Abstract
Background
Intensive Care Unit Acquired Weakness (ICUAW) is a frequent complication of critical illness due to immobility and prolonged mechanical ventilatory support.
Objectives
To describe daily peripheral muscle strength measurement in ventilated patients and explore relationships among factors that influence ICUAW.
Methods
Peripheral muscle strength of 120 ventilated ICU patients (mean age 59.8 ± 15.1; 51% female; APACHE III 61.3 ± 20.7; ICU stay 10.6 ± 8.6 days) was measured daily using a standardized hand grip dynamometry protocol. Three grip measurements for each hand were recorded in pounds-force; the mean of these three assessments was used in the analysis. Correlates of ICUAW were analyzed with mixed models to explore their relationship to grip strength (age, gender, illness severity, length of ventilatory support, medications).
Results
Median baseline grip strength was variable yet diminished (7.7; 0-102) with either a pattern of diminishing grip strength or maintenance of the baseline low grip strength over time. Controlling for days on protocol, female gender [β = −10.4(2.5); p = <.001], age [= −.24(.08); p = .004], and days receiving ventilatory support [= −.34(.12); p = .005] explained a significant amount of variance in grip strength over time.
Conclusions
Patients receiving prolonged periods of mechanical ventilatory support in this sample show marked decrements in grip strength measured by hand dynamometry, a marker for peripheral muscle strength. Hand dynamometry is a reliable method to measure muscle strength in cooperative ICU patients and can be used in future research to ultimately develop interventions to prevent ICUAW.
Keywords: mechanical ventilation, anxiety, sedation, music intervention, symptoms
Intensive care unit-acquired weakness (ICUAW), defined as the development of severe paresis related to critical illness,1 is a frequent complication of critical illness due to prolonged immobility and bed rest2 particularly in patients receiving prolonged mechanical ventilatory support.1 Respiratory and limb muscle strength are altered after 7 days of mechanical ventilation leading to delayed extubation and prolonged mechanical ventilation.3 Development of ICUAW can also contribute to physical limitations in patients who recover from critical illness.1,2
Known risk factors for ICUAW include older age, diagnosis of sepsis, electrolyte disturbances, receipt of corticosteroids and neuromuscular blocking agents, illness severity, and immobility, while an indirect link has been proposed for sedation due to reduced mobility of sedated patients.4, p. 1880 In addition, muscle mass and force of muscle contraction decrease with aging, which results in weakness that exceeds what would be expected in the presence of muscle atrophy.5 One study reported that female gender was significantly associated with ICUAW.6 Of these known risk factors, those that are not clinically modifiable include older age, gender, multisystem organ failure, sepsis and the requirement in some patients of medications needed for treatment such as corticosteroids and neuromuscular blocking agents (NMBAs).7 Modifiable risk factors include hyperglycemia and sedative medications.7
Little is known about the pattern of muscle strength during ventilatory support, or if modifiable risk factors might directly influence muscle strength in patients experiencing prolonged mechanical ventilation. Measurement of muscle strength also poses challenges in the critical care unit given that non-invasive methods require alert and cooperative patients. One objective, directly quantifiable measure of peripheral muscle strength is hand-grip dynamometry. Grip strength limited to 1-3 measurements has been used by other ICU researchers1,4 as a marker of impaired functional status with diminished grip strength linked to increased ICU mortality.1 There is a paucity of data documenting serial grip strength assessment over the course of ventilatory support. Thus, the purpose of this longitudinal study was to describe serial peripheral muscle strength measurements and to identify factors that are associated with patterns of peripheral muscle strength during mechanical ventilation.
METHODS
Design/Setting/Sample
Patients included in this descriptive, correlational study were a sub-set (n= 120) of mechanically ventilated patients enrolled in a randomized clinical trial testing anxiety self-management with preferred, relaxing music.10 Participants were recruited from 12 ICUs contained in 5 hospitals in the urban Midwest [blinded for peer review], and were receiving ventilatory support for a primary pulmonary problem.
Patients were enrolled from ICUs where care was delivered at the bedside by registered nurses in a 1:2 or 1:1 nurse-patient ratio. All of the participating ICUs had a written sedation administration protocol, however protocols varied among sites. None of the participating ICUs had progressive mobility protocols in place at the time of enrollment. Patients remained enrolled in the parent study as long as they were receiving mechanical ventilatory support, up to 30 days, were extubated, chose to withdraw, were transferred from the ICU, or died. Patients were enrolled in the parent study for 5.7 (SD 6.4) days (median 3.2; range 1-30).10
Patients met inclusion criteria if they were making their own daily care decisions, interacting appropriately with staff, were hemodynamically stable, and not currently receiving paralytic medications. Patients provided their own consent given the patient-directed nature of the intervention protocol. This study was approved by the Human Subjects’ Committee of the PI’s parent institution and the participating sites’ human subjects committees. Details on the parent study are reported elsewhere.10
Measures
Grip-strength Measurement via Hand Dynamometry
We measured peripheral muscle strength by hand-grip dynamometry using the Jamar device. The Jamar Hydraulic Hand Dynamometer (Patterson Medical, Warrenville, IL) measures the force or strength of a grip in pounds-force. It is considered to be the standard measurement of grip strength due to its high calibration accuracy at ± 3 – 5 %.11,12 The standardized normal grip strength for adult males is 101-121 pounds-force and females 57-70 pounds-force,11 providing a quantifiable measure for comparison.
Research by Mathiowetz11,13 has resulted in a standard protocol for assessing grip strength, which includes patient positioning and how to give verbal instructions for completing the assessment. Grip strength measurements are more accurate when using the mean of three grip trials as compared to either a single grip trial or the highest reading of three trials.11, 13 Mathiowetz and colleagues have shown high inter-rater reliability (right grip r = .99; left grip r = .99) and high test-retest reliability when using the mean of three grip trials (right grip r = .88; left grip r = .93).11,13 No issues with significant variability in having multiple people performing assessments have been identified.13
Given the original measurement standards were developed with healthy persons in a seated position, we worked with an occupational therapist to modify the protocol for our patients. Research nurses were trained by the occupational therapist in the Mathiowetz assessment procedure (Table 1). One Jamar device was stored at each hospital to ensure patients utilized the same device throughout the study. Baseline hand grip strength was evaluated on the day of enrollment into the parent study and then assessed daily using our protocol (Table 1). Hand-dynamometry was discontinued for that day if the patient expressed any complaints of pain or declined to complete the grip assessments.
Table 1.
Hand dynamometry Grip Strength Testing Procedure
|
| Testing Process |
|
|
After the subject is positioned appropriately, say, “Are you ready? Squeeze as hard
as you can”. As the subject begins to squeeze, say, “Harder!…Harder!… Relax”. Repeat with the same instructions for the second and third trial and on each hand. |
|
Correlates of ICU Acquired Weakness
Known correlates of ICUAW explored in our study are described below, and were limited to those available from the parent study.
Patient Characteristics and Medications
Patient characteristics included risk and protective factors such as age, diagnosis of sepsis, receipt of corticosteroids, continuous insulin infusion, any receipt of NMBAs, and illness severity. The APACHE III was used as the illness severity measure. APACHE III scores14 were calculated from the ICU admission data. The higher the score, the more ill a patient with a higher risk of ICU mortality (range 0-299).14
Sedative Exposure
Sedative exposure throughout study enrollment allowed for summarizing dose frequency (sedation frequency over 24 hours) and aggregate dose of medications [sedation intensity score (SIS)] from disparate drug classes.15 We included 8 commonly administered analgesic or sedative medications (midazolam, lorazepam, fentanyl, morphine, dexmedetomidine, hydromorphone, propofol, haloperidol) and calculated a weight-adjusted dose for each medication administered during a 4-hour time block. A patient’s mean SIS score (quotient of sum of patient’s SIS values and number of 4-hour intervals on mechanical ventilation) represents the average sedative exposure per hour relative to all other patients. Details on calculating sedative exposure can be found elsewhere.10, 15
Analysis
Descriptive statistics (frequencies for categorical data, measures of central tendency and dispersion), graphing, and mixed modeling were used. Mixed-effects models were used to analyze grip strength over time to accommodate data that are correlated, have variances that are not constant from one time point to another, and to accommodate any missing values. Using the data as is, without imputation, within a mixed model analysis has a lower type I error and higher power than any type of imputation method used for missing data which may result in biased estimates of effects and standard errors.
Possible patterns of change were explored by graphing each individual’s grip strength versus time. Sedation patterns were also explored by graphing the SIS and sedation frequency by patient over time. The patients exhibited a linear change over time with a predominantly negative slope for grip strength, although there was variation. An unconditional means model was used to assess 2 null hypotheses: (a) no change across occasions; and (b) no variation between subjects. Rejecting these null hypotheses warrants further analysis.
Determination of parameters and a final mixed effects model proceeded as follows. First, an unconditional growth model was developed with DAY added as a predictor that resulted in estimation of change coefficients. The best fitting covariance structure was the autoregressive covariance structure that assumes correlations decrease as the lag time increases. Next, a conditional growth model introduced the effect of the covariates found to be associated with grip strength and clinically important, such as an effect from sedation (SIS). An unstructured covariance structure was the best fit for this analysis.
SPSS v.17 and SAS v.9.2 were used for analyses. Level of significance was determined a priori at p ≥ .05.
RESULTS
Description of Patients
The patients in this study were mostly Caucasian females (51%) with a mean age of 59 years. The majority of indications for mechanical ventilation were respiratory failure (50%). Only 6.5% of patients had a sepsis diagnosis, 38% received continuous insulin infusions, 56% received corticosteroids and 10% received NMBAs. Patients were enrolled for a median of 4.2 days (range 1-30). Median number of days receiving ventilatory support prior to study enrollment was 6.7, with a median 9 days in the ICU prior to enrollment. Disposition of patients at ICU discharge was 92% alive and 8% deceased. Refer to Table 2 for details.
Table 2.
Demographic and Clinical Characteristics of Study Participants (n = 120)
| Variable | Frequency % | Mean (SD) | Median (Range) |
|---|---|---|---|
| Age | 52 (23-93) | ||
| Gender | 49% male 51% female |
||
| Race | Caucasian 84% Black 14% Asian 2% |
||
| APACHE III at Study Enrollment | 61.3 (20.7) | ||
| Baseline Grip Strength | 7.7 (0-102) Pounds-force |
||
| Baseline Grip strength first day | Male 13.2 (0-90) Female 13.0 (0-34) |
||
|
Length of Ventilatory Support Prior
to Enrollment (days) |
6.7 (0.2-38) | ||
|
Length of ICU stay Prior to
Enrollment (days) |
9 (1-41) | ||
| Length of Study Enrollment (Days) | 4.2 (1-30) | ||
| Indication for Mechanical Ventilation | |||
| • Respiratory failure | 50% | ||
| • Respiratory distress | 32% | ||
| • Hypoxemia | 14% | ||
| • Acute respiratory distress syndrome |
4% | ||
| ICU Admission Medical Diagnosis | |||
| • Respiratory related | 39% | ||
| • Cardiovascular | 26% | ||
| • Infectious process | 5% | ||
| • Gastrointestinal related | 7% | ||
| • Neurological | 15% | ||
| • Other; surgical admission | 8% |
Description of Grip Strength Measurement
Baseline Grip Strength
The mean baseline grip strength was quite diminished at 13, with a wide range from 0 to 102 pounds-force (Table 2). A small number of patients (n = 6) could not generate any grip strength (zero on the Jamar dial) effort despite coaching and encouragement from research staff.
Serial Grip Strength Measurement
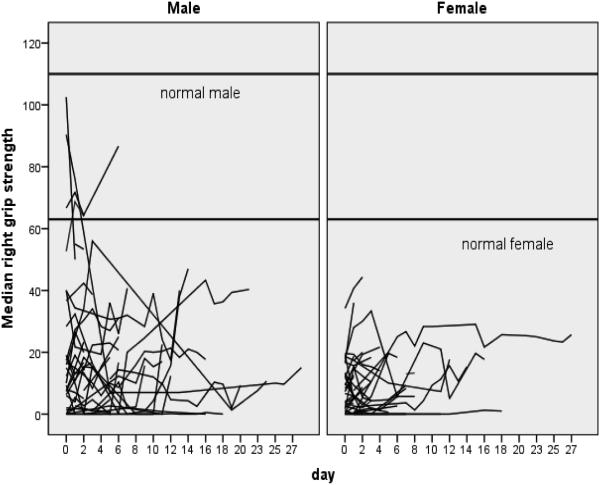
Median number of grip strength measurements for patients were 4 (0-30) with a median of 4.2 days enrolled in the study (range 1 – 30). Patients were approached for grip strength measurement each day they were enrolled in the parent study. If the patient was not able to generate any grip strength on the Jamar dial, a value of “0” was recorded for that day. If a patient was off the unit, unable to participate in measurement, or declined grip measurement, no value was recorded for that day. As evidenced by Figure 1, the pattern of grip strength during ventilatory support shows that patients either start at a higher grip-force and then decline, or they start at a lower point and either stay at that diminished level or continue to decline further over time (Figure 1). Only three patients displayed a pattern of increasing grip strength.
Figure 1.
Spaghetti Plot of Individual Patient’s Median Grip Strength by Gender Over Time
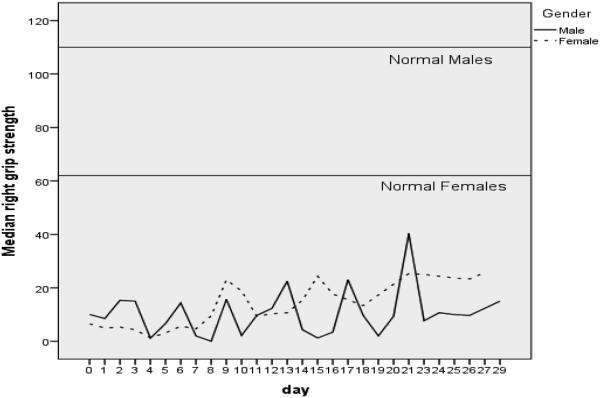
Figure 2 represents median grip strength over time by gender. The data demonstrate a fluctuating pattern of grip strength over time in both genders. By the end of the study, the data suggest a possible upward trend in grip strength as patients recover from their prolonged critical illness.
Figure 2.
Median Grip Strength by Gender over Study Period
Description of Sedative Exposure
Overall SIS was a median of 4 (range 0 – 11), dose frequency was a median of 6.4 (range 0 – 16) each study day and 26% of patients received continuous sedative/opiate infusions.
Correlates Among Grip Strength, Patient Characteristics and Sedative Exposure
Level 1 modeling indicated significant unexplained variance in both grip strength over time (z = 5.41; p < .001) and in initial grip strength (z = 5.37 p < .001), indicating further analysis being appropriate. In Level 2 modeling controlling for days on protocol, female gender [β = −10.4(2.5); p = <.001], age [= −.24(.08); p = .004], and days receiving ventilatory support [= −.34(.12); p = .005] explained a significant amount of variance in grip strength over time (Table 3). Females started with a grip strength 10.4 pounds-force lower than males. For each year older a patient was, the grip strength diminished .24 pounds-force, and for each additional day on the ventilator grip strength decreased by .34 pounds-force. APACHE III scores [= −.12(.07); p = .09], receipt of insulin, steroids, sedative exposure, or NMBAs did not significantly contribute to an explanation of variance over time in grip strength.
Table 3.
Final Models of Correlates of Peripheral Muscle Weakness (Grip Strength Assessment) N = 120
| Screening model with sedation frequency |
Screening model with sedation intensity |
Final model | ||||
|---|---|---|---|---|---|---|
| Parameter | βeta(se(β)) | p-value | βeta(se(β)) | p-value | βeta(se(β)) | p-value |
| APACHE III | −.12(.07) | .09 | −.12(.07) | .09 | ||
| Age | −.23(.09) | .01* | −.23(.09) | .02* | −.24(.08) | .004* |
| Female gender | −10.7(2.7) | <.001* | −10.6(2.7) | <.001* | −10.4(2.5) | <.001* |
| Sepsis | −1.1(4.6) | .80 | −1.1(4.6) | .81 | ||
| Continuous Insulin infusion |
−3.6(2.8) | .18 | −3.6(2.7) | .19 | ||
| Neuromuscular blockade |
−.33(4.5) | .94 | −.38(4.6) | .93 | ||
| Steroids | 2.3(2.8) | .42 | 2.2(2.8) | .43 | ||
| Total ICU Days | .20(.16) | .20 | .21(.16) | .20 | ||
|
Days on
Protocol |
−.19(.20) | .33 | −.18(.19) | .35 | −.04(.18) | .82 |
|
Days on
ventilator |
−.54(.19) | .007* | −.53(.19) | .007* | −.34(.12) | .005* |
| Sedation Frequency |
−.005(.20) | .98 | ||||
| Sedation Intensity | .09(.29) | .77 | ||||
APACHE III = Acute physiology, age, and chronic health evaluation
Sedation frequency = any sedative or analgesic administered in a 4-hour time block summed over 24 hours
Sedation intensity = aggregate score summed over 24 hours
DISCUSSION
We provide data on serial grip strength measurement in a more stable and less ill sample of ICU patients, a majority of whom were without a diagnosis of sepsis. To be eligible for the parent study, patients had to be awake and interacting appropriately with nursing staff. Thus, daily grip strength measurement was a reasonable approach for tracking peripheral muscle strength in these cooperative patients. Patients had significant decrements in peripheral muscle strength at baseline (median day 6.7 of mechanical ventilation). Baseline measures were substantially lower than adult male or female norms and patients did not show any substantial improvement in grip strength over time.
Age, female gender, and lengthy periods of ventilatory support contributed to diminished grip strength in these ICU patients, regardless of illness severity. Our sample of patients had lower severity of illness scores as compared to other studies that measured grip strength1,4 or studies that report significant and sustained weakness in ICU survivors with ARDS.5 However, our results report decrements in peripheral muscle strength regardless of diagnosis or how ill a patient was upon ICU admission. Schweikert & Hall7 offer several areas for risk factor modification in patients at risk for ICUAW. While glycemic control is one area with evidence for benefit, receipt of continuous insulin infusions was not found to impact grip strength in our study; however we did not assess overall blood glucose control. Likewise, medications linked to evidence for harm, corticosteroids and NMBs, were also not significant correlates in our study. One area of indirect evidence for the modification of ICUAW is sedation-sparing protocols. While sedative exposure did not significantly contribute to decrements in peripheral muscle strength, it could have indirectly contributed to lengthy periods of ventilatory support which was significant in our study.
Limitations
There were several limitations with our study. Generalizability of our findings are relevant only to those ICU patients with characteristics similar to those in our study. We did not obtain any data on pre-ICU disability that may have impacted grip strength assessments. It is not known if patients were already weak and had decrements in muscle strength prior to ICU admission. It is unknown when and if patients regained their muscle strength or if there were any decrements in functional domains and quality of life. Likewise, we did not obtain any measurement of respiratory muscle strength which may have influenced total ventilator days. Finally, assessment of grip strength during early stages of mechanical ventilation was challenging. We were only able to enroll patients around day 6 or 7 of mechanical ventilation, due to the parent study inclusion criteria. However, as clinical practice guidelines that call for minimizing sedation are more widely implemented, hand-grip dynamometry may be a feasible option for tracking peripheral muscle strength over the entire course of mechanical ventilation.
Implications for Practice
This paper provides additional evidence of the detrimental effects of prolonged ventilatory support and immobility in ICU patients. Older, female patients may require additional efforts to minimize ICUAW during lengthy courses of ventilatory support. Mobility programs may be able to address at least some of the decrements in peripheral muscle strength during mechanical ventilation, however, for mobility programs to be successful, patients need to be awake and interactive. Nurses will need additional training in mobility programs and alternatives to sedative medications for symptom management to promote alert and interactive patients who can be involved in maintaining or even improving peripheral muscle strength while mechanically ventilated.
Significant decrements at time of enrollment and sustained decrements without improvement in peripheral muscle strength seen in our study patients suggest even more urgency to institute activity and mobility interventions. One place to start may be to omit the “early” label and institute a culture where more awake and engaged patients is the expectation, not the exception, thereby increasing mobility intervention opportunities. Implementation of muscle strength and muscle mass sustaining or preserving interventions is needed. Given the projected increase in the number of patients who will require prolonged ventilatory support by 202016 and the financial burden of providing care for these patients, these trends have significant implications for clinicians to carefully examine ICU care processes and the significant burden of critical illness on survivors’ recovery. Innovative interventions and care processes are needed beyond progressive mobility.
Nursing needs to take the lead in managing the care for mechanically ventilated patients in several different ways. Nurses can facilitate development of mobility protocols, implementing and coordinating activity and out of bed interventions as soon as possible in a safe manner. Symptom management requires on-going intervention with both pharmacological and non-pharmacological interventions to manage the plethora of distressful patient symptoms and promote increasing patient movement. It is imperative that all members of the multidisciplinary care team take accountability and collaborate to develop and implement innovative strategies based on the best available evidence to promote muscle preservation in critically ill patients. This includes physical and occupational therapists who can optimize rehabilitation resources related to mobility as well as physicians and respiratory therapists who can optimize ventilatory management.
Implications for Future Research
The results from our study show that older, female patients with prolonged periods of ventilatory support have the greatest decrement in grip strength, a marker of peripheral muscle strength. Future longitudinal studies are needed to evaluate the feasibility of performing handgrip assessments throughout the entire course of mechanical ventilation, the predictive value of handheld dynamometry on patient functional outcomes9 and its usefulness in guiding intervention strategies.
Novel interventions need to be developed and tested to preserve muscle mass and strength in ICU patients. These strategies could include interventions that can be performed in the bed or chair to maintain or improve strength even when active mobility may not always be an option.
Innovative mobility protocols that are safe and do not induce unnecessary staff burden are needed. Given the demands of high-acuity care, staffing patterns, the increasing need for interdisciplinary support, and the aging of the nursing workforce, these and other factors need to be considered and supported when developing and testing new ICU care protocols.
Summary and Conclusions
ICUAW is a common problem in patients receiving prolonged periods of ventilatory support. This paper adds to the evidence on the detrimental influence of prolonged ventilatory support and immobility, particularly in older female patients. Understanding the etiology, pathophysiology, and risks factors of ICUAW are important for prevention.2 A recent review paper5 presents information on clinical phenotypes and possible molecular mechanisms of ICUAW which may inform innovative treatments for our patients. Multidisciplinary team efforts are needed to quantify ICUAW throughout the course of mechanical ventilation and interventions to prevent or at least minimize ICUAW.
Summary of Key Points.
Patients receiving mechanical ventilatory support are able to participate in handgrip dynamometry which can be used to monitor peripheral muscle strength
Older female patients may be a greater risk for muscle weakness
Prolonged periods of mechanical ventilatory support contribute to peripheral muscle weakness
Innovative, multi-disciplinary interventions are needed to preserve muscle strength and to minimize muscle weakness in critically ill patients
Acknowledge funding
This study was supported in part by a grant from the National Institute of Nursing Research, NIH (1 R01 NR009295, L. Chlan, Principal Investigator) and from the University of Minnesota Medical Center, Fairview Nursing Research Council (M. Tracy, PI).
Footnotes
The work was performed at the University of Minnesota. Research sites were the University of Minnesota Medical Center, Abbott-Northwestern Hospital, North Memorial Medical Center, United Hospital, Regions Hospital, and Hennepin County Medical Center.
REFERENCES
- 1.Ali N, O’Brien J, Hoffman S, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Amer J Resp Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 2.Fan E. Critical illness neuropathy and role of physical therapy and rehabilitation in critically ill patients. Resp Care. 2012;57(6):933–944. doi: 10.4187/respcare.01634. [DOI] [PubMed] [Google Scholar]
- 3.De Jonghe B, Bastuji-Garin S, Durand MC, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35(9):2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 4.Schweikert W, Pohlman M, Pohlman A, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomized controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batt J, dos Santos C, Cameron J, Herridge M. Intensive care unit-acquired weakness: Clinical phenotype and molecular mechanisms. Amer J Resp Crit Care Med. 2013;187(3):238–246. doi: 10.1164/rccm.201205-0954SO. [DOI] [PubMed] [Google Scholar]
- 6.De Jonghe B, Sharsar T, Lefaucher JP. Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 7.Schweikert W, Hall J. ICU-Acquired Weakness. CHEST. 2007;131(5):1541–1549. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 8.Barr J, Fraser G, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the Intensive Care Unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 9.Van Pee G, Segers J, Van Mechelen H, et al. The interobserver agreement of handheld dynamometry for muscle strength assessment in critically ill patients. Crit Care Med. 2011;39(8):1929–1934. doi: 10.1097/CCM.0b013e31821f050b. [DOI] [PubMed] [Google Scholar]
- 10.Chlan L, Weinert C, Heiderscheit A, et al. Effects of patient-directed music intervention on anxiety and sedative exposure in critically ill patients receiving mechanical ventilatory support: A randomized clinical trial. JAMA. 2013;309(12):2335–2344. doi: 10.1001/jama.2013.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg. 1984;9A:222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 12.Jamar Hydraulic Hand Dynamometer Owner’s Manual. N.d. Sammons Preston Rolyan; Chicago, IL: [Google Scholar]
- 13.Mathiowetz V. Reliability and validity of grip and pinch strength measurements. Physical Rehab Med. 1991;2(4):201–212. [Google Scholar]
- 14.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. CHEST. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 15.Weinert C, Calvin A. Epidemiology of sedation for mechanically ventilated patients. Crit Care Med. 2007;35:393–401. doi: 10.1097/01.CCM.0000254339.18639.1D. [DOI] [PubMed] [Google Scholar]
- 16.Zilberberg M, de Witt M, Shorr A. Accuracy of previous estimates for adult prolonged acute mechanical ventilation volume in 2020: Update using 2000-2008 data. Crit Care Med. 2012;40(1):8–20. doi: 10.1097/CCM.0b013e31822e9ffd. [DOI] [PubMed] [Google Scholar]
- 17.Tracy MF, Chlan L. Nonpharmacological interventions to manage common symptoms in patients receiving mechanical ventilation. Crit Care Nurs. 2011;31(3):19–28. doi: 10.4037/ccn2011653. [DOI] [PubMed] [Google Scholar]