Despite the known adverse effects of smoking, including lung cancer and other respiratory diseases, a significant proportion of the Canadian population continue to smoke. The global epidemic of smoking is compounded by the known effects of secondhand smoke, which exposes non-smoking individuals to a similar mix of toxic fumes and potentially adverse effects. Electronic nicotine delivery systems have emerged in the wake of governmental efforts to limit tobacco exposure and have been proposed as a possible solution. However, electronic nicotine delivery systems have not been tightly regulated or comprehensively studied. This systematic review investigated one of the central questions surrounding the increasing popularity of these devices.
Keywords: Electronic nicotine delivery system, Nicotine replacement, Quit smoking, Smoking cessation, Tobacco cessation
Abstract
BACKGROUND:
Recent studies have estimated that 21% of all deaths over the past decade are due to smoking, making it the leading cause of premature death in Canada. To date, many steps have been taken to eradicate the global epidemic of tobacco smoking. Most recently, electronic nicotine delivery systems (ENDS) have become a popular smoking cessation tool. ENDS do not burn or use tobacco leaves, but instead vapourize a solution the user then inhales. The main constituents of the solution, in addition to nicotine when nicotine is present, are propylene glycol, with or without glycerol and flavouring agents. Currently, ENDS are not regulated, and have become a controversial topic.
OBJECTIVES:
To determine whether ENDS are an effective smoking cessation tool.
METHODS:
A systematic literature search was conducted in February 2015 using the following databases: PubMed, Scopus and Web of Science Core Collection. Randomized controlled trials were the only publications included in the search. A secondary search was conducted by reviewing the references of relevant publications.
RESULTS:
After conducting the primary and secondary search, 109 publications were identified. After applying all inclusion and exclusion criteria through abstract and full-text review, four publications were included in the present literature review. A low risk of bias was established for each included study using the Cochrane Collaboration risk of bias evaluation framework.
DISCUSSION:
The primary outcome measured in all studies was self-reported abstinence or reduction from smoking. In three of the four studies, self-reported abstinence or reduction from smoking was verified by measuring exhaled carbon monoxide. In the remaining study, the primary outcome measured was self-reported desire to smoke and measured desire to smoke. All four studies showed promise that ENDS are an effective smoking cessation tool.
CONCLUSIONS:
While all publications included in the present review revealed that ENDS are effective smoking cessation aid, further evaluation of the potential health effects in long-term use of ENDS remains vital.
Abstract
HISTORIQUE :
Selon les études récentes, 21 % de tous les décès depuis dix ans sont attribuables au tabagisme, ce qui en fait la principale cause de décès prématurés au Canada. Jusqu’à présent, de nombreuses mesures ont été prises pour éradiquer l’épidémie mondiale de tabagisme. Récemment, les systèmes électroniques de délivrance de nicotine (SÉDN) sont devenus un outil populaire d’abandon du tabac. Les SÉDN ne brûlent ni n’utilisent de feuilles de tabac, mais vaporisent une solution que le consommateur inhale. Les principaux éléments de la solution, en plus de la nicotine lorsque le système en contient, sont le propylèneglycol, accompagné ou non de glycérol et de substances aromatisantes. Les SÉDN ne sont pas réglementés, mais sont devenus controversés.
OBJECTIFS :
Déterminer si les SÉDN sont un outil efficace d’abandon du tabac.
MÉTHODOLOGIE :
Les chercheurs ont effectué une analyse bibliographique systématique en février 2015 à l’aide des bases de données suivantes : PubMed, Scopus et Web of Science Core Collection. Ils ont inclus seulement les essais aléatoires et contrôlés dans leur recherche. Dans une recherche secondaire, ils ont analysé les références des publications pertinentes.
RÉSULTATS :
Après avoir terminé les recherches primaire et secondaire, les chercheurs ont extrait 109 publications. Après avoir appliqué tous les critères d’inclusion et d’exclusion à l’analyse des résumés et des textes intégraux, ils ont intégré quatre publications à la présente analyse bibliographique. À l’aide du cadre d’évaluation du risque de biais de la Collaboration Cochrane, ils ont établi que chaque étude s’associait à un faible risque de biais.
EXPOSÉ :
Le résultat clinique primaire mesuré dans les études était une abstinence ou une réduction autodéclarée du tabagisme. Dans trois des quatre études, l’abstinence ou la réduction autodéclarée du tabagisme était mesurée par le monoxyde de carbone expiré. Dans la dernière étude, le résultat clinique primaire était le désir autodéclaré de fumer et le désir mesuré de fumer. Les quatre études ont révélé que les SÉDN sont un outil efficace d’abandon du tabac.
CONCLUSIONS :
Toutes les publications incluses dans la présente analyse ont révélé que les SÉDN sont des outils d’abandon du tabac, mais il demeure essentiel d’en évaluer davantage les effets potentiels à long terme sur la santé.
It has been evident that conventional tobacco smoking is harmful to the human body since the 18th century. Although there has been a declining trend in the number of smokers over the past many years, it is estimated that 21% of deaths are due to smoking and that 5.8 million Canadians (approximately 20% of the population) smoke (1). It is well known that smoking may lead to lung cancer and other respiratory diseases (1). Statistics Canada reports that in 2008, there were 19,000 lung cancer deaths in Canada, and that the majority of lung cancer patients report being current or former smokers (1).
Smoking not only affects individuals inhaling the toxic fumes, but also affects those that are exposed to secondhand smoke. In 2013, there were 1,069,994 nonsmoking individuals who reported being exposed to secondhand smoke at home or almost every day (2). According to the WHO, “second-hand smoke exposure to adults causes serious cardiovascular and respiratory diseases, including coronary heart disease and lung cancer” (3). Secondhand smoke exposure to infants may cause sudden death. In pregnant women, it causes low birth weight (4). The WHO reports smoking as a global epidemic (3).
To deter the use of tobacco, in 2007, the WHO initiated a motion in Canada and the United States to ban tobacco advertisements, promotions and sponsorships (3). Similarly, since 2008, all provinces in Canada have illegalized smoking in private vehicles carrying children. Currently, a 33% reduction in childhood exposure to secondhand smoke has been recorded (5). Over the past 20 years, many steps have been taken to help limit tobacco exposure to the general population, as well as to reduce the attractiveness associated with smoking. Smoking cessation aids were developed as early as the 1960s (6). Recently, electronic nicotine delivery systems (ENDS) have become a popular nicotine replacement therapy.
ENDS, of which electronic cigarettes (ECs) are the most common prototype, are devices that do not burn or use tobacco leaves but instead vapourize a solution the user then inhales (6). While ENDS may not expose smokers to the harmful effects of tars and carbon monoxide (CO), the liquid chemical used in ENDS consist of propylene glycol, with or without glycerol and flavouring agent (7). The long-term health exposure to propylene glycol has not been studied, but it is known that when propylene glycol is heated to high temperatures toxic compounds such as formaldehyde and acetaldehyde are formed (8). Moreover, there is currently no research on the long-term health effects of ENDS and its use has, therefore, become a controversial topic. ENDS are currently unregulated in many countries and regulations with regard to ENDS have become a topic of significant debate. Currently, sales of ENDS used for nicotine delivery are illegal in Canada. On the other hand, ECs that are not used for nicotine delivery are available for purchase (6).
In fear of potential renormalization of smoking in the younger population and any unknown long-term health effects, it has been suggested that ENDS should be under strict regulation or even completely banned (9). It is also estimated that 30% to 50% of ENDS and EC sales are conducted on the Internet, thus targeting the younger generations (8). Without having a full understanding of the adverse effects of nicotine, young adults have turned to ENDS as what they perceive to be a safer alternative to conventional cigarette smoking (8). According to the Ontario Drug Use survey, 15% of Ontario high school students report “ever” trying e-cigars and 27% of smokers currently use ECs (4). Correspondingly, in April 2014, the United States Food and Drug Administration announced that ENDS are considered tobacco products and, therefore, limited access to individuals <18 years of age (8). Similarly, on March 2009, Health Canada published an alert advising Canadians not to use ENDS. Health Canada emphasizes that the added “nicotine is a highly addictive and toxic substance, and the inhalation of propylene glycol is a known irritant” (10).
Health Canada also advises that smoking these devices may cause poisoning and also addiction (10). de Bobadilla et al (9) state that there is “not enough evidence… the long-term effects are unknown, thus until their potential carcinogenic effect has been determined, [ENDS] should not be publicized as [carcinogen free]”. The WHO suggests that the potential for nicotine poisoning is a hazard that requires stricter regulations or a complete ban on refillable ENDS (11). ENDS that require refilling containers with liquid chemical substances increase nicotine poisoning reports, often involving children (6). It is reasonable, therefore, to consider that enhanced regulations restricting public access to ENDS is needed in light of the known adverse effects but, most importantly, research on long-term health effects.
Paradoxically, relaxed regulation with respect to ENDS has been recommended by some. Current research investigating EC used as a nicotine replacement therapy has proven a substantial lower health risk compared with smoking traditional combustible cigarettes (8,12). Sweanor and Selby (13) emphasized “people smoke for the nicotine but are dying from the smoke”. They further report that conventional smokers would want to switch to ENDS because the exposure of tar and CO is eliminated, and personalization is possible; moreover, individuals using ENDS have the option to choose both the appearance and the flavour of their ENDS (13). Individuals are still able to attain the ‘[nicotine] buzz’ and it is more cost effective compared with conventional smoking (13). ENDS also provides the “critical sensory-motor cues associated with smoking (hand-to-mouth action and visual cues)” (14). In an earlier commentary by Sweanor (15), it is emphasized that “[ENDS] have become the most popular way to try to stop smoking; the uptake of these products are associated with increased cessation”. It is of concern that strict regulations pertaining to ENDS may restrict conventional cigarette smokers from accessing a “healthier” alternative (15). Hajek (12) suggests that: “in the meantime, clinicians facing smokers who cannot or do not want to stop smoking and who follow evidence and common sense rather than ideologically and commercially driven agendas should recommend that their patients try several types of [ENDS] to see if they can find one meeting their needs”.
It is essential to understand that smoking cessation treatment not only requires the elimination of nicotine dependence, but it also requires behaviour modification. In a study conducted by Kotz et al (17), they outline that the most effective smoking cessation technique involves a combination of smoking cessation aids and also behavioural support. Moreover, “a combination of the two treatments offers almost a threefold chance of success over attempts to quit without using a cessation aid” (16). Given the controversy regarding the impact of the use of ENDS, the objective of the present systematic review was to determine whether ENDS constitute an effective smoking cessation tool. Moreover, the present review sought to determine whether ENDS should be considered as a possible adjunct to aid in smoking cessation.
METHODS
A primary search was conducted in February 2015 using the following databases: PubMed, Scopus and Web of Science Core Collection. Key words for the search included “electronic cigarette”, “e-cigarette”, “cigarette vapor”, “electronic nicotine delivery system”, “smoking cessation”, “electronic cigarette smoking cessation”, “quit smoking” and “tobacco cessation”. A secondary search was then conducted by reviewing references of topic-related articles. All searches excluded non-English publications and were limited to randomized controlled trials (RCTs). No limitations with respect to the date of publication were applied.
Study selection
Studies were included in the present systematic review only if they were RCTs in which the effectiveness of ENDS as a smoking cessation tool was investigated. All methodologies other than RCTs were excluded. With respect to the outcome measures of these RCTs, more specific inclusion/exclusion criteria were not applied to maximize sensitivity of the search to identify relevant articles. This was particularly important given the limited number of publications expected in this emerging research area.
Systematic review process
The investigators involved in the present systematic review consisted of a respiratory therapy student (CL) and a research respiratory therapist (AW). A single investigator (CL) conducted the primary search and abstract review. Data pertaining to study design, objective, population and intervention were extracted by CL and verified by AW. Articles were selected through abstract review based on confirmation of the research design and other inclusion criteria. Both investigators then independently conducted full-text reviews to exclude relevant publications that did not meet inclusion criteria. Any disagreement between the investigators were resolved by consensus.
To assess for the risk of bias in included studies, the Cochrane Collaboration risk of bias evaluation framework was used (18). Publications were individually appraised by each author in consideration of six factors: adequate sequence generation to ensure that each study allocated their study groups with a well-known sequence generation process, allocation concealment was sufficient to determine that participants could not foresee their group assignment, proper treatment blinding technique mitigating influences that would impact data outcomes, accurate reporting of incomplete data, outcome reporting that ensured researchers did not selectively report their data and evaluation of any other potential threats to validity. Quality assessment was determined solely by what was reported in each study. No attempt was made to contact authors for missing information. The characteristics of each included publication (design, objective, sample, intervention and primary findings) were then tabulated for qualitative analysis.
RESULTS
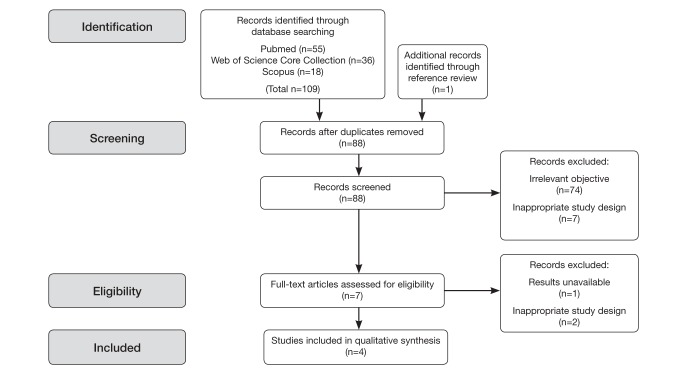
The primary search in three databases (PubMed, Scopus and Web of Science Core Collection) identified 109 articles. The secondary search reviewed the references of related articles through which one additional article was identified. Of the articles retrieved through the primary and secondary searches, 22 were determined to be duplicate citations. Through preliminary abstract review, 74 publications were determined to not be relevant to the research question, and seven publications were excluded because they did not use an RCT design. Figure 1 provides an overview of the study selection process. Each of the four included publications met all requirements for maximum scoring pursuant to the Cochrane Collaborative risk of bias evaluation framework and are summarized in Table 1.
Figure 1).
Search flow diagram outlining the process for citation identification, screening, eligibility and inclusion in the present systematic review
TABLE 1.
Characteristics of studies that are included in the present systematic review
| Author, year (reference) | Relevant objective of study | Sample type | Type of intervention, (amount of nicotine) | Primary findings |
|---|---|---|---|---|
| Adriaens et al, 2014 (14) | To investigate the efficacy of ENDS both in terms of acute craving reduction in the laboratory and in terms of sustained smoking reduction and to assess the experienced benefits and complaints | 48 participants; smoked a minimum of 10 factory-made cigarettes for the past three years and no present motive to quit smoking |
|
|
| Bullen et al, 2010 (19) | To measure the short-term effects of an electronic nicotine delivery device on desire to smoke and withdrawal symptoms | 40 participants; smoked at least 10 cigarettes a day for the past year and no present motive to quit smoking |
|
|
| Bullen et al, 2013 (20) | To assess whether ENDS with cartridges containing nicotine were more effective for smoking cessation than nicotine patches; included a blind comparison with ENDS containing no nicotine (placebo ENDS) | 657 participants; smoked ≥10 cigarettes per day for the past year with motives to quit smoking |
|
|
| Caponnetto et al, 2013 (21) | To evaluate smoking reduction, smoking abstinence and adverse events in smokers not intending to quit experimenting two different nicotine strengths of popular ENDS” | 300 participants; smoked ≥10 factory-made cigarettes per day for at least five years, and no present motivation to quit smoking |
|
|
Shenzhen Kanger Technology Co, Ltd, China;
Shenzhen Joytech Co, Ltd, China. EC Electronic cigarette; eCO Exhaled carbon monoxide; ENDS Electronic nicotine delivery systems; RD Risk difference
Characteristics of included studies
Each publication included in the present review examined the question of interest: are ENDS an effective smoking cessation aid? All participants were adults between the ages of 18 and 70 years who had a smoking history of a minimum of 10 factory-made cigarettes per day. All four studies examine the effects of ENDS using different devices that deliver 0 mg to 16 mg of nicotine. In all studies, the degree of smoking cessation is measured as a primary outcome (14,19–21). All publications identified participants as either having or not having the motivation to quit smoking. The intervention of interest in all publications included the usage of ENDS in replacement of traditional smoking cessation aids. Further details of individual study characteristics are presented in Table 1.
Summary of included studies
Adriaens et al (14) conducted an eight-month RCT with three arms. Forty-eight participants were randomly assigned into two ENDS experimental groups and one control group. The two ENDS experimental groups consisted of two groups using two different types of ENDS both containing 18 mg nicotine. The control group consisted of participants who smoked conventional cigarettes. This eight-month study was divided into two parts. For the first eight weeks, participants were asked to attend three laboratory sessions where craving and withdrawal symptoms were measured. ENDS were used after a period of 4 h abstinent from smoking and vaping. The second part of the study occurred eight weeks after the initial laboratory study was completed. The control group became the ‘switch group’, meaning these participants had the opportunity to use ENDS from there on forward (14).
In the RCT conducted by Bullen et al (19), 40 dependent smokers of ≥10 cigarettes per day were included. Participants were instructed to abstain from smoking and drinking alcohol from the night before the study. On arrival to the study centre, CO levels were measured. If the participant’s CO measured <15 parts per million (ppm), they were included in the study. Participants were randomly assigned using the Latin-square method. If participants had CO levels >15 ppm, they were asked to reschedule for a different study day. Individuals randomly assigned to the ENDS were asked to puff the device as they would a normal cigarette for 5 min. Participants left the study centre for 8 h and were instructed to use the device as needed. When participants were randomly assigned to nicotine inhalator use, they were asked to puff on the inhalator for 20 min in the first hour. Thereafter, they were instructed to use it as necessary while away from the study centre for the next 8 h. Finally, when participants were randomly assigned to smoking conventional cigarettes, they were asked to smoke as usual for 5 min in the first hour. After leaving the study centre for 8 h, the participants were instructed to smoke freely as they wished (19).
Bullen et al (20) compared ENDS with nicotine patches to determine which smoking cessation tool was more effective. In this RCT, 657 individuals were randomly assigned using computerized block randomization and block size nine by a 4:4:1 ratio of nicotine ENDS (16 mg nicotine), nicotine patches and ENDS placebos (0 mg nicotine). Participants allocated to ENDS were instructed to use them as desired from one week before until 12 weeks after their chosen quit day. Those allocated to nicotine patches were instructed to use patches daily from one week before until 12 weeks after their chosen quit day, consistent with smoking cessation guidelines. The primary outcome was self-reported continuous smoking abstinence over the entire follow-up period. Smoking <5 cigarettes total was considered to be a continuous absence from smoking (20).
Caponnetto et al (21) conducted a three-arm double-blind RCT. Three hundred participants were included in the study. They were randomly assigned into three study groups: group A received a 7.2 mg ENDS, group B used a 7.2 mg ENDS for one-half the allocated time and a 5.2 mg ENDS for the other one-half, and group C was supplied with a 0 mg ENDS. Participants were instructed to use the ENDS as desired for up to a maximum of four cartridges per day, as recommended by the manufacturer, in the anticipation of reducing the number of cigarettes smoked per day. The study consisted of a baseline visit and eight follow-up visits for a total of nine visits. The follow-up visits occurred at week 2, week 4, week 6, week 8, week 10, week 12, week 24 and week 52. At the baseline visit, smoking history and base exhaled (e) CO levels were taken. Primary measures included three categories: reducer, which included participants who self-reported ≥50% reduction in the number of cigarettes per day from baseline; quitter, which included participants who self-reported abstinence from tobacco smoking entirely with a demonstrated eCO ≤7 ppm; and failure, which included participants who failed to meet either reducer or quitter criteria.
DISCUSSION
Quit rate and smoking reduction
Adriaens et al (14) demonstrated a quit rate of 34% in the ENDS group with no significant changes in smoking behaviour in the control group. When looking at the quit rate three months after the study was completed, there was a 37% quit rate in the ENDS groups and a 38% quit rate in the ‘switch’ group (three months after initiation of ENDS). Likewise, 6% of the participants showed a reduction in cigarette usage of >80%, 10% showed a reduction of >50%, and the remaining 46% were smoking ≥50% of their baseline use of conventional cigarettes. Adriaens et al (14) also reported that the overall average number of cigarettes smoked per day in the initial (before the switch group) EC groups decreased by approximately 77%, which equates to mean (± SD) of 4.7±6.5 cigarettes compared with the baseline measurement of 20.38±8.01 cigarettes measured at intake. When examining all participants after the switch group took place, there was a >60% decrease in the number of cigarettes compared with baseline measurements.
In the study conducted by Bullen et al (20), verified abstinence at six months after the set quit day was 7.3% in the ENDS group, followed by 5.8% in the patch group and 4.1% in the placebo ENDS group. The risk difference for nicotine EC versus patches was 1.51 (95% CI −2.49 to 5.51) and for the nicotine EC group versus placebo EC group, it was 3.16 (95% CI −2.29 to 8.61). The achieved rates of abstinence were lower than anticipated by the researchers, and provided insufficient statistical power to conclude the superiority of nicotine EC versus the nicotine patches or the placebo EC. Despite these limitations, it is interesting to note that, among those participants who relapsed within 50 days, the nicotine EC group relapsed after a median time of 35 days (95% CI 15 to 56), which was more than twice as long as in the patch group (14 days, 95% CI 8 to 18, P<0.001) or placebo EC group (12 days, 95% CI 5 to 34, P=0.09) (20). The researchers also found that among the nicotine EC group, 57% of participants showed a reduction in number of daily cigarettes by at least one-half the original amount at the six-month marker. This was significantly greater in proportion compared with those in the patch group (41%; P=0.002) and nonsignificantly higher than in the placebo EC group (45%, P=0.08) (20).
Similarly, Caponnerro et al (12) demonstrated an overall reduction in smoking of 33% and a complete abstinence from smoking at week 12, and a 19.0% reduction at week 52 in all study arms. The mean overall cigarette per day consumption at baseline was 21.4 cigarettes per day, and at week 52 consumed cigarettes decreased to 13.9 cigarettes per day (P<0.0001). By completion of the study, 26.9% of quitters were still using ENDS and 73.1% of the quitters were completely free from smoking conventional cigarettes.
Desire to smoke and withdrawal symptoms
In three studies, desire to smoke and withdrawal symptoms were recorded. Adriaens et al (14) found that at the start of session 1, participants, on average, rated their craving for a cigarettes using the Minnesota Withdrawal Score (MNWS) at 7.01 (range 0 to 10). After 5 min of smoking or vaping using their allocated product, there was a clear decrease in craving compared with the start of the session (P<0.001). The degree of decreased craving was measured to be the same for all groups at 3.28±2.54. The same observations were made for session 2 and session 3, where the decrease in craving was less pronounced in the ENDS group than compared with the control group (conventional smoking). In all sessions, among all groups, there was a decrease in withdrawal symptoms from the start of the session to after 5 min of smoking or vaping (all P<0.001). The Minnesota Withdrawal Score was unchanged after 5 min of vaping or smoking to the end of all sessions (P>0.05).
Bullen et al (19) showed that after a 60 min period, participants using the 16 mg ENDS recorded a 0.81 unit less desire to smoke than the placebo ENDS (P=0.006). There was no difference in desire to smoke recorded between the 16 ENDS and inhalator. ENDS were reported as more pleasant to use compared with the inhalator (P=0.016) and produced less irritations of mouth and throat (P<0.001). A greater reduction in desire to smoke in the conventional smoking group compared with the ENDS group (P<0.0001) and nicotine inhalator group (P=0.001) was also reported. Over a 60 min period, the 16 mg ENDS group showed a greater reduction rating in irritability (P=0.48), restlessness (P=0.10) and difficulty concentrating (P=0.26) than the 0 mg ENDS group; these differences were not statistically significant when adjusted for multiple comparisons. When comparing the 16 mg ENDS with the inhalator, there was no difference in withdrawal symptoms, whereas conventional cigarette usage showed greater reduced withdrawal ratings compared with all groups.
Product satisfaction
As a secondary measurement, all four studies measured product satisfaction. Adriaens et al (14) measured product satisfaction by asking participants to list complaints and benefits of their product. When comparing the ENDS group with the control (conventional cigarette) group, comments were much more positive about the ENDS (P<0.001). Throughout the study, the ENDS group showed an upward trend in experiencing benefits as compared with the control group, where no increase in benefits were noted. Likewise, Bullen et al (19) found that there was a higher pleasantness rating when comparing the 16 mg ENDS with the inhalator (P=0.016). Based on a visual analogue score (VAS) rating, where 0 = not at all and 10 = extremely, Bullen et al found that the 16 mg ENDS group showed a higher level of recommendation to a friend (six of 10) compared with the nicotine inhalator group (four of 10) and the 0 mg ENDS group (five of 10). Also, those using ENDS, whether with 0 mg of nicotine or 16 mg of nicotine, showed a higher score for recommendation to a friend when compared with the nicotine inhalator group. However, the product satisfaction ratings based on the VAS score showed similarities between the 16 mg ENDS and the nicotine inhalators, both scoring a three of 10. ENDS were shown to be the most preferable alternative to cigarettes: “58% of participants said they preferred the ENDS, 25% preferred the inhalator and 13% liked neither.”
Similarly, Bullen et al (20) found that at the one-month marker, 88% of those participants in the ENDS group and 92% of those in the placebo ENDS would recommend their allocated device to a friend wanting to quit. Compared with the participants in the nicotine patch group, 50% would recommend their product to a friend. At the six-month marker, there was a slight variation in recommendation to 85%, 88% and 50%, respectively. In contrast, the study conducted by Caponnetto et al (21) found no significant difference for product satisfaction among the three study groups. At weeks 12, 24 and 54, median VAS values for satisfaction were 4 (group A), 4 (group B) and 3 (group C). Participants were inclined to recommend their product to friends who were thinking about quitting. Median VAS score at weeks 12, 24 and 54 were 7, 7 and 6, respectively. No significant difference was found among the three groups. Throughout the four studies, the biggest concern with ENDS was related to technical malfunction in operation or production of vapor (14,19–21).
Adverse events
No serious adverse events (eg, deaths or events requiring hospitalization) were reported by any of the included publications (14,19–21). Minor adverse events were reported in only one publication. Bullen et al (19) described that 88% of the nicotine inhalator users reported mouth and throat irritation. When comparing mouth and throat irritation among the three groups, statistical significance was noted (P<0.001). Nausea was more commonly reported after using the 16 mg ENDS, but not with any level of significance.
Limitations
In the Adriaens et al (14) study, some limitations were acknowledged. At the time of the study, ENDS could not be purchased in Belgium (where the study occurred) and participants were forced to purchase ENDS online. This limitation decreased the ease in obtaining ENDS; thus, this could be one of the possible explanations for the decrease in quit rates.
In the study conducted by Bullen et al (19), two applicable limitations were identified. Most participants saw and handled an ENDS for the first time at the study laboratory, so there was no familiarization period for participants to get use to the ENDS, and no testing was conducted for normal function of the products. This limitation could affect the participants’ rating for desire to smoke as well as product satisfaction. Second, during baseline measurements, low desire to smoke levels were reported; thus, a limited degree of observable change may have occurred (19).
In the study conducted by Bullen et al (20), one major limitation outlined by the researchers showed that participants might have agreed to participate in this study due to the interest in ENDS, but when assigned to the nicotine patch group, some participants may have withdrawn from the study due to their allocation. Likewise, another limitation outlined in this study showed that the abstinence rate might be considered inadequate due to the fact that there was inadequate nicotine replacement. Some nicotine cartridges may contain or deliver less nicotine than indicated by the label. Those that received a lower dosage may have self-reported more withdrawal symptoms compared with those receiving a greater dosage. Co-intervention bias was also noticed in this study. All participants in this study were referred to a quitline whose telephone-based behavioural support services were made available to participants. Usage of the quitline was monitored but was not reported. Those participants who did use the quitline more often may have felt more inclined to quit smoking as compared to those participants who did not use or used the support minimally.
Caponnetto et al (21) identified two key limitations. The study design was unusual in that participants had no desire to quit smoking; thus, direct comparison using this study to other smoking cessation aids is not justifiable. Secondly, the ENDS model (model 401) used in this study is discontinued from production, due to underperformance compared with other ENDS models. However, when the researchers ran their study, model 401 was the only available ENDS model.
In general, reoccurring limitations were identified in most of the studies. Among all four studies, recall bias was identified (14,19–21). Outcome measures were self-reported; thus, participants may have recalled positive memories more than negative ones, thereby favouring the results of the study, although most self-report was verified by measuring eCO level. Similarly, all four studies used some form of a survey or questionnaire to measure baseline values, desire to smoke and withdrawal symptoms. Surveys and questionnaires offer limitations where it is unknown whether participants were able to interpret the questions as intended or whether participants answered questions truthfully.
CONCLUSION
Based on the current available literature, ENDS may constitute an effective smoking cessation tool. In the studies included in the present systematic review, smoking cessation was measured through self-reported smoking reduction, complete smoking abstinance, level of desire to smoke and level of withdrawal symptoms. It is interesting to note that a majority of the studies reported in the present review included participants that initially had no desire to quit smoking. Despite this, all studies reported a significant increase in smoking reduction or complete smoking cessation.
Based on the four studies included in the present review, ENDS have the potential to eliminate the harmful effects of tobacco smoking. In interpreting these results, it is important to note that the present systematic review did not seek to develop an understanding of the long-term effects of ENDS. Health care professionals may, therefore, want to be cautious of promotion of the tool. It is also essential to be mindful that smoking addictions are not only a chemical addiction but also a behavioural issue, both of which truly comprehensive cessation strategies that involve a combination of smoking cessation aids and behavioural support typically aim to address.
Areas of investigation that could provide useful understanding on the topic of ENDS in the future include health effects related to long-term use of ENDS, and long-term population studies to discover how ENDS may influence uptake of smoking habits in younger populations and the impact of second hand exposure to ENDS smoke. With respect to the regulation of ENDS, there will be value in considering regulations that can support safe use of ENDS. ENDS have only recently become popularized; hence, a significant amount of research is required for a more thorough understanding on ENDS to support informed decision making by the public, clinicians and policy makers.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Janz T. Health at a glance: Current smoking trends. Statistics Canada. 2014. Catalogue Number 82-624-X. < www.statcan.gc.ca/pub/82-624-x/2012001/article/11676-eng.htm> (Accessed December 18, 2014)
- 2.Statistics Canada Exposure to second-hand smoke at home by age group and sex: Table 105-0501. 2014. Catalogue number 82-221-X. 2014. < www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/health95a-eng.htm> (Accessed January 12, 2015).
- 3.World Health Organization Report on the Global Tobacco Epidemic, 2013. Enforcing bans on tobacco advertising, promotion and sponsorship. 2013. < http://apps.who.int/iris/bitstream/10665/85380/1/9789241505871_eng.pdf?ua=1> (Accessed January 19, 2015).
- 4.Hammond D. Public Health Ontario; 2014. Vapourized nicotine & e-cigarettes. <www.publichealthontario.ca/en/LearningAndDevelopment/Events/Documents/Vapourized_nicotine_ecigarettes_Hammond_2014.pdf> (Accessed January 12, 2015) [Google Scholar]
- 5.Cancer Advocacy Coalition Effectiveness of bans against smoking inside private vehicles in Canada. 2013. < www.canceradvocacy.ca/reportcard/2013/Effectiveness%20of%20Bans%20Against%20Smoking%20Inside%20Private%20Vehicles%20in%20Canada.pdf> (Accessed October 18, 2014).
- 6.World Health Organization Tobacco Free Initiative: Electronic cigarettes (e-cigarettes) or electronic nicotine delivery systems. 2014. < www.who.int/tobacco/communications/statements/eletronic_cigarettes/en/> (Accessed on January 19, 2015).
- 7.Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: Effects on desire to smoke, withdrawal symptoms and cognition. Addict Behav. 2012:970–3. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Rom O, Pecorelli A, Valacchi G, Reznick R. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci. 2015:65–74. doi: 10.1111/nyas.12609. [DOI] [PubMed] [Google Scholar]
- 9.de Bobadilla JF, Dalmau R, Salto E. Cardiologists and electronic cigarettes. Rev Esp Cardiol. 2015;68:286–9. doi: 10.1016/j.rec.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Government of Canada. Healthy Canadians Health Canada Advises Canadians Not to use Electronic Cigarettes. 2009. Identification number: RA-110003348. < www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2009/13373a-eng.php> (Accessed February 12, 2015).
- 11.World Health Organization Electronic Nicotene Delivery systems. Conference of the Parties to the WHO Framework Convention on Tobacco Control. 2014. < http://apps.who.int/gb/fctc/PDF/cop6/FCTC_COP6_10Rev1-en.pdf?ua=1> (Accessed February 20, 2014)
- 12.Hajek P. Electronic cigarettes have a potential for huge public health benefit. BMC Med. 2014;12:225. doi: 10.1186/s12916-014-0225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweanor D, Selby P. Electronic Cigarettes: Implications of Regulation on Harm Reduction. 2015. < https://camh.adobeconnect.com/system/download?download-url=/_a829238269/p8gj6udsspf/output/&name=10+-+E-cigarettes+-+FINAL.pdf&session=na4breezo3hmuwgzn4v3fobq&sco_id=1212288420&ticket=6fnioe9mcqck> (Accessed on February 20, 2015)
- 14.Adriaens K, Van GD, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environment Res Public Health. 2014;11:11220–48. doi: 10.3390/ijerph111111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweanor D. Public health and electronic cigarettes. Can J Respir Ther. 2014;50:41–2. [PMC free article] [PubMed] [Google Scholar]
- 16.Zaleski R. Combined Behavioral Support and Medication Offers smokers Best Chance of Quitting. Elsevier; 2014. < www.elsevier.com/about/press-releases/research-and-journals/combined-behavioral-support-and-medication-offers-smokers-best-chance-of-quitting> (Accessed February 20, 2015) [Google Scholar]
- 17.Kotz D, Brown J, West R. Prospective cohort Study of the effectiveness of smoking cessation treatments used in the “real world”. Mayo Clin Proc. 2014;89:1360–7. doi: 10.1016/j.mayocp.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane Collaboration; 2011. < http://handbook.cochrane.org> (Accessed March 26, 2015). [Google Scholar]
- 19.Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomised cross-over trial. Tob Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 20.Bullen C, Howe C, Laugesen M, et al. Electronic Cigarettes for smoking cessation: A randomised controlled trial. Lancet. 2013;382:1629–37. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 21.Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: A prospective 12-month randomized control design study. PLoS One. 2013;8:12. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]