Abstract
Early life trauma exposure represents a potent risk factor for the development of mental illnesses such as anxiety, depression and post-traumatic stress disorder. Moreover, deleterious consequences of trauma are exacerbated in youth living in impoverished, urban environments. A priori probability maps were used to examine resting-state functional connectivity (FC) of the amygdala in 21 trauma-exposed, and 21 age- and sex-matched urban children and adolescents (youth) without histories of trauma. Intrinsic FC analyses focused on amygdala-medial prefrontal circuitry, a key emotion regulatory pathway in the brain. We discovered reduced negative amygdala-subgenual cingulate connectivity in trauma-exposed youth. Differences between groups were also identified in anterior insula and dorsal anterior cingulate to amygdala connectivity. Overall, results suggest a model in which urban-dwelling trauma-exposed youth lack negative prefrontal to amygdala connectivity that may be critical for regulation of emotional responses. Functional changes in amygdala circuitry might reflect the biological embedding of stress reactivity in early life and mediate enhanced vulnerability to stress-related psychopathology.
Keywords: adolescent, child, maltreatment, resting-state, urban
INTRODUCTION
Estimates regarding rates of exposure to traumatic events in childhood range widely from 15 to 60% (Kessler et al., 1995; Dube et al., 2001; Stein et al., 2010), with strong evidence for the highest incidence occurring in urban, inner city settings (almost 90%; Gillespie et al., 2009; Goldmann et al., 2011). Trauma and stress injure the brain, precipitate cognitive-behavioral, emotional, and somatic problems, and are strong predictors of psychiatric illness (McEwen, 2012). Trauma in early life is especially harmful—associated with ∼50% of childhood psychiatric disorders, and 30% of later-onset clinical disorders (Green et al., 2010).
Neurological evaluation of the link between trauma and psychiatric illness converges on amygdala and prefrontal brain regions. The amygdala is essential for the detection of threat and enhancement of vigilance (Zald, 2003) and is a central activator of the physiologic stress response (Dedovic et al., 2009). In contrast, the prefrontal cortex (PFC) is critical for regulation of emotion (Quirk and Beer, 2006). Animals that experience early life adversity show structural and functional changes in both the amygdala (Malter Cohen et al., 2013b for a review) and PFC (review by McEwen and Morrison, 2013). Furthermore, early life adversity may also perturb the direct bidirectional connections between these two regions (review by Tottenham and Sheridan, 2009).
Research in humans and animals consistently demonstrates altered frontoamygdala connectivity is another consequence of early life stress (Gee et al., 2013a; Malter Cohen et al., 2013a; Philip et al., 2013; Grant et al., 2014). Compromised connectivity between the amygdala and PFC has been implicated in the pathophysiology of stress-related disorders. Specifically, altered frontoamygdala connectivity has been observed in anxiety (Kim and Whalen, 2009; Roy et al., 2013), depression (Tang et al., 2013) and post-traumatic stress disorder (PTSD; Edwards et al., 2013, Stevens et al., 2013, Brown et al., 2014). It is possible that altered frontoamygdala connectivity may emerge early in life, proximal to negative traumatic experiences, and that this shift may be formative in determining healthy or deleterious outcomes for the individual. After all, frontoamygdala circuitry undergoes rapid changes across childhood and adolescence (Hare et al., 2008, Gee et al., 2013b, Gabard-Durnam et al., 2014), and thus alterations occurring during this period may have lasting effects.
Although animal research has addressed embedding of stress exposure in frontoamygdala pathways in early life, research in humans is relatively recent. Gee et al. (2013a) and Nooner et al. (2013) provided the first studies of early life stress and functional connectivity (FC) in children/adolescents. Gee et al. examined task-related variation in FC associated with orphanage rearing in 6-17 year olds during a face-processing task, whereas Nooner et al. examined how trauma-symptoms in a healthy sample of community youth relate to variations in intrinsic FC during resting-state. Both studies evidenced altered frontoamygdala FC, with orphanage-reared children showing more medial frontal and typically developing youth showing more lateral frontal amygdala FC effects. Frontoamygdala FC alterations have also been shown to relate to trauma, diurnal cortisol and internalizing symptoms in a longitudinal community sample of young adults (18 year olds; Burghy et al., 2012; Herringa et al., 2013). These studies compel the need for more research during formative years, prior to when psychopathology becomes chronic. Research that examines youth at high risk for developing clinical disorders could lead to identification of latent neural risk factors contributing to psychopathology in the aftermath of trauma exposure.
Here, we examine intrinsic resting-state neural FC in urban, low-income, minority trauma-exposed and comparison youth (ages 9-15). Past research shows not only that trauma frequency is extreme in African Americans living in impoverished urban areas, but also that negative consequences of trauma in urban, African American communities may be more severe (Alim et al., 2006). For example, while ∼20% of trauma-exposed individuals in the general population subsequently develop PTSD, African American urban residents who experience trauma are nearly two times more likely develop PTSD (Goldmann et al., 2011). In addition, lower income is a significant predictor of more severe emotional psychopathology following trauma (Lowe et al., 2014). Thus, additive effects of trauma frequency and stress burden may be particularly deleterious to healthy emotional development. Investigating the correlates of trauma exposure on the developing brain in a low income, African American urban cohort of youth provides an opportunity to identify neurological changes in those who are at highest risk for developing psychopathology.
MATERIALS AND METHODS
Participants
The current study evaluated 42 urban youth, ages 9-15 (mean = 12.6, s.d. = 2.1). Participant ages were selected to align with the emergence of puberty; puberty has been identified as a time when affective disorders frequently manifest (Angold et al., 1998). The majority of participants (n = 27) reported annual incomes of <$40 000, and only a small number (n = 6) reported incomes>$60 000. Participants were drawn from a larger study, and chosen to represent trauma-exposed (n = 21) and comparison (n = 21) groups matched on age and sex. Data from 18 participants have been reported previously (Thomason et al., 2013). Participants were recruited through advertisements posted on the Wayne State University website, Craigslist (Detroit), printed flyers, or through Metro Detroit mental health clinics. Exclusionary criteria included: English as a second language, lower than a 2nd grade reading level, history of brain injury, neurological or movement disorders or presence of magnetic resonance imaging (MRI) contraindication. Parental informed written consent and child/adolescent assent were obtained prior to participation. Demographic and behavioral measures were administered during a laboratory visit, and MRI was performed during a subsequent visit (∼2 weeks following the laboratory visit). All experimental procedures were approved by the Human Investigation Committee of Wayne State University.
Demographic data analysis
Highest level of parent/caregiver educational attainment and annual household income were coded as ordinal variables and compared between groups using Mann-Whitney U-tests. Independent samples t-tests or χ2 tests were used to test for group differences in age, sex, IQ (derived from the Kaufman Brief Intelligence Test, version 2; Kaufman and Kaufman, 2004), pubertal development (using Tanner staging; Marshall and Tanner, 1968), and parent report of child race/ethnicity. Effects were considered significant at P ≤ 0.05. Statistical analyses were two-tailed and implemented in IBM statistical package for the social sciences (SPSS) Statistics 21 (SPSS, Inc., Chicago, IL).
Trauma exposure
Utilizing both parent and child endorsements, participants that experienced at least one trauma indicated on the Children’s Trauma Assessment Center Screen Checklist were categorized as ‘trauma’ (source: Michigan Trauma Assessment Center). Forms of trauma included victimization (e.g., physical, psychological, or sexual abuse), neglect, and exposure to violence. Frequently, trauma exposed youth experienced more than one type of trauma. Number and type of endorsed traumas are provided in Table 1.
Table 1.
Demographic and clinical characteristics by group
| Variable | Trauma (n = 21) | Comparison (n = 21) | Group comparison (P-value) |
|---|---|---|---|
| Age, m (s.d.) | 12.77 (2.00) | 12.32 (2.19) | ns |
| Pubertal maturation (tanner stage), m (s.d.) | 3.75 (0.95) | 3.12 (1.38) | ns |
| Sex (female), n (%) | 15 (71.43) | 14 (66.67) | ns |
| IQ, m (s.d.) | 90.55 (10.9) | 109.94 (16.04) | <0.001 |
| Race/ethnicity, n (%) | |||
| African American | 10 (47.62) | 10 (47.62) | ns |
| Caucasian | 4 (19.05) | 10 (47.62) | |
| Hispanic | 3 (14.28) | 0 (0) | |
| Not reported | 4 (19.05) | 1 (4.76) | |
| Annual household income, n (%) | |||
| <$40 000 | 17 (81) | 10 (47.6) | 0.018 |
| $40 000 to $60 000 | 1 (4.8) | 7 (33.3) | |
| $60 000 to $80 000 | 2 (9.5) | 1 (4.8) | |
| $80 000 to $100 000 | 0 | 1 (4.8) | |
| Over $100 000 | 0 | 2 (9.5) | |
| Not reported | 1 (4.8) | 0 | |
| Highest level of parental education, n (%) | |||
| No GED/no high school diploma | 2 (9.5) | 1 (4.8) | ns |
| GED/high school diploma | 4 (19) | 1 (4.8) | |
| 2-year degree or some college | 8 (38.1) | 9 (42.9) | |
| 4-year degree | 3 (14.3) | 7 (33.3) | |
| Masters | 3 (14.3) | 2 (9.5) | |
| Doctorate | 0 | 1 (4.8) | |
| Not reported | 1 (4.8) | 0 | |
| Type of trauma endorseda, n (%) | |||
| Physical abuse | 4 (19%) | 0 | |
| Neglectful home environment | 3 (14%) | 0 | |
| Emotional abuse | 2 (10%) | 0 | |
| Exposure to domestic violence | 14 (67%) | 0 | |
| Exposure to any other violence not already identified | 11 (52%) | 0 | |
| Multiple separations from parent or caregiver | 4 (19%) | 0 | |
| Sexual abuse or exposure | 5 (24%) | 0 | |
| Anxiety symptoms (SCR)b, m (s.d.) | 22.97 (16.49) | 14.23 (11.08) | 0.05 |
| Depressive symptoms (CDI)c, m (s.d.) | 4 (5.32) | 1.8 (2) | ns |
| Motion during scan | |||
| Translational max frame-to-frame excursion (s.d.) | 0.66 (0.31) mm | 0.56 (0.25) mm | ns |
| Rotational max frame-to-frame excursion (s.d.) | 0.59 (0.27)° | 0.56 (0.33)° | |
| Translational mean movement (s.d.) | 0.13 (0.08) mm | 0.1 (0.6) mm | |
| Rotational mean movement (s.d.) | 0.11 (0.06)° | 0.12 (0.08)° | |
| Translational rms (s.d.) | 0.1 (0.05) mm | 0.08 (0.02) mm | |
| Rotational rms (s.d.) | <0.001 (<0.001)° | <0.001 (<0.001)° | |
Note. aTrauma criteria are from CTA Center Screen Checklist (Item 1) by the Michigan Trauma Asessement Center. bSCR, Screen for Child Anxiety-Related Emotional Disorders. cCDI, Children’s Depression Inventory. All P-values derived from t-tests with the exception of sex and race/ethnicity comparisons, which used χ2 tests, and income and parental education, which used Mann-Whitney U-tests. Abbreviations: m, mean; s.d., standard deviation; n, number; ns, not significant; max, maximum; rms, root mean squared (head position change during the resting-state scan).
Self-reported affect measures
Participants completed two validated self-report questionnaires: the 10-item Children’s Depression Inventory (CDI; Saylor et al., 1984) and the 41-item Screen for Childhood Anxiety-Related Disorders (SCR; Birmaher et al., 1997). The SCR was administered during the lab visit and again at the MRI visit. Lab and MRI SCR scores were highly correlated, r(42).0.76, P < 0.001. Thus, average across SCR measurements is reported. A visual analog scale (VAS) was used to obtain an average rating of fear/anxiety during the MRI visit (repeat measures at 30-min intervals) as previously described (Thomason et al., 2013).
Imaging data acquisition
MRI data were acquired using a 3.0 T Siemens Verio scanner. Participants were positioned in a 12-channel transmit-receive head coil and stabilized by padding to reduce motion-related artifacts. Participants were asked to lie quietly in the scanner with their eyes closed for the duration of the 6-min resting-state scan. For functional magnetic resonance imaging (fMRI), a total of 180 T2*-weighted blood oxygenation level-dependent (BOLD) images were acquired (interleaved ascending acquisition) using echo-planar imaging (EPI). The acquisition parameters were: repetition time [TR] = 2000 ms; echo time [TE] = 25 ms; flip angle = 90°; voxel size = 3.44 × 3.44 × 4 mm; matrix = 220 × 220 and 29 slices. Additionally, T1-weighted images were obtained for anatomical reference with the following parameters: TR = 1680 ms; TE = 3.51 ms; flip angle = 9°; voxel size = 0.7 × 0.7 × 1.3 mm; matrix = 256 × 256 and 176 slices.
Image preprocessing
Image preprocessing steps were conducted using SPM8 software (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/). After discarding the first 4 EPI volumes to allow for signal stabilization, images were slice-time corrected, realigned, spatially normalized to the Montreal Neurological Institute (MNI) template, and smoothed using an 8-mm Gaussian kernel. Frame-to-frame excursion, root mean square (rms) and head movement across the scan were calculated and averaged for translational (x, y, z) and rotational (roll, pitch, yaw) movement directions. Motion parameters were compared between groups using two-tailed independent samples t-test. Between group motion differences were considered significant at P < 0.05.
Seed-based connectivity analysis
Given prior research showing altered intrinsic FC of specific amygdala subregions in adults with PTSD (Brown et al., 2014) and anxiety (Etkin et al., 2009), connectivity of centromedial (CM) and basolateral (BL) amygdala were evaluated separately. Amygdala subregions were the leading choice for seeded ROI analyses because of their unique connectivity profiles. For more detailed discussion of amygdala subregion connectivity in humans, see (Roy et al., 2009). Seed regions were defined for CM and BL amygdala structural subdivisions (Amunts et al., 2005), following prior work (Roy et al., 2009; Qin et al., 2012). In brief, bilateral masks used stereotaxic, probabilistic maps of cytoarchitectonic boundaries defined by SPM Anatomy toolbox (Eickhoff et al., 2005). Cytoarchitectonic maps show high reliability and accuracy for guiding anatomical segmentation of amygdala subregions in children as young as 6 years of age (Kim et al., 2010; Qin et al., 2012). Given that we had no a priori lateralization hypotheses, we averaged signal from right and left amygdala masks. FC of amygdala subregions was determined by semipartial correlation using the Connectivity (CONN) FC Toolbox (ver.12.p; www.nitrc.org/projects/conn). Between group effects were considered within an anatomically defined medial prefrontal region used in prior works (Etkin and Schatzberg, 2011; Marusak et al., 2014). This mask was selected to encompass perigenual (pgACC) and subgenual (sgACC) regions of the anterior cingulate, as these regions suppress limbic reactivity through direct connections to the amygdala. Family wise error (FWE) corrected P < 0.05, significance level was used.
Motion poses a significant source of noise in FC analyses. None of the participants included in the present study had motion exceeding 1.5 mm in any direction. We addressed residual motion-related artifacts in three steps. First, Siemens MRI motion correction (MoCo) software was used during image acquisition. This procedure retrospectively measures six parameters of rigid-body translation and rotation and produces a corrected time series using affine transformation. Second, functional image volumes were realigned to the mean image in SPM8. Third, realignment parameters (with another six parameters representing their first order temporal derivatives) were removed with covariate regression analysis before computing amygdala FC. Signals from white matter and cerebral spinal fluid were also regressed out using anatomical component correction (aCompCor; Behzadi et al., 2007; Chai et al., 2012). Low overall movement levels and lack of differences between groups augment confidence that motion did not compromise observed effects.
Secondary whole-brain analyses were performed to comprehensively evaluate connectivity of the amygdala. In addition to CM and BL regions, a superficial (SF) amygdala subregion mask was generated using procedures described earlier, and connectivity from this area was also examined for possible differences between groups. Regions showing altered FC between groups were reported at a threshold of P < 0.005, cluster minimum = 10 voxels. This threshold was derived from suggested standards for whole-brain comparisons (Lieberman and Cunningham, 2009). Results that survived multiple comparisons correction for the whole brain (spatial cluster extent threshold; > 158 voxels for P < 0.05 FWE corrected) are denoted with an *asterisk in Supplementary Table S1. To plot the direction of trauma-related amygdala connectivity, individual participant beta values were extracted from peaks using 4 mm radius spheres. Pearson correlation was used to test for associations between amygdala FC and anxiety, depressive symptoms, IQ and income. All coordinates are reported in MNI convention.
To further validate our findings, data were re-analyzed using motion ‘scrubbing’ (Power et al., 2012). Specifically, movement was plotted and visually inspected for each participant using ArtRepair software (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html). Through this process we identified four trauma and three comparison participants that required censorship. They were scrubbed, and following this, maximal movement for all individuals in the sample was reduced to 0.171 mm and 0.392°. Major comparisons and study outcomes were evaluated using this alternative analytic scheme, and those data are reported as Supplementary Material.
RESULTS
Participants
Demographics and movement
As shown in Table 1, groups did not differ in age, pubertal maturation, sex, race/ethnicity, parental educational attainment or movement during the resting-state scan. Overall, residual movement was well within accepted standards (< 1.5 mm translational rms; cf. Fair et al., 2012). Relative to comparison youth, trauma-exposed participants reported lower levels of household income and IQ (Table 1), effects that were anticipated based on prior work (e.g., De Bellis, 2001; Lantz et al., 2005). Follow-up analyses controlling for income and IQ were conducted on FC data, as described later. There were significantly more females than males represented across the entire sample, [χ2(1) = 6.1, P = 0.014]. Distribution of participant race/ethnicity across the sample was representative of the study community [Wayne Country, MI; χ2(2) = 4.09, P = 0.13; www.census.gov]. Four participants (three trauma, one comparison) were on psychotropic medications (one on stimulants, one on selective serotonin reuptake inhibitors, two on serotonin-norepinephrine reuptake inhibitors and one on beta-2 adrenergic agonists). Medications were not withheld for scanning. Follow-up analyses excluding participants on medications yielded no changes to observed effects.
Self-reported affect measures
Although the trauma group was not chosen on the basis of psychopathology, participants with histories of trauma reported higher levels of anxiety (SCR) relative to comparison participants (Table 1). This is consistent with reports that childhood trauma exposure is a strong predictor of emotional psychopathology (Kessler et al., 1997). Follow-up analyses were conducted to account for effects of anxiety on connectivity (see later). Although anxiety scores reported during lab and MRI visits were significantly correlated within subject (see Methods), it is possible that variation across visits differed between groups. We therefore tested for effects of visit on anxiety levels using a Group (trauma, comparison) × Visit (lab, MRI) analysis of variance (ANOVA). Consistent with group differences reported above, a significant main effect of Group emerged, F(1, 80) = 7.08, P < 0.009. No main effect of Visit, or Group × Visit interaction was observed (P’s > 0.4) suggesting that trait anxiety was stable over a period of 2 weeks and variability between visits did not differ between groups. In contrast, average state levels of fear/anxiety during the MRI visit (VAS) did not significantly differ between groups, t(39) = 0.3, P = 0.77. Given that VAS ratings were previously shown to correlate with cortisol reactivity during the scan (Thomason et al., 2013), this result suggests that connectivity differences are not likely influenced by group differences in biologic stress responsivity during the MRI visit. Groups did not differ on trait levels of depressive symptoms, P > 0.08 (Table 1).
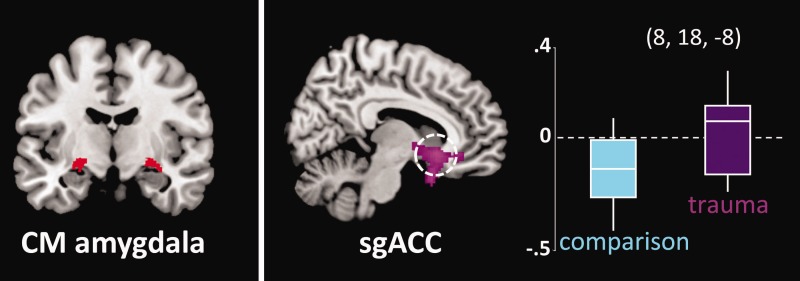
Lack of amygdala-sgACC connectivity in trauma-exposed youth
We observed significant group differences in CM amygdala-sgACC connectivity (x = 8, y = 18, z = −8, pFWE = 0.022, Z = 3.80). Extraction of average connectivity strength within this cluster revealed that the group effect resulted from the predicted negative amygdala-sgACC FC in comparison participants, which was absent in trauma-exposed youth (Figure 1). CM amygdala-sgACC FC was not related to anxiety [SCR, r(42) = 0.01, P = 0.92] or depressive [CDI, r(42) = 0.03, P = 0.83] symptoms across the sample, or within the trauma group [SCR, r(21) = −0.22, P = 0.34; CDI, r(21) = −0.15, P = 0.51]. Group differences in CM amygdala-sgACC connectivity remained significant when controlling for IQ (peak at x = −12, y = 40, z =−4, pFWE = 0.025, Z = 3.77) and income (peak at x = 10, y = 22, z = −10, pFWE = 0.027, Z = 3.74). No significant effects of trauma were observed for BL amygdala-sgACC connectivity at P < 0.05 FWE-corrected.
Fig. 1.
Absent negative CM amygdala-subgenual cingulate (sgACC) negative connectivity in trauma-exposed youth. Left: Signal was averaged across anatomically defined bilateral CM amygdala source region. Right: Tukey’s boxplots depict connectivity values by group centered on the sgACC peak (x = 8, y = 18, z = −8; MNI). The middle line indicates the median, vertical line the range and the limits of the box represent upper and lower quartiles. Results significant at a threshold of P < 0.05, FWE-corrected.
Divergent patterns of amygdala subregion FC across the sample
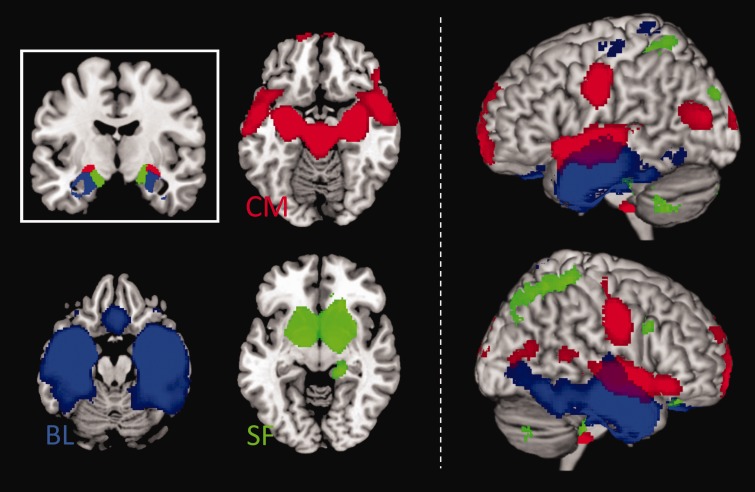
Secondary whole-brain analyses were performed to examine connectivity of major amygdala subregions. FC maps across the entire sample (Figure 2) revealed unique patterns of connectivity for bilateral CM, BL and SF amygdala subregions that are consistent with previous cytoarchitectonically based amygdala FC studies (e.g., Roy et al., 2009; Brown et al., 2014). Briefly, CM showed that signal covariation with striatal regions, whereas BL showed that signal covariation with temporal and frontal cortical regions. SF signal was strongly correlated with signal in limbic regions.
Fig. 2.
FC of amygdala subregions across the entire youth sample. Left: Inset shows anatomically defined bilateral amygdala seed regions: CM (CM; red), BL (BL; blue) and SF (SF; green). Axial slices show brain areas positively correlated with amygdala seed regions. Right: Surface renderings are used to depict separation and overlap of amygdala neural networks. Connectivity maps displayed at P < 0.005, cluster minimum = 10 voxels.
Trauma effects on amygdala whole-brain connectivity
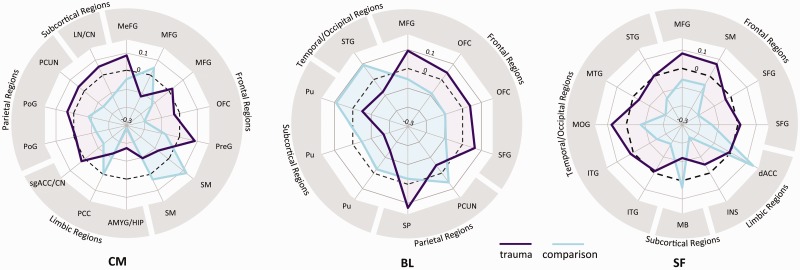
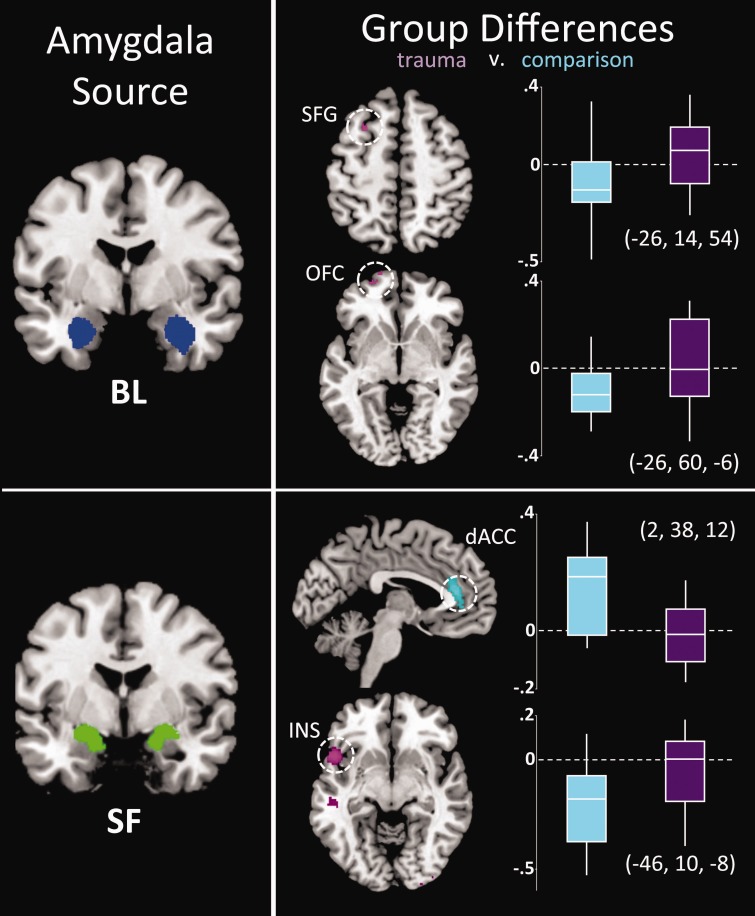
Extensive differences in amygdala FC were observed between groups (Supplementary Table S1). We also found that in a subset of areas in which groups differed, FC also related to income, IQ, anxiety or depressive symptoms. Thus, not only does amygdala FC vary across brain areas, but this variation relates to symptom severity. Data are summarized in Figure 3 as radar plots. Visual inspection of these plots suggests that, overall, individuals who have experienced trauma demonstrate increased positive connectivity, or lack of negative connectivity, across widespread brain regions. Consistency was observed across amygdala subregions in that frontoamygdala connectivity was negative in the comparison group but not in trauma-exposed youth (Figure 4). This was also true in anterior insula-amygdala connectivity, which was more negative in comparison but not in trauma-exposed participants for the SF subregion. A different effect was observed for the dorsal anterior cingulate (dACC); there, we observed increased positive amygdala connectivity in comparison but not trauma participants (Figure 4).
Fig. 3.
Differential patterns of amgydala subregion FC across the whole brain in trauma-exposed (purple) and comparison (blue) youth. Numerical values represent the average within-group correlation strength in areas where groups differed, across frontal, limbic, parietal, subcortical and temporal/occipital regions (coordinates are provided in Supplementary Table S1). Wider area on radial plots in trauma-exposed youth suggests a pattern of enhanced positive FC or diminished negative FC of the amygdala to many brain regions, particularly to areas of the frontal cortex. Dashed line indicates zero; the middle of the plot indicates negative values. Abbreviated brain regions are defined in Supplementary Table S1.
Fig. 4.
Regions showing group differences (trauma, purple; comparison: blue) in FC with BL and SF amygdala. Right: Tukey’s boxplots depict connectivity values by group. The middle line indicates the median, vertical line the range and the limits of the box represent upper and lower quartiles. Corresponding MNI coordinates of peak group difference (Supplementary Table S1) are indicated adjacent to each boxplot. Results are shown at P < 0.005, cluster minimum = 10 voxels, for display purposes.
Replication of results in scrubbed data
Data were reanalyzed utilizing censoring of high-movement frames. Following censoring, average group motion was <0.05 mm and <0.002° rms. As shown in Supplementary Figure S1, main findings of amygdala FC across the sample (Figure 2), and group differences in FC in the sgACC (Figure 1), superior frontal gyrus, dACC and insula (Figure 4) were replicated. However, the group difference in BL amygdala-orbitofrontal cortex connectivity was no longer significant.
DISCUSSION
The present study evaluates links between early life trauma and neural circuit organization, within a high sociodemographic risk youth sample. Our results indicate reduced negative amygdala-sgACC FC in trauma-exposed youth, fitting with prior observed associations between disrupted amygdala circuitry and emotional psychopathology. For example, alterations in frontoamygdala circuitry have been reported in adults with PTSD (Brown et al., 2014), and adolescents (Roy et al., 2013) and adults with generalized anxiety disorder (Etkin et al., 2009). We add to this conceptualization by discovering that experience (trauma) is associated with altered intrinsic amygdala FC in formative, developmental years. In particular, trauma-related changes were observed within amygdala-sgACC circuitry, a pathway that is critical for emotion regulation. These findings are in line with our recent study showing a reduced ability to regulate emotional conflict and an absence of negative frontoamygdala connectivity during conflict regulation in trauma-exposed youth (Marusak et al., 2014). The present findings extend this work by demonstrating that deficits in this critical emotion regulatory pathway are present even when individuals are not engaging regulatory control processes.
Four studies most similar to the current investigation are summarized in Supplementary Table S2. The most comparable study, implemented by Gee et al., evaluated FC in orphanage-reared youth during a face processing task (Gee et al., 2013a). Focusing on the same frontoamygdala circuitry emphasized here, they too found abnormalities in neural FC between groups. Interestingly, their effects were related to the type of emotional face being processed. That is, between group differences were noted during the processing of fear faces, but the direction of effects differed during processing of happy faces. This result, paired with data presented here about intrinsic FC during resting-state suggests that dynamics in frontoamygdala circuits in trauma-exposed youth may relate to processing demands, and results obtained should be considered in that light. Gee et al. also provide evidence that the age of participants being examined may interact with observed effects. They found that comparison and orphanage-reared participants exhibited different patterns of age-related change in frontoamygdala FC to fear faces. Specifically, between group differences appear to have been significant in children but not adolescents. This could be interpreted as a reduction in differences between groups with age. Though the cross-sectional nature of this sample precludes direct evaluation of this possibility, this observation can be regarded in consideration of current theory that principals of neural connectivity at one point in life may be adaptive, while at another may confer risk (Tottenham, 2013).
We interrogated amygdala FC from three amygdala subregions. CM amygdala is involved in allocating attention to relevant stimuli (Davis and Whalen, 2001) and mediating increased vigilance (Dringenberg and Vanderwolf, 1996). In contrast, BL amygdala has been linked to associative learning processes, and the SF amygdala has been implicated in social/affective processing (LeDoux, 2003; Phelps and LeDoux, 2005; Goossens et al., 2009). Given that amygdala subregions exhibit unique developmental patterns of FC across childhood and adolescence (Gabard-Durnam et al., 2014), it is likely that trauma impacts these circuits in different ways. We support this supposition by having attained commonalities and discrepancies in effects across subregions. Commonalities were observed across amygdala subregions such that youth that endured traumatic experiences tended to have diminished frontoamygdala connectivity. Discrepancies were noted for regions of the striatum, parietal regions and regions comprising the default mode brain network (e.g., precuneus and posterior cingulate). For these areas, CM and BL showed trauma-related FC effects but SF did not. In contrast, analysis of SF connectivity revealed trauma effects in regions that comprise the salience network of the brain, the insula and dACC, whereas other subregions did not. Thus, amygdala subregions critical for learning and attention and for social/affective processing demonstrate overlapping, but also distinct trauma-related alterations in connectivity.
Our results support a model of reduced emotion regulatory control in urban youth with histories of trauma. Amygdala-medial prefrontal correlations are negative at rest (Gee et al., 2013b) and during the regulation of emotional responding (Hare et al., 2008), a pattern of connectivity thought to index top-down regulatory control. Behaviorally, loss of inhibitory affective control has been associated with exposure to trauma (Pechtel and Pizzagalli, 2011) as well as presence of clinical mood disorders (Etkin et al., 2013). Prior research has also described negative attention bias and augmented vigilance in those with histories of early life trauma exposure (Pollak, 2008). Furthermore, studies using task-based fMRI show hyperactivity of the amygdala and/or hypoactivity of medial prefrontal regions in individuals with anxiety (McClure et al., 2007), PTSD (Etkin and Wager, 2007), and in children with histories of early adversity (Tottenham et al., 2011; McCrory et al., 2013), which may reflect failure of prefrontal regions to regulate amygdala reactivity. The direction of our effects (more negative frontoamygdala FC in comparison participants) suggests reduced top-down control via frontoamygdala neurocircuitry may be a consequence of early traumatic experiences.
Whole-brain exploratory analyses identified stronger amygdala-anterior insula connectivity in trauma-exposed youth. Increased FC indicates greater signal covariance between regions that detect threat and generate fear responses (i.e., amygdala; LeDoux, 2003) and process meaning and prediction of aversive bodily states (i.e., insula; Craig, 2011). Stronger amygdala-insula FC has also been reported in adults with PTSD (Rabinak et al., 2011) and adolescents with generalized anxiety disorder (GAD) (Roy et al., 2013), highlighting the relevance of this pathway for clinical disorders. Moreover, task-based fMRI studies have demonstrated increased amygdala and insula reactivity in children exposed to violence (McCrory et al., 2011) and soldiers that endure combat stress (Van Wingen et al., 2011). Increased functional covariance in the amygdala and insula at rest suggests they may be primed for rapid co-activation, or that functions subserved by these regions tend to be more coordinated.
Our results indicate significantly reduced (positive) amygdala-dACC connectivity in trauma-exposed youth. Whereas ventral aspects of the ACC (e.g., perigenual and subgenual regions) are implicated in emotion regulation, dACC is associated with the expression of fear (Etkin et al., 2011). Evidence suggests that top-down (i.e., dACC/lateral prefrontal-based) forms of emotion regulation work by recruiting the ventral ACC (vACC), which dampens limbic reactivity directly (Etkin et al., 2011). Fitting with this conceptualization, our data show congruent between group effects in dACC and sgACC. Dorsal aspects of the ACC are also implicated in sympathetic nervous arousal (Critchley, 2005). Thus, our altered amygdala-dACC FC results may reflect aberrant modulation of autonomic nervous system function. This is consistent with reports of increased arousal and fear in trauma-exposed youth, which may contribute to the development of clinical disorders (e.g., anxiety; Glaser, 2000).
We observed that differences in neuroconnectivity between trauma-exposed and control groups were associated with depression or anxiety symptomology only in select regions, including the sensorimotor cortex, middle temporal gyrus and superior parietal cortex. This is highly consistent with the observation that trauma exposure does not precisely predict emergence of psychopathology. Considerable data has shown that outcomes resulting from trauma exposure vary greatly across individuals (Cicchetti and Rogosch, 1996, Felitti et al., 1998; Tottenham and Sheridan, 2009). Thus, one would not expect that the relationship between brain measures and previously experienced trauma would be ubiquitously significantly correlated. These variables are neither orthogonal, nor perfectly correlated. Thus, having observed some but not complete overlap in significance could have been predicted. Similar consideration could explain observed associations in income and IQ, all of which are presented in superscript in Supplementary Table S1.
Given increased awareness that motion confounds interpretation of fMRI data and the resulting shift toward rigorous approaches to dealing with motion in resting-state data, we reanalyzed all data using an alternative scrubbing approach (Deen and Pelphrey, 2012; Van Dijk et al., 2012; Power et al., 2015). We saw that observed main effects were consistent, with the exception of a group difference in the orbitofrontal cortex that did not hold after scrubbing. It is possible this result did not replicate because the group difference covered a smaller territory to begin with (15 voxels), and because clusters smaller than 10 contiguous voxels are not reported as significant. The reason that in large part we saw consistent effects in scrubbed and non-scrubbed data may be due to the low overall movement profile of participants in the original analyses.
Our results underscore that traumatic stress may alter limbic circuitry in the immature brain and this may precede the onset of clinically significant conditions. There is evidence suggesting that changes in frontoamygdala circuits persist even decades later into adulthood (Dannlowski et al., 2012), emphasizing the relevance of early life exposures for lifelong socioemotional functioning. Although altered amygdala connectivity may reflect ontogenetic adaptation to an unsafe rearing environment, it is possible that these neural changes confer elevated threat vigilance, which may be detrimental to healthy emotional development. For instance, it has been suggested that increased amygdala-insula coupling mediates anxious anticipation of negative events (Carlson et al., 2011). Recalibration of neural connectivity in limbic circuitry in early life may be detrimental to evaluation of threat and safety, and compromise a child’s ability to master age-appropriate skills in social and cognitive domains. For example, altered connectivity may reflect changes in synaptic function within this emotion regulatory network or reduced priming of network components that allow for rapid control of emotional responding.
Our findings should be considered in light of limitations. First, we were not sufficiently powered to differentiate results based on onset (age) or type of trauma. Although retrospective analysis shows that trauma onset and trauma type relate to distinctive patterns of emotional functioning (English et al., 2005), prior studies also document non-specific effects of trauma type on outcomes (Arata et al., 2007; Collishaw et al., 2007) and some suggest that disentangling unique effects may result in overly narrow interpretations (Green et al., 2010). Next, participants were drawn from a larger study and were not selected on the basis of IQ or income. As a result, groups were not matched on these variables. This is not surprising given the strong association between these variables and trauma prevalence (De Bellis, 2001), but also not ideal for disentangling connectivity effects. However, the alternative is to select matched samples that may not convey the natural conditions present in trauma exposed individuals, and this could in turn impact observations about neural connectivity. Moreover, we are assured by the consistency of our findings with both animal and human research (Gee et al., 2013a; Malter Cohen et al., 2013a; Brown et al., 2014), and because follow-up analyses indicated that amygdala-sgACC results held when controlling for income and IQ. In addition, we demarcate FC effects that showed correspondence with other risk factors (i.e., poverty, IQ, anxiety, depression). Interactions between these variables are areas for future research. Although we did not find an association between frontoamygdala connectivity and anxiety or depressive symptoms, it is possible that additional symptom-brain associations would be detected with a larger sample. Along this line, an additional consideration is the sample size is relatively small (n = 42), and findings are correlational in nature—thus precluding ability to make causal attributions about changes in frontoamygdala connectivity and the experience of childhood trauma. Future longitudinal examinations of amygdala connectivity in larger samples will be necessary to better understand mechanisms contributing to neural circuit reorganization following early life stress. Finally, poor spatial resolution of standard fMRI acquisitions and susceptibility of the amygdala to EPI image distortions and draining vein effects (Merboldt et al., 2001) may lead to spatial localization errors. Furthermore, use of an 8 mm smoothing kernel to investigate small volumes such as amygdala subregions is not an optimal design. Despite these considerations, cytoarchitechtonically based amygdala FC analyses have yielded consistent, replicated delineation of differential connectivity of major amygdala subregions (Etkin et al., 2009; Roy et al., 2009; Roy et al., 2013; Brown et al., 2014). Also, the fact that patterns observed here replicate prior neuroimaging and anatomical work in animals affords further confidence in the approach.
CONCLUSIONS
Reduced negative amygdala-sgACC connectivity was observed in a sociodemographic risk sample of youth exposed to severe trauma. Isolation of these effects augments prior studies in adults obtaining similar results by evaluating intrinsic connectivity in formative years (e.g., childhood) and by measuring change in a sample at substantially increased risk for developing clinical syndromes (i.e., urban, low income, minority). Our results support the supposition that the biological embedding of adversity in early life may include changes in neural connectivity, which in turn may alter interactions with the world and susceptibility to disease.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This project was supported in part by the Merrill Palmer Skillman Institute and the Department of Pediatrics, WSU School of Medicine and by a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (M.E.T.). Further support comes from Lycaki-Young Funds, State of Michigan, Detroit Wayne Mental Health Authority, and RO1MH59299 (D.R.R.) and RO1HD075806 (M.E.T). The authors would like to thank Pavan K. Jella, for his assistance in neuroimaging data acquisition; Rita Elias, Melissa Youmans, Mallory Gardner, Timothy Lozon and Ali Daher for assistance in participant recruitment, scanning and conducting structured behavioral interviews; Matthew Carroll for consultation on statistical analyses and are grateful to those families who volunteered their time to participate in this study.
REFERENCES
- Alim TN, Charney DS, Mellman TA. An overview of posttraumatic stress disorder in African Americans. Journal of Clinical Psychology. 2006;62:801–13. doi: 10.1002/jclp.20280. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Arata CM, Langhinrichsen-Rohling J, Bowers D, O’Brien N. Differential correlates of multi-type maltreatment among urban youth. Child Abuse and Neglect. 2007;31:393–415. doi: 10.1016/j.chiabu.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–53. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Brown VM, Labar KS, Haswell CC, et al. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2014;39:361–9. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15:1736–41. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Social Cognitive and Affective Neuroscience. 2011;6:74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–8. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology: Special Issue of Development and Psychopathology. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B. Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abuse and Neglect. 2007;31:211–29. doi: 10.1016/j.chiabu.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal Of Comparative Neurology. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: the psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:539–64. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. NeuroImage. 2009;47:864–71. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Deen B, Pelphrey K. Perspective: brain scans need a rethink. Nature. 2012;491:S20. doi: 10.1038/491s20a. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Cholinergic activation of the electrocorticogram: an amygdaloid activating system. Experimental Brain Research. 1996;108:285–96. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: Findings from the adverse childhood experiences study. JAMA. 2001;286:3089–96. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, et al. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Translational Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- English DJ, Upadhyaya MP, Litrownik AJ, et al. Maltreatment’s wake: the relationship of maltreatment dimensions to child outcomes. Child Abuse and Neglect. 2005;29:597–619. doi: 10.1016/j.chiabu.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Gyurak A, O’Hara R. A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues in Clinical Neuroscience. 2013;15:419–29. doi: 10.31887/DCNS.2013.15.4/aetkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry. 2009;66:1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. The American Journal of Psychiatry. 2011;168:968–78. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, et al. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Frontiers in Systems Neuroscience. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, et al. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013a;110:15638–43. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2013b;33:4584–93. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, et al. Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry. 2009;31:505–14. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser D. Child abuse and neglect and the brain—a review. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2000;41:97–116. [PubMed] [Google Scholar]
- Goldmann E, Aiello A, Uddin M, et al. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American urban community: the Detroit neighborhood health study. Journal of Traumatic Stress. 2011;24:747–51. doi: 10.1002/jts.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Onur OA, et al. Selective processing of social stimuli in the superficial amygdala. Human Brain Mapping. 2009;30:3332–8. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, White D, Hadley J, et al. Early life trauma and directional brain connectivity within major depression. Human Brain Mapping. 2014;35:4815–26. doi: 10.1002/hbm.22514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;67:113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19119–24. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test: KBIT 2. 2004. San Antonio, TX: Pearson. [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US national comorbidity survey. Psychological Medicine. 1997;27:1101–19. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Archives of General Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kim JE, Lyoo I, Estes AM, et al. Laterobasal amygdalar enlargement in 6- to 7-year-old children with autism spectrum disorder. Archives of General Psychiatry. 2010;67:1187–97. doi: 10.1001/archgenpsychiatry.2010.148. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2009;29:11614–8. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz PM, House JS, Mero RP, Williams DR. Stress, life events, and socioeconomic disparities in health: results from the Americans’ changing lives study. Journal of Health and Social Behavior. 2005;46:274–88. doi: 10.1177/002214650504600305. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SR, Galea S, Uddin M, Koenen KC. Trajectories of posttraumatic stress among urban residents. American Journal of Community Psychology. 2014;53:159–72. doi: 10.1007/s10464-014-9634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2013a;110:18274–8. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Tottenham N, Casey BJ. Translational developmental studies of stress on brain and behavior: implications for adolescent mental health and illness? Neuroscience. 2013b;249:53–62. doi: 10.1016/j.neuroscience.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annual Review of Medicine. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2014;40:1250–58. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. The British Journal of Psychiatry : The Journal of Mental Science. 2013;202:269–76. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, et al. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21:R947–948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: how the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17180–5. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merboldt KD, Fransson P, Bruhn H, Frahm J. Functional MRI of the human amygdale? NeuroImage. 2001;14:253–7. doi: 10.1006/nimg.2001.0802. [DOI] [PubMed] [Google Scholar]
- Nooner KB, Mennes M, Brown S, et al. Relationship of trauma symptoms to amygdala-based functional brain changes in adolescents. Journal of Traumatic Stress. 2013;26:784–7. doi: 10.1002/jts.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Bloom RF, Carpenter LL. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2013;23:24–32. doi: 10.1016/j.euroneuro.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD. Mechanisms linking early experience and the emergence of emotions: illustrations from the study of maltreated children. Current Directions in Psychological Science. 2008;17:370–5. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage. 2015;105:536–51. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Supekar K, Uddin LQ, Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7941–6. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, et al. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers in Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:290–9.e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor CF, Finch AJ, Jr, Spirito A, Bennett B. The children’s depression inventory: a systematic evaluation of psychometric properties. Journal of Consulting and Clinical Psychology. 1984;52:955–67. doi: 10.1037//0022-006x.52.6.955. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Chiu WT, Hwang I, et al. Cross-national analysis of the associations between traumatic events and suicidal behavior: findings from the WHO World Mental Health Surveys. PloS One. 2010;5:e10574. doi: 10.1371/journal.pone.0010574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research. 2013;47:1469–78. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Kong L, Wu F, et al. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychological Medicine. 2013;43:1921–7. doi: 10.1017/S0033291712002759. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Tocco MA, Quednau KA, Bedway AR, Carre JM. Idle behaviors of the hippocampus reflect endogenous cortisol levels in youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:642–52.e1. doi: 10.1016/j.jaac.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Tottenham N. The importance of early experiences for neuro-affective development. Current Topics in Behavioral Neurosciences. 2013;16:109–29. doi: 10.1007/7854_2013_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry. 2011;16:664–71. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.