Abstract
Background.
In older adults admitted to intensive care units (ICUs), frailty influences prognosis. We examined the relationship between the frailty index (FI) based on deficit accumulation and early and late survival.
Methods.
Older patients (≥65 years) admitted to a specialized geriatric ICU at the Liuhuaqiao Hospital, Guangzhou, China between July–December 2011 (n = 155; age 82.7±7.1 y; 87.1% men) were followed for 300 days. The FI was calculated as the proportion present of 52 health deficits. FI performance was compared with that of several prognostic scores.
Results.
The 90-day death rate was 38.7% (n = 60; 27 died within 30 days). The FI score was correlated with the Glasgow Coma Scale, Karnofsky Scale, Palliative Performance Scale, Acute Physiology Score—APACHE II and APACHE IV (r 2 = 0.52 to 0.72, p < 0.001). Patients who died within 30 days had higher mean FI scores (0.41±0.11) than those who survived to 300 days (0.22±0.11; F = 38.91, p < 0.001). Each 1% increase in the FI from the previous level was associated with an 11% increase in the 30-day mortality risk (95% CI: 7%–15%) adjusting for age, sex, and the prognostic scores. The FI discriminated patients who died in 30 days from those who survived with moderately high accuracy (AUC = 0.89±0.03). No one with an FI score >0.46 survived past 90 days.
Conclusion.
ICU survival was strongly associated with the level of frailty at admission. An FI based on health deficit accumulation may help improve critical care outcome prediction in older adults.
Key words: Frailty, Frailty index, Specialized geriatric intensive care unit, Older patients, Survival
Everywhere in the world, with aging, people accumulate more health problems. The more health problems they accumulate, the more difficult it is to recover to a healthier state, and the greater the risk of worsening and death (1). This increased risk is not the same for every older adult (2). People at greater risk compared with others of the same age are said to be frail (1–3). As populations age, the complexity of frailty challenges healthcare systems, especially intensive care provision (4–9).
The complexity of frailty arises from both the number of problems that are active simultaneously and the tendency for intervention in one system (eg, diuretics to improve heart function) to adversely impact other systems (1,4,10). For instance, a patient with respiratory failure needs mechanical ventilation to improve oxygen supply but the high PEEP improving oxygenation can decrease return blood volume, thus inducing hypotension. Likewise, needed antibiotics can wipe out normal gut flora, predisposing to opportunistic infections. Many frail older adults can only live at home with the care provided them by family members, whose understanding and expectations can vary, making care planning difficult (11,12).
These considerations commonly challenge contemporary management of critical illness (8,9,12–16). Briefly, intensive care units (ICUs) are challenged to understand which older adults are most likely to benefit (8,9,12). In these circumstances, considering frailty might help (1,3,4,9,13). Typically, ICUs employ several prognostic scores, focusing on acute episodes, consciousness, vital signs, and disease severity on admission. Most scores include age, but do not precisely assess comorbidity and prehospital functional status or disability, or heterogeneity of health status. Just as the degree of illness severity and organ compromise are measured by standard ICU prognostic scores, so too, in other settings, does the degree of frailty appear to influence the risk of adverse outcomes (1,4,17,18). In short, measuring not just the presence of frailty, but also its severity, might add value.
A frailty index (FI) measures health deficit accumulation. It demonstrates that people are frail when they have more things wrong: on average, the more things that someone has wrong with them, the more likely they are to die (1,4,19,20). Briefly, the FI counts an individual’s health problems, broadly defined by biological and clinical characteristics, and expresses this as the ratio of the deficits present in the person to the total number of deficits considered in a given setting. The FI shows characteristic behavior: an age specific increase; strong association with mortality risk; and a quantifiable limit, beyond which score few survive (20–23). These characteristics have been verified in multiple studies (24–27). Of note, in institutionalized and clinical patients who are seriously ill, the FI score is more important than age in predicting survival (28–30).
Even though the FI has been validated in acutely ill older adults admitted to hospital (1,28–31), and in prehospital care (32), less is known about its use in ICUs. Here, we hypothesized that ICU survival and overall mortality are closely related to the degree of frailty prior to ICU admission, so that people with higher FI scores are less likely to survive. The objectives were to: (a) construct an FI to evaluate the health status of older adults admitted to the ICU; and (b) examine the relationship between the FI score and survival to 30 and 300 days in comparison with several commonly employed ICU prognostic scores.
Methods
Participants and Setting
This is a prospective cohort study of patients admitted to a specialized Geriatric ICU at the Liuhuaqiao Hospital in Guangzhou, China. Founded in 1933, it grew out of the General Hospital of Guangzhou Military Command. Since 2009, it has been open to the general public, and named “Liuhuaqiao,” meaning “flowing flowers bridge.” The hospital has 60 departments and more than 1,800 ward beds. It treats all eligible patients, from the army or the community, following the same standards. Costs for retired military patients are fully covered by the army. Community patients are covered by social and/or variable levels of private insurance.
The 16-bed specialized Geriatric ICU, established in 2007, provides critical care for older patients with serious conditions. It addresses the challenges of providing care, especially to senior military officials, in a cultural expectation that filial piety is often best demonstrated by “doing everything” without regard to a patient’s age, prior level of function, or potential outcomes (13). Over 500 patients are admitted each year, chiefly for ventilation and pressor support. The specialized geriatric ICU has significantly improved survival of older adults, for example recording a 50% mortality reduction compared to when they were cared for in the general ICU.
Here, all ICU patients aged 65+ years admitted between July 2011 and December 2011 were evaluated (n = 155; including 114 retired military officials). Mortality data were collected up to November 1, 2012 through death certificates, decedent records, and medical records, or contact with the next of kin or other caregivers. Several scores commonly used in ICU and/or geriatric care settings were recorded, including the Glasgow Coma Scale (33), Karnofsky Performance Scale (34), Palliative Performance Scale (35), Acute Physiology Score (36), and Acute Physiology and Chronic Health Evaluation—APACHE-II (37) and APACHE-IV (38).
Constructing the FI
An FI was constructed for each patient, using variables drawn from ICU admission records of acute medical conditions, chronic diseases, symptoms, signs, premorbid function (from an informant), lifestyle, health attitude, psychological health, and laboratory measures. Following a standard procedure (39), all items which met the criteria for being counted as a deficit in the FI were selected. To be counted as a deficit, a variable should: be health related; be present in the general population in at least 1% of people, but in fewer than 80%; increase in prevalence with age in the general population; be related to death or other adverse health outcomes (eg, institutionalization), and; contain <5% missing data. Any FI should have >30 variables, covering several organ systems (39). Premorbid status (eg, mobility and dependence scores) was defined as the average performance 1 month prior to admission.
Fifty-two variables satisfied these criteria (Supplementary Appendix 1). Thirty-six patients had missing values for 1–2 variables (1.9%–3.8%). Of the 13 variables with any missing values, most were missing in just 1 or 2 patients; two variables were missing a maximum of 9 cases (4.3%). Missing values were replaced using the variable’s nonmissing mean. To better understand which items most influenced FI behavior, we also subdivided it into a chronic FI (n = 23 items) and an acute FI (n = 31 items) (Supplementary Appendix 1). The variables are similar to those used in related publications (20–27). The ICU risk scores typically did not contain neuropsychiatric items, as these are not addressed in ICU guidelines (Supplementary Appendix 2).
Among the 52 variables, 15 had 2 levels (absent/present), 6 had 3 levels, 2 had 5 levels, 1 had 7 levels, and the remaining 31 were continuous. Each variable was recoded to a value between 0 and 1, that is, “1” indicating the highest level of a problem and “0” its absence. Cut-point selection for recoding was based on established criteria and the diagnostic recommendations used in the hospital (Supplementary Appendix 1). An FI value was calculated for each subject as the proportion of deficits present (ie, all deficits summed and divided by 52). Theoretically, FI values can range from 0 (no deficits present) to 1 (all deficits present). Even so, an FI > 0.7 is seldom (<1%) observed; this appears to represent a natural limit to frailty, beyond which survival is not possible (1,22,23). Higher FI values indicate a greater level of frailty, and thus worse health and greater vulnerability to adverse outcomes, and vice versa. Variable selection, grouping, and recoding were performance prior to FI construction and further statistical analyses.
Statistical Analysis
Data are presented as means and standard deviations for continuous variables and percentages for discrete variables. Analysis of variance and chi-square (χ 2) tests were used respectively to compare group differences in the means or percentages. To evaluate the relationship between each measure and survival, logistic regression was used to calculate the likelihood and 95% confidence intervals (CIs) of early death (less than 30 days) versus survival to the end of the study period (300 days). The Akaike information criterion was calculated for each score based on maximum likelihood from logistic regression to evaluate the relative quality of the model fitting. Cox proportional hazard models were employed to estimate the age/sex adjusted survival probability. FI values were graded to 0–100 integers by rounding the FI after multiplying it by 100, so as to evaluate the change in risk seen with each percent increment of the FI and other measures. To evaluate the value added by the FI, the FI and each score was tested in separate models, as well as in the same model. The all factor FI and the acute or chronic FI were also included in the Cox proportional hazard model to test which index explained more variability when adjusting for each other. Performance of the scores in classifying patients who died and who survived was assessed by the area under receiver operating characteristic curves (Supplementary Appendix 3 details the sensitivity and specificity at various cut points). Kaplan Meier survival probabilities were evaluated using each score, stratified to assay clinically useful cut-points (ie, 30% subsample with the best score, 10% with the worst, and two levels in between).
Data analyses were conducted using SPSS version 19.0 and codes developed using Matlab v8. Statistical significance level was set at p = 0.05.
Ethics
Data collection was approved by the Guangzhou Liuhuaqiao Hospital authority. Informed consent was collected from each participant or next of kin. The data analysis protocol was approved by the Research Ethics Board at the Capital District Health Authority, where the analyses took place.
Results
Most patients were married (91.6%) men (87.1%) who lived with family members (99.4%). The mean age was 82.7±7.1 years (median: 84; range: 65–103). During the 300-day follow-up, 60 patients died (38.7%), among whom 27 (45.0%) died within the first 30 days. Most (53) of the 60 deaths occurred in hospital, two in long-term care institutions, and five at home. Notably, on average people who died were not older than those who survived; instead, they had higher FIs (poorer health) and worse prognostic scores (Table 1). Most patients (n = 139, 89.7%) had multiple deficits, with sepsis, hypertension, coronary heart disease, diabetes, dementia, and renal dysfunction being most common. When considered individually, 29 of the 52 deficits used in the FI were significantly associated with death (p < 0.05). Of the remaining 23 deficits, 16 were more often present in those who died (Supplementary Appendix 1).
Table 1.
Characteristics of the Sample at Admission, by ICU Survival Outcome
| Variable | Died | Survived 300+ days | F/Chi2 | p | |
|---|---|---|---|---|---|
| In 30 d | In 31–300 d | ||||
| N | 27 | 33 | 95 | ||
| Age (year) | 82.4 (7.2) | 84.0 (6.6) | 82.3 (7.1) | 0.75 | 0.473 |
| Male (%) | 88.9 | 90.9 | 85.3 | 0.79 | 0.674 |
| Married (%) | 85.2 | 93.9 | 92.6 | 1.81 | 0.404 |
| Comorbidity (%) | 96.3 | 75.8 | 92.6 | 9.08 | 0.011 |
| Hypertension | 51.9 | 36.4 | 68.4 | 11.02 | 0.004 |
| Coronary disease | 55.6 | 30.3 | 42.1 | 3.89 | 0.143 |
| Diabetes | 25.9 | 30.3 | 27.4 | 0.16 | 0.924 |
| Hemiplegia | 22.2 | 27.3 | 17.9 | 1.36 | 0.505 |
| Chronic pulmonary disease | 25.9 | 21.2 | 13.7 | 2.61 | 0.271 |
| Chronic heart/renal insufficiency | 18.5 | 3.0 | 8.4 | 4.45 | 0.108 |
| Dementia | 11.1 | 12.1 | 4.2 | 3.12 | 0.210 |
| Cancer | 14.8 | 9.1 | 1.1 | 9.46 | 0.009 |
| Comorbidities, others | 22.2 | 12.1 | 16.8 | 1.09 | 0.581 |
| Respiratory disease | 40.7 | 63.6 | 16.8 | 26.55 | <0.001 |
| Cardiovascular disease | 29.6 | 15.2 | 31.6 | 3.36 | 0.187 |
| Cardiovascular postoperative | 7.4 | 3.0 | 27.4 | 12.29 | 0.002 |
| Sepsis | 48.1 | 21.2 | 6.3 | 26.95 | <0.001 |
| Digestive disease | 3.7 | 3.0 | 7.4 | 1.11 | 0.575 |
| Neurologic disease | 3.7 | 3.0 | 6.3 | 0.68 | 0.711 |
| Urologic disease | 7.4 | 9.1 | 2.1 | 3.41 | 0.182 |
| Other diagnose | 7.4 | 6.1 | 8.4 | 0.20 | 0.907 |
| Mechanical ventilation | 81.5 | 54.5 | 11.6 | 55.43 | <0.001 |
| Analgesia with deep vein catheter | 96.3 | 72.7 | 29.5 | 45.97 | <0.001 |
| Continuous veno-venous hemofiltration | 63.0 | 24.2 | 7.40 | 40.00 | <0.001 |
| Glasgow Coma Scale (GCS) (/15)* | 9.5 (4.0) | 11.0 (3.4) | 13.5 (2.6) | 21.06 | <0.001 |
| Karnofsky Scale KS (/100)* | 32.6 (15.3) | 43.0 (16.1) | 67.8 (20.3) | 48.03 | <0.001 |
| Palliative Performance Scale PPS (/100)* | 24.1 (15.8) | 36.1 (17.1) | 61.3 (20.0) | 52.34 | <0.001 |
| Acute Physiology Score APS (/252)† | 50.4 (25.3) | 31.7 (16.7) | 17.7 (14.4) | 40.00 | <0.001 |
| APACHE II (/71)† | 20.7 (5.9) | 16.8 (5.8) | 12.1 (5.7) | 26.57 | <0.001 |
| APACHE IV (/299) | 70.0 (25.6) | 52.3 (18.3) | 37.1 (14.6) | 38.60 | <0.001 |
| Chronic FI (23 items) (/1.00)† | 0.48 (0.15) | 0.43 (0.17) | 0.29 (0.15) | 22.88 | <0.001 |
| Acute FI (31 items) (/1.00)† | 0.36 (0.12) | 0.27 (0.08) | 0.17 (0.11) | 36.38 | <0.001 |
| All-factor FI (52 items) (/1.00)† | 0.41 (0.11) | 0.34 (0.11) | 0.22 (0.11) | 38.91 | <0.001 |
Notes: Data are presented as mean (standard deviation), unless as stated otherwise. (APACHE = Acute Physiology and Chronic Health Evaluation)
*A lower score is associated with poorer outcomes.
†A higher score is associated with poorer outcomes.
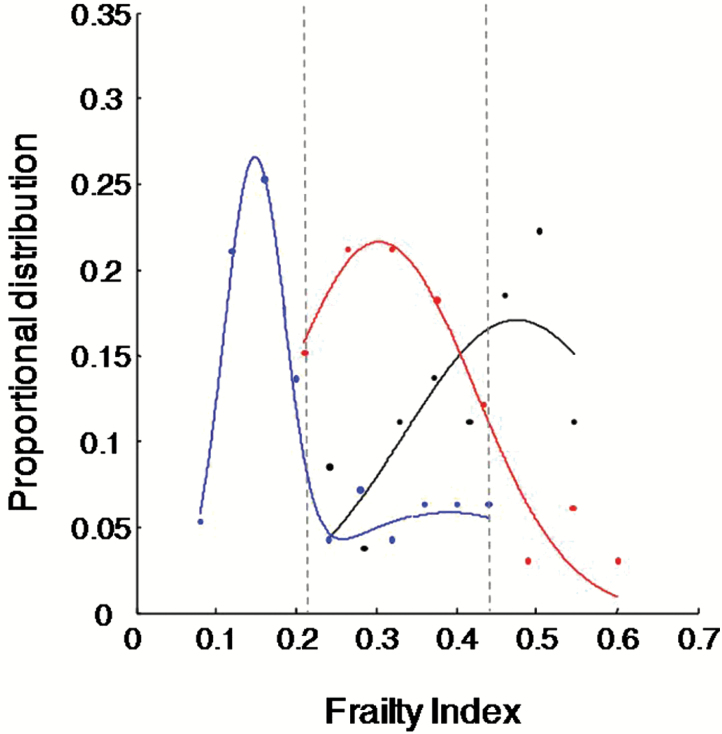
When deficits were considered collectively, the FI score ranged 0.06–0.63. The FI score was correlated with each prognostic score, that is, Glasgow Coma Scale, Karnofsky Performance Scale, Palliative Performance Scale, Acute Physiology Score, and Acute Physiology and Chronic Health Evaluation—APACHE-II and APACHE-IV (r 2 ranged between 0.52 and 0.72, ps < 0.001), and varied significantly by survival status (Figure 1): higher in patients who died within 30 days (FI = 0.41±0.11) than in the survivors (FI = 0.22±0.14, F = 38.91, p < 0.001; Table 1). All patients with FI scores < 0.22 (n = 62 or 40%) survived 30 days, whereas all patients with FI scores >0.46 (n = 15 or 10%) died within 90 days (Figure 1; maximum survival = 66 days). The AUC was 0.89 (95% CI = 0.83–0.95) for the FI in classifying individuals who died within 30 days against those who survived, comparable with the prognostic scores (Table 2). Including the FI in the same model with a prognostic score often led to an improved Akaike information criterion or AUC, or both (Table 2).
Figure 1.
Proportional distribution of the frailty index (FI) by survival status of patients admitted to the specialized geriatric ICU. Symbols (dots) represent observational data; lines represent curves fit to the data. Blue: patients who survived 300 days; red: died between 31 and 300 days; black: died within 30 days. The FI ranged from 0.06 to 0.63. Here, all patients who died had FI > 0.46 (n = 15), whereas all patients who survived 30 days had FI < 0.22 (n = 63).
Table 2.
Akaike Information Criterion (AIC) and Area Under the Curve (AUC) for the Risk of Death using Each Score with (Upper Lines) or Without (Lower Lines) Including the All-Factor Frailty Index in the Model
| Score | Died in 30 d versus survived 300+ days (n = 27 vs 95) | |||||
|---|---|---|---|---|---|---|
| AIC | AUC | Std. Error | 95% CI | p | ||
| Lower | Upper | |||||
| GCS | 115.50 | 0.79 | 0.05 | 0.69 | 0.90 | <0.001 |
| GCS + FI | 93.08 | 0.89 | 0.03 | 0.83 | 0.95 | <0.001 |
| KS | 100.34 | 0.90 | 0.03 | 0.84 | 0.96 | <0.001 |
| KS + FI | 75.96 | 0.90 | 0.03 | 0.84 | 0.96 | <0.001 |
| PPS | 94.12 | 0.91 | 0.03 | 0.85 | 0.97 | <0.001 |
| PPS + FI | 72.18 | 0.92 | 0.03 | 0.86 | 0.97 | <0.001 |
| APS | 170.37 | 0.89 | 0.03 | 0.82 | 0.95 | <0.001 |
| APS + FI | 136.59 | 0.89 | 0.03 | 0.83 | 0.95 | <0.001 |
| Acute Physiology and Chronic Health Evaluation (APACHE-II) | 158.81 | 0.86 | 0.04 | 0.79 | 0.93 | <0.001 |
| APACHE II + FI | 159.96 | 0.92 | 0.03 | 0.86 | 0.97 | <0.001 |
| APACHE-IV | 161.92 | 0.88 | 0.04 | 0.81 | 0.95 | <0.001 |
| APACHE IV + FI | 163.57 | 0.93 | 0.03 | 0.87 | 0.98 | <0.001 |
| Chronic FI (23 items) | 158.21 | 0.82 | 0.04 | 0.73 | 0.90 | <0.001 |
| Chronic FI + FI | 155.65 | 0.89 | 0.03 | 0.83 | 0.95 | <0.001 |
| Acute FI (31 items) | 165.31 | 0.87 | 0.03 | 0.81 | 0.94 | <0.001 |
| Acute FI + FI | 157.50 | 0.90 | 0.03 | 0.83 | 0.96 | <0.001 |
| All-factor FI (52 items) | 161.60 | 0.89 | 0.03 | 0.83 | 0.95 | <0.001 |
Notes: AIC = Akaike information criterion; APACHE = Acute Physiology and Chronic Health Evaluation; APS = Acute Physiology Score; AUC = Area under the ROC curve; CI = Confidence Interval; FI = the 52-item all-factor frailty index; GCS = Glasgow Coma Scale; KS = Karnofsky Scale; PPS = Palliative Performance Scale; p = level of Significance.
In an age-sex adjusted model, the FI was associated with an increased risk of death (with each 1% increase in the FI from the previous level, for example, from 0.18 to 0.19, the relative risk ratio for 30-day death =1.11, 95% CI = 1.07–1.15). Similarly, the FI was also associated with the risk of 300-day mortality (Supplementary Appendix 4). As a worked example, people of the same age and sex would be nine times more likely to die within 30 days if the FI at admission was 0.38 than if it was 0.18 (ie, e[0.11*(0.38–0.18)*100] = 9.02; Table 3, Model 1). The risk ratio for the FI remained significant when the ICU scores were also adjusted for in the models (p < 0.05), whereas age was not, nor the Glasgow Coma Scale or Acute Physiology and Chronic Health Evaluation (Table 3, Model 2). The acute and chronic FIs showed lower predictive values than did the all-factor FI (Table 3).
Table 3.
Cox Proportional Hazard Ratio for the Risk Using the Frailty Index and the Prognostic Scores
| Model 1. Each score adjusted by age and sex | ||||||
|---|---|---|---|---|---|---|
| Score | Died in 30 d versus survived 300+ days (n = 27 vs 95) | |||||
| B | Exp (B) | Wald | 95% CI | p | ||
| Lower | Upper | |||||
| GCS | −0.24 | 0.78 | 27.42 | 0.72 | 0.86 | <0.001 |
| KS | −0.07 | 0.93 | 32.74 | 0.91 | 0.96 | <0.001 |
| PPS | −0.07 | 0.94 | 34.52 | 0.91 | 0.96 | <0.001 |
| APS | 0.05 | 1.05 | 50.75 | 1.04 | 1.07 | <0.001 |
| APACHE II | 0.15 | 1.17 | 29.55 | 1.10 | 1.23 | <0.001 |
| APACHE IV | 0.05 | 1.05 | 50.75 | 1.04 | 1.07 | <0.001 |
| Chronic FI (23 items) | 0.06 | 1.06 | 23.98 | 1.04 | 1.09 | <0.001 |
| Acute FI (31 items) | 0.09 | 1.09 | 36.42 | 1.06 | 1.13 | <0.001 |
| All-factor FI (52 items) | 0.10 | 1.11 | 34.13 | 1.07 | 1.15 | <0.001 |
| Model 2. Each score, adjusted by age, sex, and the frailty index | ||||||
| Models | Died in 30 d versus survived 300+ days (n = 27 vs 95) | |||||
| B | Exp (B) | Wald | 95% CI | p | ||
| Lower | Upper | |||||
| Age | −0.02 | 0.98 | 0.61 | 0.94 | 1.03 | 0.435 |
| Sex | 0.03 | 1.03 | 0.00 | 0.28 | 3.81 | 0.960 |
| GCS | −0.07 | 0.93 | 1.34 | 0.82 | 1.05 | 0.247 |
| All-factor FI (52 items) | 0.09 | 1.09 | 19.12 | 1.05 | 1.14 | 0.000 |
| Age | −0.02 | 0.98 | 1.00 | 0.93 | 1.02 | 0.316 |
| Sex | 0.00 | 1.00 | 0.00 | 0.28 | 3.66 | 0.994 |
| KS | −0.05 | 0.95 | 13.79 | 0.92 | 0.97 | 0.000 |
| All-factor FI (52 items) | 0.05 | 1.05 | 6.55 | 1.01 | 1.10 | 0.011 |
| Age | −0.03 | 0.97 | 1.32 | 0.93 | 1.02 | 0.251 |
| Sex | 0.04 | 1.04 | 0.00 | 0.28 | 3.79 | 0.954 |
| PPS | −0.05 | 0.95 | 16.26 | 0.92 | 0.97 | 0.000 |
| All-factor FI (52 items) | 0.05 | 1.05 | 7.24 | 1.01 | 1.10 | 0.007 |
| Age | 0.00 | 1.00 | 0.03 | 0.95 | 1.04 | 0.868 |
| Sex | −0.15 | 0.86 | 0.06 | 0.24 | 3.09 | 0.813 |
| APS | 0.03 | 1.03 | 6.72 | 1.01 | 1.05 | 0.010 |
| All-factor FI (52 items) | 0.06 | 1.06 | 5.07 | 1.01 | 1.11 | 0.024 |
| Age | −0.02 | 0.98 | 0.65 | 0.94 | 1.03 | 0.419 |
| Sex | −0.14 | 0.87 | 0.04 | 0.23 | 3.32 | 0.842 |
| APACHE-II | 0.05 | 1.05 | 1.88 | 0.98 | 1.14 | 0.170 |
| All-factor FI (52 items) | 0.08 | 1.09 | 14.09 | 1.04 | 1.14 | 0.000 |
| Age | −0.02 | 0.99 | 0.47 | 0.94 | 1.03 | 0.494 |
| Sex | −0.13 | 0.88 | 0.04 | 0.24 | 3.16 | 0.840 |
| Apache-IV | 0.03 | 1.03 | 7.01 | 1.01 | 1.06 | 0.008 |
| All-factor FI (52 items) | 0.05 | 1.06 | 4.64 | 1.00 | 1.11 | 0.031 |
| Age | −0.01 | 0.99 | 0.07 | 0.95 | 1.04 | 0.794 |
| Sex | −0.17 | 0.85 | 0.06 | 0.22 | 3.23 | 0.806 |
| Chronic FI (23 items) | −0.03 | 0.97 | 1.22 | 0.92 | 1.02 | 0.269 |
| All-factor FI (52 items) | 0.13 | 1.14 | 15.97 | 1.07 | 1.22 | 0.000 |
| Age | −0.01 | 0.99 | 0.11 | 0.95 | 1.04 | 0.739 |
| Sex | −0.11 | 0.90 | 0.02 | 0.24 | 3.42 | 0.877 |
| Acute FI (31 items) | 0.03 | 1.03 | 0.64 | 0.96 | 1.09 | 0.424 |
| All-factor FI (52 items) | 0.08 | 1.08 | 4.59 | 1.01 | 1.16 | 0.032 |
Notes: APACHE = Acute Physiology and Chronic Health Evaluation; APS = Acute Physiology Score; CI = Confidence Interval; FI = the 52-item all-factor frailty index; GCS = Glasgow Coma Scale; KS = Karnofsky Scale; PPS = Palliative Performance Scale; p = level of Significance.
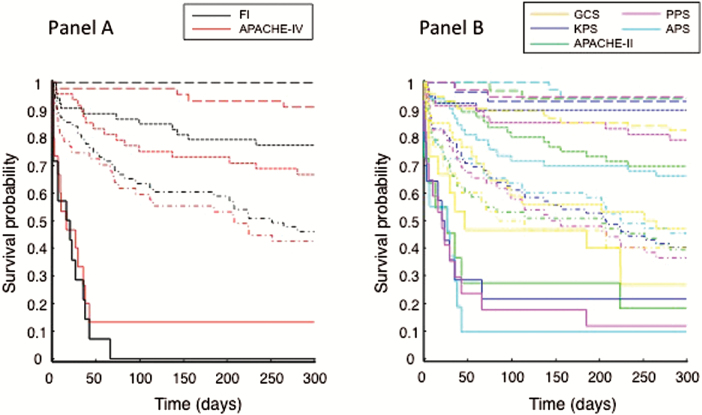
A robust dose-responsive survival probability was observed with the FI, as with most of the prognostic scores (Figure 2A,B). The 15 patients with FI > 0.46 and 100% mortality were not so identified using other scores (Figure 2A,B). Five of these 15 patients did not also have the worst APACHE-IV scores, that is, the two best-performing scores identified different subsets of patients with the highest risks of death.
Figure 2.
Kaplan Meier survival probability (KM) of patients admitted to the specialized geriatric ICU as a function of time (days). Each color represents the survival curves of a separate score. Panel A: black—frailty index; red—APACHE-IV (Acute Physiology and Chronic Health Evaluation IV). Panel B: yellow—GCS (Glasgow Coma Scale); blue—KPS (Karnofsky Performance Scale); purple—PPS (Palliative Performance Scale); cyan—APS (Acute Physiology Score); green—APACHE-II (Acute Physiology and Chronic Health Evaluation). For each score, survival data were stratified into four clinically useful groups; ie, 30% subsample with the best score (dashed lines), 10% with the worst score (solid lines), and two levels in between representing 30% subsamples each (dotted lines and dot-dashed lines).
Discussion
We evaluated whether frailty was related to survival in older people admitted to a specialized geriatric ICU, and compared an FI, with several commonly used ICU prognostic scores. All were strongly associated with survival, and the performance of the FI was at least as good as any ICU score. In addition, the FI identified a group of people who were least frail (with FI < 0.22; corresponding to 40% of the sample) all of whom survived 30 days, and a least fit group (FI > 0.46; 10% of the sample), none of whom no one survived beyond 66 days. In short, the degree of frailty, as estimated by both acute deficits and information gathered on function, mobility, and health attitude 1 month prior to admission, was closely related to critical care survival in older adults.
Our data contribute to understanding whether knowledge about the extent of premorbid deficit accumulation adds prognostic value in acutely ill patients (28–32). An important part of understanding prognosis is in needing to advise people about near-term outcomes of hospital admission for frail older adults who are acutely ill (28–30,40). For this reason, examining older patients who died within days to months is of particular interest. Here, the FI showed that the subset patients with the worst FI scores all died, whereas the subset with the best scores all survived. Such complete mortality was not predicted using any other scores. Clinically, it is often the prediction of the best and worst outcomes that is most valuable in knowing how to gauge expectations. By contrast, considerable uncertainty typically accords any intermediate score, so impacts less on care options. That the high-risk subsets from the FI and APACHE-IV (the “best” performing prognostic scores) were not identical, suggests that older adults who died quickly could have heterogeneous profiles, not always identified now. For example, here, an APACHE-IV score as low as 60 (indicating low risk) was observed in two people whose FI > 0.46 (indicating high risk) and who did not survive. This brings to attention the potential value in taking frailty into account when assessing critically ill older patients.
By using the FI and the risk scores in the same model, we were able to address whether the FI added value to the ICU scores. The FI allows many pieces of data to contribute information, even when some deficits are not individually significant (1). Further, the all-item FI includes 33 items that were not considered by APACHE IV, and 35 items not considered by the Acute Physiology Score. Although the standard ICU scores shared certain items with the FI, they notably omitted essential information about premorbid function. Here, the AUC changed variably with use of the FI, to a high level between 0.89 and 0.93. While an increase of AUC was observed in most cases, the Kaplan Meier AUC remained 0.90 even though Akaike information criterion was lower. This likely suggests the following: (a) FI helps improve performance, especially when it was not high originally; (b) to better understand the data, different analysis approaches may allow examinations from different angles; and (c) information imbedded in the data can determine the accuracy limit of a model, reflected by the <100% accuracy by using multiple models.
The redundancy of the human body makes it unlikely that two summary scores would assess risk and be completely independent, even with no overlap in their items. Indeed, this redundancy allows for different versions of the FI—using differing items, differing numbers of items, and items of differing natures (eg, self-reported vs clinical assessments)—to give similar results (41). Likewise, the items that make up an FI can be sampled at random without loss of explanatory power (42). The results suggest that the all-item FI explained more variability compared to the partial ones; by taking into account the accumulation of a large number of deficits, each can reflect an aspect of the system and thus add information to understanding the data.
How to best incorporate frailty in the ICU setting is challenging, given that older patients needing critical care typically have complex health conditions, and that multiple, interacting problems can vary between individuals. Short-term mortality in older survivors arises in relation not just to their acute illness, but to their overall state of health, which is what the FI quantifies (1,3,43,44). Multiple deficits in the same individuals challenge modern healthcare systems, which have experienced enormous progress (and train people for, are reimbursed and evaluated) using a paradigm of single system illness and “most responsible” diagnoses (3,45). Surrogate measures of physiologic age offer the ability to stratify the risk for rapid deterioration related to a major event. Here, the FI combined the effects of multiple problems in a single, graded variable that was easily operationalized from routine ICU assessments and combined them with clinically relevant information that often is known by informants. Using such instruments is part of a shift in how prognostic factors for ICU outcomes are evaluated (9). Similarly, some measures now commonly employed in ICUs might inform acute care of frail older adults in geriatric medicine (43). Such interplay is part of the rationale for specialized geriatric intensive care, which offers a range of approaches—for example early mobilization (15) and playing attention to sleep (16)—that can make routine care less hazardous.
Our data also contribute to understanding frailty. Here, despite the high prevalence of critical illness, the FI remained closely related to the risk of death, consistent with other observations in seriously ill patients (1,28–30). The limit to frailty (ie, FI < 0.7) notably held. In short, this study joins virtually all others, including from community-dwelling older Chinese adults (22,24), in demonstrating a quantifiable extent to how many things an individual can have wrong with them and survive. Such a submaximal limit is shared with many other frailty measures, suggesting that locally adapted scales can capture this important feature. This warrants further study, especially when frailty is measured across cultures.
Our study has important limitations. First, this is a single centre study; whether the conclusion is generalizable warrants multicentre investigations. Second, reflecting historical and social circumstances, the sample consists mostly of men, and many from the military. Previous studies have shown close relationships between age and the FI and between FI and mortality in both men and women (1). Third, our analysis has centred on 30-day mortality, and even though the 300-day data demonstrated similar trends, these might not hold for other follow-up periods. Constructing an FI can be a significant amount of work, especially recoding variables. Furthermore, as is common even with research studies, there were missing values for some variables, which can affect generalizability. Here, our data showed consistent features in other ICU samples; for example, the high proportion with comorbidities was also seen in a large study where 79.4% of ICU patients had comorbidities (46).
Even though our study suggests that the FI can help improve the performance of the various common ICU scores, further research will be needed to address which scores are most valid for mortality prediction in ICU. Here, the Karnovsky performance scale was compared with the FI because, unlike the other scores, in many parts of China it used routinely.
We focused on understanding 30-day death chiefly because short-term survival is most critical for ICU outcome evaluation. For survival outcome analysis, the most updated health status has been shown to be more important than prior health states, making 30-day mortality a way to understand overall outcomes in this group, where there is net decline: that is, on average, decline outweighs recovery (21). Recognizing that any net positive score is likely to be early, we have also examined mortality over 300 days in relation to the risk scores and the trend of the results held true (Supplementary Appendix 4). Note too, although death was readily verifiable, survival might be less so, given that some families have a financial interest in not reporting death immediately, as older adults receive social benefits. As such, the actual long-term death rate could be higher. This is a less serious concern for short-term mortality as most patients (n = 138, 89.0%) remained in hospital/institution, and deaths were recorded immediately as they occurred.
With its very large population of older adults, understanding frailty in China is important in its own right. Health deficits, even when including acute illness, are only one part of what puts people at risk: the social and physical environment is also important, something not directly measured here. Previous research has suggested that the FI is robust to exactly which variables are used in its construction (1). Even so, in the critical care setting, this must be established formally through larger studies.
Conclusion
Older patients needing critical care are typically both critically ill and also often frail, which collectively determine their outcomes. Our study examined the value of the health deficit accumulation-based FI in a specialized geriatric ICU. The data showed that the greater the degree of frailty, the greater the risk of death. Here, the frailest group of patients (with the highest FI scores) showed 100% mortality, some of whom were not identified by the other commonly used measures, which do not focus on older adults. Our study also verified that the empirical limit to deficit accumulation (FI ~ 0.7) also applies to seriously ill older adults: when the limit of frailty is reached, death occurs within days to months. In short, our data showed a close association between frailty and ICU survival, and suggest that the FI can add value to commonly used ICU prognostic scores. Paying attention to premorbid health and function can help care providers better estimate the likelihood of benefit from ICU admission. In particular, since it is often difficult to make people better than they were a month before they became ill, that level of function might serve as a benchmark for individual care planning. Both considerations are motivating further inquires by our group.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This research was partially supported by the China-Canada Joint Health Research Initiative Grant from the Canadian Institutes of Health Research (CIHR CCI-92216), and operating grants from the National Natural Science Foundation of China (E0603-59976016; 61300107), Chinese Military Medicine and Health Foundation (119-10BJZ09), Natural Science Foundation of Guangdong (S2012010010212), Science and Technology Planning Project of Guangdong, China (2012A061400010), Science and Technology Planning Project of Guangzhou, China (201505031501397; 201504301341059), and Guangdong Key Laboratory of Geriatric Infection and Organ Function. Additional funding for analysis came from the Fountain Innovation Fund of the Queen Elizabeth II Health Sciences Research Foundation, Halifax, Canada.
Supplementary Material
References
- 1. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi:10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 2. Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. doi:10.2307/2061224 [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/Gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi:10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray SL, Anderson ML, Hubbard RA, et al. Frailty and incident dementia. J. Gerontol A Biol Sci Med Sci. 2013;68:1083–1090. doi:10.1093/gerona/glt013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E95–102. doi:10.1503/cmaj.130639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Maguet P, Roquilly A, Lasocki S, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi:10.1007/s00134-014-3253-4 [DOI] [PubMed] [Google Scholar]
- 8. McDermid RC, Bagshaw SM. Scratching the surface: the burden of frailty in critical care. Intensive Care Med. 2014;40:740–742. doi:10.1007/s00134-014-3246-3 [DOI] [PubMed] [Google Scholar]
- 9. Baldwin MR. Measuring and predicting long-term outcomes in older survivors of critical illness. Minerva Anestesio. 2014 Jun 13. [Epub ahead of print]. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4375061/ [PMC free article] [PubMed] [Google Scholar]
- 10. Bibas L, Levim M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–143. doi:10.1016/j.pcad.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 11. Rockwood K, Guo Z, Song X. The frail older adult and the intensive care unit. Chin J Geriat. 2013;32:3–8. [Google Scholar]
- 12. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi:10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 13. Ricou B, Merlani P. What limits for acute care in the elderly? Curr Opin Anaesthesiol. 2008;21:380–385. doi:10.1097/ACO.0b013e3283007b91 [DOI] [PubMed] [Google Scholar]
- 14. Balas MC, Burke WJ, Gannon D, et al. Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU pain, agitation, and delirium guidelines. Crit Care Med. 2013;41(9 Suppl 1):S116–S127. doi:10.1097/CCM.0b013e3182a17064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Engel HJ, Needham DM, Morris PE, Gropper MA. ICU early mobilization: from recommendation to implementation at three medical centers. Crit Care Med. 2013;41(9 Suppl 1):S69–S80. doi:10.1097/CCM.0b013e3182a240d5 [DOI] [PubMed] [Google Scholar]
- 16. Sterniczuk R, Rusak B, Rockwood K. Sleep disturbance in older ICU patients. Clin Interv Aging. 2014;9:969–977. doi:10.2147/CIA.S59927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sourial N, Bergman H, Karunananthan S, et al. Implementing frailty into clinical practice: a cautionary tale. J Gerontol A Biol Sci Med Sci. 2013;68:1505–1511. doi:10.1093/gerona/glt053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hubbard JM, Jatoi A. Incorporating biomarkers of frailty and senescence in cancer therapeutic trials. J Gerontol A Biol Sci Med Sci. 2014 Apr 26. [Epub ahead of print]. doi:10.1093/gerona/glu046 [DOI] [PubMed] [Google Scholar]
- 19. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. doi:10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59:M627–M632. doi:10.1093/genora/59.6.M627 [DOI] [PubMed] [Google Scholar]
- 21. Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183:E487–E494. doi:10.1503/cmaj.101271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennett S, Song X, Mitnitski A, Rockwood K. A limit to frailty in very old, community-dwelling people: a secondary analysis of the Chinese longitudinal health and longevity study. Age Ageing. 2013;42:372–377. doi:10.1093/ageing/afs180 [DOI] [PubMed] [Google Scholar]
- 23. Armstrong JJ, Mitnitski A, Launer LJ, White LR, Rockwood K. Frailty in the Honolulu-Asia aging study: deficit accumulation in a male cohort followed to 90% mortality. J Gerontol A Biol Sci Med Sci. 2015;70:125–131. doi:10.1093/gerona/glu089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi J, Yang Z, Song X, et al. Sex differences in the limit to deficit accumulation in late middle-aged and older Chinese people: results from the Beijing longitudinal study of Aging. J Gerontol A Biol Sci Med Sci. 2014;69:702–709. doi:10.1093/gerona/glt143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular health study. J Am Geriatr Soc. 2008;56:898–903. doi:10.1111/j.1532-5415.2008.01656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Theou O, Brothers TD, Rockwood MR, Haardt D, Mitnitski A, Rockwood K. Exploring the relationship between national economic indicators and relative fitness and frailty in middle-aged and older Europeans. Age Ageing. 2013;42:614–619. doi:10.1093/ageing/aft010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang C, Song X, Mitnitski A, et al. Gender differences in the relationship between smoking and frailty: results from the Beijing longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2013;68:338–346. doi:10.1093/gerona/gls166 [DOI] [PubMed] [Google Scholar]
- 28. Krishnan M, Beck S, Havelock W, Eeles E, Hubbard RE, Johansen A. Predicting outcome after hip fracture: using a frailty index to integrate comprehensive geriatric assessment results. Age Ageing. 2014;43:122–126. doi:10.1093/ageing/aft084 [DOI] [PubMed] [Google Scholar]
- 29. Evans SJ, Sayers M, Mitnitski A, Rockwood K. The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing. 2014;43:127–132. doi:10.1093/ageing/aft156 [DOI] [PubMed] [Google Scholar]
- 30. Dent E, Chapman I, Howell S, Piantadosi C, Visvanathan R. Frailty and functional decline indices predict poor outcomes in hospitalised older people. Age Ageing. 2014;43:477–484. doi:10.1093/ageing/aft181 [DOI] [PubMed] [Google Scholar]
- 31. Pilotto A, Rengo F, Marchionni N, et al. ; FIRI-SIGG Study Group. Comparing the prognostic accuracy for all-cause mortality of frailty instruments: a multicentre 1-year follow-up in hospitalized older patients. PLoS One. 2012;7:e29090. doi:10.1371/journal.pone.0029090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldstein J, Travers A, Hubbard R, Moorhouse P, Andrew MK, Rockwood K. Assessment of older adults by emergency medical services: methodology and feasibility of a care partner comprehensive geriatric assessment (CP-CGA). CJEM. 2014;16:1–14. http://www.ncbi.nlm.nih.gov/pubmed/24456751 [PubMed] [Google Scholar]
- 33. Bastos PG, Sun X, Wagner DP, Wu AW, Knaus WA. Glasgow coma scale score in the evaluation of outcome in the intensive care unit: findings from the acute physiology and chronic health evaluation III study. Crit Care Med. 1993;21:1459–1465. doi:10.1097/00003246-199310000-00012 [DOI] [PubMed] [Google Scholar]
- 34. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–193. http://www.ncbi.nlm.nih.gov/pubmed/6699671 [DOI] [PubMed] [Google Scholar]
- 35. Olajide O, Hanson L, Usher BM, Qaqish BF, Schwartz R, Bernard S. Validation of the palliative performance scale in the acute tertiary care hospital setting. J Palliat Med. 2007;10:111–117. doi:0.1089/jpm.2006.0125 [DOI] [PubMed] [Google Scholar]
- 36. Le Gall JR, Loirat P, Alperovitch A, et al. A simplified acute physiology score for ICU patients. Crit Care Med. 1984;12:975–977. doi:10.1097/00003246-198411000-00012 [DOI] [PubMed] [Google Scholar]
- 37. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. http://www.ncbi.nlm.nih.gov/pubmed/3928249 [PubMed] [Google Scholar]
- 38. Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute physiology and chronic health evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310. doi:10.1097/01.CCM.0000215112.84523.F0 [DOI] [PubMed] [Google Scholar]
- 39. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi:10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mazzola P, Bellelli G, Perego S, et al. The sequential organ failure assessment score predicts 30-day mortality in a geriatric acute care setting. J Gerontol A Biol Sci Med Sci. 2013;68:1291–1295. doi:10.1093/gerona/glt020 [DOI] [PubMed] [Google Scholar]
- 41. Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi:10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]
- 42. Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi:10.1111/j.1532-5415.2006.00738.x [DOI] [PubMed] [Google Scholar]
- 43. Gu D, Sautter J, Huang C, Zeng Y. Health inputs and cumulative health deficits among the older Chinese. Soc Sci Med. 2011;72:806–814. doi:10.1016/j.socscimed.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Drubbel I, de Wit NJ, Bleijenberg N, Eijkemans RJ, Schuurmans MJ, Numans ME. Prediction of adverse health outcomes in older people using a frailty index based on routine primary care data. J Gerontol A Biol Sci Med Sci. 2013;68:301–308. doi:10.1093/gerona/gls161 [DOI] [PubMed] [Google Scholar]
- 45. Oakley R, Pattinson J, Goldberg S, et al. Equipping tomorrow’s doctors for the patients of today. Age Ageing. 2014;43:442–447. doi:10.1093/ageing/afu077 [DOI] [PubMed] [Google Scholar]
- 46. Sprung CL, Artigas A, Kesecioglu J, et al. The Eldicus prospective, observational study of triage decision making in European intensive care units. Part II: intensive care benefit for the elderly. Crit Care Med. 2012;40:132–138. doi:10.1097/CCM.0b013e318232d6b0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.