Abstract
Background and Aims: Although most ovarian cancers express estrogen (ER), progesterone (PR), and androgen (AR) receptors, they are currently not applied in clinical decision making. We explored the prognostic impact of sex steroid hormone receptor protein and mRNA expression on survival in epithelial ovarian cancer. Methods: Immunohistochemical stainings for ERα, ERβ, PR, and AR were assessed in relation to survival in 118 serous and endometrioid ovarian cancers. Expression of the genes encoding the four receptors was studied in relation to prognosis in the molecular subtypes of ovarian cancer in an independent data set, hypothesizing that the expression levels and prognostic impact may differ between the subtypes. Results: Expression of PR or AR protein was associated with improved 5-year progression-free (P = .001 for both) and overall survival (P < .001 for both, log-rank test). ERα and ERβ did not provide prognostic information. Patients whose tumors coexpressed PR and AR had the most favorable prognosis, and this effect was retained in multivariable analyses. Analyses of the corresponding genes using an independent data set revealed differences among the molecular subtypes, but no clear relationship between high coexpression of PGR and AR and prognosis. Conclusions: A favorable outcome was seen for patients whose tumors coexpressed PR and AR. Gene expression data suggested variable effects in the different molecular subtypes. These findings demonstrate a prognostic role for PR and AR in ovarian cancer and support that tumors should be stratified based on molecular as well as histological subtypes in future studies investigating the role of endocrine treatment in ovarian cancer.
Introduction
Epithelial ovarian cancer accounts for about 3% of female cancers and is the leading cause of death from gynecologic malignancy. Although a somewhat decreased incidence and slightly improved survival have been noted during the last decades, the majority of tumors are diagnosed at advanced stages and the relative 5-year survival is less than 50% [1]. New treatment concepts have shown promising results in clinical trials, but predictive markers are needed for refined therapeutic strategies and will likely need to be stratified in relation to histopathological and molecular subtypes of ovarian cancer [2], [3].
Endocrine factors play key roles in ovarian cancer development, with risk reduction related to multiparity and use of oral contraceptives [4], [5]. Likewise, estrogen (ER), progesterone (PR), and androgen (AR) receptors represent prognostic markers and therapeutic targets in, e.g., breast cancer and prostate cancer [6], [7], [8]. Estrogen regulates growth and differentiation in the normal ovaries and has been demonstrated to have mutagenic effects. Progesterone, on the other hand, induces apoptosis and decreases cell membrane permeability, leading to decreased invasive potential. Progesterone may however stimulate growth at low concentrations, whereas higher concentrations seem to have growth inhibitory effects [9], [10]. The majority of ovarian cancer cases are diagnosed in perimenopausal and postmenopausal women [1]. After menopause, when the estradiol level decreases, androgens are still produced and also seem to influence ovarian cancer development. Androgens promote cell proliferation, and androgen levels are decreased by the use of oral contraceptives [11]. Although the majority of ovarian cancers express ER, antiestrogen treatment has not been successful in ovarian cancer. Several studies have assessed the prognostic value of endocrine receptor expression in ovarian cancer, concluding that expression of PR is prognostically favorable, whereas the results on ERα and ERβ are contradictory. Likewise, the association with other clinical risk factors is variable [12], [13], [14], [15], [16], [17]. A review and meta-analysis by Zhao et al. including in total 35 studies, of which 23 considered the prognostic value of ER, did not find any evidence of an effect of ER on prognosis [18]. Recently though, a multinational study including almost 3000 women with invasive epithelial ovarian cancer showed that ER expression was associated with improved disease-specific survival in endometrioid tumors, whereas PR expression was prognostic in serous tumors [19]. Furthermore, AR expression has been suggested to be associated with a favorable prognosis in serous ovarian tumors and is hypothesized to predict response to antiandrogen treatment [20], [21]. Overall, the frequency of ER, PR, and AR expression seems to decrease with increasing malignant potential in ovarian tumors, but reports regarding covariation of the receptors are contradictory [12], [16], [20], [22], [23]. In general, however, studies of endocrine responsiveness in ovarian cancer are limited by the relatively small number of cases in each study and have not yet led to clinical application.
Ovarian cancer is a highly heterogeneous disease, and increasing evidence suggests that the different subtypes respond differently to targeted treatments and also that prognostic and predictive biomarkers may be subtype specific. In addition to the histopathological classification of ovarian cancers, gene expression profiling has revealed intrinsic molecular subtypes with additional prognostic information [24], [25], [26]. Apart from outlining the prognostic value of ER (both the α and β isoforms), PR, and AR in serous and endometrioid ovarian cancer, we aimed to explore the potential additional effect of coexpression of two or more of these receptors. We also sought to compare the immunohistochemical findings with the mRNA levels of the genes encoding each receptor in relation to the previously published molecular subtypes of ovarian cancer, using an independent data set and hypothesizing that the expression and prognostic impact of the genes encoding the sex hormone receptors may vary between the molecular subtypes. To the best of our knowledge, no reports so far have stratified for molecular subtype in relation to endocrine receptor expression in ovarian cancer, either on the mRNA or on the protein level, and this approach has the potential to further increase our knowledge of endocrine signaling in ovarian cancer.
Materials and Methods
Tumor Material
One hundred eighteen patients with epithelial serous (n = 87) and endometrioid (n = 31) ovarian cancer were included in the present study. The patients were recruited from a consecutive cohort study in the southern Swedish health care region between June 1998 and June 2000 (n = 128 patients with ovarian cancer, outlined in Malander et al. [27]) and at the oncogenetic counseling at Lund University Hospital from 1981 to 1997 (n = 18 patients). The small number of patients recruited via oncogenetic counseling reflects the limited extent of counseling service at that time. Of the 146 eligible patients, 4 patients with clear cell tumors and 10 patients with mucinous tumors were excluded because these tumors are generally not expected to express sex steroid hormone receptors [10], [13]. Another 14 patients with tumors of unknown primary, mixed histologies, or undifferentiated carcinomas were excluded to reduce external factors, which may potentially bias the analyses. Of the 118 included patients, 30 (25%) had a verified BRCA1 (n = 26) or BRCA2 (n = 4) mutation. Detailed clinical features of included tumors are outlined in Supplementary Table S1. Tumor samples were collected at primary surgery, and the patients had not received chemotherapy before this. Information on amount of residual disease after surgery was not available. Fifty-nine of 118 (50%) patients received postoperative carboplatin (AUC5) and paclitaxel (175 mg/m2) treatment, 18/118 (15%) received carboplatin (AUC5) and cyclophosphamide (500 mg/m2), and 17/118 (14%) with varying disease stages were reported not to have received any postoperative chemotherapy. Information on chemotherapy treatment was missing for 24/118 (21%) patients, whereas no information on hormonal treatment was available. Eleven of 30 (37%) of the patients with BRCA mutations were also diagnosed with breast cancer either before or after the ovarian cancer diagnosis. All deaths within the follow-up time, however, were related to ovarian cancer. Histopathological subtypes were reviewed by a gynecological pathologist (A. M.). The histologic subtype and grade were determined according to Silverberg and WHO 2003, and hematoxylin and eosin–stained slides were used to assess tumor grade [28], [29]. All tumors were staged according to the International Federation of Gynecology and Obstetrics criteria [30]. Ethical approval for the study was granted by the Lund University ethics committee (Sweden), and all patients had given their written informed consent to participate in the study.
Immunohistochemical Stainings
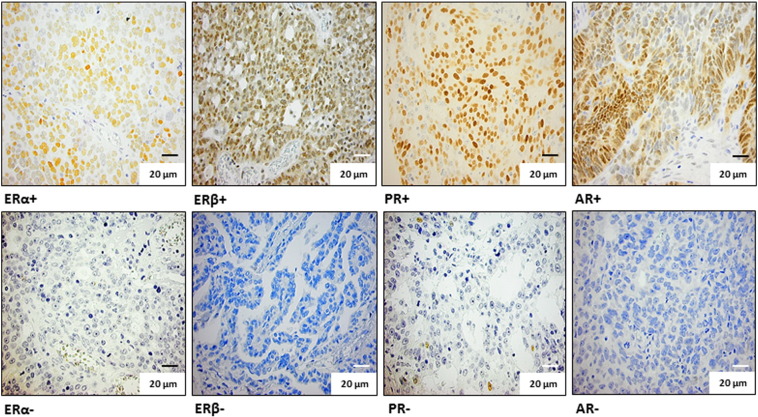
Existing tissue micro array (TMA) blocks were used for evaluation of protein expression by immunohistochemical staining of ERα, ERβ, PR, and AR. The construction of the TMA blocks is outlined in Malander et al. [27]. Three mouse monoclonal antibodies (ERα, DAKO A/S, Glostrup, Denmark, clone 1D5, cat. #M7047, dilution 1:100; ERβ, DAKO, M7292, dilution 1:10; AR, DAKO, M3562, dilution 1:100) and one rabbit polyclonal antibody (PR, DAKO, cat. #A0098, dilution 1:50) were used. For ERα and PR stainings, antigen retrieval was achieved by microwave treatment in 10 mM citrate buffer (pH 6.0, 15 minutes). Antigen retrieval for ERβ and AR stainings was achieved using a pressure cooker and DAKO’s solutions (ERβ pH 6, cat. #S1699, AR pH 9, cat. #S2367), following the manufacturer’s instructions (DAKO). All immunohistochemical stainings were performed using an automated immunostainer (Techmate 500, DAKO), following the manufacturer’s instructions, with application of the Envision systems for visualization (DAKO). Breast cancer tissue known to be positive for the respective receptors was used as positive controls, and ovarian cancer tissue with removal of the primary antibodies was used as negative controls. The ERα and PR nuclear stainings were evaluated independently by authors S. M. and M. N. and ERβ and AR by J. M. J. and N. A. At least three cores per tumor (diameter 0.6 mm) were used to determine the staining pattern. In general, antibodies showed homogenous staining across cores within individual tumors (> 80%), but in cases where heterogeneous staining was observed, the staining pattern representing the majority of the tumor cells was used. Stainings were evaluated regarding percent tumor cells with stained nuclei and staining intensity, where 1 = weak, 2 = moderate, and 3 = strong staining intensity. In line with the Swedish national guidelines for breast cancer, 10% stained tumor cells was used as cutoff for positive versus negative stainings [31]. Thus, for statistical comparisons, only the prevalence of stained tumor cells was taken into account, and < 10% stained tumor cells were dichotomized as 0 and ≥ 10% stained tumor cells as 1. Examples of positive and negative nuclear stainings for the respective receptors are shown in Figure 1. Positive versus negative stainings were studied in relation to 5-year progression-free survival (PFS) and overall survival (OS).
Figure 1.
Examples of immunohistochemical stainings for ERα, ERβ, PR, and AR. Top row from left to right: positive immunohistochemical stainings (≥ 10% stained cells) for ERα, ERβ, PR, and AR. Bottom row from left to right: negative immunohistochemical stainings (< 10% stained cells) for ERα, ERβ, PR, and AR. Magnification, 40 ×.
Sex Steroid Hormone Receptor Gene Expression in Relation to Molecular Subtype
To expand the hypothesis, we aimed to outline if the association between a favorable prognosis and receptor expression at the protein level could be detected on the mRNA level. For this purpose, an independent, publicly available data set consisting of 285 high-grade serous and endometrioid, borderline as well as low-grade serous and endometrioid ovarian tumors, fallopian tube, and primary peritoneal cancers was used for studying the expression of the ESR1, ESR2, PGR, and AR genes, coding for ERα, ERβ, PR, and AR, respectively [24]. Gene probes specific for ESR1, ESR2, PGR, and AR were identified and aligned with the reference genome assembly human build 19 (GRCh37/hg19, released February 2009) using the online BLAST-Like Alignment Tool, identifying DNA sequences with ≥ 95% similarity of ≥ 25 bases. When multiple probe sets identified the same gene, the probe set with the highest number of probes identifying the specific gene was chosen and used for further analyses. The data set was originally used for transcriptional subtyping of ovarian cancers, revealing six different subtypes (C1-C6) of which the four subtypes representing high-grade serous ovarian cancer are now commonly termed “mesenchymal” (C1), “immunoreactive” (C2), “differentiated” (C4), and “proliferative” (C5) [25], [26]. C3 represents borderline and a few serous tumors of varying differentiation grade, and C6 represents low-grade endometrioid tumors [24]. High versus low expression of the respective genes was studied in relation to PFS and OS in the different molecular subtypes using the median mRNA expression level across all the samples as cutoff. Because of limited follow-up time in this data set, 5-year PFS and OS were not evaluable in all subtypes, and therefore 3-year PFS and OS were used as end points.
Statistical Analyses
The prognostic value of immunohistochemical expression of ERα, ERβ, PR, and AR was investigated using PFS time and OS time, both censored at 5 years, as end points. PFS time was defined as the time interval between date of diagnosis and the first sign of disease recurrence (clinical and/or radiological) or death of any cause, whichever came first. OS time was defined as the time interval between date of diagnosis and death of any cause, obtained from medical records and the Swedish civil registration register. No patients were lost to follow-up before 5 years, and all patients who died before 5 years of diagnosis died of ovarian cancer, i.e., had persistent or recurrent disease. In the publicly available gene expression data set, high versus low expression of ESR1, ESR2, PGR, and AR in the respective molecular ovarian cancer subtypes, using the median as cutoff, was investigated using PFS time and OS time as end points, here censored at 3 years because of limited follow-up time [24]. PFS time and OS time were defined as mentioned above. For PFS, disease progression or death of any cause was considered an event, and patients lost to follow-up were censored at the time of last notification. For OS, deaths within 3 years were considered as events, and patients lost to follow-up were censored at the time of last notification. Of the patients who died before 3 years of diagnosis, all but two died of ovarian cancer. OS and PFS were for both data sets estimated using the Kaplan-Meier method and compared between groups using the log-rank test. For protein expression, hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated in univariable and multivariable analyses using Cox proportional hazards regression, adjusted for clinical factors known to influence ovarian cancer survival (stage, age at diagnosis, histological grade, histology, and BRCA mutation status) [32], [33], [34]. A previous Gynecologic Oncology Group study has shown an association between increasing age at diagnosis and tumor progression or death, with the greatest risk among patients ≥ 70 years at diagnosis [32]. This cutoff was therefore used in the present study. Stage (III-IV vs I-II), age at diagnosis (< 70 vs ≥ 70 years), histology (serous versus endometrioid), and BRCA mutation status (BRCA mutation versus wild type) were treated as binary factors and histological grade as a categorical factor on three levels with grade 3 as reference. Chemotherapy was not adjusted for in the multivariable analyses because treatment data were missing for 21% of the patients, and all patients reported to have received chemotherapy were given platinum-containing combinations. Instead, a stability analysis, i.e., a separate multivariable analysis including the variable postoperative chemotherapy (platinum-containing chemotherapy versus no treatment), was performed for 5-year PFS and OS. To account for histology-dependent differences in receptor expression, which may not be captured by adjusting for histology in the multivariable analysis due to the uneven histological distribution, the independent effect of the receptors on survival was also assessed in serous and endometrioid tumors separately. In the external data set, comparisons between mRNA levels in the different molecular subtypes were performed using Kruskal-Wallis test. Associations between receptor protein expression and clinical parameters were assessed using Fisher exact test, except for the ordinal variables stage and grade where the Mann-Whitney test was used. Statistical analyses of protein expression data were performed in SPSS statistics version 22, and analyses on gene expression data were performed in R version 3.1.0. All P values were two-sided.
Results
Expression of Sex Steroid Hormone Receptors and Relation to Outcome
The expression and the prognostic value were first assessed individually for each marker, with ERα positivity detected in 52/118 (44%), ERβ positivity in 102/117 (82%), PR positivity in 36/118 (31%), and AR positivity in 52/118 (44%) tumors (Supplementary Table S1, Figure 1). The representation of stage, grade, age at diagnosis, histology, BRCA mutation status, and postoperative chemotherapy in relation to receptor status is outlined in Table 1. PR negativity was associated with both advanced stage and advanced grade (P = .006 and P = .001, respectively), and AR negativity was associated with high grade (P = .025) and older age at diagnosis (≥ 70 years, P = .017). ERβ positivity was associated with high grade (P = .016, Table 1). No evidence of associations between ERα and stage, grade, or age at diagnosis was found. Likewise, no associations between protein expression of any of the receptors and histological subtype or BRCA mutation status were observed. Expression of either PR or AR alone was associated with improved 5-year PFS and OS (PFS, HR 0.42 [0.24-0.71], P = .001 and OS, HR 0.34 [0.19-0.62], P < .001 for PR + versus PR −; PFS, HR 0.48 [0.30-0.75], P = .001 and OS, HR 0.38 [0.23-0.63], P < .001 for AR + versus AR −, respectively; Table 2). In contrast, ERα or ERβ as single markers influenced neither the 5-year PFS nor the OS (PFS, HR 1.2 [0.77-1.8], P = .4 and OS, HR 1.1 [0.69-1.7], P = .7 for ERα + versus ERα −; PFS, HR 1.2 [0.62-2.2], P = .6 and OS, HR 1.2 [0.60-2.3], P = .6 for ERβ + versus ERβ −, respectively; Table 2; Supplementary Supplementary Figure S1, Supplementary Figure S2). The effect of combined expression of the receptors on survival was then explored. Both PR positivity and AR positivity signified an improved 5-year PFS and OS irrespective of ERα or ERβ status (data not shown). Patients with PR-positive tumors had a median 5-year PFS of > 60 months and patients with AR-positive tumors had a median PFS of 42 months compared with 20 and 18 months, respectively, for patients whose tumors were PR- or AR-negative. The median 5-year OS for patients with both PR-positive and AR-positive tumors was > 60 months compared with 40 and 37 months, respectively, for PR- or AR-negative cases (Figure 2, Figure 3). The survival curves for patients with discordant (i.e., PR +/AR − or PR −/AR +) or absent (i.e., PR −/AR −) protein expression of PR and AR were almost identical and, when combined, showed a 5-year median PFS of 21 months and median OS of 41 months compared with > 60-month median PFS and OS for patients whose tumors were double positive for PR and AR (Figure 4). Because ERα and ERβ expression did not influence survival, they were excluded from the multivariable analyses. The favorable prognosis seen among patients with PR +/AR + tumors compared with patients whose tumors had discordant or absent PR and AR expression remained after adjustment for stage, grade, age at diagnosis, BRCA mutation status, and histology (PFS, HR 0.32 [0.13-0.79], P = .014; OS, HR 0.24 [0.080-0.70], P = .009; Table 3). The additional effect of coexpression of PR and AR was evident after adjustment for the same clinical factors as mentioned above (PFS, P for interaction = .004; OS, P for interaction = .016, data not shown).
Table 1.
Clinical Characteristics of Patients Included in the Study and Stratified for Hormone Receptor Expression Status
| PR |
AR |
ERα |
ER⇠|
Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + |
− |
+ |
− |
+ |
− |
+ |
− |
||||||
|
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
|||||
| 36 | 82 | P† | 52 | 66 | P | 52 | 66 | P | 102 | 15 | P | 118 | |
| 5-Year OS⁎ | |||||||||||||
| Events/person years | 13/148 | 62/269 | 22/209 | 53/209 | 34/181 | 41/237 | 65/352 | 10/61 | 75/418 | ||||
| 5-Year OS (%) | 63.9 | 24.4 | 57.7 | 19.7 | 34.6 | 37.9 | 36.3 | 33.3 | 36.4 | ||||
| 5-Year PFS | |||||||||||||
| Events/person years | 17/124 | 67/196 | 29/170 | 55/150 | 39/134 | 45/187 | 73/270 | 11/46 | 84/320 | ||||
| 5-Year PFS (%) | 52.8 | 18.3 | 44.2 | 16.7 | 25.0 | 31.8 | 28.4 | 26.7 | 28.8 | ||||
| Histology | |||||||||||||
| Serous | 23 (64) | 64 (78) | .12 | 39 (75) | 48 (73) | .8 | 40 (77) | 47 (71) | .5 | 78 (76) | 9 (60) | .2 | 87 (74) |
| Endometrioid | 13 (36) | 18 (22) | 13 (25) | 18 (27) | 12 (23) | 19 (29) | 24 (23) | 6 (40) | 31 (26) | ||||
| Stage | |||||||||||||
| I | 10 (28) | 5 (6) | .006 | 11 (21) | 4 (6) | .090 | 5 (10) | 10 (15) | .6 | 12 (12) | 2 (13) | .18 | 15 (13) |
| II | 5 (14) | 11 (13) | 5 (10) | 11 (17) | 6 (11) | 10 (15) | 11 (11) | 5 (33) | 16 (14) | ||||
| III | 18 (50) | 52 (63) | 31 (60) | 39 (59) | 35 (67) | 35 (53) | 64 (63) | 6 (40) | 70 (59) | ||||
| IV | 3 (8) | 14 (17) | 5 (10) | 12 (18) | 6 (11) | 11 (17) | 15 (15) | 2 (13) | 17 (14) | ||||
| Grade | |||||||||||||
| 1 | 12(33) | 5 (6) | .001 | 13 (25) | 4 (6) | .025 | 9 (17) | 8 (12) | .6 | 13 (13) | 3 (20) | .016 | 17 (14) |
| 2 | 8 (22) | 16 (19) | 10 (19) | 14 (21) | 10 (19) | 14 (21) | 18 (18) | 6 (40) | 24 (20) | ||||
| 3 | 13 (36) | 47 (57) | 23 (44) | 37 (56) | 26 (50) | 34 (51) | 57 (56) | 3 (20) | 60 (51) | ||||
| Missing | 3 (8) | 14 (17) | 6 (11) | 11 (17) | 7 (13) | 10 (15) | 14 (14) | 3 (20) | 17 (14) | ||||
| Age at diagnosis | |||||||||||||
| Median (years) | 56 | 59.5 | 55.5 | 63 | 60 | 56 | 58 | 63 | 58 | ||||
| Range (years) | 40-78 | 26-83 | 26-79 | 41-83 | 43-83 | 26-80 | 26-83 | 41-80 | 26-83 | ||||
| ≥ 70 years | 7 (19) | 22 (27) | .5 | 7 (13) | 22 (33) | .017 | 16 (31) | 13 (20) | .2 | 25 (24) | 4 (27) | 1.0 | 29 (25) |
| < 70 years | 29 (81) | 60 (73) | 45 (86) | 44 (67) | 36 (69) | 53 (80) | 77 (75) | 11 (73) | 89 (75) | ||||
| BRCA mutation | |||||||||||||
| BRCA1 | 6 (17) | 20 (24) | .4 | 14 (27) | 12 (18) | .5 | 12 (23) | 14 (21) | .8 | 24 (23) | 2 (13) | .12 | 26 (22) |
| BRCA2 | 2 (6) | 2 (2) | 1 (2) | 3 (4) | 1 (2) | 3 (4) | 2 (2) | 2 (13) | 4 (3) | ||||
| Wild type | 28 (78) | 60 (73) | 37 (71) | 51 (77) | 39 (75) | 49 (74) | 76 (74) | 11 (73) | 88 (75) | ||||
| Postoperative chemotherapy | |||||||||||||
| Carboplatin/paclitaxel | 15 (42) | 44 (54) | .2 | 24 (46) | 35 (53) | .4 | 29 (56) | 30 (45) | .15 | 51 (50) | 8 (53) | .2 | 59 (50) |
| Carboplatin/cyclophosphamide | 8 (22) | 10 (12) | 8 (15) | 10 (15) | 9 (17) | 9 (14) | 18 (18) | 0 (0) | 18 (15) | ||||
| No chemotherapy | 7 (19) | 10 (12) | 10 (19) | 7 (11) | 4 (8) | 13 (20) | 16 (16) | 1 (7) | 17 (14) | ||||
| Missing | 6 (17) | 18 (22) | 10 (19) | 14 (21) | 10 (19) | 14 (21) | 17 (17) | 6 (40) | 24 (20) | ||||
Survival analyses are performed using the Kaplan-Meier method.
P values are calculated using Fisher exact test, except for the ordinal variables stage and grade where the Mann-Whitney test is used.
ERβ expression is missing for one endometrioid sample.
Table 2.
| 5-Year PFS Univariable Cox |
5-Year OS Univariable Cox |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (Events) | HR | 95% CI | P | n (Events) | HR | 95% CI | P | |||
| PR | ||||||||||
| (+ vs −) | 118 (84) | 0.42 | 0.24 | 0.71 | .001 | 118 (75) | 0.34 | 0.19 | 0.62 | < .001 |
| AR | ||||||||||
| (+ vs −) | 118 (84) | 0.48 | 0.30 | 0.75 | .001 | 118 (75) | 0.38 | 0.23 | 0.63 | < .001 |
| ERα | ||||||||||
| (+ vs −) | 118 (84) | 1.2 | 0.77 | 1.8 | .4 | 118 (75) | 1.1 | 0.69 | 1.7 | .7 |
| ERβ | ||||||||||
| (+ vs −) | 117 (84) | 1.2 | 0.62 | 2.2 | .6 | 117 (75) | 1.2 | 0.60 | 2.3 | .6 |
| Combined PR and AR expression | ||||||||||
| (PR +/AR + vs PR − and/or AR −) | 118 (84) | 0.29 | 0.15 | 0.59 | .001 | 118 (75) | 0.21 | 0.092 | 0.49 | < .001 |
| Stage | ||||||||||
| (III-IV vs I-II) | 118 (84) | 4.4 | 2.3 | 8.3 | < .001 | 118 (75) | 4.0 | 2.0 | 7.8 | < .001 |
| Grade | ||||||||||
| Grade 1 vs grade 3 | 101 (69) | 0.25 | 0.10 | 0.64 | 101 (64) | 0.21 | 0.074 | 0.58 | ||
| Grade 2 vs grade 3 | 101 (69) | 0.99 | 0.57 | 1.7 | 101 (64) | 0.81 | 0.45 | 1.4 | ||
| Grade 3 | 101 (69) | 1.0 | .014⁎ | 101 (64) | 1.0 | .010⁎ | ||||
| Age at diagnosis | ||||||||||
| (< 70 vs ≥ 70 years) | 118 (84) | 0.66 | 0.42 | 1.1 | .083 | 118 (75) | 0.43 | 0.27 | 0.70 | .001 |
| Histology | ||||||||||
| (Serous vs endometrioid) | 118 (84) | 1.7 | 1.0 | 2.9 | .036 | 118 (75) | 1.6 | 0.92 | 2.8 | .094 |
| BRCA mutation | ||||||||||
| (BRCA mutation vs wild type) | 118 (84) | 0.64 | 0.39 | 1.1 | .092 | 118 (75) | 0.59 | 0.33 | 1.0 | .066 |
P for grade on 2 degrees of freedom.
Supplementary Figure S1.
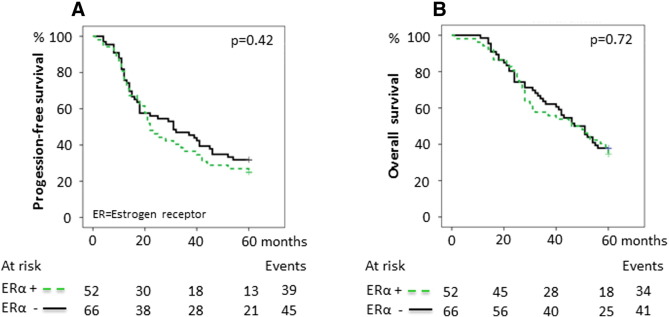
Survival in relation to estrogen receptor α expression. (A) 5-Year PFS for ERα expression. The 5-year PFS was 25.0% ([95% confidence interval] 13.2%-36.8%) for ERα + tumors compared with 31.8% (20.6%-43.0%) for ERα − tumors. (B) 5-Year OS for ERα expression. The 5-year OS was 34.6% (21.7%-47.5%) for ERα + tumors compared with 37.9% (26.1%-49.7%) for ERα − tumors. P values were calculated using the log-rank test.
Supplementary Figure S2.
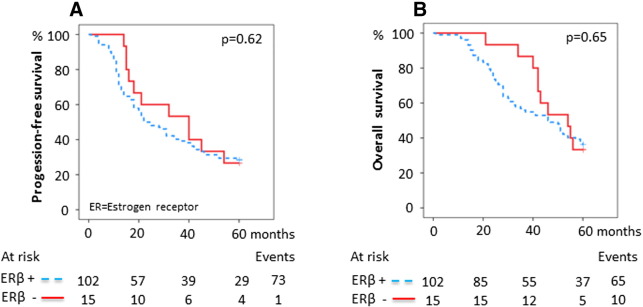
Survival in relation to estrogen receptor β expression. (A) 5-Year PFS for ERβ expression. The 5-year PFS was 28.4% ([95% confidence interval] 19.6%-37.2%) for ERβ + tumors compared with 26.7% (4.4%-49.0%) for ERβ − tumors. (B) 5-Year OS for ERβ expression. The 5-year OS was 36.3% (26.9%-45.7%) for ERβ + tumors compared with 33.3% (9.4%-57.2%) for ERβ − tumors. P values were calculated using the log-rank test.
Figure 2.
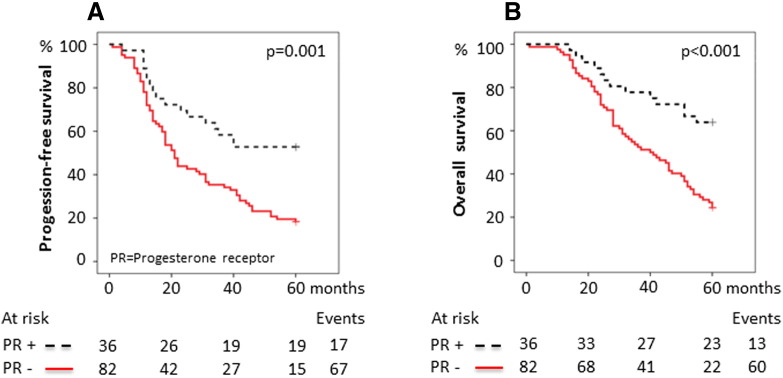
Survival in relation to PR expression. (A) 5-Year PFS for PR expression. The 5-year PFS was 52.8% ([95% confidence interval] 36.5%-69.1%) for PR-positive tumors compared with 18.3% (9.9%-26.7%) for PR-negative tumors. (B) 5-Year OS for PR expression. The 5-year OS was 63.9% (48.2%-79.6%) for PR-positive tumors compared with 24.4% (15.2%-33.6%) for PR-negative tumors. P values were calculated using the log-rank test.
Figure 3.
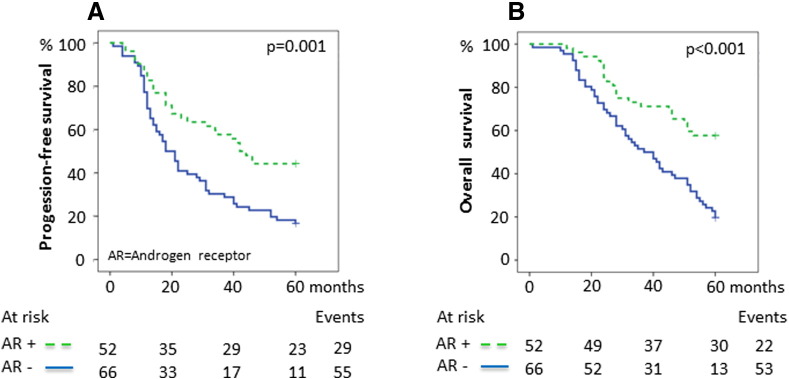
Survival in relation to AR expression. (A) 5-Year PFS for AR expression. The 5-year PFS was 44.2% ([95% confidence interval] 30.7%-57.7%) for AR-positive tumors compared with 16.7% (7.7%-25.7%) for AR-negative tumors. (B) 5-Year OS for AR expression. The 5-year OS was 57.7% (44.2%-71.2%) for AR-positive tumors compared with 19.7% (10.1%-29.3%) for AR-negative tumors. P values were calculated using the log-rank test.
Figure 4.
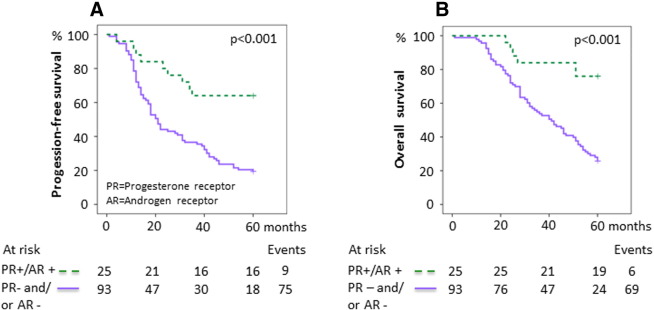
Survival in relation to combined PR and AR expression. (A) Five-year PFS for dual positive versus dual negative or discordant PR and AR expression. The 5-year PFS was 64.0% ([95% confidence interval] 45.2%-82.8%) for PR +/AR + tumors compared with 19.4% (11.4%-27.4%) for tumors with dual negative or discordant receptor expression (PR −/AR −, PR +/AR −, or PR −/AR +). (B) 5-Year OS for dual positive versus dual negative or discordant PR and AR expression. The 5-year OS was 76.0% (59.3%-92.7%) for PR +/AR + tumors compared with 25.8% (17.0%-34.6%) for tumors with dual negative or discordant receptor expression. P values were calculated using the log-rank test.
Table 3.
| 5-Year PFS Multivariable Cox |
5-Year OS Multivariable Cox |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (Events) | HR | 95% CI | P | n (Events) | HR | 95% CI | P | |||
| Combined PR and AR expression | ||||||||||
| (PR +/AR + vs PR − and/or AR −) | 101 (69) | 0.32 | 0.13 | 0.79 | .014 | 101 (64) | 0.24 | 0.080 | 0.70 | .009 |
| Stage | ||||||||||
| (III-IV vs I-II) | 101 (69) | 5.7 | 2.4 | 13.7 | < .001 | 101 (64) | 10.4 | 3.7 | 28.9 | < .001 |
| Grade | ||||||||||
| Grade 1 vs grade 3 | 101 (69) | 0.53 | 0.18 | 1.6 | 101 (64) | 0.58 | 0.17 | 2.0 | ||
| Grade 2 vs grade 3 | 101 (69) | 0.82 | 0.46 | 1.5 | 101 (64) | 0.66 | 0.36 | 1.2 | ||
| Grade 3 | 101 (69) | 1.0 | .5⁎ | 101 (64) | 1.0 | .3⁎ | ||||
| Age at diagnosis | ||||||||||
| (< 70 vs ≥ 70 years) | 101 (69) | 0.90 | 0.54 | 1.5 | .7 | 101 (64) | 0.60 | 0.35 | 1.0 | .059 |
| BRCA mutation | ||||||||||
| (BRCA mutation vs wild type) | 101 (69) | 0.38 | 0.20 | 0.72 | .003 | 101 (64) | 0.34 | 0.17 | 0.67 | .002 |
| Histology | ||||||||||
| (Serous vs endometrioid) | 101 (69) | 0.57 | 0.28 | 1.2 | .13 | 101 (64) | 0.31 | 0.13 | 0.72 | .007 |
P for grade on 2 degrees of freedom.
Stability analyses including postoperative chemotherapy for 5-year PFS and OS did not affect the independent effect of coexpression of PR and AR, but the evidence of such an effect was weaker (PFS, HR 0.40 [0.15-1.0], P = .064, n = 84 patients [61 events]; OS, HR 0.32 [0.10-1.0], P = .050, n = 84 patients [57 events]; data not shown). Analyses without the chemotherapy treatment variable were therefore consistently used throughout this study. The favorable prognosis of PR +/AR + tumors also remained when assessed in serous and endometrioid tumors separately, although the evidence of such an effect was weaker (OS, HR 0.32 [0.081-1.2], P = .097, n = 73 patients [49 events] for serous tumors; OS, HR 0.15 [0.018-1.2], P = .073, n = 28 patients [15 events] for endometrioid tumors; data not shown). Analyses including all tumors and adjusted for histology were therefore consistently used throughout this study.
Receptor Encoding Genes and Correlations with Molecular Subtypes
Next, the mRNA levels of ESR1, ESR2, PGR, and AR were explored in relation to the molecular (transcriptionally defined) subtypes of ovarian cancer, i.e., “mesenchymal” (C1), “immunoreactive” (C2), “borderline” (C3), “differentiated” (C4), “proliferative” (C5), and “low-grade endometrioid” (C6). PGR was upregulated in the subtypes consisting of borderline (C3) and low-grade endometrioid tumors (C6) (P < .001). The same tendency was seen for ESR2 (P < .001). Slightly higher median levels of ESR1 were observed in the immunoreactive (C2), differentiated (C4), and low-grade endometrioid (C6) subtypes (P < .001), whereas AR levels were more similar across subtypes (P = .057; Supplementary Figure S3). Expression of the individual genes in the whole cohort did not predict OS or PFS, but stratification for molecular subtype revealed a tendency toward an association between high levels of PGR and improved 3-year OS in the immunoreactive subtype (C2) (OS, HR 0.33 [0.084-1.3], P = .11; data not shown). Interestingly, despite protein expression of ERα not influencing survival in our in-house TMA cohort, weak evidence of an association between high levels of ESR1 and both improved 3-year PFS and OS in the proliferative (C5) subtype was seen (PFS, HR 0.45 [0.19-1.0], P = .058; OS, HR 0.19 [0.041-0.89], P = .035; data not shown). In contrast, low levels of PGR appeared to be associated with improved 3-year OS in this subtype, although the evidence of such an effect was very weak (OS, HR 3.0 [0.80-11.5], P = .10; data not shown). Finally, weak evidence of an effect of combined high expression of PGR and AR was seen for 3-year PFS, but not OS, in the immunoreactive subtype (C2) (PFS, HR 0.43 [0.17-1.0], P = .063; Figure 5).
Supplementary Figure S3.
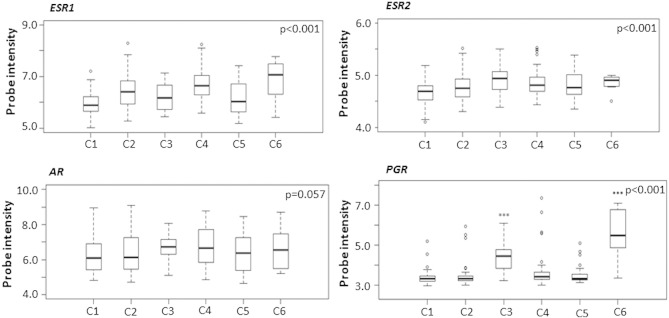
Hormone receptor mRNA levels in relation to molecular subtypes. Representation of ESR1, ESR2, AR, and PGR mRNA levels in the respective molecular ovarian cancer subtypes (C1-C6) in an independent, publicly available data set consisting of ovarian tumors (Tothill et. al., Clin Cancer Res 2008). Normalized probe intensities are outlined on the Y-axes. P values were calculated using Kruskal-Wallis test. C1 = Mesenchymal subtype, C2 = immunoreactive subtype, C3 = borderline subtype, C4 = differentiated subtype, C5 = proliferative subtype, C6 = low-grade endometrioid subtype.
Figure 5.
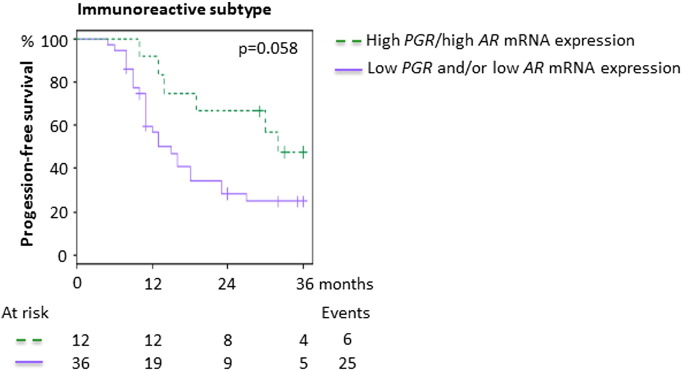
Progression-free survival in relation to PGR and AR mRNA expression levels in the immunoreactive subtype. 3-Year PFS for dual high versus dual low or discordant mRNA levels of PGR and AR in the immunoreactive subtype in an independent, publicly available data set consisting of ovarian tumors (Tothill et al., Clin Cancer Res 2008*). The 3-year PFS was 47.6% ([95% confidence interval] 25.7%-88.2%) for tumors with dual high PGR and AR mRNA levels (high PGR/high AR) compared with 24.7% (13.5%-45.4%) for tumors with dual low or discordant PGR and AR mRNA levels (low PGR/low AR, high PGR/low AR, or low PGR/high AR). The median mRNA value across the samples was used as cutoff for high versus low expression. The P value was calculated using the log-rank test. Crosses represent censored cases. *The data set consisted of 285 ovarian tumors, of which 251 were originally separated into 6 different molecular subtypes. The 251 tumors with assigned subtypes were used in all gene expression analyses in this study.
Discussion
The prognostic value of sex steroid hormone receptor expression in ovarian cancer is not fully defined. In this study, we can however demonstrate that expression of PR and AR predicts 5-year PFS and OS, which is in line with two previous publications [19], [20]. Furthermore, coexpression of PR and AR revealed an even better 5-year PFS and OS, and the independent effect of such an interaction remained after adjustment for known prognostic factors such as stage, grade, age at diagnosis, histology, and BRCA mutation status in multivariable analyses. Data on amount of residual disease after surgery, which is a very strong prognostic factor for ovarian cancer survival, were unfortunately not available, a limitation in this retrospective study [32], [35].
In the present study, no information on use of endocrine treatment was available, precluding analyses of possible effects of endocrine treatment on the findings reported. However, endocrine treatment is not standard in ovarian cancer and is unlikely to have been administered to the study cohort to such an extent that it has influenced the results [14], [19]. The present cohort is limited in size and therefore did not allow thorough analyses in the separate histologic subgroups because of lack of power. Nevertheless, stability analyses stratified for histology showed that the effect of coexpression of PR and AR was present in both serous and endometrioid tumors, thus justifying the combination of these two histologies in the present study.
The link between endocrine receptor expression and prognosis may relate to tumor differentiation, with reduced receptor levels being reported in high-grade, aggressive tumors in both our study and others [13], [20]. This may at least partly explain why the presence of endocrine receptors is prognostically favorable. BRCA1-associated breast cancers in general lack expression of ER and PR, whereas BRCA2-dependent breast cancers show more variability [36]. Likewise, the majority of BRCA-associated ovarian tumors are high grade (and of serous histology). A previous, small study did not find any differences in expression of ERα, ERβ, PR, or AR between BRCA1-associated and matched sporadic ovarian cancer cases, in line with our findings [37]. As has previously been shown, though, presence of a BRCA mutation remained a strong predictor of outcome in this study [33], [37].
Although increasing evidence supports a prognostic effect of PR and AR expression in ovarian cancer, endocrine therapy has so far only produced modest results with response rates of (at best) up to 18% for tamoxifen and 20%–40% for aromatase inhibitors [38], [39], [40], [41]. Likewise, the effect of the antiprogesterone and antiglucocorticoid agent mifepristone is questionable, as the initial encouraging results have not been confirmed [42], [43]. Promising effects have been suggested in pilot studies of adjuvant treatment with medroxyprogesterone acetate plus platinum-containing chemotherapy in advanced ovarian cancer, with the best response among patients whose tumors showed high expression of PR [44]. Confirmatory clinical trials are however lacking. The above-mentioned results stand in contrast to breast cancer, where expression of ER and PR predicts response to endocrine treatment [6]. Of note, however, the response to endocrine treatment may be related to the intrinsic molecular subtypes in both tumor types.
The finding that aromatase inhibition appears slightly more effective than tamoxifen in ovarian cancer likely reflects the more efficient hormone inhibition of aromatase inhibitors. In support of this notion, epidemiological studies indicate that reduced circulating levels of androgens decrease the risk of developing ovarian cancer, but clinical studies have shown only limited effects of androgen deprivation [21], [45], [46]. At present, clinical efficacy data are lacking, but the effect of endocrine treatment in ovarian cancer may be underestimated because of the limited size of the studies performed to date, inclusion of heavily pretreated patients, and a mixture of different histologic types of ovarian cancer where receptor status was not always assessed up front. The observation that AR expression decreases following chemotherapy may also suggest that endocrine treatment could be more effective in the adjuvant setting [21]. The presence and prognostic value of AR expression in ovarian cancer vary in different studies, but increased AR expression seems to generally be associated with a favorable prognosis, as is also the case in the present study [14], [20], [23]. In this study, the expression pattern for AR (44% AR +) and PR (31% PR +) is in line with previous reports, whereas the frequency of ERα positivity (44% ERα +) is lower [14], [16], [19], [20]. In contrast, ERβ positivity (82% ERβ +) was observed at a higher frequency [17]. Overall, the results vary somewhat between studies, which can probably be explained by antibody specificity and the use of different cutoff levels, not least for the less well-studied receptor ERβ [47]. For example, we used an ERβ antibody binding to the functional, ligand binding isoform, ERβ1. Taken together, this stresses the problem with less studied markers, with lack of consensus on choice of antibody and scoring. As presented in this study, double positivity for PR and AR predicts both a better 5-year PFS and a better 5-year OS than either PR or AR alone. The biological background for this is not clear, and to the best of our knowledge, no functional interactions between PR and AR have been described, but the most obvious common denominator is ER(α). PR mediates the apoptotic and cell-differentiating effects of progesterone on the ovaries and acts as a tumor suppressor, and estradiol is also known to regulate the expression of PR. Thus, the function of the receptors is probably of greater importance than the levels of receptor expression [10]. In line with this, a recent study reported novel functional interactions between PR and ERα, whereby progesterone modulates ERα chromatin-binding events and transcriptional activity in breast cancer cells [48]. One may therefore hypothesize that the interaction described in this study may at least to some extent relate to the genomic function of ERα, although this could not be captured in the present analyses. Such a hypothesis is in line with the results from previous studies, reporting an association between AR and ER(α) expression in mixed ovarian cancer cohorts [20], [23]. Likewise, androstenedione constitutes a link between progesterone and testosterone, which may be linked to our finding of a positive predictive effect of dual expression of PR and AR.
The various ovarian cancer subtypes have been suggested to signify different disease entities, justifying separate assessment of the role of endocrine factors in the molecular subtypes of ovarian cancer [25], [26], [49]. Previous studies have investigated the differences in sex steroid hormone receptor expression on both the protein and mRNA levels in normal ovarian surface epithelium and ovarian cancer cells of varying malignant potential and, although not completely consistent, in general noted a decrease in receptor expression levels in malignant compared with normal cells [17], [50], [51]. ERα, but not ERβ, protein expression has also been shown to correlate well with corresponding mRNA levels [17]. We investigated whether the prognostically favorable effect seen in tumors expressing PR and AR, and especially in those coexpressing both receptors, was paralleled by a similarly favorable prognosis in tumors with high mRNA levels of the encoding genes. We also hypothesized that the effect may vary in the different molecular subtypes and therefore used an independent, publicly available data set already stratified into subtypes to address this question. As may be expected because of the great heterogeneity among ovarian cancers, we could not establish any associations on the mRNA level between ESR1, ESR2, PGR, or AR and PFS or OS in this large independent data set of serous and endometrioid tumors. We could however demonstrate a tendency toward different directions of the associations between mRNA levels of PGR and ESR1 expression and the immunoreactive and proliferative subtypes, but a clear prognostic effect of dual high expression of PGR and AR was not observed. This contrasts with the close relationship between, e.g., high ER protein expression and high ESR1 gene expression and prognosis seen in ER-positive breast cancer. It is therefore possible that the regulation of sex steroid hormone receptor expression differs between breast and ovarian cancer, but this study was not designed to address that question. The intrinsic relationships and actions of sex steroid hormone receptors may also be better captured by measurement of modules of coregulated genes and/or microRNAs involved in endocrine signaling. Based on the present findings, however, we conclude that the variable effects of sex steroid hormone receptor gene expression seen in the distinct molecular subtypes of ovarian cancer support that intrinsic subtypes are considered also in future validation studies and clinical treatment trials. Other factors involved in endocrine signaling, in addition to the ones assessed in the present study, may also reveal pertinent information regarding the true prognostic value of sex steroid hormone receptors in ovarian cancer.
Conclusions
In conclusion, we demonstrate a prognostic role of PR and AR expression in ovarian cancer, with independent effects on PFS and OS and the best outcome for patients whose tumors displayed coexpression of PR and AR. We also reveal different expression levels for the genes encoding ERα, ERβ, PR, and AR in the different molecular subtypes of ovarian cancer in an independent data set, but the relationship between mRNA levels and prognosis is uncertain. Our data define a basis for further evaluation of the role of sex steroid hormone receptors, and in the future possibly endocrine treatment, in ovarian cancer and support that such studies may be subtype specific to comprehensively evaluate the potential clinical benefit.
The following are the supplementary data related to this article.
Clinical Characteristics Stratified For Histopathologic Subtype
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.tranon.2015.09.002.
Acknowledgements
We would like to acknowledge Kristina Lövgren and Anna Ebbesson for generous assistance with the laboratory work.
Footnotes
Conflict of interest: The authors declare no conflicts of interests.
Financial support: Financial support was granted from a governmental funding of clinical research within the National Health Services (ALF), the Swedish Cancer Society, the G Nilsson Cancer Foundation, the B Kamprad Foundation, the Swedish Cancer and Allergy Foundation, King Gustaf V’s Jubilee Foundation, the Lund University Hospital Research Foundation and an unrestricted educational grant from the Swedish Society for Gynecologic Oncology sponsored by Roche. Funding agencies have no influence on the study design, data collection or analysis, or manuscript writing.
References
- 1.Howlander N., Noone A., Krapcho M., Garshell J., Miller D., Altekrused S., Kosary C., Yu M., Ruhl J., Tatalovich Z. SEER Cancer Statistics Review, 1975-2011. 2014. http://seercancergov/csr/1975_2011/
- 2.Perren T.J., Swart A.M., Pfisterer J., Ledermann J.A., Pujade-Lauraine E., Kristensen G., Carey M.S., Beale P., Cervantes A., Kurzeder C. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 3.Liu J.F., Barry W.T., Birrer M., Lee J.M., Buckanovich R.J., Fleming G.F., Rimel B., Buss M.K., Nattam S., Hurteau J. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–1214. doi: 10.1016/S1470-2045(14)70391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Beral V., Doll R., Hermon C., Peto R., Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 5.Purdie D.M., Bain C.J., Siskind V., Webb P.M., Green A.C. Ovulation and risk of epithelial ovarian cancer. Int J Cancer. 2003;104:228–232. doi: 10.1002/ijc.10927. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 7.Mason B.H., Holdaway I.M., Mullins P.R., Yee L.H., Kay R.G. Progesterone and estrogen receptors as prognostic variables in breast cancer. Cancer Res. 1983;43:2985–2990. [PubMed] [Google Scholar]
- 8.D'Amico A.V., Chen M.H., Renshaw A.A., Loffredo M., Kantoff P.W. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 9.McDonnel A.C., Van Kirk E.A., Isaak D.D., Murdoch W.J. Inhibitory effects of progesterone on plasma membrane fluidity and tumorigenic potential of ovarian epithelial cancer cells. Exp Biol Med. 2003;228:308–314. doi: 10.1177/153537020322800310. [DOI] [PubMed] [Google Scholar]
- 10.Ho S.M. Estrogen, progesterone and epithelial ovarian cancer. Reprod Biol Endocrinol. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coenen C.M., Thomas C.M., Borm G.F., Hollanders J.M., Rolland R. Changes in androgens during treatment with four low-dose contraceptives. Contraception. 1996;53:171–176. doi: 10.1016/0010-7824(96)00006-6. [DOI] [PubMed] [Google Scholar]
- 12.Arias-Pulido H., Smith H.O., Joste N.E., Bocklage T., Qualls C.R., Chavez A., Prossnitz E.R., Verschraegen C.F. Estrogen and progesterone receptor status and outcome in epithelial ovarian cancers and low malignant potential tumors. Gynecol Oncol. 2009;114:480–485. doi: 10.1016/j.ygyno.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindgren P.R., Cajander S., Backstrom T., Gustafsson J.A., Makela S., Olofsson J.I. Estrogen and progesterone receptors in ovarian epithelial tumors. Mol Cell Endocrinol. 2004;221:97–104. doi: 10.1016/j.mce.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Lee P., Rosen D.G., Zhu C., Silva E.G., Liu J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecol Oncol. 2005;96:671–677. doi: 10.1016/j.ygyno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Burges A., Bruning A., Dannenmann C., Blankenstein T., Jeschke U., Shabani N., Friese K., Mylonas I. Prognostic significance of estrogen receptor alpha and beta expression in human serous carcinomas of the ovary. Arch Gynecol Obstet. 2010;281:511–517. doi: 10.1007/s00404-009-1185-y. [DOI] [PubMed] [Google Scholar]
- 16.Hogdall E.V., Christensen L., Hogdall C.K., Blaakaer J., Gayther S., Jacobs I.J., Christensen I.J., Kjaer S.K. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the 'MALOVA' ovarian cancer study. Oncol Rep. 2007;18:1051–1059. [PubMed] [Google Scholar]
- 17.Chan K.K., Wei N., Liu S.S., Xiao-Yun L., Cheung A.N., Ngan H.Y. Estrogen receptor subtypes in ovarian cancer: a clinical correlation. Obstet Gynecol. 2008;111:144–151. doi: 10.1097/01.AOG.0000296715.07705.e9. [DOI] [PubMed] [Google Scholar]
- 18.Zhao D., Zhang F., Zhang W., He J., Zhao Y., Sun J. Prognostic role of hormone receptors in ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2013;23:25–33. doi: 10.1097/IGC.0b013e3182788466. [DOI] [PubMed] [Google Scholar]
- 19.Sieh W., Kobel M., Longacre T.A., Bowtell D.D., deFazio A., Goodman M.T., Hogdall E., Deen S., Wentzensen N., Moysich K.B. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013;14:853–862. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nodin B., Zendehrokh N., Brandstedt J., Nilsson E., Manjer J., Brennan D.J., Jirstrom K. Increased androgen receptor expression in serous carcinoma of the ovary is associated with an improved survival. J Ovarian Res. 2010;3:14. doi: 10.1186/1757-2215-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elattar A., Warburton K.G., Mukhopadhyay A., Freer R.M., Shaheen F., Cross P., Plummer E.R., Robson C.N., Edmonson R.J. Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer. Gynecol Oncol. 2012;124:142–147. doi: 10.1016/j.ygyno.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Munstedt K., Steen J., Knauf A.G., Buch T., von Georgi R., Franke F.E. Steroid hormone receptors and long term survival in invasive ovarian cancer. Cancer. 2000;89:1783–1791. doi: 10.1002/1097-0142(20001015)89:8<1783::aid-cncr19>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Cardillo M.R., Petrangeli E., Aliotta N., Salvatori L., Ravenna L., Chang C., Castagna G. Androgen receptors in ovarian tumors: correlation with oestrogen and progesterone receptors in an immunohistochemical and semiquantitative image analysis study. J Exp Clin Cancer Res. 1998;17:231–237. [PubMed] [Google Scholar]
- 24.Tothill R.W., Tinker A.V., George J., Brown R., Fox S.B., Lade S., Johnson D.S., Trivett M.K., Etemadmoghadam D., Locandro B. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 25.The Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konecny G.E., Wang C., Hamidi H., Winterhoff B., Kalli K.R., Dering J., Ginther C., Chen H.W., Dowdy S., Cliby W. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malander S., Rambech E., Kristoffersson U., Halvarsson B., Ridderheim M., Borg A., Nilbert M. The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol. 2006;101:238–243. doi: 10.1016/j.ygyno.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Silverberg S.G. Histopathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol. 2000;19:7–15. doi: 10.1097/00004347-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Tavassoli F.A., Devilee P. IARC Press; Lyon: 2003. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. [Google Scholar]
- 30.Heintz A.P., Odicino F., Maisonneuve P., Quinn M.A., Benedet J.L., Creasman W.T., Ngan H.Y., Pecorelli S., Beller U. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 31.Ryden L., Jonsson P.E., Chebil G., Dufmats M., Ferno M., Jirstrom K., Kallstrom A.C., Landberg G., Stal O., Thorstenson S. Two years of adjuvant tamoxifen in premenopausal patients with breast cancer: a randomised, controlled trial with long-term follow-up. Eur J Cancer. 2005;41:256–264. doi: 10.1016/j.ejca.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 32.Winter W.E., III, Maxwell G.L., Tian C., Carlson J.W., Ozols R.F., Rose P.G., Markman M., Armstrong D.K., Muggia F., McGuire W.P. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 33.Zhong Q., Peng H.L., Zhao X., Zhang L., Hwang W.T. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res. 2015;21:211–220. doi: 10.1158/1078-0432.CCR-14-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal R., Kaye S.B. Prognostic factors in ovarian cancer: how close are we to a complete picture? Ann Oncol. 2005;16:4–6. doi: 10.1093/annonc/mdi104. [DOI] [PubMed] [Google Scholar]
- 35.Winter W.E., III, Maxwell G.L., Tian C., Sundborg M.J., Rose G.S., Rose P.G., Rubin S.C., Muggia F., McGuire W.P. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 36.Foulkes W.D., Metcalfe K., Sun P., Hanna W.M., Lynch H.T., Ghadirian P., Tung N., Olopade O.I., Weber B.L., McLennan J. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: the influence of age, grade, and histological type. Clin Cancer Res. 2004;10:2029–2034. doi: 10.1158/1078-0432.ccr-03-1061. [DOI] [PubMed] [Google Scholar]
- 37.Aghmesheh M., Edwards L., Clarke C.L., Byth K., Katzenellenbogen B.S., Russell P.J., Friedlander M., Tucker K.M., de Fazio A. Expression of steroid hormone receptors in BRCA1-associated ovarian carcinomas. Gynecol Oncol. 2005;97:16–25. doi: 10.1016/j.ygyno.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 38.Markman M., Webster K., Zanotti K., Peterson G., Kulp B., Belinson J. Phase 2 trial of carboplatin plus tamoxifen in platinum-resistant ovarian cancer and primary carcinoma of the peritoneum. Gynecol Oncol. 2004;94:404–408. doi: 10.1016/j.ygyno.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Smyth J.F., Gourley C., Walker G., MacKean M.J., Stevenson A., Williams A.R., Nafussi A.A., Rye T., Rye R., Stewart M. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen receptor-positive patients. Clin Cancer Res. 2007;13:3617–3622. doi: 10.1158/1078-0432.CCR-06-2878. [DOI] [PubMed] [Google Scholar]
- 40.Hatch K.D., Beecham J.B., Blessing J.A., Creasman W.T. Responsiveness of patients with advanced ovarian carcinoma to tamoxifen. A Gynecologic Oncology Group study of second-line therapy in 105 patients. Cancer. 1991;68:269–271. doi: 10.1002/1097-0142(19910715)68:2<269::aid-cncr2820680209>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.Bowman A., Gabra H., Langdon S.P., Lessells A., Stewart M., Young A., Smyth J.F. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Res. 2002;8:2233–2239. [PubMed] [Google Scholar]
- 42.Rocereto T.F., Brady W.E., Shahin M.S., Hoffman J.S., Small L., Rotmensch J., Mannel R.S. A phase II evaluation of mifepristone in the treatment of recurrent or persistent epithelial ovarian, fallopian or primary peritoneal cancer: a gynecologic oncology group study. Gynecol Oncol. 2010;116:332–334. doi: 10.1016/j.ygyno.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 43.Rocereto T.F., Saul H.M., Aikins J.A., Jr., Paulson J. Phase II study of mifepristone (RU486) in refractory ovarian cancer. Gynecol Oncol. 2000;77:429–432. doi: 10.1006/gyno.2000.5789. [DOI] [PubMed] [Google Scholar]
- 44.Niwa K., Onogi K., Wu Y., Mori H., Harrigan R.C., Tamaya T. Clinical implication of medroxyprogesterone acetate against advanced ovarian carcinoma: a pilot study. Eur J Gynaecol Oncol. 2008;29:252–255. [PubMed] [Google Scholar]
- 45.Levine D., Park K., Juretzka M., Esch J., Hensley M., Aghajanian C., Lewin S., Konner J., Derosa F., Spriggs D. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer. 2007;110:2448–2456. doi: 10.1002/cncr.23072. [DOI] [PubMed] [Google Scholar]
- 46.Du B A., Meier W., Luck H.J., Emon G., Moebus V., Schroeder W., Costa S., Bauknecht T., Olbricht S., Jackisch C. Chemotherapy versus hormonal treatment in platinum- and paclitaxel-refractory ovarian cancer: a randomised trial of the German Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) Study Group Ovarian Cancer. Ann Oncol. 2002;13:251–257. doi: 10.1093/annonc/mdf038. [DOI] [PubMed] [Google Scholar]
- 47.Wu X., Subramaniam M., Negron V., Cicek M., Reynolds C., Lingle W.L., Goetz M.P., Ingle J.N., Spelsberg T.C., Hawse J.R. Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. J Cell Biochem. 2012;113:711–723. doi: 10.1002/jcb.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohammed H., Russell I.A., Stark R., Rueda O.M., Hickey T.E., Tarulli G.A., Serandour A.A., Birrell S.N., Bruna A., Saadi A. Progesterone receptor modulates ERalpha action in breast cancer. Nature. 2015;523:313–317. doi: 10.1038/nature14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237–249. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 50.Lau K.M., Mok S.C., Ho S.M. Expression of human estrogen receptor-alpha and -beta, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc Natl Acad Sci U S A. 1999;96:5722–5727. doi: 10.1073/pnas.96.10.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akahira J., Suzuki T., Ito K., Kaneko C., Darnel A.D., Moriya T., Okamura K., Yaegashi N., Sasano H. Differential expression of progesterone receptor isoforms A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors. Jpn J Cancer Res. 2002;93:807–815. doi: 10.1111/j.1349-7006.2002.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical Characteristics Stratified For Histopathologic Subtype