Abstract
Objective
The purpose of this study was to examine the relationship between fasting serum leptin and adiponectin levels with bone mineral density (BMD) and body composition in pre-menopausal, middle-aged Hispanic and Caucasian women.
Objective
Participants’ (68 Hispanic and 36 Caucasian) BMD and bone mineral content were measured by dual-energy X-ray absorptiometry, and body density was measured by hydrodensitometry. Serum leptin was determined by enzyme immunoassay and adiponectin by ELISA.
Results
Hispanic women had significantly higher leptin, BMD, and fat mass (FM), and lower adiponectin than Caucasian women. There was no significant correlation between leptin and BMD for Hispanic or Caucasian women; adiponectin was inversely correlated with BMD in Caucasian women only (p = 0.01). In both Hispanic and Caucasian women, lean body mass and adiponectin best explained the variance in BMD (r2 = 0.25, p < 0.001).
Conclusion
These data demonstrate no significant relationship between leptin and BMD of pre-menopausal, middle-aged Hispanic and Caucasian women, and a significant inverse relationship between adiponectin and BMD in Caucasian women. The role of adipocytokines in the regulation of BMD remains inconclusive and may vary across ethnic groups.
Keywords: BMD, ethnicity, hormone, leptin, adiponectin
INTRODUCTION
Osteoporosis, “a disease characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility and a consequent increase in fracture risk” (1), is the most common metabolic bone disease and responsible for approximately 1.5 million fractures per year (2). Approximately 40% of non-Hispanic White women over the age of 50 will experience a hip, spine, or wrist fracture at some point during the remainder of their lives (2). In comparison, the lifetime risk for men and non-White women is less for all fracture types, yet recent research indicates it may be rising for some populations, to include Hispanic women (2). Bone mineral density (BMD) is a significant predictor of fracture risk and therefore is used clinically to define osteoporosis and osteopenia (2). The development of dual-energy X-ray absorptiometry (DXA) allows for measurement of BMD with low radiation exposure, and DXA has become the most used clinical tool for the determination of osteoporosis.
Osteoporosis has been classically considered a disorder of post-menopausal women of Northern European descent because they generally achieve a lower peak bone mass and demonstrate a greater rate of decline than their male Caucasian counterparts (2). The age-adjusted prevalence of osteoporosis at the hip of post-menopausal women is approximately 17% for non-Hispanic Whites compared to 6% for non-Hispanic Blacks (2). However, the risk of fracture among other populations is escalating most likely due to increased life expectancy and a decline in regular physical activity (3).
Body mass is one of the strongest predictors of bone mass for both males and females regardless of age. In fact, increased body mass is associated with a decreased risk of osteoporotic vertebral and hip fractures, and considered the best predictor of BMD in both males and females (4). Glauber et al. (5) found that body mass was a strong determinant of bone density, explaining 6–20% of overall bone density variability in post-menopausal Caucasian women. Further, individuals with a body mass index (BMI) greater than 33 kg/m2 are at only 1% risk for developing osteoporosis (6). In general, Hispanic women have an overall greater BMD than non-Hispanic Caucasian women (7); additionally, lean body mass (LBM) was found to be a stronger contributor to BMD than fat mass (FM) among Hispanic (7) and Caucasian (8) post-menopausal women. This association between BMD and factors of adiposity appear to be dependent upon the site of measurement and sex. Felson et al. (9) found that in elderly post-menopausal women, body mass and BMI contributed up to 20% of the variance in BMD measured at weight-bearing and non-weight-bearing sites. In males, however, body mass and BMI explained approximately 3–7% of the variance in BMD for weight-bearing sites only (9).
Leptin is the product of the ob gene and is synthesized and secreted by adipocytes. An elevated serum leptin concentration is characteristic of obese humans. Leptin has traditionally been defined as a regulator of appetite and energy expenditure. Recent research has demonstrated that leptin may have a role in bone formation by inhibiting the development of osteoclasts (10). Because leptin receptors are expressed on bone cells (10–12), leptin may affect bone by the inhibition of bone resorption (10); however, this research is inconclusive. Some researchers have reported a positive correlation between serum leptin levels and BMD (10, 13, 14); whereas other researchers have reported a negative correlation (15, 16) or no significant association (17–20) between leptin and BMD.
Also secreted by adipocytes, adiponectin affects glucose regulation and insulin sensitivity, with significantly lower adiponectin concentrations associated with type 2 diabetic, insulin-resistant, and obese individuals (21–24). Adiponectin has been reported to be negatively correlated with BMD (25–29), whereas others have indicated no significant correlation (18, 30–33). The affect of adiponectin on BMD may be modulated through osteoblast-derived osteocalcin (34).
The decrease in BMD noted with age may be a result of an age-related decline in skeletal growth factors (3), and leptin (10) and adiponectin (34) may have a role in bone formation; therefore, these adipocytokines may contribute to the difference in BMD between Hispanic and Caucasian women. The purpose of this study was to examine the relationship between fasting serum leptin and adiponectin levels with BMD in pre-menopausal Hispanic and Caucasian women.
METHODS
Participants
Sixty-eight Hispanic and 36 Caucasian pre-menopausal women between the ages of 35 and 50 years from El Paso, Texas, and surrounding communities volunteered to participate in this study. A woman was disqualified from participation in this study if she was pregnant, lactating, had irregular menstrual cycles, or amenorrhea. Women were also excluded from the study for diagnosed diabetes, thyroid disorders, or if they were taking any medications known to affect bone metabolism. Prior to data collection, a detailed explanation of the study procedures and potential risks and benefits was given, and each participant provided written consent to participate on an informed consent document approved by the University of Texas at El Paso Institutional Review Board.
Study Protocol
Each participant reported to the laboratory in a fasted state between 0600 and 0800 hours; and was instructed to refrain from alcohol and caffeine consumption, and exercise 24 hours prior to testing. All laboratory procedures were completed during a single testing session and upon arrival to the laboratory, each participant completed the associated study documents and was asked to void. Body mass was measured to the nearest 0.01 kg using a calibrated load cell scale (Tanita Corporation, Tokyo, Japan); height was measured to the nearest 0.1 cm using a stadiometer (Seca Corp., Hamburg, Germany); and BMI was calculated as body mass (kg) divided by height (m) squared. A fasting blood sample was then collected followed by body composition assessments by DXA and hydrostatic densitometry.
Leptin and Adiponectin Assessment
For each participant, approximately 5 mL of blood was collected by venipuncture from an antecubital vein into a blank serum vacuum tube. The serum tube was allowed to clot and then centrifuged for 20 min. Serum aliquots were separated into cryule vials (Wheaton, Millville, NJ, USA) and frozen at −80°C for later analysis of leptin and adiponectin. Serum leptin concentration (ng/mL) was determined by enzyme immunoassay (LDN, Nordhorn, Germany). Literature published by the manufacturer of the leptin assay kit used for this study indicated an expected serum leptin concentrations for women of 7.36 ± 3.73 ng/mL. The sensitivity of the assay was 0.2 ng/mL. For low (5 ng/mL), medium (10 ng/mL), and high (21 ng/mL) controls, the inter-assay reproducibility (CV%) was 3.6, 8.6, and 7.8%, respectively, and the intra-assay reproducibility (CV%) was 5.4, 4.3, and 4.1%, respectively. Serum total adiponectin concentration (μg/mL) was determined by ELISA (Linco, St. Charles, MO, USA) (intra-assay CV%: 7.4, 0.9, and 1.8%; inter-assay CV%: 8.4, 2.4, and 6.2% for low, medium, and high adiponectin standards, respectively). Absorbance for all assays was assessed using a microtiterplate reader (SpectraMAX 190, Molecular Devices, Sunnyvale, CA, USA). All participant samples were measured in duplicate and the average of the two measures was recorded as the leptin or adiponectin concentration.
Dual-Energy X-Ray Absorptiometry
Total body BMD and bone mineral content (BMC) of each participant were assessed using DXA (Lunar DPX-NT, GE Lunar Corp., Madison, WI, USA). A quality assurance (QA) test, which calibrates and verifies the correct operation of the densitometer, was performed at the start of each testing day to examine the functionality, accuracy, and precision of the system. The CV% for the DXA system used was 0.23% based on 254 QA test procedures and control measurements. All measurements were performed in accordance with manufacturer specifications. Participants were asked to remove all jewelry and other accessories, and were measured in a standard set of gym shorts and T-shirt provided by the investigators. During the approximately 15-minute whole-body X-ray scan procedure participants lie supine on a padded table.
Hydrostatic Densitometry
Immediately prior to the hydrostatic weighing procedure, residual lung volume was determined using the modified O2 dilution method described by Wilmore (35). Participants repeated the procedure until achieving three measured values within 50 mL, and the average of these trials was recorded as the residual lung volume.
Body density of each participant was determined by the hydrostatic densitometry method (36). The electronic strain gauge scale was calibrated using a two-point calibration technique and the gain was set using a 4 kg lead weight. While supported by the platform suspended from the scale, each participant was asked to perform a maximal exhalation while completely submerged in water. The underwater weight of each participant was recorded while completely submerged and at maximal exhalation. Participants were asked to perform the submersion procedure a minimum of five trials and the heaviest three values within 100 g were averaged together and used for the determination of body density. Body density was corrected for measured residual lung volume and gastrointestinal gas (0.1 L), and body composition was calculated using a three-component model (37). For the three-component model equation (37), total body BMC determined from DXA was multiplied by the constant 1.25 to estimate total body mineral content (38). FM was calculated by multiplying total body mass and percentage body fat (BF); LBM was calculated as total body mass minus FM minus BMC; and fat-free mass was calculated as total body mass minus FM.
Statistical Analysis
Statistical analyses were conducted using the software package SPSS v14.0 (SPSS, Inc., Chicago, IL, USA). Descriptive data were compared between groups using a one-way analysis of variance. Preliminary analyses for violations of the assumption of normality, linearity, and homoscedasticity revealed that the leptin and adiponectin data were not normally distributed; therefore, the leptin data were transformed using the equation (39) and a Log10 transformation was used to transform adiponectin (p > 0.20) data to reflect normality. Pearson’s correlation analysis and multiple stepwise linear regression were used to examine the relationships between BMD and leptin, adiponectin, BF, body mass, BMI, FM, and LBM; and to identify those variables with the greatest predictive influence of BMD. Collinearity between independent variables was assessed using variance inflation factor and a value above 10.0 indicated multicollinearity. Fat-free mass was found to have a variance inflation factor of 241.0 and therefore was excluded from the stepwise regression model. Potential outliers were identified by Mahalanobis distances with a critical value of 13.82. Significance was set at an alpha level of ≤0.05.
RESULTS
Descriptive characteristics of the pre-menopausal Hispanic and Caucasian women of this study are presented in Table 1. In general, Hispanic women were of shorter stature (p = 0.002) and had a greater BMI (p = 0.004), BF (p = 0.001), and FM (p = 0.007) than Caucasian women. Hispanic women also had a significantly higher serum leptin concentration (p = 0.004) and BMD (p = 0.03) compared to Caucasian women, and significantly lower serum adiponectin concentration (p = 0.005). There was no significant difference between Hispanic and Caucasian women for age (p = 0.64), body mass (p = 0.11), or LBM (p = 0.45).
TABLE 1.
Descriptive Characteristics of Pre-Menopausal Hispanic and Caucasian Women (Mean ± SE)
| Variables | Hispanic (N = 68) | Caucasian (N = 36) | Combined (N = 104) |
|---|---|---|---|
| Age (years) | 42.9 ± 0.5 | 43.4 ± 0.7 | 43.1 ± 0.4 |
| Height (cm) | 161.4 ± 0.7* | 165.1 ± 0.9 | 162.6 ± 0.6 |
| Body mass (kg) | 71.7 ± 1.8 | 66.9 ± 2.2 | 70.0 ± 1.4 |
| BMI (kg/m2) | 27.5 ± 0.6* | 24.5 ± 0.7 | 26.5 ± 0.5 |
| Body Fat (%) | 39.5 ± 1.0* | 34.2 ± 1.2 | 37.7 ± 0.8 |
| Leptin (ng/mL) | 9.2 ± 0.9* | 5.9 ± 0.6 | 8.1 ± 0.6 |
| Adiponectin (μg/mL) | 9.2 ± 0.5* | 12.4 ± 0.8 | 10.3 ± 0.4 |
| Bone mineral density (g/cm2) | 1.215 ± 0.009* | 1.177 ± 0.015 | 1.202 ± 0.008 |
| Fat mass (kg) | 29.1 ± 1.3* | 23.4 ± 1.5 | 27.2 ± 1.0 |
| Lean body mass (kg) | 39.9 ± 0.8 | 40.9 ± 1.1 | 40.3 ± 0.6 |
Significantly different than Caucasian (p ≤ 0.03).
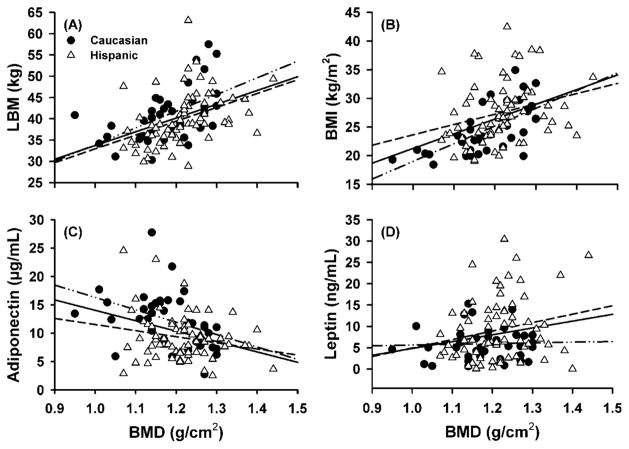
Pearson correlation coefficients revealed low to moderate relationships between variables with all participants combined; however, when the groups were evaluated individually, some of these relationships were lost (Table 2). With all participants combined, there was a significant moderate correlation between BMD and LBM (r = 0.44, p < 0.001) (Figure 1A), BMD and body mass (r = 0.43, p < 0.001), and BMD and BMI (r = 0.42, p < 0.001) (Figure 1B). A lesser, but still significant overall correlation was observed between BMD and adiponectin (r = −0.33, p = 0.001) (Figure 1C), BMD and FM (r = 0.32, p = 0.001), BMD and leptin (r = 0.18, p = 0.04) (Figure 1D), and BMD and BF (r = 0.17, p = 0.05). For Hispanic women, there was a moderate correlation between BMD and LBM (r = 0.42, p < 0.001) (Figure 1A), BMD and body mass (r = 0.31, p = 0.005), and a low correlation between BMD and BMI (r = 0.26, p = 0.01) (Figure 1B). There was no significant correlation between BMD and leptin (Figure 1D), adiponectin (Figure 1C), FM, or BF for Hispanic women. A moderate correlation was observed between BMD and BMI (r = 0.64, p < 0.001) (Figure 1B), BMD and body mass (r = 0.63, p < 0.001), BMD and LBM (r = 0.54, p < 0.001) (Figure 1A), BMD and FM (r = 0.52, p = 0.001), and BMD and adiponectin (r = −0.40, p = 0.015) (Figure 1C) for Caucasian women. For Caucasian women, there was a low correlation between BMD and BF (r = 0.33, p = 0.03), whereas a non-significant correlation was observed between BMD and leptin (Figure 1D).
TABLE 2.
Pearson Correlation Coefficients Between Bone Mineral Density (BMD), Leptin, Adiponectin, Percentage Body Fat (BF), Body Mass (BM), Body Mass Index (BMI), Fat Mass (FM), and Lean Body Mass (LBM) of All Pre-Menopausal Women Combined, and Hispanic and Caucasian Women
| Pearson correlation | BMD | Leptin | Adiponectin | BF | BM | BMI | FM |
|---|---|---|---|---|---|---|---|
| All women combined | |||||||
| Leptin | 0.176* | ||||||
| Adiponectin | −0.330** | −0.161 | |||||
| BF (%) | 0.165* | 0.356** | −0.238* | ||||
| BM (kg) | 0.433** | 0.477** | −0.268* | 0.658** | |||
| BMI | 0.415** | 0.505** | −0.328** | 0.735** | 0.932** | ||
| FM (kg) | 0.316** | 0.444** | −0.283* | 0.891** | 0.920** | 0.923** | |
| LBM (kg) | 0.436** | 0.359** | −0.182 | 0.024 | 0.758** | 0.605** | 0.444** |
| Hispanic women | |||||||
| Leptin | 0.170 | ||||||
| Adiponectin | −0.200 | −0.262* | |||||
| BF (%) | −0.011 | 0.411** | −0.140 | ||||
| BM (kg) | 0.309* | 0.515** | −0.187 | 0.672** | |||
| BMI | 0.265* | 0.523** | −0.240* | 0.727** | 0.938** | ||
| FM (kg) | 0.167 | 0.484** | −0.164 | 0.885** | 0.932** | 0.925** | |
| LBM (kg) | 0.418** | 0.403** | −0.164 | 0.092 | 0.790** | 0.662** | 0.514** |
| Caucasian women | |||||||
| Leptin | 0.071 | ||||||
| Adiponectin | −0.401* | 0.286 | |||||
| BF (%) | 0.328* | 0.017 | −0.173 | ||||
| BM (kg) | 0.629** | 0.287* | −0.377* | 0.594** | |||
| BMI | 0.641** | 0.313* | −0.316 | 0.668** | 0.933** | ||
| FM (kg) | 0.515** | 0.168 | −0.327* | 0.882** | 0.897** | 0.894** | |
| LBM (kg) | 0.541** | 0.356* | −0.307 | −0.035 | 0.775** | 0.651** | 0.417* |
Analyzed using transformed leptin and adiponectin values.
p ≤ 0.05;
p ≤ 0.001.
FIGURE 1.
Relationship between bone Mineral Density (BMD) and a) lean body mass (LBM), b) body mass index (BMI), c) Adiponectin, and d) Leptin of pre-menopausal Hispanic and Caucasian women. Regression lines for all women (——; panel a: r = 0.436, P < 0.001; panel b: r = 0.415, P < 0.001; panel c: r = −0.330, P = 0.001; panel d: r = 0.176, P = 0.037), Hispanic women (– – – –; panel a: r = 0.418, P < 0.001; panel b: r = 0.265, P = 0.014; panel c: r = −0.200, P = 0.103; panel d: r = 0.170, P = 0.083), and Caucasian women (– . . –; panel a: r = 0.541, P < 0.001; panel b: r = 0.641, P < 0.001; panel c: r = −0.401, P = 0.015; panel d: r = 0.071, P = 0.340).
A multiple stepwise regression model was created to identify which variables (leptin, adiponectin, body mass, BF, BMI, FM, and LBM) best explained the variance of BMD (Table 3). With all participants included in the model, the results of the regression analysis determined that 25.5% of the total BMD variance was explained by the combined individual and shared variance of LBM and adiponectin (R2 = 0.255, p < 0.001). Excluding shared variance, LBM and adiponectin explained 14.7% (R2 = 0.147, p < 0.001) and 6.5% (R2 = 0.065, p = 0.004) of the total variance, respectively. When Hispanic and Caucasian women were analyzed separately, the regression model determined that LBM was the only significant predictor of BMD for Hispanics (R2 = 0.175, p < 0.001), and BMI was the only significant predictor of BMD for Caucasians (R2 = 0.411, p < 0.001). Leptin did not significantly contribute to the regression model for all the study participants, Hispanic, or Caucasian women (Table 3).
TABLE 3.
Stepwise Multiple Regression Analysis Examining Predictors of Bone Mineral Density in Pre-Menopausal Hispanic and Caucasian Women
| Dependent variable | Step no. | Predicting variable | Beta | R2 | p-Value |
|---|---|---|---|---|---|
| Overall BMD | Total | LBM, adiponectin | 0.25 | <0.001 | |
| 1 | LBM | 0.39 | 0.15 | <0.001 | |
| 2 | Adiponectin | −0.26 | 0.07 | 0.004 | |
| Hispanic BMD | 1 | LBM | 0.42 | 0.17 | <0.001 |
| Caucasian BMD | 1 | BMI | 0.64 | 0.41 | <0.001 |
For the model, the dependent variable was bone mineral density (BMD); predicting variables were leptin (transformed), adiponectin (transformed), body mass index (BMI), percentage body fat, fat mass, lean body mass (LBM), and body mass. Total represents the combined individual and shared variance of LBM and BMI.
DISCUSSION
The purpose of this study was to examine the relationship between fasting serum leptin and adiponectin levels, BMD, and body composition in pre-menopausal Hispanic and Caucasian women. The major findings of this study were that (a) fasting serum leptin was weakly associated (r = 0.176) with BMD with all women combined, but had no statistically significant association with BMD when women were divided by ethnic group; (b) fasting serum leptin was not a significant predictor of BMD in this group of pre-menopausal Hispanic and Caucasian women; (c) fasting serum adiponectin was inversely associated with BMD for all women combined (r = −0.330) and for Caucasian women (r = −0.401); (d) fasting serum adiponectin was a significant predictor of BMD only when all women were combined; (e) 17.5% of the BMD variance for Hispanic women was explained by LBM; and (f) 41.1% of the BMD variance was explained by BMI for Caucasian women. The Hispanic women of this study had a significantly greater BF, greater serum leptin, and lower adiponectin concentrations than the Caucasian women (Table 1). Overall, the fasting serum leptin concentrations of our study were correlated with BF (Table 2), which is in agreement with previous reports (40–42). When analyzed by group, the relationship between serum leptin and BF remained for Hispanic women, although the relationship between serum leptin and BF was not significant for Caucasian women.
Although this study found no association between serum leptin concentration and BMD when women were divided into ethnic groups, and that leptin was not a significant predictor of BMD, in vitro investigations and studies in animals suggest that leptin may have a role in bone formation and/or bone turnover. Leptin receptors are expressed on bone cells (10–12). In vitro studies have suggested that leptin inhibits osteoclast generation, making leptin a possible inhibitor of bone resorption, a major contributor to osteoporosis (10). In vitro administration of leptin in a human marrow stromal cell line appeared to enhance the osteoblast maturation pathway while suppressing the adipocyte maturation pathway leading to an increase in the mineralization of the extracellular matrix (12). Conversely, Ducy et al. (15) found that leptin had an inhibitory effect on bone formation in mice. Mice that were leptin-deficient and leptin receptor-deficient were found to have an increased bone mass despite hypogonadism and hypocortisolism (15). When intracerebroventricular infusion of leptin was administered to these mice, a decrease in bone mass was observed. The authors suggested that the control of bone formation is a central, leptin-dependent, function in mice (15). This is supported by Iwaniec et al. (43) who administered leptin in the hypothalamus via recombinant adeno-associated virus while not changing peripheral leptin concentrations. Leptin-deficient mice treated with the hypothalamic leptin improved total bone volume and femoral length so that by 15 weeks of treatment these variables were not different from wild-type mice (43). The results of these studies (10–12,15,43) suggest that the effects of leptin on bone could be a combination of multiple pathways and dependent upon bone site and compartment.
It is possible that the relationship between leptin and bone does not affect BMD in humans. Similar to our study, most data on the association between leptin and bone in humans are from cross-sectional samples and are inconclusive. A 10-year longitudinal study reported that high serum leptin levels were predictive of low risk for nontraumatic fracture in older men and women (14). Yet, leptin was found to not correlate with BMD or biomarkers of bone formation in post-menopausal osteoporotic women (20). An analysis of 302 elderly men and women observed that plasma leptin was a weak predictor of BMD and only significant at the femoral neck in women (17). The authors suggested that bone mass was more dependent on factors affecting adiposity than a direct association of leptin on bone cell function (17). A report of 1906 pre-menopausal women found leptin to be associated with BMD; however, the association was lost when the model was adjusted for BMI and age (19) further suggesting that adiposity-related factors has greater influence on BMD than leptin. The results of our study, similar to that of previous investigations, indicate that BMD is most associated with variables reflective of body mass (i.e., LBM and BMI). It is possible, however, that leptin has an indirect role in regulating BMD through its association with BF.
Leptin, acting through the central and sympathetic nervous systems, has been suggested to indirectly regulate adiponectin by influencing osteoblastic secretion of osteocalcin (15) and has been discussed in two reviews (34, 44). Adiponectin may impact BMD because adiponectin and adiponectin receptors are expressed in bone-forming cells, potentially providing a signaling pathway between fat and BMD (45). Adiponectin appears to indirectly increase osteoclast formation by stimulating receptor activator of nuclear factor-B ligand (RANKL) pathway and inhibiting osteoprotegerin production in osteoblasts (46). An inverse relationship between adiponectin and BMD has been reported among post-menopausal women (27, 29, 47–49) and men (27, 28, 50), but there are confounding reports for pre-menopausal women (30, 31, 51) and men (32, 52). In our study, adiponectin was a significant predictor of BMD for all women, but not independently for Hispanic or Caucasian women.
Obese individuals typically have greater BMD and leptin values (20), and lower adiponectin concentrations (21, 23, 24) than non-obese individuals. Body mass has been shown to be a strong predictor of bone mass in both adults and children (9). However, there is considerable debate as to which of the components of body mass, FM or LBM, is a stronger determinant of bone mass (53). Therefore, the association between leptin, adiponectin, and BMD may be a secondary effect to variables that affect adiposity, such as body mass, BF, FM, or LBM.
Within the literature, the relationship between BMD and leptin and adiponectin is inconclusive. For this group of Hispanic and Caucasian pre-menopausal women, BMD was influenced more by the LBM and BMI of an individual than leptin, adiponectin, body mass, BF, or FM, and this relationship varied slightly between the different ethnic groups. Multiple regression revealed that once these predictive variables were in the model, the addition of leptin did not improve the correlation. The observational nature of this study does not allow inference into the physiological mechanisms that may be associated with adipocytokines, body composition, and BMD. It is possible that leptin and/or adiponectin are one of a cascade of signaling hormones for BMD and indirectly influences BMD. Additional research is necessary to further elucidate the relationship between leptin, adiponectin, BMD, and body composition.
Acknowledgments
This study was funded by a grant from the National Institutes of Health (NIH), National Center on Minority Health and Health Disparities (NCM-HHD) (P 20 MD000548) through the Hispanic Health Disparities Research Center of the University of Texas at El Paso; and in part supported by Grant Number 5G12RR008124 (to the Border Biomedical Research Center (BBRC)/University of Texas at El Paso) from the National Center for Research Resources (NCRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCMHHD, NCRR, or NIH. The authors thank Dr. Kristin Gosselink for laboratory support and Charlie Potter, Clare Spence, Carlos G. Sifuentes, Bernadette Franco, and Misty Babbey for assistance with data collection.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Peck WA, Burckhardt P, Christiansen C, et al. Consensus development conference—diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004. [Google Scholar]
- 3.Raisz LG, Kream BE, Lorenzo JA. Metabolic bone disease. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. 10. Philadelphia, PA: Saunders; 2003. pp. 1373–1410. [Google Scholar]
- 4.Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138(3):160–169. doi: 10.1093/oxfordjournals.aje.a116842. [DOI] [PubMed] [Google Scholar]
- 5.Glauber HS, Vollmer WM, Nevitt MC, Ensrud KE, Orwoll ES. Body weight versus body fat distribution, adiposity, and frame size as predictors of bone density. J Clin Endocrinol Metab. 1995;80(4):1118–1123. doi: 10.1210/jcem.80.4.7714079. [DOI] [PubMed] [Google Scholar]
- 6.Bigler JM, Abetel G, Krieg MA, et al. Importance of clinical profile in the postmenopausal osteoporosis by densitometry. Schweiz Med Wochenschr. 1996;126(31–32):1347–1351. [PubMed] [Google Scholar]
- 7.Taaffe DR, Villa ML, Holloway L, Marcus R. Bone mineral density in older non-Hispanic Caucasian and Mexican-American women: relationship to lean and fat mass. Ann Hum Biol. 2000;27(4):331–344. doi: 10.1080/03014460050044829. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Lohman TG, Stini WA, Ritenbaugh C, Aickin M. Fat or lean tissue mass: which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res. 1997;12(1):144–151. doi: 10.1359/jbmr.1997.12.1.144. [DOI] [PubMed] [Google Scholar]
- 9.Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8(5):567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 10.Holloway WR, Collier FM, Aitken CJ, et al. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17(2):200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 11.Thomas T. Leptin and fragility fracture: evidence for a protective effect in humans. Am J Med. 2004;117(12):966–968. doi: 10.1016/j.amjmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulou F, Krassas GE, Kalothetou C, Koliakos G, Constantinidis TC. Serum leptin values in relation to bone density and growth hormone-insulin like growth factors axis in healthy men. Arch Androl. 2004;50(2):97–103. [PubMed] [Google Scholar]
- 14.Schett G, Kiechl S, Bonora E, et al. Serum leptin level and the risk of nontraumatic fracture. Am J Med. 2004;117(12):952–956. doi: 10.1016/j.amjmed.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 15.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, Takeda N, Sarui H, et al. Association between serum leptin concentrations and bone mineral density, and biochemical markers of bone turnover in adult men. J Clin Endocrinol Metabol. 2001;86(11):5273–5276. doi: 10.1210/jcem.86.11.8020. [DOI] [PubMed] [Google Scholar]
- 17.Dennison EM, Syddall HE, Fall CH, et al. Plasma leptin concentration and change in bone density among elderly men and women: the Hertfordshire Cohort Study. Calcif Tissue Int. 2004;74(5):401–406. doi: 10.1007/s00223-002-0017-x. [DOI] [PubMed] [Google Scholar]
- 18.Huang KC, Cheng WC, Yen RF, Tsai KS, Tai TY, Yang WS. Lack of independent relationship between plasma adiponectin, leptin levels and bone density in nondiabetic female adolescents. Clin Endocrinol. 2004;61(2):204–208. doi: 10.1111/j.1365-2265.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 19.Ruhl CE, Everhart JE. Relationship of serum leptin concentration with bone mineral density in the United States population. J Bone Miner Res. 2002;17(10):1896–1903. doi: 10.1359/jbmr.2002.17.10.1896. [DOI] [PubMed] [Google Scholar]
- 20.Shaarawy M, Abassi AF, Hassan H, Salem ME. Relationship between serum leptin concentrations and bone mineral density as well as biochemical markers of bone turnover in women with postmenopausal osteoporosis. Fertil Steril. 2003;79(4):919–924. doi: 10.1016/s0015-0282(02)04915-4. [DOI] [PubMed] [Google Scholar]
- 21.Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13(1):51–59. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Heidemann C, Sun Q, van Dam RM, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Int Med. 2008;149(5):307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2007;9:282–289. doi: 10.1111/j.1463-1326.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 24.Wolf G. Adiponectin: a regulator of energy homeostasis. Nutr Rev. 2003;61(8):290–292. doi: 10.1301/nr.2003.aug.290-292. [DOI] [PubMed] [Google Scholar]
- 25.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Yano S, Sugimoto T. Relationships between serum adiponectin levels versus bone mineral density, bone metabolic markers, and vertebral fractures in type 2 diabetes mellitus. Eur J Endocrinol. 2009;160(2):265–273. doi: 10.1530/EJE-08-0642. [DOI] [PubMed] [Google Scholar]
- 26.Jurimae J, Rembel K, Jurimae T, Rehand M. Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res. 2005;37(5):297–302. doi: 10.1055/s-2005-861483. [DOI] [PubMed] [Google Scholar]
- 27.Lenchik L, Register TC, Hsu FC, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33(4):646–651. doi: 10.1016/s8756-3282(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 28.Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta. 2008;387(1–2):31–35. doi: 10.1016/j.cca.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92(4):1517–1523. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- 30.Jurimae J, Jurimae T. Plasma adiponectin concentration in healthy pre- and postmenopausal women: relationship with body composition, bone mineral, and metabolic variables. Am J Physiol-Endocrinol Metab. 2007;293(1):E42–E47. doi: 10.1152/ajpendo.00610.2006. [DOI] [PubMed] [Google Scholar]
- 31.Kontogianni MD, Dafni UG, Routsias JG, Skopouli FN. Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. J Bone Miner Res. 2004;19(4):546–551. doi: 10.1359/JBMR.040107. [DOI] [PubMed] [Google Scholar]
- 32.Oh KW, Lee WY, Rhee EJ, et al. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol (Oxf) 2005;63(2):131–138. doi: 10.1111/j.1365-2265.2005.02312.x. [DOI] [PubMed] [Google Scholar]
- 33.Sodi R, Hazell MJ, Durham BH, Rees C, Ranganath LR, Fraser WD. The circulating concentration and ratio of total and high molecular weight adiponectin in post-menopausal women with and without osteoporosis and its association with body mass index and biochemical markers of bone metabolism. Clin Biochem. 2009;42(13–14):1375–1380. doi: 10.1016/j.clinbiochem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310(1–2):21–29. doi: 10.1016/j.mce.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilmore JH. A simplified method for determination of residual lung volumes. J Appl Physiol. 1969;27(1):96–100. doi: 10.1152/jappl.1969.27.1.96. [DOI] [PubMed] [Google Scholar]
- 36.Katch F, Michael ED, Horvath SM. Estimation of body volume by underwater weighing: description of a simple method. J Appl Physiol. 1967;23(5):811–813. doi: 10.1152/jappl.1967.23.5.811. [DOI] [PubMed] [Google Scholar]
- 37.Lohman TG. Applicability of body composition techniques and constants for children and youths. Exer Sport Sci Rev. 1986;14:325–357. [PubMed] [Google Scholar]
- 38.Salamone LM, Fuerst T, Visser M, et al. Measurement of fat mass using DEXA: a validation study in elderly adults. J Appl Physiol. 2000;89(1):345–352. doi: 10.1152/jappl.2000.89.1.345. [DOI] [PubMed] [Google Scholar]
- 39.Ott RL, Longnecker M. An Introduction to Statistical Methods and Data Analysis. 5. Pacific Grove, CA: Duxbury; 2001. Inferences about more than two population central values; p. 404. [Google Scholar]
- 40.Ceddia RB. Direct metabolic regulation in skeletal muscle and fat tissue by leptin: implications for glucose and fatty acids homeostasis. Int J Obes. 2005;29(10):1175–1183. doi: 10.1038/sj.ijo.0803025. [DOI] [PubMed] [Google Scholar]
- 41.Janecková R. The role of leptin in human physiology and pathophysiology. Physiol Res. 2001;50(5):443–459. [PubMed] [Google Scholar]
- 42.McCargar LJ. Leptin response and body weight regulation in humans. J Clin Biochem Nutr. 1999;26(2):77–84. [Google Scholar]
- 43.Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides. 2007;28(5):1012–1019. doi: 10.1016/j.peptides.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf G. Energy regulation by the skeleton. Nutr Rev. 2008;66(4):229–233. doi: 10.1111/j.1753-4887.2008.00027.x. [DOI] [PubMed] [Google Scholar]
- 45.Berner HS, Lyngstadaas SP, Spahr A, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35(4):842–849. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Luo XH, Guo LJ, Xie H, et al. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21(10):1648–1656. doi: 10.1359/jbmr.060707. [DOI] [PubMed] [Google Scholar]
- 47.Araneta MRG, von Muhlen D, Barrett-Connor E. Sex differences in the association between adiponectin and BMD, bone loss, and fractures: the Rancho Bernardo Study. J Bone Miner Res. 2009;24(12):2016–2022. doi: 10.1359/JBMR.090519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jurimae J, Jurimae T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26(6):618–623. doi: 10.1007/s00774-008-0861-5. [DOI] [PubMed] [Google Scholar]
- 49.Zoico E, Zamboni M, Di Francesco V, et al. Relation between adiponectin and bone mineral density in elderly post-menopausal women: role of body composition, leptin, insulin resistance, and dehydroepiandrosterone sulfate. J Endocrinol Invest. 2008;31(4):297–302. doi: 10.1007/BF03346361. [DOI] [PubMed] [Google Scholar]
- 50.Bozic B, Loncar G, Prodanovic N, et al. Relationship between high circulating adiponectin with bone mineral density and bone metabolism in elderly males with chronic heart failure. J Card Fail. 2010;16(4):301–307. doi: 10.1016/j.cardfail.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Chanprasertyothin S, Saetung S, Payattikul P, Rajatanavin R, Ongphiphadhanakul B. Relationship of body composition and circulatory adiponectin to bone mineral density in young premenopausal women. J Med Assoc Thai. 2006;89(10):1579–1583. [PubMed] [Google Scholar]
- 52.Gonnelli S, Caffarelli C, Del Santo K, et al. The relationship of ghrelin and adiponectin with bone mineral density and bone turnover markers in elderly men. Calcif Tissue Int. 2008;83(1):55–60. doi: 10.1007/s00223-008-9149-y. [DOI] [PubMed] [Google Scholar]
- 53.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31(5):547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]