Abstract
BACKGROUND
In a phase 1–2 trial of albumin-bound paclitaxel (nab-paclitaxel) plus gemcitabine, substantial clinical activity was noted in patients with advanced pancreatic cancer. We conducted a phase 3 study of the efficacy and safety of the combination versus gemcitabine monotherapy in patients with metastatic pancreatic cancer.
METHODS
We randomly assigned patients with a Karnofsky performance-status score of 70 or more (on a scale from 0 to 100, with higher scores indicating better performance status) to nab-paclitaxel (125 mg per square meter of body-surface area) followed by gemcitabine (1000 mg per square meter) on days 1, 8, and 15 every 4 weeks or gemcitabine monotherapy (1000 mg per square meter) weekly for 7 of 8 weeks (cycle 1) and then on days 1, 8, and 15 every 4 weeks (cycle 2 and subsequent cycles). Patients received the study treatment until disease progression. The primary end point was overall survival; secondary end points were progression-free survival and overall response rate.
RESULTS
A total of 861 patients were randomly assigned to nab-paclitaxel plus gemcitabine (431 patients) or gemcitabine (430). The median overall survival was 8.5 months in the nab-paclitaxel–gemcitabine group as compared with 6.7 months in the gemcitabine group (hazard ratio for death, 0.72; 95% confidence interval [CI], 0.62 to 0.83; P<0.001). The survival rate was 35% in the nab-paclitaxel–gemcitabine group versus 22% in the gemcitabine group at 1 year, and 9% versus 4% at 2 years. The median progression-free survival was 5.5 months in the nab-paclitaxel–gemcitabine group, as compared with 3.7 months in the gemcitabine group (hazard ratio for disease progression or death, 0.69; 95% CI, 0.58 to 0.82; P<0.001); the response rate according to independent review was 23% versus 7% in the two groups (P<0.001). The most common adverse events of grade 3 or higher were neutropenia (38% in the nab-paclitaxel–gemcitabine group vs. 27% in the gemcitabine group), fatigue (17% vs. 7%), and neuropathy (17% vs. 1%). Febrile neutropenia occurred in 3% versus 1% of the patients in the two groups. In the nab-paclitaxel–gemcitabine group, neuropathy of grade 3 or higher improved to grade 1 or lower in a median of 29 days.
CONCLUSIONS
In patients with metastatic pancreatic adenocarcinoma, nab-paclitaxel plus gemcitabine significantly improved overall survival, progression-free survival, and response rate, but rates of peripheral neuropathy and myelosuppression were increased. (Funded by Celgene; ClinicalTrials.gov number, NCT00844649.)
Pancreatic cancer is the fourth leading cause of cancer-related death in Europe and the United States.1,2 Since 1997, gemcitabine therapy has been the standard first-line treatment for patients with unresectable locally advanced or metastatic pancreatic cancer.3 Among patients with metastatic disease, the 5-year survival rate is only 2%,1 and 1-year survival rates of 17 to 23% have been reported with gemcitabine.3-5 Numerous phase 2 studies involving patients with advanced pancreatic cancer have shown promising results; however, most subsequent large phase 3 studies have not shown significantly improved survival,6-16 with the exception of a study involving patients who received combination therapy with gemcitabine plus erloti nib, which was associated with a significant improvement in overall survival (median increase, 2 weeks),5 and a phase 2–3 trial conducted by a French consortium study group involving patients who received oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) therapy, which was associated with a median increase in overall survival of 4.3 months.4
In preclinical studies, albumin-bound paclitaxel particles (nab-paclitaxel [Abraxane], Celgene) showed antitumor activity as a single agent and synergistic activity in combination with gemci tabine in murine models of pancreatic cancer.17,18 In particular, nab-paclitaxel improved the intratumoral concentration of gemcitabine.17,18 On the basis of preclinical evidence, a phase 1–2 clinical trial was conducted that involved previously untreated patients with metastatic pancreatic adenocarcinoma. In that study, the maximum dose of nab-paclitaxel that was associated with an acceptable level of adverse events was 125 mg per square meter of body-surface area, which was administered in combination with gemcitabine, at a dose of 1000 mg per square meter, on days 1, 8, and 15 every 4 weeks.17 The efficacy was promising, with a median survival of 12.2 months and a manageable safety profile. In a phase 3 study, we investigated the efficacy and safety of this combination therapy.
METHODS
STUDY OVERSIGHT
The study was approved by the independent ethics committee at each participating institution and was conducted in accordance with the International Conference on Harmonisation E6 requirements for Good Clinical Practice and with the ethical principles outlined in the Declaration of Helsinki.19 All the patients provided written informed consent before the initiation of the study.
All the authors vouch for the adherence of the study to the protocol (available with the full text of this article at NEJM.org). The first draft of the manuscript was written by the first author, with input from the trial investigators, and by clinical researchers and a biostatistician employed by the sponsor (Celgene), all of whom are authors. The authors were assisted by a medical writer who was employed by the sponsor. No one who is not an author or who is not otherwise acknowledged contributed to the manuscript. The first author made the decision to submit the manuscript for publication, which was agreed on by all the authors.
The sponsor monitored the study and provided the study drugs at no charge. The protocol was designed by the first author in collaboration with the sponsor. Data were collected by the investigators and analyzed by a statistician, employed by the sponsor, who is also an author and who vouches for the accuracy and completeness of the data reported.
PATIENTS
Eligible adults (≥18 years of age) had a Karnofsky performance-status score of 70 or more (on a scale from 0 to 100, with higher scores indicating better performance status), had not previously received chemotherapy for metastatic disease, and had histologically or cytologically confirmed metastatic adenocarcinoma of the pancreas that was measurable according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0.20 Metastatic disease had to have been diagnosed within 6 weeks before randomization.
Eligible patients could have received treatment with fluorouracil or gemcitabine as a radiation sensitizer in the adjuvant setting if the treatment had been received at least 6 months before randomization. Patients who had received cytotoxic doses of gemcitabine or any other chemotherapy in the adjuvant setting and those with islet-cell neoplasms or locally advanced disease were excluded. Patients had to have adequate hematologic, hepatic, and renal function (including an absolute neutrophil count of ≥1.5×109 per liter, a hemoglobin level of ≥9 g per deciliter, and a bilirubin level at or below the upper limit of the normal range, according to the standards at the central laboratory).
STUDY DESIGN AND TREATMENT
In this international, multicenter, open-label, randomized, phase 3 study, we randomly assigned eligible patients, in a 1:1 ratio, to receive a 30-to-40–minute intravenous infusion of nab-paclitaxel at a dose of 125 mg per square meter, followed by an infusion of gemcitabine according to the gemcitabine label at a dose of 1000 mg per square meter, on days 1, 8, 15, 29, 36, and 43, or to receive gemcitabine alone at a dose of 1000 mg per square meter weekly for 7 of 8 weeks (cycle 1). In subsequent cycles, all patients were administered treatment on days 1, 8, and 15 every 4 weeks.
Patients were stratified according to performance status, presence or absence of liver metastases, and geographic region. Treatment continued until disease progression or until there was an unacceptable level of adverse events. Per protocol, crossover was not allowed at any time after randomization.
ASSESSMENTS
The investigators evaluated the tumor response in patients every 8 weeks by means of spiral computed tomography or magnetic resonance imaging. In addition, all scans were independently assessed by two readers and one adjudicator, all of whom were unaware of the treatment assignments, with the use of RECIST, version 1.0. Serial measurements of the carbohydrate antigen 19-9 (CA19-9) level were performed at baseline and every 8 weeks thereafter.
Safety was monitored by means of an assessment by the investigators of treatment-related adverse events and serious adverse events, weekly laboratory testing performed at a central laboratory, and the rates of dose modifications, dose delays, and premature discontinuations of the study drug. Patients were followed for survival until death or study closure.
STUDY END POINTS
The primary efficacy end point was overall survival. The secondary end points were progression-free survival and the response rate as assessed by means of independent radiographic review. Progression-free survival and response rates were also analyzed by means of investigator assessments. Additional efficacy end points included the rate of disease control (defined as stable disease for ≥16 weeks, confirmed complete response, or confirmed partial response) and the time to treatment failure. The percentages of patients with a maximum reduction in the CA19-9 level of at least 20% and at least 90% were also calculated for each treatment group.
Treatment-related adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf) and were coded and summarized according to the preferred terms in the Medical Dictionary for Regulatory Activities, version 15.0.
STATISTICAL ANALYSIS
All efficacy analyses were carried out in the intention-to-treat population (i.e., all patients who underwent randomization). Overall survival, which was the primary efficacy end point, was analyzed with the use of the Kaplan–Meier method and a stratified log-rank test. We calculated that with a sample of 842 patients with 608 events the study would have 90% power to detect a hazard ratio for death with nab-paclitaxel plus gemcitabine versus gemcitabine monotherapy of 0.769 at a two-sided alpha level of 0.049. The power was increased from 80 to 90% in a protocol amendment before any interim analyses were performed. One planned interim efficacy analysis to assess futility was performed when at least 200 patients had been followed for 6 months or more. For the final analysis, the survival status of all patients was updated within 1 month before the data-cutoff date (September 17, 2012). Data from patients who were alive were censored for the survival analysis (see the statistical analysis plan, which is available with the protocol).
A multivariate analysis of survival was performed with the use of a Cox proportional-hazard model to evaluate the treatment effect with adjustment for stratification factors. The comparison of the response rates between the treatment groups was performed with the use of the chi-square test. The correlation between changes in serum levels of CA19-9 and survival was evaluated by means of a Cox regression model.
RESULTS
PATIENTS AND TREATMENT GROUPS
A total of 861 patients in North America (63%), eastern Europe (15%), Australia (14%), and western Europe (9%) underwent randomization during the period from May 2009 through April 2012 at 151 community and academic centers in 11 countries. A total of 431 patients were randomly assigned to nab-paclitaxel plus gemcitabine, and 430 to gemcitabine alone (intention-to-treat population). A total of 421 patients received nab-paclitaxel plus gemcitabine, and 402 received gemcitabine (treated population) (Fig. S1 in the Supplementary Appendix, available at NEJM.org). All demographic and clinical characteristics at baseline were well balanced between the two groups (Table 1).
Table 1. Characteristics of the Patients at Baseline*.
| Characteristic | nab-Paclitaxel plus Gemcitabine (N = 431) |
Gemcitabine Alone (N = 430) |
Total (N = 861) |
|---|---|---|---|
| Age | |||
| No. of yr | |||
| Median | 62 | 63 | 63 |
| Range | 27–86 | 32–88 | 27–88 |
| Distribution — no. (%) | |||
| <65 yr | 254 (59) | 242 (56) | 496 (58) |
| ≥65 yr | 177 (41) | 188 (44) | 365 (42) |
| Sex — no. (%) | |||
| Female | 186 (43) | 173 (40) | 359 (42) |
| Male | 245 (57) | 257 (60) | 502 (58) |
| Race or ethnic group — no.(%)† | |||
| Asian | 8 (2) | 9 (2) | 17 (2) |
| Black | 16 (4) | 16 (4) | 32 (4) |
| White | 378 (88) | 375 (87) | 753 (87) |
| Hispanic | 25 (6) | 26 (6) | 51 (6) |
| Other | 4 (1) | 4 (1) | 8 (1) |
| Region — no. (%) | |||
| Australia | 61 (14) | 59 (14) | 120 (14) |
| Eastern Europe | 64 (15) | 62 (14) | 126 (15) |
| North America | 268 (62) | 271 (63) | 539 (63) |
| Western Europe | 38 (9) | 38 (9) | 76 (9) |
| Karnofsky performance-status score — no./total no. (%)‡ | |||
| 100 | 69/429 (16) | 69/429 (16) | 138/858 (16) |
| 90 | 179/429 (42) | 199/429 (46) | 378/858 (44) |
| 80 | 149/429 (35) | 128/429 (30) | 277/858 (32) |
| 70 | 30/429 (7) | 33/429 (8) | 63/858 (7) |
| 60 | 2/429 (<1) | 0/429 | 2/858 (<1) |
| Pancreatic tumor location — no. (%) | |||
| Head | 191 (44) | 180 (42) | 371 (43) |
| Body | 132 (31) | 136 (32) | 268 (31) |
| Tail | 105 (24) | 110 (26) | 215 (25) |
| Unknown | 3 (1) | 4 (1) | 7 (1) |
| Site of metastatic disease — no. (%) | |||
| Liver | 365 (85) | 360 (84) | 725 (84) |
| Lung | 153 (35) | 184 (43) | 337 (39) |
| Peritoneum | 19 (4) | 10 (2) | 29 (3) |
| No. of metastatic sites — no. (%) | |||
| 1 | 33 (8) | 21 (5) | 54 (6) |
| 2 | 202 (47) | 206 (48) | 408 (47) |
| 3 | 136 (32) | 140 (33) | 276 (32) |
| >3 | 60 (14) | 63 (15) | 123 (14) |
| Level of carbohydrate antigen 19-9 — no./total no. (%) | |||
| Normal§ | 60/379 (16) | 56/371 (15) | 116/750 (15) |
| ULN to <59× ULN | 122/379 (32) | 120/371 (32) | 242/750 (32) |
| ≥59× ULN | 197/379 (52) | 195/371 (53) | 392/750 (52) |
| Carbohydrate antigen 19-9 — U/ml¶ | |||
| Median | 2293.7 | 2759.2 | 2469.7 |
| Range | 1.9–6,159,233.0 | 0.3–12,207,654.2 | 0.3–12,207,654.2 |
| Previous therapy — no. (%) | |||
| Radiation therapy | 19 (4) | 11 (3) | 30 (3) |
| Chemotherapy | 23 (5) | 12 (3) | 35 (4) |
| Whipple procedure | 32 (7) | 30 (7) | 62 (7) |
| Biliary stent | 80 (19) | 68 (16) | 148 (17) |
There were no significant between-group differences at baseline. The term nab-paclitaxel denotes 130-nm albumin-bound paclitaxel, and ULN upper limit of the normal range.
Race or ethnic group was self-reported.
Karnofsky performance-status scores range from 0 to 100, with higher scores indicating better performance status. Two patients in the nab-paclitaxel–gemcitabine group had a score of 70 or more at the screening visit but a score of 60 at the baseline visit on day 1 of cycle 1.
The normal range was 0 to 35 U per milliliter. Approximately 10 to 15% of patients with pancreatic cancer do not have Lewis antigens and thus do not have the ability to secrete carbohydrate antigen 19-9.
Data were missing for 52 patients in the nab-paclitaxel–gemcitabine group and for 59 in the gemcitabine group.
EFFICACY
Overall Survival
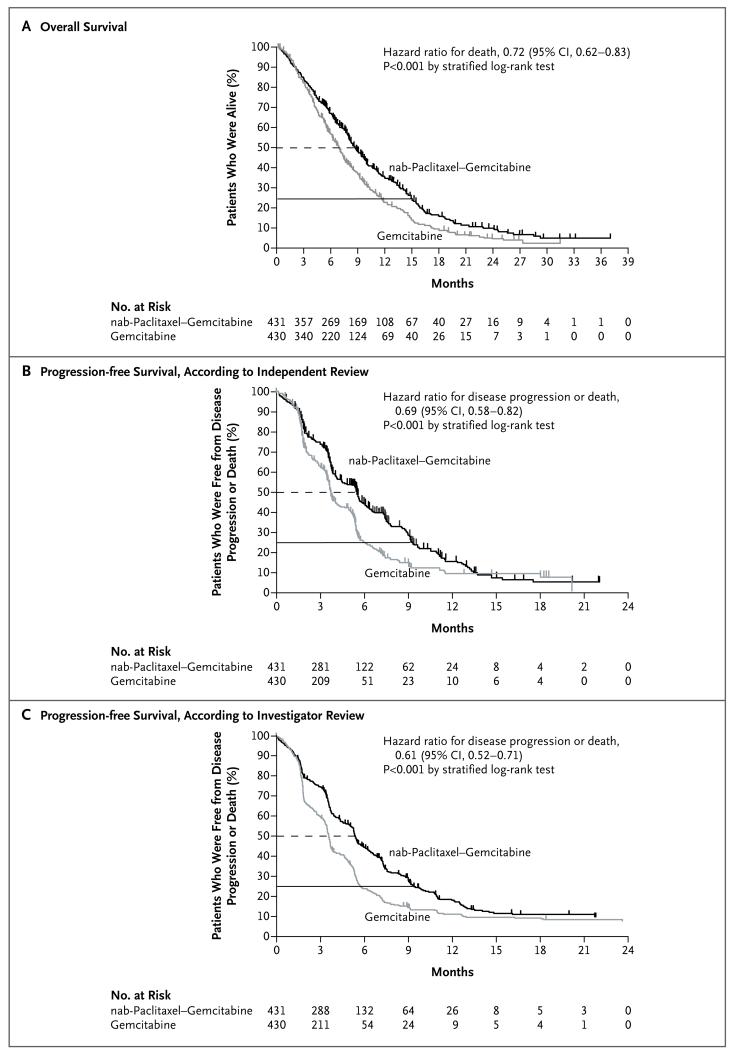
The survival analysis was based on 692 deaths (80% of patients), including 333 in the nab-paclitaxel–gemcitabine group (77%) and 359 in the gemcitabine group (83%). In the intention-to-treat population, the median survival was 8.5 months (95% confidence interval [CI], 7.89 to 9.53) with nab-paclitaxel plus gemcitabine, as compared with 6.7 months (95% CI, 6.01 to 7.23) with gemcitabine (hazard ratio for death, 0.72; 95% CI, 0.62 to 0.83; P<0.001) (Fig. 1A).
Figure 1. Kaplan–Meier Curves for Survival and Progression-free Survival in the Intention-to-Treat Population.
The dashed line indicates the median and the solid line the time point at which 25% of the patients were alive. The term nab-paclitaxel denotes 130-nm albumin-bound paclitaxel.
At the time point at which 25% of the patients were alive, survival was longer in the nab-paclitaxel–gemcitabine group than in the gemcitabine group (14.8 months vs. 11.4 months). Data were censored if the patients were alive at the time of the analysis or had been lost to follow-up. Data for 23% of the patients were censored for survival in the nab-paclitaxel–gemcitabine group, as compared with data for 17% of the patients in the gemcitabine group, with a median follow-up of 9.1 months (range, 0.1 to 36.9) and 7.4 months (range, 0.0 to 31.3), respectively.
The 1-year and 2-year survival rates were significantly higher with nab-paclitaxel plus gemcitabine than with gemcitabine (Table 2). A Cox regression analysis of survival with the stratification factors as covariates was performed. In addition to a significant treatment effect with nab-paclitaxel plus gemcitabine, with a hazard ratio for death of 0.71 (95% CI, 0.61 to 0.83; P<0.001), the Karnofsky performance-status score and the presence or absence of liver metastases were independent predictors of survival.
Table 2. Overall Survival, Progression-free Survival, and Response Rates in the Intention-to-Treat Population.
| Efficacy Variable | nab-Paclitaxel plus Gemcitabine (N = 431) |
Gemcitabine Alone (N = 430) |
Hazard Ratio or Response-Rate Ratio (95% CI)* |
P Value |
|---|---|---|---|---|
| Overall survival | ||||
| Median overall survival — mo (95% CI) | 8.5 (7.9–9.5) | 6.7 (6.0–7.2) | 0.72 (0.62–0.83) | <0.001 |
| Survival rate — % (95% CI) | ||||
| 6 mo | 67 (62–71) | 55 (50–60) | <0.001 | |
| 12 mo | 35 (30–39) | 22 (18–27) | <0.001 | |
| 18 mo | 16 (12–20) | 9 (6–12) | 0.008 | |
| 24 mo | 9 (6–13) | 4 (2–7) | 0.02 | |
| Progression-free survival | ||||
| Median progression-free survival — mo (95% CI) | 5.5 (4.5–5.9) | 3.7 (3.6–4.0) | 0.69 (0.58–0.82) | <0.001 |
| Rate of progression-free survival — % (95% CI) | ||||
| 6 mo | 44 (39–50) | 25 (20–30) | ||
| 12 mo | 16 (12–21) | 9 (5–14) | ||
| Response | ||||
| Rate of objective response | ||||
| Independent review | ||||
| No. of patients with a response | 99 | 31 | 3.19 (2.18–4.66) | <0.001 |
| % (95% CI) | 23 (19–27) | 7 (5–10) | ||
| Investigator review | ||||
| No. of patients with a response | 126 | 33 | 3.81 (2.66–5.46) | <0.001 |
| % (95% CI) | 29 (25–34) | 8 (5–11) | ||
| Rate of disease control† | ||||
| No. of patients | 206 | 141 | 1.46 (1.23–1.72) | <0.001 |
| % (95% CI) | 48 (43–53) | 33 (28–37) | ||
| Best response according to independent review — no. (%) |
||||
| Complete response | 1 (<1) | 0 | ||
| Partial response | 98 (23) | 31 (7) | ||
| Stable disease | 118 (27) | 122 (28) | ||
| Progressive disease | 86 (20) | 110 (26) | ||
| Could not be evaluated‡ | 128 (30) | 167 (39) |
The hazard ratio for death is provided for overall survival, and the hazard ratio for progression or death is provided for progression-free survival, with a hazard ratio of less than 1 favoring the nab-paclitaxel–gemcitabine group. The response-rate ratios are provided for the response rates, with a response-rate ratio of more than 1 favoring the nab-paclitaxel–gemcitabine group. The 95% confidence interval for response-rate ratios was calculated according to the asymptotic 95% confidence interval of the relative risk in the nab-paclitaxel–gemcitabine group, as compared with the gemcitabine group.
Disease control included confirmed complete response, confirmed partial response, and stable disease for 16 weeks or more.
Included are 72 patients (17%) in the nab-paclitaxel–gemcitabine group and 87 (20%) in the gemcitabine group who did not have an assessment after the baseline visit.
Second-Line Therapy
The rate of the use of subsequent anticancer therapy was balanced between the treatment groups: 38% in the nab-paclitaxel–gemcitabine group and 42% in the gemcitabine group. A total of 27 patients (6%) in the gemcitabine group crossed over to receive a regimen that included nab-paclitaxel. When the data for survival were censored at the time of the initiation of subsequent therapy, there was significantly longer survival with nab-paclitaxel plus gemcitabine than with gemcitabine (median survival, 9.4 months vs. 6.8 months; hazard ratio for death, 0.68; 95% CI, 0.56 to 0.82; P<0.001).
Progression-free Survival
In the analysis of progression-free survival according to independent assessment, 542 patients (63%) had progression of disease or died, including 64% of the patients in the nab-paclitaxel–gemcitabine group and 62% in the gemcitabine group. There was significantly longer progression-free survival in the nab-paclitaxel–gemcitabine group than in the gemcitabine group, with a median of 5.5 months (95% CI, 4.5 to 5.9) versus 3.7 months (95% CI, 3.6 to 4.0) (hazard ratio for disease progression or death, 0.69; 95% CI, 0.58 to 0.82; P<0.001) (Fig. 1B and Table 2). The rate of progression-free survival at 1 year was 16% in the nab-paclitaxel–gemcitabine group, as compared with 9% in the gemcitabine group. The median progression-free survival according to investigator assessment was 5.3 months (95% CI, 4.4 to 5.5) with nab-paclitaxel plus gemcitabine versus 3.5 months (95% CI, 3.2 to 3.6) with gemcitabine (hazard ratio for disease progression or death, 0.61; 95% CI, 0.52 to 0.71; P<0.001) (Fig. 1C) — a finding that was similar to that for progression-free survival according to independent review.
Time to Treatment Failure
The median time to treatment failure, according to independent review, was 5.1 months (95% CI, 4.1 to 5.5) in the nab-paclitaxel–gemcitabine group, as compared with 3.6 months (95% CI, 3.5 to 3.9) in the gemcitabine group (hazard ratio, 0.70; 95% CI, 0.60 to 0.80; P<0.001).
Overall Response Rates
The response rate according to independent review was significantly higher with nab-paclitaxel plus gemcitabine than with gemcitabine (23% [95% CI, 19 to 27] vs. 7% [95% CI, 5 to 10]; P<0.001; response-rate ratio, 3.19 [95% CI, 2.18 to 4.66]) (Table 2). Similarly, the response rate that was based on investigator assessment was significantly higher with nab-paclitaxel plus gemcitabine than with gemcitabine (29% [95% CI, 25 to 34] vs. 8% [95% CI, 5 to 11]; P<0.001; response-rate ratio, 3.81 [95% CI, 2.66 to 5.46]).
The rate of disease control (confirmed response or stable disease for ≥16 weeks), according to independent assessment, was 48% (95% CI, 43 to 53) in the nab-paclitaxel–gemcitabine group and 33% (95% CI, 28 to 37) in the gemcitabine group (rate ratio for disease control, 1.46; 95% CI, 1.23 to 1.72; P<0.001) (Table 2).
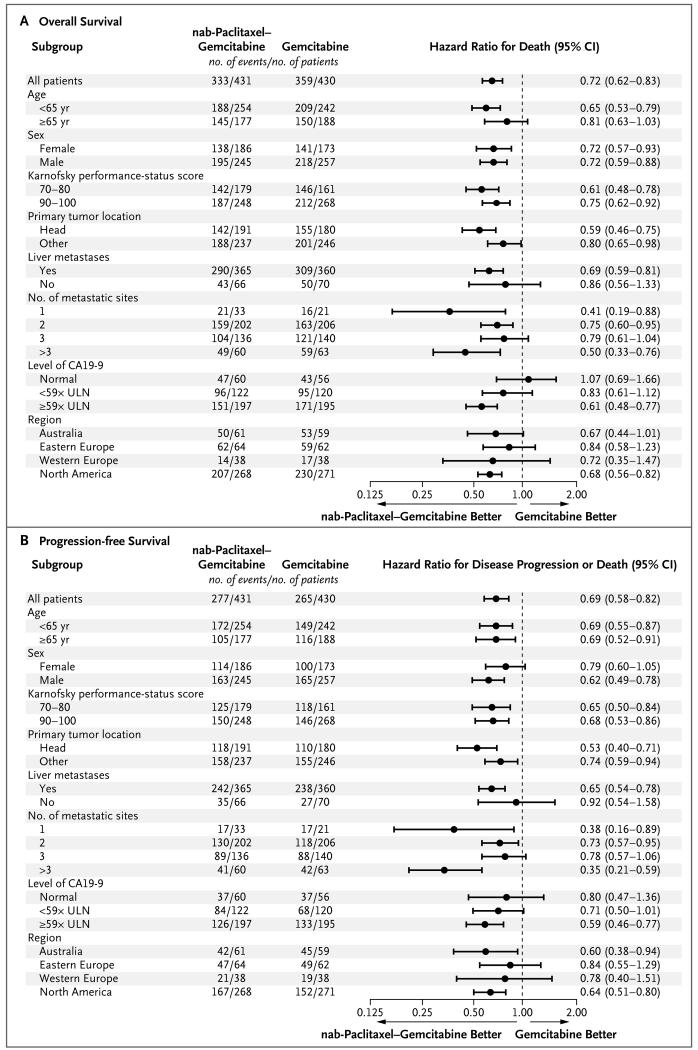
Subgroup Analyses
The treatment effect consistently favored the nab-paclitaxel–gemcitabine group across the majority of prespecified subgroups. In general, the patients with more advanced disease — those with poorer performance status (Karnofsky performance-status score of 70 or 80), the presence of liver metastasis, more than three sites of metastatic disease, metastatic pancreatic cancer at the initial diagnosis, or a CA19-9 level that was 59 times the upper limit of the normal range or higher — had the greatest reduction in the risk of death (Fig. 2A). Similar trends were observed for progression-free survival according to subgroup (Fig. 2B).
Figure 2. Forest Plots of the Treatment Effect on Survival and Progression-free Survival in Prespecified Subgroups.
Karnofsky performance-status scores range from 0 to 100, with higher scores indicating better performance status. CA19-9 denotes carbohydrate antigen 19-9, and ULN upper limit of the normal range.
CA19-9
A total of 379 patients in the nab-paclitaxel–gemcitabine group and 371 patients in the gemcitabine group had a baseline CA19-9 measurement. A total of 61% of the patients in the nab-paclitaxel–gemcitabine group, as compared with 44% of those in the gemcitabine group, had a decrease from baseline of at least 20% (P<0.001), and 31% versus 14% had a decrease of at least 90% (P<0.001). Patients in the two treatment groups who had a decrease of at least 90% in the CA19-9 level had a median survival of 13.5 months, as compared with 8.2 months among those with a decrease of less than 90% (hazard ratio for death, 0.53; 95% CI, 0.43 to 0.67; P<0.001).
TREATMENT EXPOSURE
The median duration of treatment was 3.9 months (range, 0.1 to 21.9) in the nab-paclitaxel–gemcitabine group and 2.8 months (range, 0.1 to 21.5) in the gemcitabine group, with 32% and 15% of patients, respectively, receiving treatment for at least 6 months. In the nab-paclitaxel-gemcitabine group, 41% of the patients had reductions in the nab-paclitaxel dose and 47% had reductions in the gemcitabine dose. In total, 71% of all nab-paclitaxel doses administered during the study were at the full dose of 125 mg per square meter. The median relative dose intensity (the proportion of the administered cumulative dose relative to the planned cumulative dose) in the nab-paclitaxel–gemcitabine group was 81% for nab-paclitaxel and 75% for gemcitabine.
In the gemcitabine group, 33% of patients had dose reductions, resulting in a median relative dose intensity of 85%. The median cumulative dose of gemcitabine delivered was greater in the nab-paclitaxel–gemcitabine group than in the gemcitabine group (11,400 mg per square meter vs. 9000 mg per square meter); this difference was related to the increased duration of treatment in the nab-paclitaxel–gemcitabine group.
SAFETY
In the nab-paclitaxel–gemcitabine group, the most frequently reported nonhematologic adverse events related to treatment were fatigue (in 54% of patients), alopecia (in 50%), and nausea (in 49%). Treatment-related adverse events of grade 3 or higher that were reported more often in the nab-paclitaxel–gemcitabine group than in the gemcitabine group were neutropenia, leukopenia, fatigue, and peripheral neuropathy (Table 3). The incidences of anemia and thrombocytopenia were similar in the two groups. The incidence of febrile neutropenia was low and was similar in the two treatment groups. The incidence of peripheral neuropathy (all grades) leading to the discontinuation of nab-paclitaxel was 8%, and the incidence leading to a dose reduction was 10%.
Table 3. Common Adverse Events of Grade 3 or Higher and Growth-Factor Use*.
| Event | nab-Paclitaxel plus Gemcitabine (N = 421) |
Gemcitabine Alone (N = 402) |
|---|---|---|
| Adverse event leading to death — no. (%) | 18 (4) | 18 (4) |
| Grade ≥3 hematologic adverse event — no./total no. (%)† | ||
| Neutropenia | 153/405 (38) | 103/388 (27) |
| Leukopenia | 124/405 (31) | 63/388 (16) |
| Thrombocytopenia | 52/405 (13) | 36/388 (9) |
| Anemia | 53/405 (13) | 48/388 (12) |
| Receipt of growth factors — no./total no. (%) | 110/431 (26) | 63/431 (15) |
| Febrile neutropenia — no. (%)‡ | 14 (3) | 6 (1) |
| Grade ≥3 nonhematologic adverse event occurring in >5% of patients — no. (%)‡ |
||
| Fatigue | 70 (17) | 27 (7) |
| Peripheral neuropathy§ | 70 (17) | 3 (1) |
| Diarrhea | 24 (6) | 3 (1) |
| Grade ≥3 peripheral neuropathy | ||
| Median time to onset — days | 140 | 113 |
| Median time to improvement by one grade — days | 21 | 29 |
| Median time to improvement to grade ≤1 — days | 29 | NR |
| Use of nab-paclitaxel resumed — no./total no. (%) | 31/70 (44) | NA |
NA denotes not applicable, and NR not reached.
Assessment of the event was made on the basis of laboratory values.
Assessment of the event was made on the basis of investigator assessment of treatment-related adverse events.
Peripheral neuropathy was reported on the basis of groupings of preferred terms defined by standardized queries in the Medical Dictionary for Regulatory Activities.
None of the patients had grade 4 neuropathy. Among patients who received treatment for 4 months (the average treatment duration), the rate of grade 3 neuropathy was 7%. In the nab-paclitaxel–gemcitabine group, the median time to the first occurrence of grade 3 neuropathy was 140 days, and the median time to improvement from grade 3 to grade 2 was 21 days and to grade 1 or resolution of the event was 29 days. Of the patients who had grade 3 peripheral neuropathy, 44% resumed treatment at a reduced dose of nab-paclitaxel within a median of 23 days after the onset of a grade 3 event.
The proportion of patients with serious adverse events was similar in the two treatment groups (50% with nab-paclitaxel plus gemcitabine and 43% with gemcitabine). Fatal events were reported for 4% of the patients in each treatment group. Sepsis (all grades) was reported more often in the nab-paclitaxel–gemcitabine group than in the gemcitabine group (5% vs. 2%), as was pneumonitis (4% vs. 1%).
DISCUSSION
This large, randomized, international, phase 3 study showed that nab-paclitaxel plus gemcitabine led to a significant improvement in survival at all time points. In particular, the survival curves separated early, with a median improvement of 1.8 months and an improvement of 3.4 months at the time point when 25% of the patients were alive (Fig. 1A). The rate of survival was significantly higher in the nab-paclitaxel–gemcitabine group than in the gemcitabine group — by 59% at 1 year (35% vs. 22%) and by more than 100% at 2 years (9% vs. 4%). A sensitivity analysis of survival showed that the difference between the treatment groups could not be attributed to the use of second-line therapy. The treatment effect consistently favored the nab-paclitaxel–gemcitabine group across the majority of prespecified subgroups.
With respect to the secondary end points (progression-free survival and response rate) and all other efficacy end points, there were consistent, significant improvements with nab-paclitaxel plus gemcitabine, supporting the results of the primary analysis of overall survival. The improvement in progression-free survival corresponded to a 31% reduction in the risk of progression or death with nab-paclitaxel plus gemcitabine, as compared with gemcitabine. The response rate according to independent review was tripled with nab-paclitaxel plus gemcitabine. The results with respect to progression-free survival and response rate as assessed by the investigators were consistent with those as assessed by independent review. A higher percentage of patients in the nab-paclitaxel–gemcitabine group than in the gemcitabine group had a reduction of at least 90% in the CA19-9 level, which has been reported to be associated with an improvement in survival.21
Adherence to treatment and dose intensity were high with both agents and in both treatment groups. The addition of nab-paclitaxel to gemcitabine increased the cumulative delivery of gemcitabine. The longer treatment duration and greater cumulative dose in the nab-paclitaxel–gemcitabine group, as compared with the gemcitabine group, showed that this combination can be administered effectively. The suitability of the dosing regimen was confirmed by the observations that the majority of patients did not require a dose reduction and that 71% of the nab-paclitaxel doses were delivered at the starting dose of 125 mg per square meter.
The safety profile for both regimens was consistent with that in previous reports.3,17,22 The rate of serious life-threatening adverse events was not increased with nab-paclitaxel plus gemcitabine, as compared with gemcitabine alone; adverse events were generally grade 3 or lower and resolved without specific treatment. The most notable difference in adverse events between the two treatment groups was observed with respect to peripheral neuropathy, which was cumulative and rapidly reversible in most patients with temporary discontinuation of nab-paclitaxel and a subsequent reduction in the dose. The incremental risks of sepsis and pneumonitis were managed by protocol amendments to increase awareness; early diagnosis and treatment of these events reduced the risk of fatal outcomes. A limitation of the study was that quality of life was not measured.
This international study was carried out at academic and community centers in North America, Europe, and Australia. The dose used in this trial was established in the phase 1–2 trial on the basis of the greatest efficacy and an acceptable adverse-event profile, and all efficacy analyses presented here were prespecified and were carried out in the intention-to-treat population. The use of randomization and the large sample resulted in well-balanced treatment groups, both overall and within strata. The estimated medians for overall survival, progression-free survival, and the response rates that were observed in the gemcitabine group fell within the ranges reported in large, phase 3 studies that have evaluated chemotherapy for the treatment of adenocarcinoma of the pancreas.3-6,8-10,13,15,16
Many agents that have shown promising results in phase 2 trials of pancreatic cancer fail to improve survival in phase 3 trials.6-16 Although this phase 3 trial showed a clinically significant improvement in survival, the median survival in the nab-paclitaxel–gemcitabine group in the current trial was more than 3 months shorter than the survival observed at the same dose level in the phase 1–2 trial.17 It should be noted that the preceding phase 1–2 study was conducted in only 4 U.S. treatment centers, whereas this multi national, phase 3 study enrolled patients at 151 centers in 11 countries.
The phase 2–3 trial of FOLFIRINOX versus gemcitabine4 also showed a clinically meaningful improvement in survival among patients with pancreatic adenocarcinoma. The FOLFIRINOX study differed from the current study in several aspects. It pooled data from the phase 2 and 3 portions and excluded patients older than 75 years of age. In our study, 10% of the patients were at least 75 years of age. The FOLFIRINOX study also excluded patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 2 (on a scale from 0 to 5, with 0 indicating no symptoms and full activity and higher scores indicating increasing levels of disability), whereas 8% of the patients in our trial had a poor performance status, corresponding to an ECOG performance status of 2.23 The relevance of these differences is highlighted by the results of a multivariate Cox regression analysis, in which performance status was an independent predictor of survival. Nevertheless, FOLFIRINOX improved median survival by 4.3 months over gemcitabine and is clearly an active regimen.
In conclusion, nab-paclitaxel combined with gemcitabine is superior to gemcitabine alone but causes more myelosuppression and peripheral neuropathy; however, these side effects appear to be reversible.
Supplementary Material
Acknowledgments
We thank all the patients and their families who participated in this phase 3 study; Amanda Johnson and Tammy Davis of Celgene for coordination of the clinical trial sites; Alfredo Romano, M.D., and Brian Lu, M.D., Ph.D., of Celgene for medical monitoring; and Anita N. Schmid, Ph.D., of Celgene for medical writing assistance.
Supported by Celgene.
Footnotes
Presented in part at the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium, San Francisco, January 24–26, 2013; and the ASCO Annual Meeting, Chicago, May 31–June 4, 2013.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Cancer facts and figures 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- 2.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792–800. doi: 10.1093/annonc/mdt010. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 6.Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645–51. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 7.Gonçalves A, Gilabert M, François E, et al. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23:2799–805. doi: 10.1093/annonc/mds135. [DOI] [PubMed] [Google Scholar]
- 8.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–22. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–8. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 10.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–85. doi: 10.1200/JCO.2008.20.9007. [Erratum, J Clin Oncol 2009;27:5859.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abou-Alfa GK, Letourneau R, Harker G, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol. 2006;24:4441–7. doi: 10.1200/JCO.2006.07.0201. [DOI] [PubMed] [Google Scholar]
- 12.Stathopoulos GP, Syrigos K, Aravantinos G, et al. A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95:587–92. doi: 10.1038/sj.bjc.6603301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639–45. doi: 10.1093/annonc/mdi309. [Erratum, Ann Oncol 2006;17:535.] [DOI] [PubMed] [Google Scholar]
- 14.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer: definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–9. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 15.Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–83. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 16.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–10. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–54. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frese KK, Neesse A, Cook N, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260–9. doi: 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good clinical practice research guidelines reviewed, emphasis given to responsibilities of investigators: second article in a series. J Oncol Pract. 2008;4:233–5. doi: 10.1200/JOP.0854601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Reni M, Cereda S, Balzano G, et al. Carbohydrate antigen 19-9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer. 2009;115:2630–9. doi: 10.1002/cncr.24302. [DOI] [PubMed] [Google Scholar]
- 22.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 23.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135–41. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.