Abstract
Wound healing is impaired in diabetes resulting in significant morbidity and mortality. Neutrophils are the main leukocytes involved in the early phase of healing. As part of their anti-microbial defense, neutrophils form extracellular traps (NETs) by releasing decondensed chromatin lined with cytotoxic proteins1. NETs, however, can also induce tissue damage. Here we show that neutrophils isolated from type 1 and type 2 diabetic humans and mice were primed to produce NETs (a process termed, NETosis). Expression of peptidylarginine deiminase 4 (PAD4), an enzyme important in chromatin decondensation, was elevated in neutrophils from individuals with diabetes. When subjected to excisional skin wounds, wild-type (WT) mice produced large quantities of NETs in wounds, but this was not observed in Padi4−/− mice. In diabetic mice, higher levels of citrullinated histone H3 (H3Cit, a NET marker) were found in their wounds and healing was delayed. Wound healing was accelerated in Padi4−/− mice as compared to WT mice, and was not compromised by diabetes. DNase 1, which disrupts NETs, accelerated wound healing in diabetic and normoglycemic WT mice. Thus, NETs impair wound healing, particularly in diabetes where neutrophils are more susceptible to NETosis. Inhibiting NETosis or cleaving NETs may improve wound healing and reduce NET-driven chronic inflammation in diabetes.
NETs were originally recognized as a host defense mechanism in which neutrophils release their nuclear and granular contents to contain and kill pathogens1. Bacterial endotoxins, such as lipopolysaccharides (LPS), stimulate the release of NETs that form extensive webs of DNA coated with cytotoxic histones and microbicidal proteases1. A prerequisite for NETosis is modification of arginine residues of histones to citrulline by PAD4, which changes the charge of the histones, leading to massive chromatin decondensation2, 3. NETs also form during sterile inflammation4. NETs are a key scaffold in pathologic thrombi and fuel cardiovascular, inflammatory and thrombotic diseases in mice and humans4, 5.
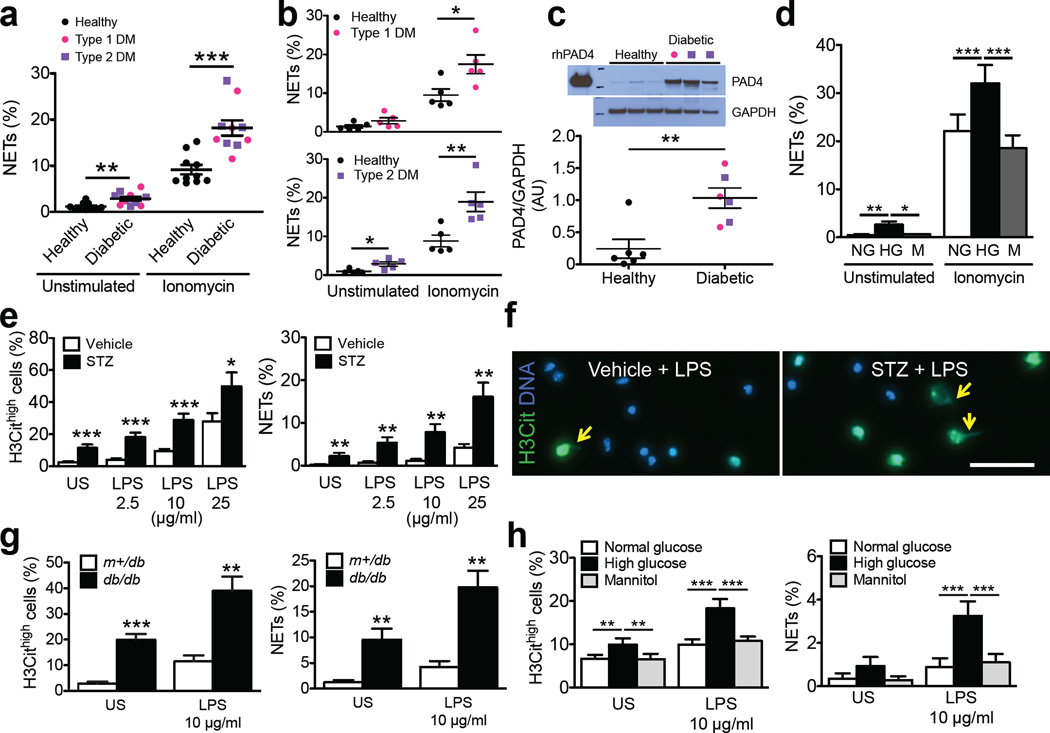
Under diabetic conditions, neutrophils produce more superoxide6 and cytokines7. Tumor necrosis factor-α, which primes neutrophils for NETosis8, is increased in diabetic individuals9. The diabetic microenvironment may thus favor NETosis. To test whether diabetes predisposes neutrophils to NETosis, we isolated neutrophils from the fresh whole blood obtained from individuals with either type 1 or type 2 diabetes whose glycated hemoglobin (HbA1c) was >6.5%, indicating mild prolonged hyperglycemia (Supplementary Fig. 1a). Neutrophils from these individuals were indeed more susceptible to NETosis when stimulated with the calcium ionophore, ionomycin (Fig. 1a,b). PAD4 is a calcium-dependent enzyme10 that is key in mediating NETosis11. Western blotting revealed a 4-fold upregulation of PAD4 protein expression in the neutrophils from individuals with diabetes as compared to healthy controls (Fig. 1c), which should favor chromatin decondensation12. Neutrophils from Type 2 diabetics have elevate basal calcium levels13. A direct correlation between intracellular calcium levels and fasting serum glucose levels has also been reported13. Calcium flux is necessary for efficient NET formation14 as it promotes production of reactive oxygen species (ROS) and PAD4-mediated chromatin citrullination10, 14. In addition, NETosis was shown to metabolically require glucose15. Therefore, elevated glucose, as seen in diabetes, may participate in NETosis at many levels. Our present findings are complemented by a recent report showing that circulating NET-related biomarkers, nucleosomes, cell-free double-stranded DNA and neutrophil elastase, are increased in the sera of individuals with type 2 diabetes, and nucleosomes positively correlate with these individuals’ HbA1c levels16.
Figure 1.
Diabetes or high glucose concentrations in vitro prime human and mouse neutrophils to undergo NETosis. (a,b) Percentage of NET production by unstimulated and ionomycin-stimulated peripheral neutrophils isolated from fresh whole blood of healthy individuals (black circle, n = 10) and individuals with diabetes mellitus (DM) (pink circle, type 1 DM, n = 5; purple square, type 2 DM, n = 5). (c) Western blot analysis of PAD4 expression in neutrophils from healthy or diabetic individuals (top) and quantification of PAD4 expression, normalized to GAPDH expression (bottom). AU, arbitrary units. n = 6 for healthy control, n = 6 for diabetic individuals. (d) Percentage of NET production by neutrophils from healthy individuals that were exposed to normal glucose (NG, 5.5 mM), high glucose (HG, 22 mM) and mannitol (M, 16.5 mM plus 5.5 mM glucose) in vitro. n = 5 per condition. (e,g,h) Percentage of cells that were hypercitrullinated at histone H3 (H3Cithigh, left panel) and produced NETs (right panel) in neutrophils isolated from (e) streptozotocin (STZ)-induced diabetic mice (n = 12 for vehicle, n = 10 for STZ), (g) db/db diabetic mice (n = 7 for m+/db; n = 8 for db/db) and (h) normoglycemic WT mice whose neutrophils were exposed to different glucose concentrations in vitro (n = 10 per medium condition). US, unstimulated. (f) Representative immunofluorescence images of isolated neutrophils from vehicle- or STZ-treated mice. Neutrophils were exposed to LPS (25 ~g/ml) for 2.5 h. Yellow arrows indicate NETs. Scale, 50 ~m. *P<0.05, **P<0.01, ***P<0.001. (a–c,g) Mann-Whitney test; (d,h) repeated measures ANOVA followed by Bonferroni's post test; (e) Student’s t test.
Because frequent hyperglycemia is common to both type 1 and type 2 diabetes, as indicated by the higher HbA1c in the diabetic cohorts compared to the healthy controls (Supplementary Fig. 1a, Supplementary Table 1), we hypothesized that high glucose alone may contribute to neutrophil priming. We therefore isolated neutrophils from healthy donors and pre-incubated them in media with normal (5.5 mM) or high (22 mM) glucose concentrations prior to stimulation with ionomycin or phorbol 12-myristate 13-acetate (PMA), which triggers ROS production. Both ionomycin and PMA stimulated more of the high glucose-exposed neutrophils to produce NETs compared to pre-incubation with normal glucose or equal concentrations of the non-metabolizable sugar alcohol, mannitol (Fig. 1d, Supplementary Fig. 1b). Thus, the increased susceptibility of diabetic neutrophils to NETosis is at least in part due to elevations in blood glucose. Our observations differ from earlier reports17, 18 possibly due to different methods of neutrophil isolation. Using the less activating Histopaque and Percoll gradients compared to dextran sedimentation19–21, we found a clear priming effect by diabetes or hyperglycemia on NETosis.
We then examined the susceptibility to NETosis in diabetic mouse models, and the role of PAD4 and impact of NETs on diabetic wound healing. Immunostaining of fresh blood cells from streptozotocin (STZ)-induced diabetic mice (a model of type 1 diabetes) (Supplementary Fig. 2a–c) revealed an approximately 4 fold increase in H3Cit+ neutrophils when compared to normoglycemic mice (Supplementary Fig. 3). About 4.5 fold more isolated neutrophils from diabetic mice were H3Cithigh and ~2% produced NETs after incubation in vitro without stimulation, while <0.2% NETs were seen in the normoglycemic controls (Fig. 1e). LPS further stimulated more neutrophils from the STZ-induced diabetic mice to be H3Cithigh and form NETs compared to vehicle-treated normoglycemic mice (Fig. 1e,f). Thus, similar to humans, diabetes has inflammatory or metabolic components that predispose mouse neutrophils to NETosis. Although there is no specific anti-mouse PAD4 antibody to evaluate whether PAD4 protein expression is increased by diabetes, neutrophil priming could also be attributable to an increased PAD4 activity as indicated by elevated histone H3 citrullination3 (Fig. 1e, Supplementary Fig. 3). Similar NETosis assays were performed with neutrophils from genetically modified db/db mice (Supplementary Fig. 4), a model of type 2 diabetes. A higher proportion of these neutrophils were H3Cithigh and formed NETs when compared to the neutrophils from normoglycemic control m+/db mice (Fig. 1g), indicating enhanced NETosis is a common phenomenon in mouse diabetes regardless of the type or etiology as we observed in the human condition. LPS stimulated more high glucose-exposed neutrophils from normoglycemic WT mice to become H3Cithigh and produce NETs when compared to those exposed to normal glucose or mannitol (Fig. 1h), indicating a possible priming role of high glucose. Thus the mouse models of diabetes represent well the human condition in respect to susceptibility to NETosis and induction of PAD4 activity.
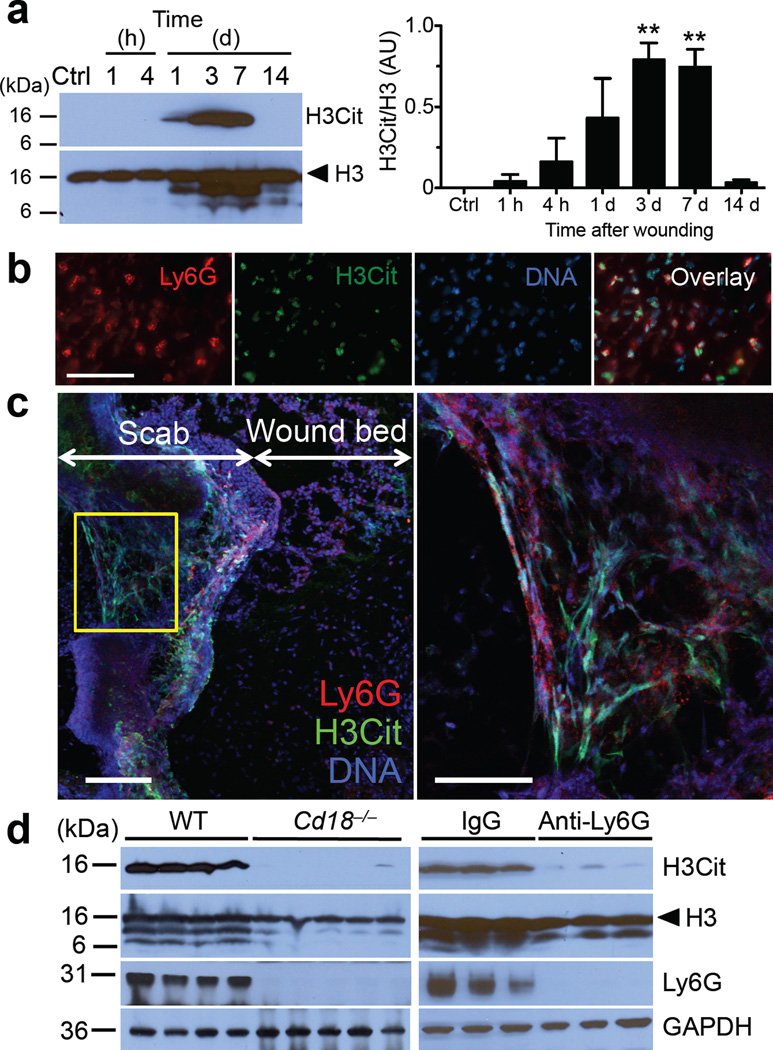
Depletion of neutrophils in mice was previously shown to accelerate re-epithelialization of uninfected diabetic wounds22. Because NETs can be injurious to tissues23, we asked whether NETs form in wounds and impact healing. We examined excisional wounds24 from normoglycemic WT mice. H&E staining confirmed that recruitment of leukocytes, mainly neutrophils, overlaps with the keratinocyte proliferation stage that leads to re-epithelialization (Supplementary Fig. 5). Therefore, neutrophils or NETs could interfere with healing. Analysis of wounds revealed increased amount of H3Cit that peaked from 3 to 7 days after wounding (Fig. 2a). Immunofluorescence images of wounds 3 days after injury showed that H3Cit+ neutrophils were present in the wound bed immediately beneath the scab (Fig. 2b; Supplementary Fig. 6). Confocal microscopy substantiated the presence of NETs in skin wounds. Externalized DNA colocalized with H3Cit in areas associated with intense staining of the neutrophil membrane marker, Ly6G (Fig. 2c). Of note, H3Cit and neutrophils were absent in the surface layers of unwounded skin (Supplementary Fig. 6). Skin expresses PAD isoforms 1–3 (ref. 25) which could citrullinate extracellular proteins in the scab. To verify the cellular source of H3Cit, we subjected CD18 (β2 integrin)-deficient (Cd18−/−) mice, which are defective in leukocyte recruitment, to wounding. In these mice, both H3Cit and Ly6G were undetectable by Western blotting in wounds 3 days after injury (Fig. 2d, left panels), a time when H3Cit was maximal in the WT wounds (Fig. 2a), suggesting H3Cit is of leukocyte origin. H&E staining and immunofluorescence microscopy showed that the few Cd18−/− neutrophils present in these wounds were H3Cit+ and produced NETs (Supplementary Fig. 7a,b). Indeed, Cd18−/− neutrophils produced NETs efficiently in vitro (Supplementary Fig. 7c), showing that β2 integrins were not required for NETosis. Wounds from WT mice with depleted neutrophils also showed markedly reduced H3Cit (Fig. 2d, right panels). Thus, our data indicate that neutrophils are the source of the H3Cit present in the wounds.
Figure 2.
NETs are present in the wounds of WT mice. (a) Representative Western blot of the time course for H3Cit appearance in wounds after skin injury (left) and quantification of levels of H3Cit to histone H3 (right). AU, arbitrary units. Ctrl, control unwounded skin; H3, histone H3. **P<0.01 versus Ctrl, Student’s t test, n = 3 for Ctrl, 1 and 4 h, n = 5 for 1, 3, 7 and 14 d. (b) Immunofluorescence images of the wound bed immediately beneath the scab 3 days after injury. Scale, 50 ~m. (c) Representative confocal images of wounds 3 days after injury. Area enclosed by the yellow box is magnified and shown on the right. Scale, 100 ~m (left panel), 50 ~m (right panel). (d) Western blots of wounds collected 3 days after injury from mice with defective leukocyte recruitment (Cd18−/−, left) and mice depleted of neutrophils using an anti-Ly6G antibody (right, representative of n = 7). IgG, IgG isotype control for the anti-Ly6G antibody.
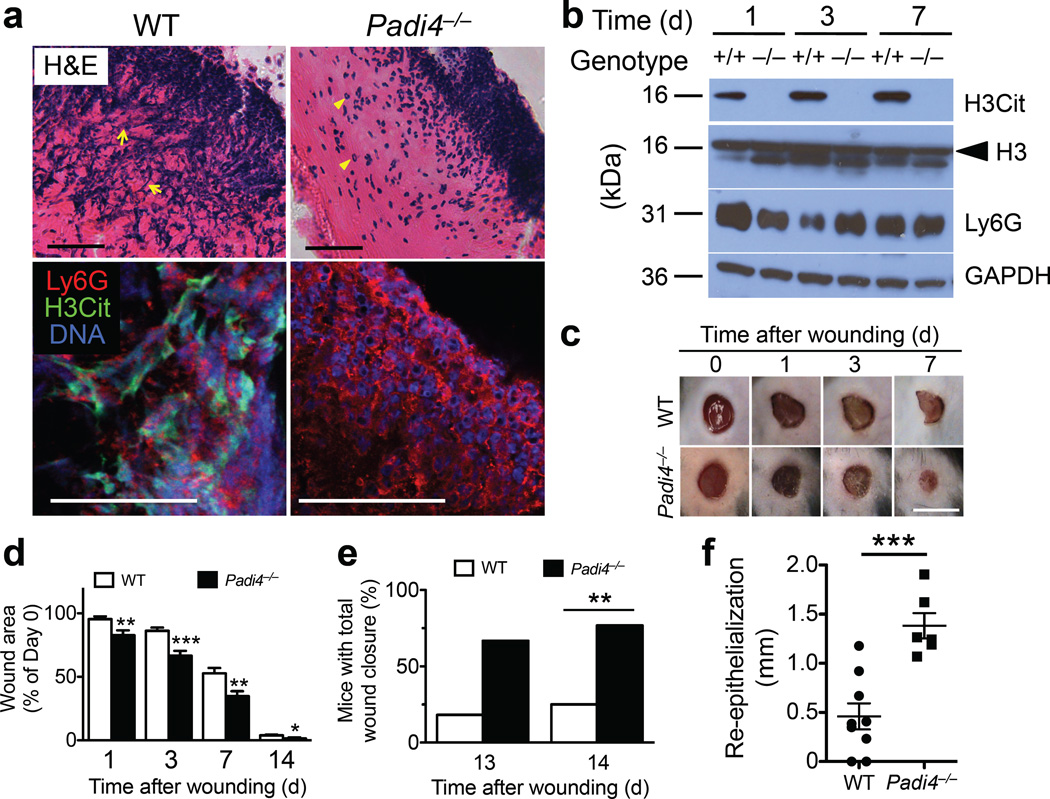
To establish the role of NETs in wound healing, we compared wounds of WT to Padi4−/− mice. Prominent extracellular DNA structures observed by H&E were absent in Padi4−/− scabs (Fig. 3a, upper panels), as were the H3Cit and extracellular chromatin patterns seen in WT mice by confocal microscopy (Fig. 3a, lower panels). In contrast to the robust H3Cit signals in WT wounds, no H3Cit was detectable in wounds from Padi4−/− mice despite normal neutrophil recruitment (Fig. 3b; Supplementary Fig. 8). Unlike neutrophil recruitment-defective P-/E-selectin double mutants that have opportunistic infections26 and impaired wound healing24, wounds in Padi4−/− mice did not show overt signs of infection (Fig. 3c) and healed faster than wounds in WT mice (Fig. 3c,d). This is likely because other neutrophil functions such as phagocytosis11, degranulation and ROS production27 are intact in Padi4−/− neutrophils so that these neutrophils are fully capable of performing other host defense mechanisms. About 80% of Padi4−/− mice had all wounds healed on day 14 compared to only 25% of WT controls (Fig. 3e). The beneficial effect of PAD4 deficiency on wound healing was observed very early after injury (Fig. 3d), indicating that NETs might impair the onset of initial healing processes such as re-epithelialization. In line with this hypothesis, re-epithelialization progressed 3-fold faster in Padi4−/− mice compared to WT mice (Fig. 3f, Supplementary Fig. 9). Immunofluorescence staining of wounds for Ki67 (a proliferation marker) and TUNEL (an indicator of apoptosis) was not different between WT and Padi4−/− mice 3 days after wounding (data not shown). It is thus possible that keratinocyte migration is affected and further investigation is needed to prove it. Although WT and Padi4−/− neutrophils also express PAD2 and PAD3 (ref. 11), our data demonstrate that PAD4, the only nuclear PAD, is essential for the histone H3 citrullination and NETosis in skin wounds. Coudane et al.28 reported that PAD4 is the main PAD isoform detected in scabs of wounds from WT mice, and that PAD2 is unnecessary for citrullination of scab proteins as observed in PAD2-deficient mice, further strengthening the unique deimination role of PAD4 in the wounds.
Figure 3.
PAD4 deficiency facilitates wound repair in normoglycemic mice. (a) Images of H&E staining (upper panels) and confocal microscopy (lower panels) of wounds from WT and Padi4−/− mice 3 days after injury. Scale, 50 ~m. Presence of extracellular DNA (blue streaks) in the scab of WT mice are indicated by yellow arrows, while intact neutrophils in that of Padi4−/− mice are indicated by yellow arrowheads in the H&E images. (b) Representative Western blots of wounds from WT (+/+) and Padi4−/− (−/−) mice. See Supplementary Fig. 8 for quantifications. (c) Photographs of healing wounds of WT and Padi4−/− mice up to 7 days after wounding. Scale, 5 mm. (d) Changes in wound area compared to day 0. Per order in the bar chart, n = 16, 16, 15, 12 for WT groups, n = 12, 12, 12, 9 for Padi4−/− groups, *P<0.05, **P<0.01, ***P<0.001 versus WT, Student’s t test. (e) Percent of WT and Padi4−/− mice that completed wound healing on day 13 and 14 after injury. Day 13: WT (2/11) vs Padi4−/− (6/9), P=0.065; Day 14: WT (4/16) vs Padi4−/− (10/13), **P<0.01; two-tailed Fisher's exact test. (f) Re-epithelialization as determined from H&E staining on wounds from WT and Padi4−/− mice 3 days post wounding. See Supplementary Fig. 9 for histology of wounds. n = 9 for WT, n = 6 for Padi4−/−, ***P<0.001, Student’s t test.
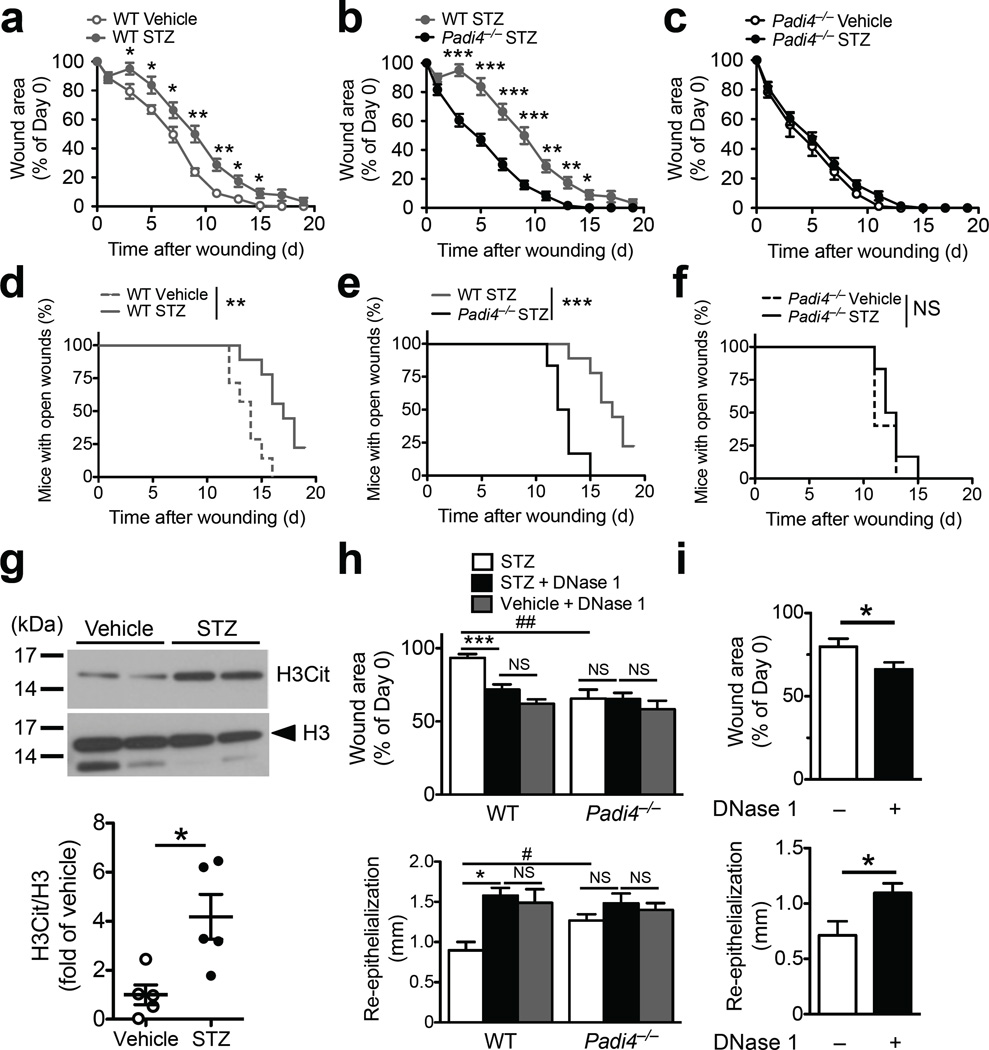
We next examined whether NETs interfere with diabetic wound healing. Type 1 diabetes was induced in WT and Padi4−/− mice by STZ and 8 weeks later these mice were subjected to wounding. Changes in body weight, fed blood glucose and diabetes induction rate were similar between the two genotypes (Supplementary Fig. 2d–f). As expected, diabetic WT mice healed more slowly than normoglycemic controls (Fig. 4a). All normoglycemic WT mice healed by day 16, while ~20% of diabetic mice still had open wounds on day 19 (Fig. 4d). On day 7, diabetic Padi4−/− mice healed >35% faster than diabetic WT mice (Fig. 4b). By day 15, all diabetic Padi4−/− mice were completely healed (Fig. 4e). Notably, diabetes did not impair wound healing in Padi4−/− mice (Fig. 4c,f), which underscores NETs as the major determinant delaying healing in the diabetic mice. Higher H3Cit levels were detected in wounds of STZ-induced diabetic mice compared to the normoglycemic WT mice 1 day post wounding (Fig. 4g). Enhanced NETosis in diabetic animals recapitulates our in vitro observations (Fig. 1e,f), further supporting the role of NETs in the delay in diabetic wound repair. Antibiotics, provided to mimic the medical regimen of diabetic patients with chronic wounds, did not abolish the beneficial effect of PAD4 deficiency (Supplementary Fig. 10).
Figure 4.
PAD4 deficiency or DNase 1 treatment enhances wound healing in diabetic mice. (a–f) Data from all groups were obtained simultaneously in multiple experiments but split into three graphs (a–c and d–f) to facilitate comparison. n = 7 for WT vehicle, n = 9 for WT STZ, n = 5 for Padi4−/− vehicle, n = 6 for Padi4−/− STZ, *P<0.05, **P<0.01, ***P<0.001 and NS, non-significant between groups on respective post-wounding day (a–c, Student’s t test) or between curves (d–f, log-rank test). (a–c) Changes in wound area compared to day 0. (d–f) Percentage of mice with open wounds recorded after injury up to day 19. (g) Representative Western blots of H3Cit levels in wounds one day post wounding from vehicle-treated normoglycemic and STZ-induced diabetic mice (top) and quantification (compared to mean of vehicle after normalization to respective H3 level) (bottom). n = 5 per group, *P<0.05, Mann-Whitney test. (h, i) Wound area reduction (upper panel) and re-epithelialization (lower panel) with DNase 1 (dornase alfa) treatment in (h) diabetic WT and Padi4−/− mice and (i) normoglycemic WT mice. (h) Per order in the bar chart, n = 6, 8, 9 for the WT groups, n = 5, 6, 8 for the Padi4−/− groups, *P<0.05, ***P<0.001 and NS, non-significant using Kruskal-Wallis test followed by Dunn's post test, # P<0.05, ##P<0.01 using Mann-Whitney test, (i) n = 9 without DNase 1, n = 10 with DNase 1, *P<0.05, Student’s t-test.
NETs and histones directly induce epithelial and endothelial damage23. A high concentration of neutrophil elastase, a component of NETs1, can cause degradation of the wound matrix and delay healing29. Such a toxic environment produced by NETs may explain the slower keratinocyte repopulation in the wound beds of WT mice. Because PAD4 is not expressed in the skin25, its negative effect on wound healing is most likely due to infiltrating neutrophils. Using NETosis to defend against microbes may not be very effective during wound healing as Staphylococcus species, which are very abundant in diabetic wounds30, degrade NETs to escape trapping31.
Pre-digestion of NETs with DNase 1 accelerated their clearance by macrophages in vitro32. We thus tested whether systemic DNase 1 treatment could accelerate wound healing in diabetic mice that were maintained on antibiotics. Without DNase 1 treatment, wound healing was faster in diabetic Padi4−/− mice, as assessed by 28% more reduction in wound area (Fig. 4h, upper panel) and 41% more re-epithelialization (Fig. 4h, lower panel) compared to the diabetic WT mice as examined on day 3 post wounding. Administration of DNase 1 reduced wound area faster by >20% and enhanced re-epithelialization by >75% in diabetic WT mice, an extent similar to that of DNase 1-treated normoglycemic WT mice (Fig. 4h). DNase 1 treatment did not further improve wound healing in diabetic Padi4−/− mice (Fig. 4h). These data indicate that NETs are the major source of extracellular DNA that hinders wound healing. Such beneficial effects of DNase 1 were not confined to diabetic wounds. Three days post wounding, wound areas in normoglycemic mice treated with DNase 1 were smaller than in those treated with vehicle (Fig. 4i, upper panel). Re-epithelialization was also enhanced by 54% in the DNase 1-treated group (Fig. 4i, lower panel), while neutrophil recruitment was not affected (data not shown).
Our results indicate that plasma DNase 1 activity may regulate wound healing. Less functional polymorphisms of DNase I exist in the human population33, 34. These polymorphisms or the presence of inhibitors impairing DNase 1 function35 predisposes individuals to cardiovascular and autoimmune disease, likely because DNA (NETs) are not dismantled and removed in a timely manner33–35. Wound healing could be similarly affected in individuals with decreased DNase 1 activity. Topical treatment with an ointment containing fibrinolysin and DNase (Elase) is sometimes used clinically for wound debridement. In addition to removing necrotic tissue, our findings suggest that the DNase component may also cleave NETs to enhance wound recovery.
In summary, our data demonstrate that diabetes activates neutrophils to overproduce PAD4 and NETs and identify NETs as a key factor delaying wound healing. PAD4 inhibition and cleavage of NETs by DNase 1 could be novel therapeutic approaches to wound resolution, not only in diabetes, but also to wounds resulting from aseptic procedures such as surgeries of normoglycemic patients. We further validate the importance of PAD4 in human disease, and report the upregulation of PAD4 in individuals with diabetes, thus providing new rationale to develop specific PAD4 inhibitors36. Although NETs were first postulated to limit infection1, a lack of NETs did not worsen bacteremia in PAD4-deficient mice which were subjected to polymicrobial sepsis27, indicating that NET inhibition will not likely render the host vulnerable to bacterial infections. Because PAD4 and NET formation contribute to inflammatory and thrombotic diseases4, 5 that are prominent in diabetics37, 38, anti-NET therapy could have additional benefits. The increased NETosis in diabetes suggests that NETs may fuel these disorders and inhibiting NETosis or cleavage of NETs may lessen them.
METHODS
Methods and associated references are available in the online version of the paper.
Supplementary Material
ACKNOWLEDGMENTS
We thank H. Ferris for advice on diabetes protocols; L. DeVita for selection of diabetic patients; P. Forbes (The Harvard Clinical and Translational Science Center, NIH Award UL1 TR001102) for statistical advice; J. E. Cabral and S. Cifuni for valuable technical support; and L. Cowan for manuscript preparation assistance. This study was supported by the American Diabetes Association (Innovation Award 7-13-IN-44, D.D.W.), National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL102101, D.D.W.), National Cancer Institute (R01CA136856, Y.W.), National Institute of Diabetes and Digestive and Kidney Diseases (R01DK031036, C.R.K) and a GlaxoSmithKline/Immune Disease Institute Alliance Fellowship (S.L.W.).
Footnotes
AUTHOR CONTRIBUTIONS
S.L.W. designed the study, performed the majority of the experiments, analyzed the data and wrote the manuscript; M.D. and K.M. performed experiments and analyzed data; M.G. provided expert technical assistance; Y.W. provided Padi4−/− mice and critical discussion of the work; A.B.G. provided clinical advice and selected diabetic patients for in vitro NETosis assays; C.R.K. provided helpful suggestions on experimental design and critical reading of the manuscript; D.D.W. designed the study, supervised the project and co-wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- 4.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 5.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karima M, et al. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol. 2005;78:862–870. doi: 10.1189/jlb.1004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanses F, Park S, Rich J, Lee JC. Reduced neutrophil apoptosis in diabetic mice during staphylococcal infection leads to prolonged TNFα production and reduced neutrophil clearance. PLoS One. 2011;6:e23633. doi: 10.1371/journal.pone.0023633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas GM, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandraki KI, et al. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J Clin Immunol. 2008;28:314–321. doi: 10.1007/s10875-007-9164-1. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y, et al. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochemistry. 2006;45:11727–11736. doi: 10.1021/bi061180d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leshner M, et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexiewicz JM, Kumar D, Smogorzewski M, Klin M, Massry SG. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995;123:919–924. doi: 10.7326/0003-4819-123-12-199512150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One. 2014;9:e97088. doi: 10.1371/journal.pone.0097088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Espinosa O, Rojas-Espinosa O, Moreno-Altamirano MM, Lopez-Villegas EO, Sanchez-Garcia FJ. Metabolic requirements for neutrophil extracellular traps (NETs) formation. Immunology. 2015;145:213–224. doi: 10.1111/imm.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menegazzo L, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2014 doi: 10.1007/s00592-014-0676-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Joshi MB, et al. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013;587:2241–2246. doi: 10.1016/j.febslet.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 18.Riyapa D, et al. Neutrophil extracellular traps exhibit antibacterial activity against burkholderia pseudomallei and are influenced by bacterial and host factors. Infect Immun. 2012;80:3921–3929. doi: 10.1128/IAI.00806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebecchi IM, Ferreira Novo N, Julian Y, Campa A. Oxidative metabolism and release of myeloperoxidase from polymorphonuclear leukocytes obtained from blood sedimentation in a Ficoll-Hypaque gradient. Cell Biochem Funct. 2000;18:127–132. doi: 10.1002/(SICI)1099-0844(200006)18:2<127::AID-CBF865>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp. 2010:1724. doi: 10.3791/1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–455. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 23.Saffarzadeh M, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramaniam M, et al. Role of endothelial selectins in wound repair. Am J Pathol. 1997;150:1701–1709. [PMC free article] [PubMed] [Google Scholar]
- 25.Nachat R, et al. Peptidylarginine deiminase isoforms 1–3 are expressed in the epidermis and involved in the deimination of K1 and filaggrin. J Invest Dermatol. 2005;124:384–393. doi: 10.1111/j.0022-202X.2004.23568.x. [DOI] [PubMed] [Google Scholar]
- 26.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 27.Martinod K, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125:1948–1956. doi: 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coudane F, et al. Deimination and expression of peptidylarginine deiminases during cutaneous wound healing in mice. Eur J Dermatol. 2011;21:376–384. doi: 10.1684/ejd.2011.1394. [DOI] [PubMed] [Google Scholar]
- 29.Herrick S, et al. Up-regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers are associated with matrix degradation. Lab Invest. 1997;77:281–288. [PubMed] [Google Scholar]
- 30.Grice EA, et al. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc Natl Acad Sci U S A. 2010;107:14799–14804. doi: 10.1073/pnas.1004204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berends ET, et al. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191:2647–2656. doi: 10.4049/jimmunol.1300436. [DOI] [PubMed] [Google Scholar]
- 33.Bodano A, et al. Study of DNASE I gene polymorphisms in systemic lupus erythematosus susceptibility. Ann Rheum Dis. 2007;66:560–561. doi: 10.1136/ard.2006.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumamoto T, et al. Association of Gln222Arg polymorphism in the deoxyribonuclease I (DNase I) gene with myocardial infarction in Japanese patients. Eur Heart J. 2006;27:2081–2087. doi: 10.1093/eurheartj/ehl177. [DOI] [PubMed] [Google Scholar]
- 35.Hakkim A, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis HD, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol. 2015;11:189–191. doi: 10.1038/nchembio.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 38.Morel O, Jesel L, Abbas M, Morel N. Prothrombotic changes in diabetes mellitus. Semin Thromb Hemost. 2013;39:477–488. doi: 10.1055/s-0033-1343888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.