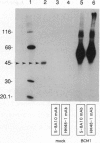
Abstract
We have recently described a murine lymphocyte protein, provisionally termed sgp-60, which is expressed on virtually all lymphocytes of both T- and B-cell origin. A hamster monoclonal antibody to sgp-60 can inhibit interleukin-2 (IL-2) production, IL-2-receptor expression, and T-cell proliferation, events normally observed after stimulation of T cells with an antibody to the T-cell receptor/CD3 complex or with the lectin concanavalin A. Our previous studies did not reveal the molecular nature of the sgp-60 antigen. Purification of sgp-60 and protein sequencing demonstrate that sgp-60 is identical to the CD48 antigen, a ligand for the CD2 antigen, which is also called Blast-1 in humans, BCM1 in mice, and OX-45 in rats. The identity of sgp-60 and CD48 was independently confirmed in gene transfection experiments. The anti-sgp-60 monoclonal antibody was selectively reactive with COS-7 cells transfected with a BCM1 cDNA clone but not with Kb-transfected controls. The results of the present report, together with our previous functional studies, may have implications for the role of CD2 and CD48 in murine T-cell activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvieux J., Willis A. C., Williams A. F. MRC OX-45 antigen: a leucocyte/endothelium rat membrane glycoprotein of 45,000 molecular weight. Mol Immunol. 1986 Sep;23(9):983–990. doi: 10.1016/0161-5890(86)90129-x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Freeman G. J., Gray G. S., Gimmi C. D., Lombard D. B., Zhou L. J., White M., Fingeroth J. D., Gribben J. G., Nadler L. M. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991 Sep 1;174(3):625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORER P. A. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950 Dec;4(4):372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- González Cabrero J., Reiser H. The sgp-60 molecule is linked to the plasma membrane via a glycosylphosphatidylinositol anchor. Eur J Immunol. 1991 Aug;21(8):1899–1903. doi: 10.1002/eji.1830210818. [DOI] [PubMed] [Google Scholar]
- Haustein D., Marchalonis J. J., Harris A. W. Immunoglobulin of T lymphoma cells. Biosynthesis, surface representation, and partial characterization. Biochemistry. 1975 May 6;14(9):1826–1834. doi: 10.1021/bi00680a004. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kato K., Koyanagi M., Okada H., Takanashi T., Wong Y. W., Williams A. F., Okumura K., Yagita H. CD48 is a counter-receptor for mouse CD2 and is involved in T cell activation. J Exp Med. 1992 Nov 1;176(5):1241–1249. doi: 10.1084/jem.176.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen N., Moessner R., Arvieux J., Willis A., Williams A. F. The MRC OX-45 antigen of rat leukocytes and endothelium is in a subset of the immunoglobulin superfamily with CD2, LFA-3 and carcinoembryonic antigens. EMBO J. 1988 Oct;7(10):3087–3091. doi: 10.1002/j.1460-2075.1988.tb03174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane W. S., Galat A., Harding M. W., Schreiber S. L. Complete amino acid sequence of the FK506 and rapamycin binding protein, FKBP, isolated from calf thymus. J Protein Chem. 1991 Apr;10(2):151–160. doi: 10.1007/BF01024778. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Razi-Wolf Z., Freeman G. J., Galvin F., Benacerraf B., Nadler L., Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4210–4214. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H., Benacerraf B. Costimulatory signal provided by a B-lymphoblastoid cell line and its Ia-negative variant. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10069–10073. doi: 10.1073/pnas.86.24.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H., Coligan J., Palmer E., Benacerraf B., Rock K. L. Cloning and expression of a cDNA for the T-cell-activating protein TAP. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2255–2259. doi: 10.1073/pnas.85.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H. sgp-60, a signal-transducing glycoprotein concerned with T cell activation through the T cell receptor/CD3 complex. J Immunol. 1990 Oct 1;145(7):2077–2086. [PubMed] [Google Scholar]
- Salinovich O., Montelaro R. C. Reversible staining and peptide mapping of proteins transferred to nitrocellulose after separation by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1986 Aug 1;156(2):341–347. doi: 10.1016/0003-2697(86)90263-0. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Fisher R. C., LeBeau M. M., Lawrence J. B., Barton D. E., Francke U., Dustin M., Thorley-Lawson D. A. Blast-1 possesses a glycosyl-phosphatidylinositol (GPI) membrane anchor, is related to LFA-3 and OX-45, and maps to chromosome 1q21-23. J Exp Med. 1989 Mar 1;169(3):1087–1099. doi: 10.1084/jem.169.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. W., Williams A. F., Kingsmore S. F., Seldin M. F. Structure, expression, and genetic linkage of the mouse BCM1 (OX45 or Blast-1) antigen. Evidence for genetic duplication giving rise to the BCM1 region on mouse chromosome 1 and the CD2/LFA3 region on mouse chromosome 3. J Exp Med. 1990 Jun 1;171(6):2115–2130. doi: 10.1084/jem.171.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita H., Nakamura T., Karasuyama H., Okumura K. Monoclonal antibodies specific for murine CD2 reveal its presence on B as well as T cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):645–649. doi: 10.1073/pnas.86.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Staunton D., Fisher R., Amiot M., Fortin J. J., Thorley-Lawson D. A. Expression of the Blast-1 activation/adhesion molecule and its identification as CD48. J Immunol. 1991 Apr 1;146(7):2192–2200. [PubMed] [Google Scholar]