neurorehabil Neural Repair. 2013;27(1):24–34. doi: 10.1177/1545968311434552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, De Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J Neurotrauma. 2007;24(6):1000–1012. doi: 10.1089/neu.2006.0233. [DOI] [PubMed] [Google Scholar]
- 41.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke. 2010;41(1):129–135. doi: 10.1161/STROKEAHA.109.563247. [DOI] [PubMed] [Google Scholar]
- 42.Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp brain Res. 2006;168(3):368–383. doi: 10.1007/s00221-005-0097-8. [DOI] [PubMed] [Google Scholar]
- 43.Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. [Accessed February 18, 2015];Arch Phys Med Rehabil. 2003 84(10):1486–1491. doi: 10.1016/s0003-9993(03)00349-6. http://www.ncbi.nlm.nih.gov/pubmed/14586916. [DOI] [PubMed] [Google Scholar]


