Abstract
The neuronal ceroid lipofuscinoses (NCLs) are lysosomal storage disorders and together are the most common degenerative brain diseases in childhood. They are a group of disorders linked by the characteristic accumulation of abnormal storage material in neurons and other cell types, and a degenerative disease course. All NCLs are characterized by a combination of dementia, epilepsy, and motor decline. For most childhood NCLs, a progressive visual failure is also a core feature. The characteristics of these symptoms can vary and the age at disease onset ranges from birth to young adulthood. Genetic heterogeneity, with fourteen identified NCL genes and wide phenotypic variability render diagnosis difficult. A new NCL classification system based on the affected gene and the age at disease onset allows a precise and practical delineation of an individual patient’s NCL type. A diagnostic algorithm to identify each NCL form is presented here. Precise NCL diagnosis is essential not only for genetic counseling, but also for the optimal delivery of care and information sharing with the family and other caregivers. These aspects are challenging because there are also potential long term complications which are specific to NCL type. Therefore care supported by a specifically experienced team of clinicians is recommended. As the underlying pathophysiological mechanism is still unclear for all NCL forms, the development of curative therapies remains difficult. This article is part of a Special Issue entitled: The neuronal ceroid lipofuscinoses or Batten Disease.
Keywords: Batten, Ceroid, NCLs, Disease classification, Diagnostic algorithm
1. Introduction and definition
Diagnosis of childhood dementia represents a huge challenge. The neuronal ceroid lipofuscinoses (NCLs) are the most common cause of dementia in children. They are a group of diverse disorders linked by the characteristic accumulation of abnormal storage material in neurons and other cell types, and a degenerative disease course. They form a heterogeneous group of incurable lysosomal storage diseases which lead to dementia, epilepsy, blindness (usually) and motor deterioration [1,2]. The number of different NCL causing genes is high with significant variability within and across forms.
The authors of this article, clinicians with particular interest and experience of the NCLs, want to show that it is possible to diagnose NCL disease in an economical manner and give hints for the management of disease-specific problems.
2. New nomenclature of NCL diseases
Traditionally, NCL diseases were classified according to the age at disease onset (congenital, infantile, late infantile, juvenile, adult) and sometimes also according to the respective authors (Haltia-Santavuori, Jansky-Bielschowsky, Batten, Spielmeyer-Vogt, Kufs) [2].
NCL diseases are however much more genetically heterogeneous than initially thought. Mutations in the same gene may also lead to very different disease courses [3,4]. Other designations such as “Finnish” or “Turkish” NCL variant are outdated, as mutations in the respective genes in fact occur worldwide [5]. Therefore, the hitherto existing nomenclature is obsolete.
An internationally developed new NCL nomenclature clearly identifies each NCL disease both genetically and clinically (Table 1) [2,6]: it classifies both the defective gene as well as the age at disease onset (congenital, infantile, late infantile, juvenile or adult). An exact diagnosis is essential for genetic counseling, sharing information regarding prognosis and future disease course, and for optimal symptom care.
Table 1.
Genetic spectrum and new nomenclature of NCL diseases.
| Disease | MIM number/reference | Gene | Protein |
|---|---|---|---|
| CLN1 disease, infantile | #256730 | CLN1/PPT1 | PPT1a |
| CLN1 disease, late-infantile | |||
| CLN1 disease, juvenile | |||
| CLN1 disease, adult | |||
| CLN2 disease, late-infantile | #204500 | CLN2/TPP1 | TPP1a |
| CLN2 disease, juvenile | |||
| CLN3 disease, juvenile | #204200 | CLN3 | Transmembrane protein |
| CLN4 disease, adult (AD inheritance) | #162350 | CLN4/DNAJC5 | Soluble cysteine string protein α |
| CLN5 disease, late-infantile | #256731 | CLN5 | Soluble lysosomal protein |
| CLN5 disease, juvenile | |||
| CLN5 disease, adult | |||
| CLN6 disease, late-infantile | #601780 | CLN6 | Transmembrane protein |
| CLN6 disease, adult (Kufs type A) | |||
| CLN7 disease, late-infantile | #610951 | CLN7/MFSD8 | Transmembrane protein |
| CLN8 disease, late-infantile | #600143 | CLN8 | Transmembrane protein |
| CLN8 disease, EPMR | |||
| CLN10 disease, congenital | #610127 | CLN10/CTSD | Cathepsin Da |
| CLN10 disease, late-infantile | |||
| CLN10 disease, juvenile | |||
| CLN10 disease, adult | |||
| CLN11 disease, adult | [9] | CLN11/GRN | Progranulinb |
| CLN12 disease, juvenile | [10] | CLN12/ATP13A2 | ATPase type 13A2c |
| CLN13 disease, adult (Kufs type B) | [13] | CLN13/CTSF | Cathepsin Fa |
| CLN14 disease, infantile | [12] | CLN14/KCTD7 | Potassium channel tetramerization domain containing protein type 7d |
Lysosomal enzymes.
GRN mutations also in Frontotemporal lobar degeneration with TDP43 inclusions MIM #607485.
ATP13A2 mutations also in Kufor–Rakeb syndrome (KRS, Parkinson disease 9) MIM #606693.
KCTD7 mutations also seen in progressive myoclonic epilepsy type 3 (EPM3) MIM #611726.
3. The genetic spectrum of NCL diseases
To date, fourteen different NCL forms have been described (Table 1) [3,7–13]. More NCL genes remain to be identified as in some patients mutations cannot be demonstrated in any of the known NCL genes although they present with the typical NCL symptoms and characteristic lysosomal storage material.
Intracellular localisation and function (where known) of the defective NCL proteins are different: four NCL types are caused by defects in lysosomal enzymes (CLN1, CLN2, CLN10, CLN13), others by defects in transmembrane proteins (CLN3, CLN6, CLN7, CLN8) [7]. Mutations in an ATPase gene (CLN12) [10] and a potassium channel gene (CLN14) [12] also cause NCL disease. The recently identified CLN4 gene (DNAJC5) codes for a protein with putative function in synapses [8]. How these genetic defects lead to neurodegeneration is still not understood.
Clinically, the different NCL diseases have much in common despite their heterogeneity. This is important both for diagnosis and (palliative) treatment. To date, there is no disease-modifying or curative treatment for any of the NCLs.
4. The clinical spectrum of NCL diseases
In almost all NCL forms the patients are initially healthy and have a normal developmental profile. The main alerting symptoms are the combination of two or more of dementia, visual loss, epilepsy, and motor deterioration. The age at disease onset can range from birth to adulthood. The order in which symptoms occur is variable and depends both on age at onset and on genetic form. In a young child, first symptoms are developmental slowing followed by standstill, then later regression of psychomotor development, or epilepsy. In a school child, first symptoms are usually visual loss and behavior change, followed by dementia [2]. The different disease courses are described as follows [2]:
4.1. Congenital onset NCL
Congenital CLN10 disease is the only NCL form where patients are already severely affected at birth. Intrauterine or immediate postnatal onset of epileptic seizures as well as congenital microcephaly should lead to the suspected diagnosis. The disease leads to death in early infancy. It is associated with the deficiency of the lysosomal enzyme cathepsin D. Confirmation of the diagnosis is based on demonstration of the enzymatic deficiency and a mutation in the CLN10 gene [14].
4.2. Infantile onset NCL
4.2.1. CLN1 disease
In patients with this NCL form [15], early development appears normal until 6–18 months of age. At onset, there is typically decreased tone and decreased social interaction followed by rapidly progressive psychomotor regression, myoclonus, seizures, and visual failure. By 2 years of age, there is blindness with optic atrophy and macular and retinal changes but no pigment aggregation. Fulminant brain atrophy leads to progressive microcephaly. The electroencephalogram becomes flat. There is also early extinction on the electroretinogram. Seizures in infantile NCL may not be as prominent as in later-onset forms. Ultimately, spasticity develops and patients become vegetative [16]. The disease is associated with the deficiency of the lysosomal enzyme palmitoyl protein thioesterase 1 (PPT1) and is caused by mutations in CLN1. Diagnosis is based on the enzyme deficiency and mutation of the gene.
4.2.2. CLN14 disease
Two infant siblings have been reported who presented with myoclonus, developmental regression and visual failure. A mutation in KCTD7, a gene responsible for the function of a potassium channel, was found [12].
4.3. Late-infantile onset NCL
The classic late infantile onset NCL is caused by mutations in CLN2, but many other forms also present between ages 1 and 4 years.
4.3.1. CLN2 disease
Patients with this classic late infantile NCL [17] typically present with slowing of development and psychomotor regression, usually in the second or third year of life. Epilepsy typically develops between 2 and 4 years of age. Epilepsy takes many forms in this NCL form and is often refractory to medical treatment. Vision loss is associated with abnormal ERG and visual evoked potential (VEP). Retinal degeneration is most visible in the macula. The general decay of psychomotor functions is rapid and uniform between the third and fifth birthday [18]. Children with later onset (after 4 years) tend to have milder course with more prominent ataxia and less prominent epilepsy. The disease is associated with the deficiency of the lysosomal enzyme tripeptidyl peptidase 1 (TPP1) and is caused by mutations in CLN2, the demonstration of both of which serves for diagnosis.
4.3.2. CLN1 disease
CLN1 disease is characterized by visual and cognitive decline followed by ataxia and myoclonus [19].
4.3.3. CLN5 disease
This form of late-infantile NCL [20] has been called the “Finnish variant,” but it occurs world-wide [5]. Age at onset is more variable than in classic CLN2 disease, a mean age at onset of 5.6 years (range 4–17 years). Clinical features include psychomotor regression, ataxia, myoclonic epilepsy, and visual failure. Vision loss may be the presenting sign. Diagnosis depends on analysis of CLN5.
4.3.4. CLN6 disease
CLN6 disease [21] has a variable age at onset ranging from 18 months to 8 years. Clinical features include motor delay, dysarthria, ataxia, vision loss, and seizures. Seizures are an early feature, starting before age 5 years in the majority of patients. Early vision loss occurs in about half of patients with this disease. Deterioration is rapid and death usually occurs between 5 and 12 years of age. Diagnosis depends on analysis of CLN6.
4.3.5. CLN7 disease
CLN7 disease [22] has also been called the “Turkish” variant but has been shown to occur world-wide. Age at onset is 2–7 years. The initial symptom is typically seizures followed by progressive motor decline, myoclonus, cognitive changes, and vision loss. Diagnosis is through gene analysis.
CLN8 disease occurs in two primary forms [23]. The late-infantile form is characterized by vision loss, myoclonic seizures, progressive motor and cognitive decline, and early death. Age at onset is 5–10 years of age. CLN8 mutations have also been associated with “Northern Epilepsy” which is a form of progressive myoclonus epilepsy but does not have the vision loss associated with NCL [24]. Diagnosis is through gene analysis.
4.4. Juvenile onset NCL
Classic juvenile NCL is due to mutations in the CLN3 gene, but juvenile onset has been reported in other forms.
4.4.1. CLN3 disease
This classic juvenile NCL form [25] presents between ages 4 and 7 years with insidious onset of blindness caused by retinal degeneration (Fig. 1). Progressive cognitive decline and behavioral problems follow. The behavior problems are typically characterized as angry outbursts, physical violence, and anxiety with features of depression. Seizures develop slightly later. Generalized tonic–clonic seizures are the most common in CLN3 disease, but are typically well-controlled with medication at least initially. The movement disorder in CLN3 disease is parkinsonism, that is sometimes responsive to L-DOPA. CLN3 disease has a severe, characteristic dysarthria that is most prominent after 10 years of age. In addition to the neurologic features, CLN3 patients manifest a cardiac conduction abnormality in the second decade of life. Age at death is usually in the third decade. Diagnosis relies on the demonstration of vacuolated lymphocytes in peripheral blood and on mutations in CLN3, where a typical deletion is found in the wide majority of cases.
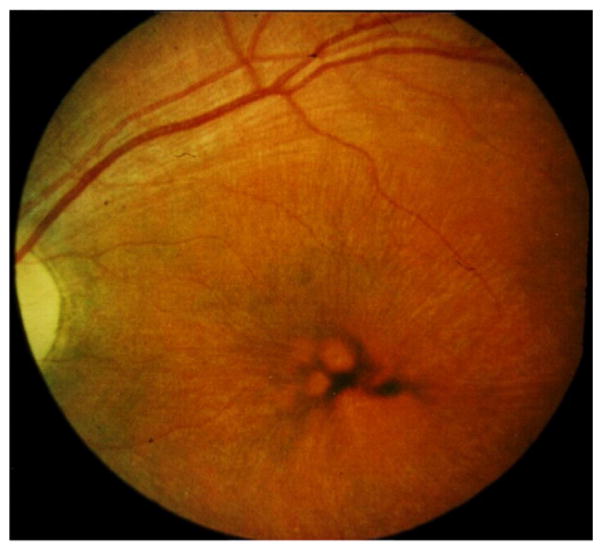
Fig. 1.
Fundoscopic appearance of the retina of a patient with juvenile CLN3 disease. Irregular pigment distribution and thin blood vessels are visible.
4.4.2. CLN1 disease
Juvenile-onset NCL due to CLN1 mutations [26] differs from both CLN3 disease and classic infantile NCL [15]. Age at onset is 5–10 years with cognitive decline as the earliest symptom in most. Seizures occur in multiple forms. Motor decline occurs, but there is typically neither parkinsonism nor myoclonus. Spasticity and ataxia may develop. Vision loss is late in this form, occurring usually between ages 10 and 14 years. Diagnosis is as for the infantile form of CLN1 disease.
4.5. Adult onset NCL
Classic adult onset NCL (Kufs disease) had been categorized as CLN4 disease, but it is now apparent that there are multiple causes of adult-onset NCL.
4.5.1. CLN4 disease
This has recently been reclassified to refer to “Parry disease” associated with mutations in DNAJC5 [11]. This is the only autosomal dominant NCL. Symptom onset occurs after age 30 years. Clinical features include ataxia, progressive dementia, seizures, and myoclonus with no visual loss. Diagnosis depends on mutations in CLN4 (DNAJC5).
4.5.2. CLN6 disease
The Kufs type A variant of adult-onset NCL [27] was recently associated with mutations in CLN6 [8]. This form of NCL has onset around 30 years of age with progressive myoclonic epilepsy followed by development of dementia and ataxia. Dysarthria is prominent. There is no vision loss in this disease. Death typically occurs within 10 years. Kufs Type B is clinically similar, but without the epilepsy and dysarthria. Mutations in the CLN6 gene are diagnostic.
4.5.3. CLN13 disease
The Kufs type B variant [27] presents in the same age range as type A with dementia as well as cerebellar and/or extra-pyramidal signs. The disease is associated with a deficiency of the lysosomal enzyme cathepsin F. Mutations in the responsible gene [13] allow diagnosis.
4.5.4. CLN1 disease
Adult onset NCL due to CLN1 mutations is characterized by onset after 18 years of age [28]. Cognitive decline and depression are the initial manifestations followed by development of ataxia, parkinsonism, and vision loss. Diagnosis, as in other forms of CLN1 disease, is through testing of PPT1 activity and mutation analysis.
The NCLs described above have several features in common suggesting biological similarities or functional interactions between involved genes. The clinical manifestations of the different NLCs differ in age at onset, order of progression, and specific symptoms. This variability suggests either differential gene expression within the CNS or differential vulnerability of different neuronal populations. Knowledge of clinical presentations can help guide diagnostic evaluation in patients with features of NCL. However, more quantitative study of the natural history in NCLs is required to inform neurobiological investigations and development of outcome measures for clinical trials.
5. Strategy for the diagnosis of NCL types
The clinical approach to diagnosis of an NCL disorder starts at the age at which symptoms appear. Suggestive situations can be divided in four typical groups: (1) very young infants, including newborns with congenital epilepsy and microcephaly, (2) young children with developmental standstill or regression and severe epilepsy, (3) school children with visual loss, followed by dementia and epilepsy, and (4) young adults with non-specific mental, motor or behavioral abnormalities. In each of these groups, a characteristic set of NCL types can be expected, caused by variable mutations in the known NCL genes.
Table 2 provides an algorithm of diagnostic investigations once an NCL is considered in a person with suggestive symptoms. Initial laboratory studies comprise enzymatic tests, light and electron microscopic investigations of intracellular storage that, when performed in the logical order shown, lead straight and economically to adequate molecular genetic confirmation.
Table 2.
Diagnostic algorithm for NCL diseases.
| Clinical presentation | Necessary diagnostic | Possibly affected genes |
|---|---|---|
| Newborn with epilepsy and microcephaly | Enzyme testing for cathepsin D (CtsD) (leucocytes of fibroblasts). | |
| CTSD deficient: | CLN10 | |
| Young child (>6 months) with developmental standstill or regression and/or newly occurring severe epilepsy of unknown cause | Enzyme testing for PPT1 and TPP1 (dry blood spots or leucocytes or fibroblasts) | |
| PPT1 deficient: | CLN1 | |
| TPP1 deficient: | CLN2 | |
| If PPT1 and TPP1 enzyme activities are normal: Electron microscopic examination (skin biopsy or lymphocytes). | ||
| If storage material is present: genetic testing. | CLN5, CLN6, CLN7, CLN8, CLN14 | |
| School child with visual loss and/or dementia and epilepsy | Search for lymphocyte vacuoles (light microscopy of blood smear, Fig. 2). | |
| If lymphocyte vacuoles are present: | CLN3 | |
| If no lymphocyte vacuoles, enzyme testing for PPT1, TPP1 and CtsD (see above) | ||
| PPT1 deficient: | CLN1 | |
| TPP1 deficient: | CLN2 | |
| CTSD deficient: | CLN10 | |
| If PPT1 and TPP1 enzyme activities are normal: Electron microscopic examination (skin biopsy or lymphocytes). | ||
| If storage material is present: | CLN5, CLN6, CLN7, CLN8, CLN12 | |
| Young adult with non-specific mental, motor or behavioral abnormalities. | Enzyme testing for PPT1, TPP1 and CtsD (see above) | |
| PPT1 deficient: | CLN1 | |
| TPP1 deficient: | CLN2 | |
| CTSD deficient: | CLN10 | |
| CTSF deficient: | CLN13 | |
| If enzyme activities are normal: Electron microscopic examination (skin biopsy or lymphocytes). | ||
| If storage material is present: genetic testing (eventually in special cases even without detection of storage material), consider possible mode of inheritance. | If autosomal dominant: CLN4 If autosomal recessive: CLN6, CLN11, CLN13 |
6. Specialized palliative therapies in NCL
Palliative therapies in NCL diseases represent a significant challenge due to multiple symptom complexes and affected body systems. Moreover, treatment is difficult as most patients have severe visual impairment and may not be able to communicate verbally with caregivers. Collaboration with a team of clinicians with NCL experience is recommended in order to improve palliative medication: Table 3 is based on experience collected by such teams and gives an overview of medication found to be helpful in treating the most common symptoms of NCLs.
Table 3.
Overview on medication for palliative therapies in NCL.
| Symptom | Substance | Comment |
|---|---|---|
| Epilepsya | Valproate | Advantage: mood stabilizing effect, useful in juvenile NCL patients with psychotic symptoms |
| Lamotrigine | ||
| Topiramate | Increase dosage slowly to minimize side effects such as speech disturbance (starting dose 0.5 mg/kg/d). Agitation may be a side effect. In this case discontinue the drug. | |
| Levetiracetame | Severe agitation is a possible side effect in juvenile NLC. | |
| Diazepam, lorazepam | Acute therapy of prolonged grand mal seizures | |
| Myoclonus | Levetiracetame | Also effective as anti-epileptic medication (especially in late infantile NCL) |
| Zonisamide | ||
| Piracetame | High dosage required (300–350 mg/kg/d) | |
| Spasticity | Baclofen (1st choice) | Frequently high dosage required |
| Tizanidine (2nd choice) | Good effect also against dyskinesia | |
| Tetrahydrocannabinol | “Add-on” medication, increase dosage slowly up to 0.07 mg/kg/d, | |
| Botulinum toxin | Local application by injection to muscles; always accompanied by physical therapy |
Avoid overtreatment, as most seizures in NCL are treatment-resistant.
Epilepsy in NCL is therapy-resistant in most cases. Progressive brain degeneration leads to changes in the effectiveness of medication and to unexpected toxicity. Therefore, the following rules should be followed for treating seizures in NCL:
Neither complete absence of seizures nor normalization of electroencephalogram (EEG) is realistic goal of treatment.
The EEG in NCL is mainly for monitoring. Therapy should be adjusted to clinical symptoms.
Therapy with more than two anticonvulsants may result in increased side effects rather than reduction of seizures.
Some anticonvulsants are recommendable for NCL patients (valproate and lamotrigine), others may have negative effects on the disease course and should be avoided (carbamazepine, phenytoin, vigabatrin). See Table 3.
Use only as many drugs as necessary and as few as possible.
Myoclonus is a symptom difficult to treat. However, it is often more distressing for caregivers than to the patients. Levetiracetam and piracetam are at least partially effective treatments (Table 3). Some antiepileptic drugs can aggravate myoclonus and should be avoided (carbamazepine, gabapentin and lamotrigine in late infantile NCL types). Reduction or rationalization of a patient’s medication load may sometimes improve myoclonus.
Spasticity, when it causes discomfort, should be treated with physical therapy and medication. Baclofen and tizandine are effective. Tetrahydrocannabinol has also been used. Benzodiazepines may be effective but have frequent side effects, lose their efficacy with time, and sometimes appear to have a negative effects on the general disease course. Underlying painful events (see below) may trigger terrifying spastic exacerbations and must be recognized.
Episodes of apparent or suspected pain are frequent and are caused by a great variety of factors. Some of them are related to chronic immobility such as pathological skeletal fractures, renal calculi, or venous thrombosis. Abdominal pain may be caused by constipation due to disease-related intestinal hypomotility or malnutrition. Recognition and management of pain in NCL patients requires particular skills [29].
Diagnosis and treatment of tormenting psychopathological symptoms such as sleep disturbance, fear, aggressive behavior, depression, and hallucinations represent a particular challenge. Careful and painstaking documentation of these symptoms, recognition of context and possible triggers can be extremely helpful in managing environmental exacerbating factors. Psychopharmacologic medication should be given only after careful consideration and in consultation with child neurologists and child psychiatrists, parents, and caregivers. Drugs to consider for psychotic symptoms in juvenile NCL are risperidone for hallucinations or panic attacks and fluvoxamine for anxiety and depression.
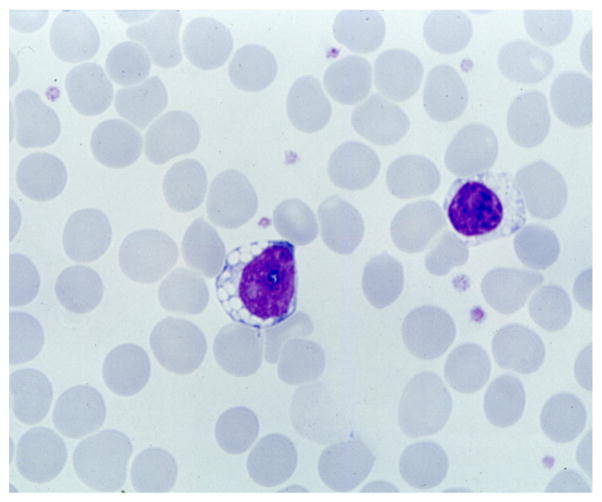
Fig. 2.
Vacuoles in the cytoplasm of a peripheral blood lymphocyte from a patient with juvenile CLN3 disease. Routine blood smear.
Acknowledgments
We thank the children and families from whom we have learned so much. The work leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 281234.
Footnotes
This article is part of a Special Issue entitled: The Neuronal Ceroid Lipofuscinoses or Batten Disease.
References
- 1.Haltia M. The neuronal ceroid-lipofuscinoses: from past to present. Biochim Biophys Acta. 2006;1762:850–856. doi: 10.1016/j.bbadis.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Mole SE, Williams R, Goebel HH. Contemporary Neurology Series. Oxford University Press; Oxford: 2011. The neuronal ceroid lipofuscinoses (Batten disease) p. 480. [Google Scholar]
- 3.Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat. 2011;33:42–63. doi: 10.1002/humu.21624. [DOI] [PubMed] [Google Scholar]
- 4.Lebrun AH, Moll-Khosrawi P, Pohl S, Makrypidi G, Storch S, Kilian D, Streichert T, Otto B, Mole SE, Ullrich K, Cotman S, Kohlschutter A, Braulke T, Schulz A. Analysis of potential biomarkers and modifier genes affecting the clinical course of CLN3 disease. Mol Med. 2011;17:1253–1261. doi: 10.2119/molmed.2010.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebrun AH, Storch S, Ruschendorf F, Schmiedt ML, Kyttala A, Mole SE, Kitzmuller C, Saar K, Mewasingh LD, Boda V, Kohlschutter A, Ullrich K, Braulke T, Schulz A. Retention of lysosomal protein CLN5 in the endoplasmic reticulum causes neuronal ceroid lipofuscinosis in Asian sibship. Hum Mutat. 2009;30:E651–E661. doi: 10.1002/humu.21010. [DOI] [PubMed] [Google Scholar]
- 6.Williams RE, Mole SE. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology. 2012;79:183–191. doi: 10.1212/WNL.0b013e31825f0547. [DOI] [PubMed] [Google Scholar]
- 7.Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793:697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Arsov T, Smith KR, Damiano J, Franceschetti S, Canafoglia L, Bromhead CJ, Andermann E, Vears DF, Cossette P, Rajagopalan S, McDougall A, Sofia V, Farrell M, Aguglia U, Zini A, Meletti S, Morbin M, Mullen S, Andermann F, Mole SE, Bahlo M, Berkovic SF. Kufs disease, the major adult form of neuronal ceroid lipofuscinosis, caused by mutations in CLN6. Am J Hum Genet. 2011;88:566–573. doi: 10.1016/j.ajhg.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, Rossi G, Pareyson D, Mole SE, Staropoli JF, Sims KB, Lewis J, Lin WL, Dickson DW, Dahl HH, Bahlo M, Berkovic SF. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bras J, Verloes A, Schneider SA, Mole SE, Guerreiro RJ. Mutation of the Parkinson-ism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum Mol Genet. 2012;21:4240–4246. doi: 10.1093/hmg/dds089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noskova L, Stranecky V, Hartmannova H, Pristoupilova A, Baresova V, Ivanek R, Hulkova H, Jahnova H, van der Zee J, Staropoli JF, Sims KB, Tyynela J, Van Broeckhoven C, Nijssen PC, Mole SE, Elleder M, Kmoch S. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am J Hum Genet. 2011;89:241–252. doi: 10.1016/j.ajhg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staropoli JF, Karaa A, Lim ET, Kirby A, Elbalalesy N, Romansky SG, Leydiker KB, Coppel SH, Barone R, Xin W, MacDonald ME, Abdenur JE, Daly MJ, Sims KB, Cotman SL. A homozygous mutation in KCTD7 links neuronal ceroid lipofuscinosis to the ubiquitin–proteasome system. Am J Hum Genet. 2012;91:202–208. doi: 10.1016/j.ajhg.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith KR, Dahl HH, Canafoglia L, Andermann E, Damiano J, Morbin M, Bruni AC, Giaccone G, Cossette P, Saftig P, Grotzinger J, Schwake M, Andermann F, Staropoli JF, Sims KB, Mole SE, Franceschetti S, Alexander NA, Cooper JD, Chapman HA, Carpenter S, Berkovic SF, Bahlo M. Cathepsin F mutations cause Type B Kufs disease, an adult-onset neuronal ceroid lipofuscinosis. Hum Mol Genet. 2013;22:1417–1423. doi: 10.1093/hmg/dds558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J, Lehesjoki AE, Tyynela J. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006;129:1438–1445. doi: 10.1093/brain/awl107. [DOI] [PubMed] [Google Scholar]
- 15.Autti T, Cooper JD, Van Diggelen OP, Haltia M, Jalanko A, Kitzmüller C, Kopra O, Lönnqvist T, Lyly A, Mole SE, Rapola J, Vanhanen SL. CLN1. In: Mole SE, Williams RE, Goebel HH, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Oxford University Press; Oxford: 2011. pp. 55–79. [Google Scholar]
- 16.Santavuori P, Vanhanen SL, Sainio K, Nieminen M, Wallden T, Launes J, Raininko R. Infantile neuronal ceroid-lipofuscinosis (INCL): diagnostic criteria. J Inherit Metab Dis. 1993;16:227–229. doi: 10.1007/BF00710250. [DOI] [PubMed] [Google Scholar]
- 17.Chang M, Cooper JD, Davidson BL, van Diggelen OP, Elleder M, Goebel HH, Golabek AA, Kida E, Kohlschütter A, Lobel P, Mole SE, Schulz A, Sleat DE, Warburton M, Wisniewski KE. CLN2. In: Mole SE, Williams RE, Goebel HH, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Oxford University Press; Oxford: 2011. pp. 80–109. [Google Scholar]
- 18.Steinfeld R, Heim P, Von Gregory H, Meyer K, Ullrich K, Goebel HH, Kohlschütter A. Late infantile neuronal ceroid lipofuscinosis: quantitative description of the clinical course in patients with CLN2 mutations. Am J Med Genet. 2002;112:347–354. doi: 10.1002/ajmg.10660. [DOI] [PubMed] [Google Scholar]
- 19.Bonsignore M, Tessa A, Di Rosa G, Piemonte F, Dionisi-Vici C, Simonati A, Calamoneri F, Tortorella G, Santorelli FM. Novel CLN1 mutation in two Italian sibs with late infantile neuronal ceroid lipofuscinosis. Eur J Paediatr Neurol. 2006;10:154–156. doi: 10.1016/j.ejpn.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Aberg L, Autti T, Cooper JD, Elleder M, Haltia M, Jalanko A, Kitzmüller C, Kopra O, Mole S, Nuutila A, Peltonen L, Punkari ML, Rapola J, Tyynela J. CLN5. In: Mole SE, Williams RE, Goebel HH, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Oxford University Press; Oxford: 2011. pp. 140–158. [Google Scholar]
- 21.Alroy J, Braulke T, Cismondi IA, Cooper JD, Creegan D, Elleder M, Kitzmüller C, Kohan R, Kohlschütter A, Mole SE, Noher de Halac I, Pfannl R, Quitsch A, Schulz A. CLN6. In: Mole SE, Williams RE, Goebel HH, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Oxford University Press; Oxford: 2011. pp. 159–175. [Google Scholar]
- 22.Elleder M, Kousi M, Lehesjoki AE, Mole S, Siintola E, Topçu M. CLN7. In: Mole SE, Williams RE, Goebel HH, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Oxford University Press; Oxford: 2011. pp. 176–188. [Google Scholar]
- 23.Aiello C, Cannelli N, Cooper JD, Haltia M, Herva R, Lahtinen U, Lehesjoki AE, Mole S, Santorelli FM, Siintola E, Simonati A. CLN8. In: Mole SE, Williams RE, Goebel HH, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Oxford University Press; Oxford: 2011. pp. 189–202. [Google Scholar]
- 24.Herva R, Tyynela J, Hirvasniemi A, Syrjakallio-Ylitalo M, Haltia M. Northern epilepsy: a novel form of neuronal ceroid-lipofuscinosis. Brain Pathol (Zurich, Switzerland) 2000;10:215–222. doi: 10.1111/j.1750-3639.2000.tb00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Åberg L, Autti T, Braulke T, Cooper JD, van Diggelen OP, Jalanko A, Kenrick S, Kitzmüller C, Kohlschütter A, Kyttälä A, Mitchison HM, Mole SE, Niezen-de Boer R, Punkari ML, Schulz A, Talling M, Williams RE. CLN3. In: Mole SE, Williams RE, Goebel HH, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Oxford University Press; Oxford: 2011. pp. 110–139. [Google Scholar]
- 26.Kalviainen R, Eriksson K, Losekoot M, Sorri I, Harvima I, Santavuori P, Jarvela I, Autti T, Vanninen R, Salmenpera T, van Diggelen OP. Juvenile-onset neuronal ceroid lipofuscinosis with infantile CLN1 mutation and palmitoyl-protein thioesterase deficiency. Eur J Neurol. 2007;14:369–372. doi: 10.1111/j.1468-1331.2007.01668.x. [DOI] [PubMed] [Google Scholar]
- 27.Berkovic SF, Andermann F, Andermann E, Carpenter S, Wolfe L. Kufs disease: clinical features and forms. Am J Med Genet Suppl. 1988;5:105–109. doi: 10.1002/ajmg.1320310614. [DOI] [PubMed] [Google Scholar]
- 28.Ramadan H, Al-Din AS, Ismail A, Balen F, Varma A, Twomey A, Watts R, Jackson M, Anderson G, Green E, Mole SE. Adult neuronal ceroid lipofuscinosis caused by deficiency in palmitoyl protein thioesterase 1. Neurology. 2007;68:387–388. doi: 10.1212/01.wnl.0000252825.85947.2f. [DOI] [PubMed] [Google Scholar]
- 29.Hauer J. Identifying and managing sources of pain and distress in children with neurological impairment. Pediatr Ann. 2010;39:198–205. doi: 10.3928/00904481-20100318-04. (quiz 232-194) [DOI] [PubMed] [Google Scholar]