Abstract
Although body ownership—i.e. the feeling that our bodies belong to us—modulates activity within the primary somatosensory cortex (S1), it is still unknown whether this modulation occurs within a somatotopically defined portion of S1. We induced an illusory feeling of ownership for another person’s finger by asking participants to hold their palm against another person’s palm and to stroke the two joined index fingers with the index and thumb of their other hand. This illusion (numbness illusion) does not occur if the stroking is performed asynchronously or by the other person. We combined this somatosensory paradigm with ultra-high field functional magnetic resonance imaging finger mapping to study whether illusory body ownership modulates activity within different finger-specific areas of S1. The results revealed that the numbness illusion is associated with activity in Brodmann area (BA) 1 within the representation of the finger stroking the other person’s finger and in BA 2 contralateral to the stroked finger. These results show that changes in bodily experience modulate the activity within certain subregions of S1, with a different finger-topographical selectivity between the representations of the stroking and of the stroked hand, and reveal that the high degree of somatosensory specialization in S1 extends to bodily self-consciousness.
Keywords: somatotopy, ownership, self, touch, 7T fMRI
INTRODUCTION
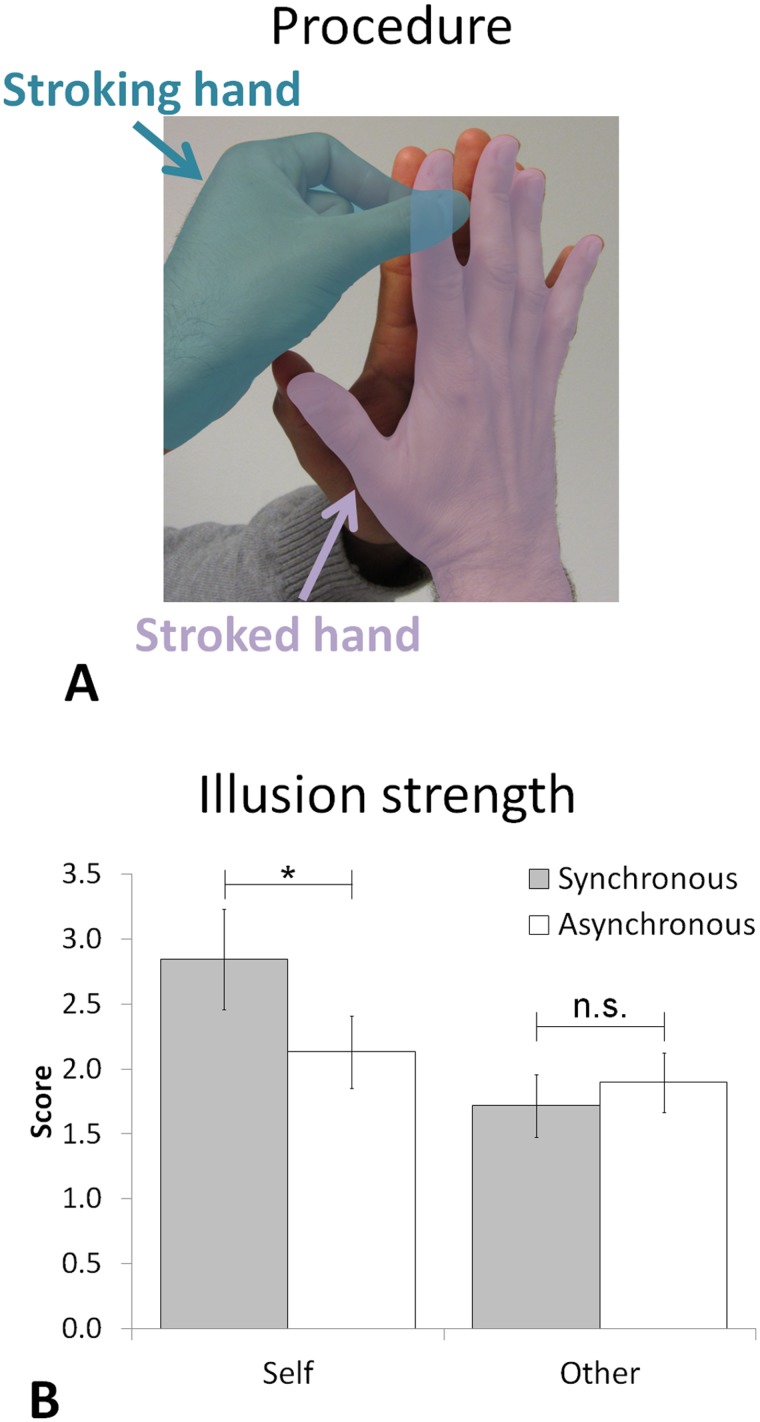
Body ownership is a fundamental aspect of self-consciousness (Gallagher, 2000; Jeannerod, 2007; Tsakiris et al., 2007b, 2010; Blanke, 2012), referring to the sense that our body belongs to us and is distinct from those of other persons. Body ownership originates from congruent multisensory signals (van den Bos and Jeannerod, 2002). Therefore, certain incongruent conditions of visual, tactile and proprioceptive stimulation can induce errors or illusions of body ownership for a fake hand (e.g. Botvinick and Cohen, 1998) or for a virtual body (Ehrsson, 2007; Lenggenhager et al., 2007). Body ownership has been most often studied by manipulating visuo-tactile inputs (Schaefer et al., 2006; Lenggenhager et al., 2007; Ionta et al., 2011; Blanke, 2012). However, the presence of a visual component is not always necessary, as simple tactile-proprioceptive stimulations creating a mismatch between where the touch is applied and where it is predicted (e.g. by crossing the hands or by interposing another person’s finger) have also been found to induce ownership for a fake hand (Ehrsson et al., 2005; Pozeg et al., 2014) or for another person’s finger (Boulware, 1951; Arnold, 1952; Dieguez et al., 2009), without any concomitant visual stimulation. We used a paradigm involving tactile and proprioceptive stimulation that induces an alteration of finger experience and ownership, i.e. the so-called numbness illusion (NI; Dieguez et al., 2009). The NI arises when one person holds his/her palm against another person’s palm and strokes with the index and thumb of his/her other hand the two joined index fingers (his/her and the other person’s; Figure 1A). During this simple procedure, participants report different alterations of body perception, such as a sensation of numbness and widening of their own finger, as well as a feeling of owning the other person’s index finger (Dieguez et al., 2009). None of these previous studies investigating the NI reported any change in sensation related to the stroking hand.
Fig. 1.
Experimental procedure. (A) Procedure to induce the NI: subject’s stroked hand (indicated in violet) is held against another person’s palm and the subject strokes with the index and thumb of his other free hand (i.e. stroking hand indicated in blue) the two joined index fingers. The stroking was performed either by the subjects (self condition) or by the experimenter (other condition). (B) The illusion strength shows an Agent × Synchrony interaction. Gray and white bars indicate the synchronous and the asynchronous stroking conditions, respectively; error bars indicate the standard error of the mean (SEM). The asterisk indicates a statistically significant difference (P < 0.05).
Although the sense of ownership has traditionally been associated with brain activity within the premotor cortex, the posterior parietal cortex and the insula (Ehrsson et al., 2004, 2005; Tsakiris et al., 2007a; Kammers et al., 2009; Ionta et al., 2011; Petkova et al., 2011; Zeller et al., 2011; Evans and Blanke, 2013; Gentile et al., 2013), some studies have also demonstrated the contribution of primary somatosensory (S1) cortex in processing and integrating visuo-tactile information related to one’s own and to a fake or another person’s hand (Schaefer et al., 2006; Kanayama et al., 2007; Tsakiris et al., 2007a; Dieguez et al., 2009; Schaefer et al., 2009; Cardini et al., 2011; Lenggenhager et al., 2011; Aspell et al., 2012; Evans and Blanke, 2013; Kuehn et al., 2013). In particular, by inducing the NI while recording somatosensory evoked potentials (SEPs) provoked by median nerve stimulation, Dieguez et al. (2009) showed that illusory finger ownership was associated with a modulation of the earliest cortical component (i.e. the N20 component) of the SEP, which is a marker of S1 activity [predominantly of Brodmann area (BA) 3b, see below; Allison et al., 1989; Baumgartner et al., 1998]. However, SEPs, as in Dieguez et al. (2009), have limited spatial resolution involving multiple fingers, and do not allow a precise localization of the activity changes induced by illusory ownership in different portions of S1. Additionally, Dieguez et al. (2009) suggested that the NI, which is inherently based on double touch, arises from the combination of tactile information from the stroking and stroked fingers, although the SEP analysis did not allow the authors to investigate the NI effects for the representation of the stroking hand.
S1 includes a somatotopic representation of the contralateral body (Penfield and Boldrey, 1937) and consists of four cytoarchitectonic regions, namely BAs 3a, 3b, 1 and 2, as shown in both non-human primates (Kaas et al., 1979) and humans (Geyer et al., 1999, 2000; Grefkes et al., 2001). In recent years, the increased spatial resolution provided by ultra-high field functional magnetic resonance imaging (fMRI) has allowed for mapping of the representation of each finger in S1 in individual subjects (Sanchez-Panchuelo et al., 2010, 2012; Stringer et al., 2011; Martuzzi et al., 2014) and to separate S1 representations across BAs 3b, 1 and 2 (Sanchez-Panchuelo et al., 2012; Martuzzi et al., 2014). Consistent with what has been observed in non-human primates, these imaging studies showed that within the representation of a body part (e.g. a finger), BA 3b responds selectively to the stimulation of that specific body area, whereas BA 1 and 2 have larger receptive fields and respond also to the stimulation of neighboring regions (Besle et al., 2014; Martuzzi et al., 2014). Although it has been shown that S1 activity is modulated by finger ownership (Dieguez et al., 2009), it is currently unclear whether finger ownership is associated with a somatotopically specific pattern of S1 activity, as can be found within BA 3b, or whether it requires the integration of tactile information from multiple digits, as observed within BA 1 and 2, therefore suggesting a finger-unspecific ownership effects.
The aim of this study is to apply ultra-high field fMRI to study the contribution of the different subregions of S1 to bodily experience and to body ownership in particular. Among the different illusory conditions that have been experimentally used to study body-part ownership, the NI is very well suited for understanding the contribution of S1 to finger ownership, because it is a tactile-proprioceptive illusion, inducing changes in bodily experience and body ownership with effects that are experienced within the fingers involved in the task bilaterally—i.e. the index finger of the stroked hand and the thumb and the index of the stroking hand. As these fingers, as well as the others, are precisely represented within S1, the NI allows us to investigate whether finger ownership is specifically associated with the cortical representation of the fingers for which the illusion is experienced or whether it encompasses also the neighboring finger representations within S1. No illusion is experienced when the two fingers are stroked asynchronously or if another person performs the stroking. Therefore, these conditions (i.e. asynchronous stroking and stroking performed by another person), which provide the same tactile input without altering bodily experience (Dieguez et al., 2009), were used as control conditions to study the cortical effects of the NI on S1 representations.
Thus, in this article we used ultra-high field fMRI mapping to compare brain activity evoked in S1 by the different conditions of tactile stimulation used to induce the NI. In a 2 × 2 factorial design we compared S1 activity when the participant directly stroked her/his finger and an experimenter’s finger (Self) with her/his left index and thumb, or while the experimenter stroked his own and the participant’s finger (Other), and when tactile stimulation was synchronously or asynchronously applied to the two fingers. In particular, we tested whether a different pattern of activity is evoked by synchronous self-administered stimulation, i.e. the condition inducing the NI, specifically in the cortical representation of the fingers for which the illusion is experienced or whether the illusion encompasses other portions of the somatosensory cortex, including the representations of the neighboring fingers. We also analyzed the different activation patterns for the S1 representations of the stroked and of the stroking hand. Based on the nature of sensory–motor conflicts inducing the NI, which involve the integration of predictive signals and somatosensory feedback specific to different fingers of the two hands, we hypothesized that the NI-related changes in body ownership should be associated with activity modulations within the BAs containing overlapping finger representations, such as BA 1 and BA 2, more so than in BA 3b, where individual digit representations are specific and spatially segregated.
MATERIALS AND METHODS
Participants and experimental procedures
Twelve right-handed male adults aged between 18 and 44 years (mean ± s.d.: 26.6 ± 8.4 years) participated in the study. All the participants were naïve to the purposes of the study. Handedness was assessed with the Edinburgh Oldfield Handedness Inventory (Oldfield, 1971), and all participants provided written informed consent. All procedures were approved by the Ethics Committee of the Faculty of Biology and Medicine of the University of Lausanne and the study was conducted in accordance with the Declaration of Helsinki.
The experimental procedure included three separate steps (see below for a detailed description of each step): first, a pre-scan training session during which participants familiarized themselves with the task outside the scanner; second, the acquisition of the functional localizers of individual finger representations in S1 for the right and the left hand; third, the actual NI experiment.
Pre-scan training session
Before entering the scanner participants familiarized themselves with the experimental conditions of the NI experiment and with the somatosensory sensations related to them. Subjects were not formally debriefed about the nature of the NI until after the scanning session. Following Experiment 1 described in Dieguez et al. (2009), participants were asked to place the palm of their right hand against the left-hand palm of one experimenter (Figure 1A). Keeping this posture, either the participant (self) or the experimenter (other) stroked the two joined index fingers with the thumb and the index of the other (free) hand. The stroking was self-paced at approximately a frequency of 1 Hz and performed on the dorsal side of two distal phalanges of the two index fingers either synchronously (synchronous—i.e. both fingers stroked simultaneously) or asynchronously (asynchronous—i.e. the participant’s and the experimenter’s fingers were stroked at approximately the same frequency but alternatively, one at the time). This led to four experimental conditions—namely other-synchronous, other-asynchronous, self-synchronous and self-asynchronous. During training, special attention was paid to achieve a consistent stroking frequency and pressure across participants (the participating experimenter was always the same trained individual). After each condition, participants were asked to rate five items relative to the NI associated with the stroked index finger (see Dieguez et al., 2009), namely strangeness of the sensation, feeling of widening of the finger, feeling of numbness, feeling that the two index fingers merged into one big finger and feeling of owning the other person’s finger. Rating was performed on a scale from 1 (no effect) to 5 (strong effect). After this training, participants were allowed to freely discuss the sensations they experienced until an agreement could be reached that any such sensation should give rise to a single rating of the NI during the scanning period.
Functional localizer of individual finger representations
Prior to the main experiment, the representations of each single finger for both the right and the left hand were mapped. The finger mapping procedure was the same as the one described by Martuzzi et al. (2014). The only difference was that the number of stimulation blocks (one run including six blocks instead of two runs of four blocks each) was reduced to shorten scanning time. The mapping procedure was the same for both hands, with the right (i.e. the stroked hand in the NI experiment) mapped first, followed by the left hand (i.e. the stroking hand in the NI experiment).
During the acquisition of the functional localizer data, participants kept their arm comfortably stretched along the magnet bore with the palm up and their fingers were manually stroked one at the time, from the proximal to the distal portion of the finger, by an experimenter positioned at the entrance of the bore. The experimenter performed the stroking along the palmar side of the two distal phalanges of each finger using his own index finger at a frequency of approximately 1 Hz. Each finger was stroked for 20 s, followed by 10 s of rest (no stroking) in the following order: D1 (thumb)–D3 (middle)–D5 (little)–D2 (index)–D4 (ring). This sequence was repeated six times, keeping the order of fingers being stroked fixed, in accordance to the mapping procedure proposed by Martuzzi et al. (2014). To reduce the variability of stimulus pace as well as of pressure on the fingers, all the stroking procedures were performed by the same experimenter. The rationale for using the human touch as a stimulus is 2-fold: first, the stimulus is very similar in nature to the stimulus used during the NI; second, human touch provides not only localized touch but also several features such as motion, texture, and temporal variability and therefore inducing an activation of the entire S1, encompassing BA 3b, 1 and 2 (Martuzzi et al., 2014).
NI experiment
In the NI experiment, an experimenter (standing at the entrance to the magnet bore) held his left palm against the participant’s right palm (as he/she was lying in the scanner) for the entire duration of the acquisition run. The participant’s right hand was actively held up, with the forearm kept in an approximately vertical position with the palm supported against the experimenter’s palm. To avoid changes in the hand position during the experiment, particular care was used in finding a comfortable position for the participant.
Each trial began with a 4 s cue indicating which type of stroking the participant and the experimenter would perform in the upcoming stimulation block (i.e. ‘synchronous’, ‘asynchronous’ or ‘no stroking’). During the cue period, if needed, the subject performed the reaching movement of the stroking hand toward the stroked one. Participant and experimenter were independently cued via visual and auditory instructions, respectively, and then the stroking condition was performed for 20 s. At the end of the stroking, participants had 4 s to rate the NI perceived during the stroking period on a scale from 1 (very weak) to 5 (very strong) via manual signaling with their free, left hand. After 12 s of rest, a new cycle started. Identical to the pre-scanning training step, the NI experiment included four experimental conditions: other-synchronous, other-asynchronous, self-synchronous and self-asynchronous. Participants were instructed and trained to perform the stroking by moving only their wrist and to minimize the movement of their fingers, hand and elbow, in order to minimize the motor-related activity within the finger representations and also to minimize stimulus-correlated head movements. During the other-synchronous and other-asynchronous conditions, participants kept their left hand on their belly, in proximity to their right hand, in order to minimize the reaching movement during the preparation of the self stroking conditions. Experimental conditions were pseudo-randomly intermixed across trials. Two functional runs were acquired, each including two repetitions per experimental condition.
MR data acquisition
Images were acquired on a short-bore head-only 7T scanner (Siemens Medical, Germany) with an eight-channel Tx/Rx rf-coil (Rapid Biomedical, Germany). Functional images were acquired using a sinusoidal readout echo-planar images (EPI) sequence (Speck et al., 2008) and comprised 28 axial slices for the functional localizers and 24 slices for the NI experiment. Slices were placed over the postcentral gyrus (approximately orthogonal to the central sulcus) in order to cover the representations of both hands within the primary somatosensory cortex. The orientation of the slices was kept constant between the functional localizers and the NI experiment (in-plane resolution 1.3 × 1.3 mm2; slice thickness 1.3 mm; gap 0.13 mm; matrix size 160 × 160, FOV = 210 mm, TE = 27 ms, GRAPPA = 2) and the TR was set to 2.5 s for the functional localizers and 2 s for the NI experiment (an example of slice selection for three representative subjects is shown in Supplementary Figure S1). One functional localizer series per hand was acquired, each comprising 361 volumes. In the NI experiment two functional runs were acquired, comprising 329 volumes each.
To aid coregistration, a single whole-brain EPI volume with 64 slices (1.3 × 1.3 × 1.3 mm3 resolution) was also acquired, keeping the same slice orientation used in the functional volumes. To aid in the delineation of the BAs, an anatomical volume was acquired using the MP2RAGE sequence (Marques et al., 2010), with TE = 2.63 ms, TR = 7.2 ms, TI1 = 0.9 s, TI2 = 3.2 s, TRmprage = 5 s (1 × 1 × 1 mm3 resolution).
Prior to acquisition of each functional or anatomical run, B0 shimming was performed using the manufacturer’s shim algorithm, with multiple repetitions and careful verification of the results before starting the EPI sequence.
Behavioral data analysis
For each participant independently, we first computed the individual mean and standard deviation of the responses across all the experimental conditions. Then, data were normalized by subtracting the mean value and dividing the demeaned scores by the standard deviation. Normalization transformed the rating data in Z-score with a normal distribution allowing the use of parametric tests.
The normalized ratings were analyzed by means of a two-way repeated measures analysis of variance (ANOVA) with Agent (self, other) and Synchrony (synchronous and asynchronous) as within-subject factors. Post hoc analyses were conducted using the Tukey Honestly Significant Difference (HSD) test thresholded at P < 0.05.
fMRI data analysis
Statistical analyses were conducted using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK). Functional volumes were temporally realigned to the first slice acquired, spatially realigned to the first volume acquired, and smoothed with an isotropic Gaussian kernel (FWHM = 2 mm).
The MP2RAGE volume was coregistered with the whole brain EPI image by means of rigid body transformations and both were subsequently coregistered to the mean EPI functional volume.
Statistical analyses were performed using a General Linear Model (GLM) with the canonical hemodynamic response function (HRF) and its time derivative as basis functions. The model included two regressors (the boxcar convoluted with the HRF and the temporal derivative thereof) per experimental condition and the motion parameters as nuisance regressors. All the analyses were conducted in the individual subject space (i.e. in the space of the functional acquisitions).
For each participant, somatotopic mapping was performed independently for the right and the left hand following the procedure described in Martuzzi et al. (2014). Briefly, we computed an F-contrast including the HRF regressors of all the five fingers and five independent t-tests, one for each HRF regressor of individual finger stimulations. The result of the F-contrast (P < 0.001 uncorrected) identified all the voxels responding to the stimulation of at least one finger and was used as an S1 mask. Within the S1 mask, each voxel was independently labeled as representing the digit demonstrating the highest t-value for that particular voxel.
Following the guidelines identified in cytoarchitectonic studies (Geyer et al., 2000; Grefkes et al., 2001), for each single subject BA 3b was delineated as the region on the anterior wall of the postcentral gyrus, BA 1 as the region in the crown of the postcentral gyrus, adjacent to BA 3b, and BA 2 as the region on the posterior wall of the postcentral gyrus, adjacent to BA 1 (masks of BA 3b, 1 and 2 for three representative subjects are shown in Supplementary Figure S2). Thanks to the high spatial resolution and the high signal-to-noise ratio of the EPI, BAs were manually delineated on the mean EPI of each participant. To delineate the finger representations within each of the three BAs, the three BA masks were combined (simple conjunction) with the somatotopic maps and a 5-voxel cluster threshold was independently applied on the conjunctions of each BA and S1 masks. The mapping procedure elicited significant BOLD responses within BA 2, but single finger responses were highly overlapped and did not yield reliable somatotopic maps. Therefore, we could only identify BA 2 as a whole, without separating single finger representations. BA 3a was not investigated because the functional localizer is based on gentle touch applied on the skin and hence it does not elicit reliable activations within BA 3a, which receives proprioceptive information from muscles and joints (Friedman and Jones, 1981).
In summary, we first used an F-contrast (P < 0.001 uncorrected) to compute the S1 mask and applied a winner-takes-all method based on the individual finger t-contrasts to create the somatotopic maps. Then, we manually delineated the masks for BA 3b, 1 and 2, following the guidelines identified in cytoarchitectonic studies. Finally, we computed the conjunction maps between the S1 and each of the three BA masks and applied a 5-voxel cluster threshold, independently on each of these three maps to define the finger representations within each BA.
Single subject analysis of the illusion data was carried on using a GLM including the models of the response to each of the experimental conditions, to the cue/reach period (modeled separately for the self and the other conditions) and to the response. Motion parameters were also included as nuisance regressors.
For visualization purposes only, the anatomical images and the results of the statistical analyses were normalized to the MNI space using the diffeomorphic registration algorithm (DARTEL; Ashburner, 2007) and resampled to a resolution of 1 × 1 × 1 mm3. Cortical reconstruction and data projection onto the cortical surface were performed using CARET (Van Essen et al., 2001; http://brainmap.wustl.edu/caret/).
Voxel-wise group analysis
Voxel-wise group-level statistics were obtained by means of second-level analyses. The individual contrast images associated with the four experimental conditions were first normalized to the MNI space using the DARTEL algorithm (Ashburner, 2007) and resampled to a resolution of 1 × 1 × 1 mm3. The normalized volumes were then analyzed using a second-level factorial design analysis, with Agent and Synchrony as factors.
Tests were conducted to determine the active regions in each experimental condition (P < 0.001, uncorrected; 200 voxel spatial-extent threshold) and to investigate the regions showing a main effect of the Agent and of the Synchrony as well as an Agent × Synchrony interaction.
Group analysis within individual finger representations
Group analysis was intended to identify the effect of the NI over each finger representation and BA. To avoid the blurring effect induced by the functional and anatomical variability of finger representations across participants (Martuzzi et al., 2014), individual data were pooled across participants according to their functional location as identified by the somatotopic functional localizer.
Within each finger representation within BA 3b and 1, as identified by the somatotopic mapping analysis, and for BA 2 bilaterally, we computed for each experimental condition the average BOLD responses vs baseline (represented by the average HRF beta values estimated in the GLM analysis) by averaging over all voxels in each representation. This step resulted in four values per subject and region representing the four mean responses over the area to the four experimental conditions with respect to baseline (i.e. the rest condition). These average responses were first tested to assess whether these areas showed a significant positive beta value to the experimental conditions using a one-tailed t-test, because we expected that within the contralateral S1 our stimulation would induce an increase in the neuronal activity, compared with baseline. The significance level was set to P < 0.05 corrected for multiple comparisons (88, as we tested four experimental conditions within the representations of each of the five fingers for both BAs 3b and 1 and for the two BA 2), leading to an effective P < 0.00057. We then tested whether these responses differed across the four experimental conditions by means of a two-way repeated measures ANOVA with Agent (self, other) and Synchrony (synchronous and asynchronous) as within-subject factors. Post hoc test was conducted using the Tukey HSD test. The significance level was set to P < 0.05 corrected for multiple comparisons (22, as we computed one ANOVA for each of the 20 representation and for BA 2 bilaterally), leading to an effective P < 0.0023 for both the ANOVA and the Tukey HSD test. Finally, for the representations and BAs showing a significant Agent × Synchrony interaction, we computed the Pearson correlation between the synchrony-induced changes in beta values and synchrony-induced changes in the normalized ratings for both the self and the other stroking conditions. To evaluate the finger-specificity of the observed effects, each of the tests was also performed for the entire BA, which included all five finger representations.
RESULTS
Numbness illusion
The average ratings indicating the strength of the NI during the scanning session were 1.7, 1.9, 2.8 and 2.1 for the other-synchronous, other-asynchronous, self-synchronous and self-asynchronous conditions, respectively (Figure 1B; see also the Supplementary Material for the scores acquired during the pre-scan training session). As predicted, they revealed a main effect of Agent (F1,11 = 8.46; P = 0.014) and an interaction Agent × Synchrony (F1,11 = 16.28; P = 0.002). Post hoc tests highlighted that during the synchronous self-stroking condition participants had a stronger NI than in any of the other conditions (all P < 0.005), whereas no differences were observed among the other three experimental conditions (all P > 0.2). These results show that illusory body ownership (as induced in the NI) was successfully induced in the MR environment, similar to previous findings (Dieguez et al., 2009). Despite not being explicitly tested, no subjective changes of the stroking hand were reported by any of the tested subjects during the interview after the training phase or during any other phase of the experiment.
fMRI activation maps: general description of individual S1 activations
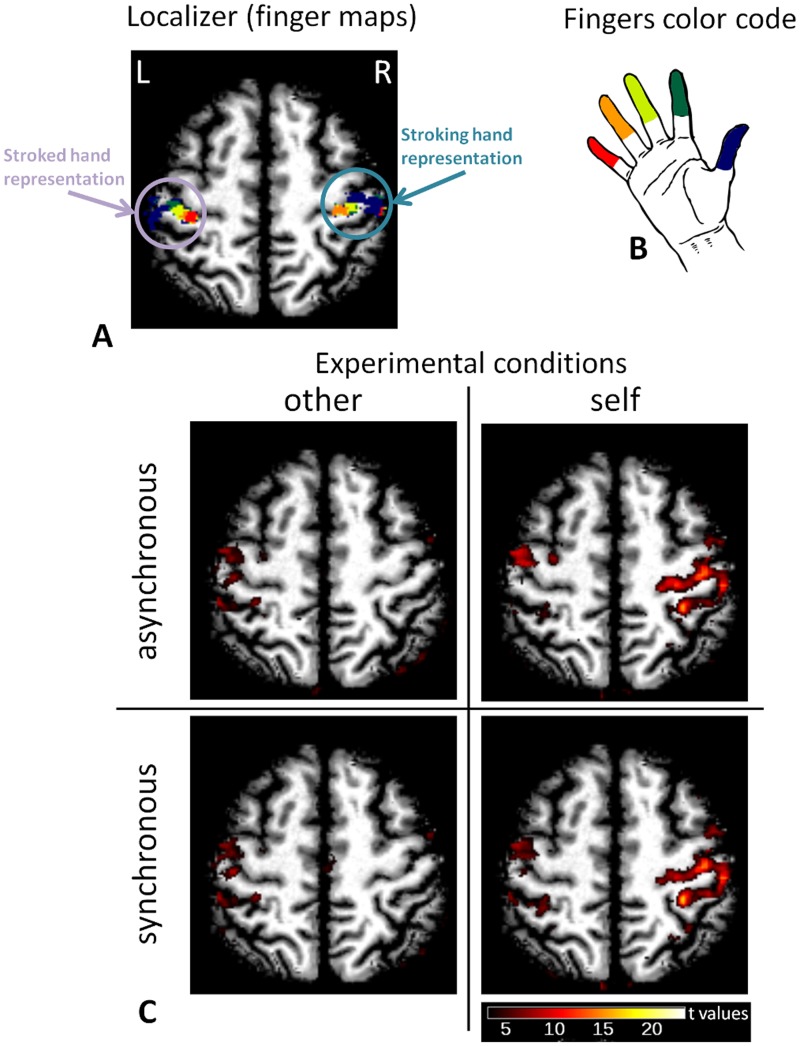
Activation maps and finger representations for the left and right S1 from a representative subject are shown in a representative axial slice in Figure 2 and on an inflated brain in Figure 3. Visual inspection of single subject results (P < 0.001 uncorrected) qualitatively showed that activations within the left S1 (stroked hand) were well co-localized with the representation of the index finger for all four experimental conditions, but this activation was larger when the experimenter performed the stroking when the experimenter performed the stroking (i.e. ‘other’ conditions’). Moreover, when the subject performed the stroking (i.e. ‘self’ conditions) we observed a large activation within the right S1 (stroking hand) that was not limited to the representation of the thumb and index fingers in right S1 (i.e. the two stroking fingers) but spanned all five finger representations.
Fig. 2.
Individual results on a selected axial plane (neurological convention). (A) The finger maps obtained in the localizer sessions for both the stroked and the stroking hand are indicated for a representative subject. R and L indicate the right and left hemisphere, respectively. (B) Pictorial representation of the color code used to indicate finger representations. (C) Brain activity in the four experimental conditions (P < 0.001 uncorrected) for the same subject and slice shown in panel (A). These results show the reduced activity in response to self vs other touch within the representation of the stroked (right) hand and also show that self touch elicits a widespread response in the area of the representation of the stroking (left) hand encompassing all finger representations.
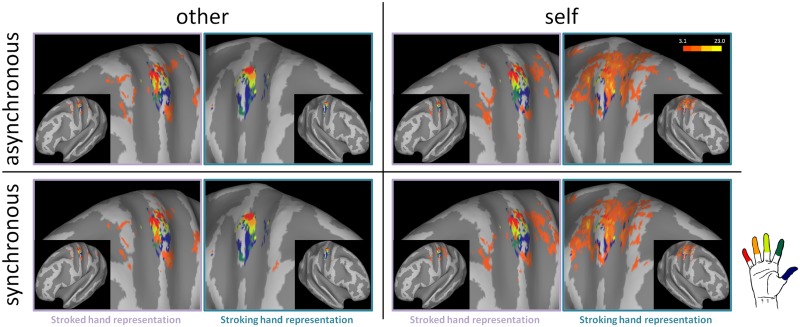
Fig. 3.
Individual results on inflated brain (P < 0.001 uncorrected). The figure shows the finger representation and the activation across the four experimental conditions for the same representative subject shown in Figure 2.
fMRI activation maps: voxel-wise group analysis
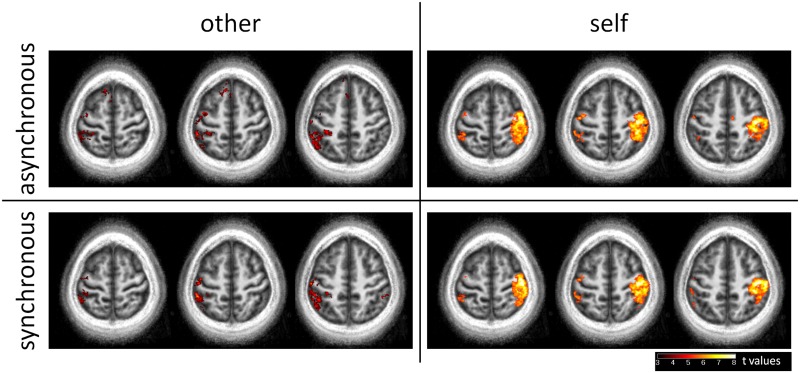
The results of the voxel-wise group analysis for the four experimental conditions are shown in representative axial slices in Figure 4.
Fig. 4.
Results of the voxel-wise group analysis overlaid to the participant’s mean anatomical volume (Z = 62, 58 and 54 mm in the MNI space). Because of the somatotopic specificity of the activations and the inter-subjects variability of finger representations, no experimental modulation of the activations can be statistically observed within the postcentral gyrus contralateral to the stroked hand.
In the other-synchronous and other-asynchronous conditions, we observed activations within the left primary somatosensory cortex (stroked hand). Additional activations were observed in the left inferior parietal lobule, and the left precentral gyrus (BA 6). In the other-synchronous condition we observed an activated area within the right supramarginal gyrus, whereas in the other-asynchronous condition we observed an activated area in the left Supplementary Motor Area.
In the self-synchronous and the self-asynchronous conditions, we observed in the right hemisphere (stroking hand) a large activated cluster including the precentral gyrus (BAs 4 and 6), the postcentral gyrus (BAs 3b, 1 and 2) and the superior parietal lobule (BA 7). We also observed activations within the left postcentral gyrus (i.e. the primary somatosensory cortex representing the stroked hand), the left inferior parietal lobule and the left precentral gyrus (BA 6). In the self-asynchronous condition, we also observed an activated cluster within the right middle cingulate cortex.
The contrast across the four experimental conditions only revealed a main effect of Agent within the right primary somatosensory, right motor and right premotor cortices (i.e. contralateral to the stroking left hand). No other significant effects (main effect of Synchrony, or Agent × Synchrony interaction) were observed in the voxel-wise group analysis.
BOLD responses within individual finger representations in BAs 3b and 1
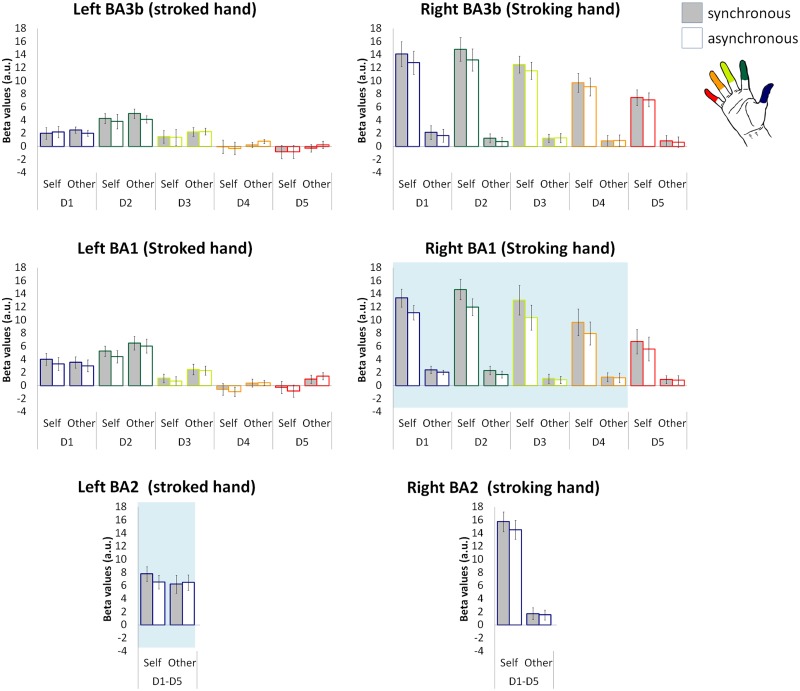
To account for the functional and anatomical variability of S1 across participants, we extracted the beta values for each finger representation in BAs 3b and 1, independently mapped for each participant (i.e. following Martuzzi et al., 2014), and tested whether these areas showed a significant positive response to the experimental conditions. The average beta values for each finger representation within left and right S1 are shown in Figure 5.
Fig. 5.
Beta values across fingers and BAs. Gray and white bars indicate the synchronous and the asynchronous stroking conditions, respectively; error bars indicate the SEM. Note that in the left BA 3b and 1, activations were focal and localized within the representation of the stroked finger (i.e. D2). The shaded area in the right BA 1 and left BA 2 panels highlights the finger representations exhibiting a significant Agent × Synchrony interaction.
Within the right BA 3b—the representation of the stroking left hand—we observed significantly positive responses within the representations of all five fingers in the self-synchronous and self-asynchronous conditions—i.e. when the participants performed the stimulation themselves (all P < 5 × 10−5).
Within the right BA 1, we also observed significantly positive BOLD responses to the self-synchronous and self-asynchronous conditions for the representations of D1, D2, D3 and D4 (all P < 4.5 × 10−4) but not for those of D5 (P = 0.006), and to the other-asynchronous condition within the representation of D1 (P = 1 × 10−4).
Within the right BA 2, we observed a significant BOLD response to the self-synchronous and self-asynchronous conditions (all P < 5 × 10−7).
Within left BA 3b—the representation of the stroked right hand—the representation of D2 (i.e. the finger being touched during all experimental conditions) showed a positive BOLD response in the other-synchronous, other-asynchronous and self-synchronous conditions (all P < 1 × 10−4), but not for the self-asynchronous condition (P = 0.003). A positive BOLD response was also observed with the representation of D1 during the other-synchronous and other-asynchronous conditions (P < 5.5 × 10−4) and within the representation of D3 during the other-asynchronous condition (P = 3.5 × 10−4). However, the responses within the other finger representations and experimental conditions were not significant after correction for multiple comparisons (all P > 0.003 uncorrected).
The analysis of the left BA 1 revealed positive BOLD responses only within the representation of D2 for the other-synchronous, other-asynchronous and self-synchronous conditions (all P < 3.5 × 10−4) and for the representation of D1 during the self-synchronous condition (P = 5.5 × 10−4). However, the responses within the other finger representations and experimental conditions were not significant after correction for multiple comparisons (all P > 0.001 uncorrected).
Within the left BA 2, we observed significant BOLD responses to all the experimental conditions (all P < 5 × 10−4).
NI modulation of the BOLD response within BAs 3b, 1 and 2
Within the finger representations of BA 3b and 1, as well as within the region of BA 2 responding to the stimulation of any finger, we tested whether the BOLD responses differed across the four experimental conditions by means of a 2 × 2 repeated measures ANOVA with Agent (self, other) and Synchrony (synchronous and asynchronous) as within-subject factors.
Within the right BA 3b—the representation of the stroking hand—a significant main effect of Agent was observed for each of the five finger representations (all P < 5 × 10−5; with higher response for self than for other), whereas a main effect of Synchrony was observed only for the representations of the two stroking fingers that are D1 and D2 (all P < 0.001; with higher response for the synchronous than for the asynchronous conditions). No Agent × Synchrony interaction was observed within any finger representation. The analysis of the average BOLD response of the right BA 3b—i.e. taking all five finger representations together—showed a significant main effect of Agent (P < 2 × 10−5) but not a significant a main effect of Synchrony (P = 0.010) or an Agent × Synchrony interaction (P = 0.124).
Within the right BA 1, D1, D2 and D3 representations showed a main effect of Agent (all P < 0.004; with higher response for self than for other) and D1 and D2 representations showed a main effect of Synchrony (all P < 0.003; with higher response synchronous than for the asynchronous). More importantly, the representations of D1, D2, D3 and D4 showed a significant Agent × Synchrony interaction (all P < 0.001), suggesting that they are involved in ownership, and this interaction was not observed for any of the other analyzed regions. Post hoc analysis (Tukey HSD test) showed that for all four finger representations, the response to the synchronous and asynchronous stimulations was significantly different for the self (P < 2 × 10−4) but not for the other (P > 0.2) conditions. The analysis of the average BOLD response of the entire right BA 1 revealed the same pattern of results, with a significant main effect of Agent and of Synchrony and a significant Agent × Synchrony interaction (all P < 2 × 10−4). Post hoc analysis showed the same patterns of interaction observed for the representations of D1, D2, D3 and D4.
Within the right BA 2, we observed a main effect of Agent (P < 3 × 10−7; with higher response for self than for other), but not a main effect of Synchrony nor an Agent × Synchrony interaction (all P > 0.06).
Within left BA 3b—the representation of the stroked hand—no finger representation showed a significant main effect of Agent of or Synchrony, or an Agent × Synchrony interaction (all P > 0.08). The same results were observed when all five finger representations were analyzed together (all P > 0.5).
Within the left BA 1, no finger representation showed a significant main effect of Agent or Synchrony or an Agent × Synchrony interaction (all P < 0.008). The average BOLD response of BA 1—i.e. taking together all the five finger representations—did not show any significant effect (all P > 0.15).
The left BA 2 did not show a significant main effect of Agent or Synchrony (all P > 0.25), but showed a significant Agent × Synchrony interaction (P < 0.002), Post hoc analysis showed that the response to the synchronous and asynchronous stimulations was significantly different (with the synchronous condition yielding a larger response) for the self (P < 0.003) but not for the other (P > 0.75) conditions.
In summary, all finger representations within the right BA 3b (stroking hand) and the representations of D1, D2, D3 and D4 within the right BA 1 showed significantly positive responses to stimulation. However, only the representations of D1, D2, D3 and D4 within BA 1 showed a significant Agent × Synchrony interaction indicating that activity in these four regions reflects the sense of ownership associated with the NI. The analyses also showed that within the left BA 3b and 1 (stroked hand), activations were focal and localized within the representation of the stroked finger—i.e. D2—but the intensity of these activations was not modulated by the sense of ownership. Conversely, the left BA 2 showed a significant Agent × Synchrony interaction indicating that the activity within these regions is modulated by the illusion.
Correlation analysis
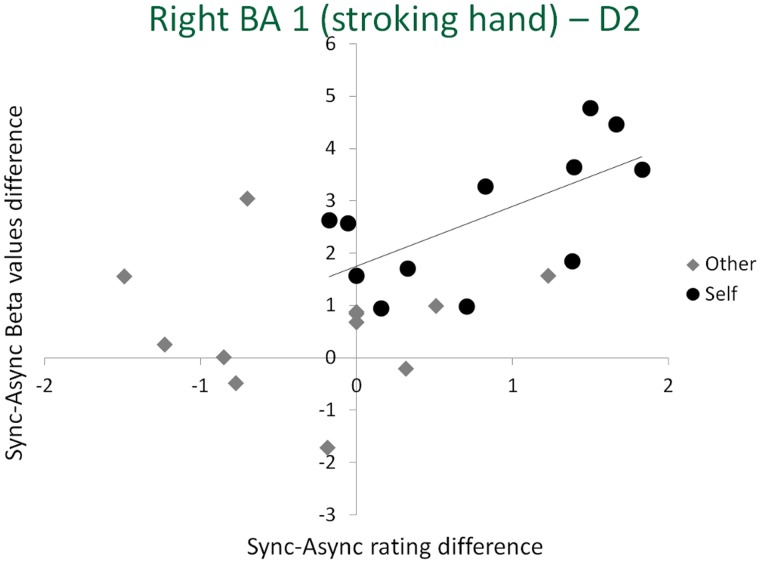
To further analyze these changes, for each of the three finger representations that showed a significant Agent × Synchrony interaction (i.e. D1, D2, D3 and D4 representations within the right BA 1), as well as for the left BA 2, we computed the Pearson correlation between the synchrony-induced changes in the BOLD response and the synchrony-induced changes in normalized NI ratings for the self and for the other conditions. This analysis revealed a single significant correlation between the changes in BOLD signal amplitude and subjective NI ratings, found within the D2 representation of the right BA 1 (Figure 6) and only for the illusion condition (i.e. for the synchrony-induced changes in the self stroking condition: r = 0.670, P = 0.017). We note that D2 in right BA 1 is the cortical representation of the stroking finger that touches the finger of the other person (i.e. the location where numbness and illusory ownership is experienced).
Fig. 6.
Correlation between synchrony-induced changes in beta values and illusion rating within the right BA 1 (i.e. representing the stroking hand) for the representation of D2, the only the representation showing a significant correlation between the changes in rating and in BOLD activity. The solid line represents the estimated linear regression for the self condition (i.e. the only condition showing a significant correlation).
There was no significant correlation within the D2 representation (or any of the other finger representations) when the other person performed the stroking (r = 0.074, P = 0.819). No significant correlation was observed within the D1, D3 and D4 representations of the right BA 1 and within the left BA 2 (all P > 0.3).
DISCUSSION
In this study, we used a tactile-proprioceptive illusion (i.e. the NI) in combination with somatotopic finger mapping and ultra-high field fMRI to investigate how changes in bodily experience, and ownership in particular, are reflected in the activity of the different finger representations in S1. Our results revealed that, in line with Dieguez et al. (2009), synchronous self-administered stimulation of one’s own and another person finger induces changes in the experience of one’s own finger and body ownership for the other person’s finger, these changes do not occur when the stroking is performed asynchronously or by another person. These synchrony- and agent-dependent experiential changes were associated with specific and selective modulations of neural activity in different finger representations in the different BAs within S1. In particular, the NI effects were reflected in a modulation of the activity of the BA 2 representation of the stroked hand and of the BA 1 representation of the stroking hand. Within BA 2 contralateral to the stroked hand, the illusion modulated S1 activity without any finger specificity. Within BA 1 contralateral to the stroking hand, the activity modulation was only found for the representation of the finger stroking the other person’s finger (D2) and extended to representations of the adjacent fingers (D1, D3 and D4). However, only for the representations of the stroking finger D2, changes in BOLD signal correlated with subjective ratings of the illusion intensity. However, no effect of the illusion was found in BA 3b for the stroking or stroked finger representations. The high specificity in the modulation of the different S1 maps due to changes in body ownership shown in this study is a new finding in the field of neural correlates of body perception.
Using different illusion paradigms and imaging modalities, previous studies have shown that S1 activity is modulated by body-part ownership. Tsakiris et al. (2007a), for instance, showed that changes in ownership for a fake hand, as induced by the rubber-hand illusion, were associated with a modulation of S1 activity. Other studies revealed gamma-band synchrony over parietal areas as a correlate of crossmodal integration linked to fake hand ownership (Kanayama et al., 2007, 2009), mu-band suppression over fronto-parietal regions related to the felt ownership of a virtual hand (Evans and Blanke, 2013), and alpha band modulations over sensorimotor cortex linked to self-identification for a virtual body (Lenggenhager et al., 2011). Despite methodological differences among the cited studies, collectively they suggest that S1 activity is linked to body ownership. However, the limited spatial resolution of these studies, using electroencephalography (EEG), evoked potentials and non-high-field fMRI, did not allow investigating whether the observed S1 modulation related to body ownership was specific to the cortical representation of the stimulated body part, and whether this activity was localized to any of the four S1 sub-regions specifically or recruited S1 globally. Our results allow a fine-grained analysis of the mechanisms of body ownership in the different finger representation and within single S1 sub-regions.
Tactile information processing is organized hierarchically across the BAs that form S1. Signals from skin receptors first reach BA 3b, which hosts neurons with mainly finger-specific receptive fields, then BA 1, which includes many neurons with multi-finger receptive fields, and finally arrive in BA 2, which contains neurons with even larger receptive field, covering all fingers (Iwamura et al., 1983; Gardner, 1988). Previous high-resolution fMRI data from our own and other groups and activation maps obtained in this study confirm that in humans, finger representations are more specific in BA 3b than in BA 1, and that BA 2 responds to the stimulation of all fingers (Besle et al., 2014; Martuzzi et al., 2014). Additionally, BA 1 and 2 have been associated with bilateral tactile integration (Tame et al., 2012).
In the NI protocol, multi-finger neurons responding to the stimulation of the index finger are activated, both for the stroking and the stroked hand, and changes in body ownership induced by the NI were associated with changes in activity in BAs containing multi-finger receptive fields and integrating bilateral stimuli, that is in BA 1 and BA 2, but not BA 3b. The pattern of activity modulation in the different S1 areas induced by the NI varied as a function the sensory-motor stimulation induced by the different conditions. Whenever we perform a movement, S1 generates an internal prediction of the somatosensory consequences of such movement, allowing the brain to differentiate self- from externally induced sensory stimulations (Jeannerod, 1988; Wolpert et al., 1995; Decety, 1996; Wolpert, 1997; Cullen, 2004). In the case of self-stroking, as administered during the NI paradigm, such prediction may concern the S1 representations of both the stroking and the stroked hand. However, during the critical condition inducing the NI, i.e. the self-synchronous condition, participants touched their own and someone else’s fingers at the same time. Due to the finger arrangement, a prediction that the stroking thumb and the stroking index are touching the same object is generated, i.e. the index finger of one’s own stroked hand. However, this prediction turns out to be false, as the touch is applied over another person’s finger, which sends no somatosensory feedback, therefore yielding a conflict between the predicted and received somatosensory feedback. Such sensory–motor conflict in turn generates a feeling of numbness for one’s own finger and ownership for an unresponsive finger (Dieguez et al., 2009). Results from this study show how the complex modulation of neural activity in different areas of the primary somatosensory cortex reflects the NI.
Within the representation of the right stroked hand (i.e. the left S1), the modulation of activity in BA 2 reflected the interaction between the person administering touch and the synchrony of the stimulation. The Agent × Synchrony interaction was explained by a stronger response in the self-touch synchronous condition and this modulation within BA 2 of the stroked hand might reflect the observed changes in the feeling of finger ownership. The sensory–motor conflict eliciting the NI requires the integration of tactile signals from the two hands. The finding that the critical modulation of S1 activity for the representation of the stroked hand was found in BA 2 is consistent with converging evidence from studies in non-human primates and humans suggesting that BA 2 integrates somatosensory inputs bilaterally. Indeed, BA 2 has neurons with bilateral receptive fields (Iwamura et al., 2001, 2002) and has relatively dense callosal connections with the contralateral S1 (Killackey et al., 1983) and, together with BA 1, is involved in the integration of bilateral tactile signals (Tame et al., 2012). We also note that within the different representations and BAs contralateral to the stroked hand, we did not observe any significant effect of synchrony or of the agent. Therefore, we consider it unlikely that the interaction observed within the left BA 2 simply originated from differences in the intensity of stimulation across blocks and participants, but rather reflects the sensory–motor conflict induced in the critical self-synchronous stimulation condition.
Within the representations of the left stroking hand (i.e. the right S1), activity in BA 1 showed a significant Agent × Synchrony interaction. Such modulation was specific for the representation of D2, i.e. the finger receiving the strongest conflict signals in the self-synchronous condition, and extended to the representations of D1, D3 and D4. In addition, only for D2 such activity modulation was also correlated with the strength of the NI as reported by participants’ ratings. It is worth noting that the correlation between BOLD activity and subjective rating was computed only for those regions that effectively reacted to the NI, as indicated by a significant Agent × Synchrony interaction in the BOLD signal. Hence, the results of the correlation analysis are to be considered only for exploratory purposes. The left hand effectively touched the stroked hand only in the self conditions. Although this observation can partially explain the observed Agent × Synchrony interaction, it cannot explain the correlation between changes in BOLD response and strength of the illusion. Thus, we propose that the higher response for D2 representation in the self-synchronous condition is associated with the detection of self-other conflict during the NI.
Taken together data from the S1 representations of both hands suggest that the NI is characterized by a specific modulation of activity of the different S1 subregions, such that the BA 1 representation of the stroking finger and the BA 2 representation of the stroked hand more strongly respond to the combination of self-touch and synchronous conflicting feedback. These BAs have a somatotopic representation of the fingers that also respond to the stimulation of the neighboring fingers and integrate the tactile information originated in neighboring body parts. Therefore, the BA-specific activation pattern induced by the NI is most likely associated with finger-unspecific modulations of S1 activity.
It is import to note that the present results do not imply that the NI arises exclusively from the modulation of S1 activity. Because the NI stimulation procedure involves both hands simultaneously, and is based on tactile, proprioceptive and motor conflicts, other areas, containing bilateral and ipsilateral hand representations may participate in the effect, such as S2 (Whitsel et al., 1969) or BA 5 (Sakata et al., 1973) or M1.
The pattern of brain activation reflecting the NI was observed only in the finger-specific analysis and not in the voxel-wise group analysis. This is due to the inter-subject variability of the finger regions, which is comparable in size with the size of finger representations (Martuzzi et al., 2014). The effects were precisely localized within the representations of the fingers involved in the task and the inter-subject variability likely rendered these effects undetectable in EEG or low-resolution fMRI studies. Inter-subject variability, in conjunction with the high spatial resolution and the small smoothing kernel used in the study, could also explain why we did not observe effects in the premotor and parietal cortex, as reported elsewhere (e.g. Ehrsson et al., 2004, 2005; Kammers et al., 2009; Petkova et al., 2011; Zeller et al., 2011; Evans and Blanke, 2013). Indeed, differences in brain activity within these regions were observed at the individual subject level, but not in the group analysis. This highlights the usefulness of precisely localizing body-part representations in studies investigating cognitive and perceptual aspects of bodily awareness at high spatial resolution.
In conclusion, data from ultra-high field fMRI finger mapping revealed that the NI was associated with a modulation of activity in the fingers representations within the different BAs forming S1. In particular, the NI was associated with higher activation in BA 1 contralateral to the stroking hand and in BA 2 contralateral to the stroked hand. These results show the highly selective anatomical and functional brain mechanisms of bodily experience that are limited to specific BAs within S1, extending the high degree of somatosensory specialization in S1 to bodily self-consciousness.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
CONFLICT OF INTEREST
None declared.
Supplementary Material
Acknowledgments
This study was supported by the Swiss National Science Foundation (SNSF 513225 and 31003A_153070), the Bertarelli Foundation, the Centre d’Imagerie BioMédicale of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenaards and Louis-Jeantet Foundations.
REFERENCES
- Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. Journal of Neurophysiology. 1989;62(3):694–710. doi: 10.1152/jn.1989.62.3.694. [DOI] [PubMed] [Google Scholar]
- Arnold HL., Jr Japanese illusion. Science. 1952;115(2995):577. doi: 10.1126/science.115.2995.577-a. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Aspell JE, Palluel E, Blanke O. Early and late activity in somatosensory cortex reflects changes in bodily self-consciousness: an evoked potential study. Neuroscience. 2012;216:110–22. doi: 10.1016/j.neuroscience.2012.04.039. [DOI] [PubMed] [Google Scholar]
- Baumgartner U, Vogel H, Ellrich J, Gawehn J, Stoeter P, Treede RD. Brain electrical source analysis of primary cortical components of the tibial nerve somatosensory evoked potential using regional sources. Evoked Potentials-Electroencephalography and Clinical Neurophysiology. 1998;108(6):588–99. doi: 10.1016/s0168-5597(98)00040-9. [DOI] [PubMed] [Google Scholar]
- Besle J, Sanchez-Panchuelo RM, Bowtell R, Francis S, Schluppeck D. Event-related fMRI at 7T reveals overlapping cortical representations for adjacent fingertips in S1 of individual subjects. Human Brain Mapping. 2014;35(5):2027–43. doi: 10.1002/hbm.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nature Reviews. Neuroscience. 2012;13(8):556–71. doi: 10.1038/nrn3292. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391(6669):756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Boulware JT. Numbness, body-image, and the Japanese illusion. Science. 1951;114(2970):584–5. doi: 10.1126/science.114.2970.584. [DOI] [PubMed] [Google Scholar]
- Cardini F, Longo MR, Haggard P. Vision of the body modulates somatosensory intracortical inhibition. Cerebral Cortex. 2011;21(9):2014–22. doi: 10.1093/cercor/bhq267. [DOI] [PubMed] [Google Scholar]
- Cullen KE. Sensory signals during active versus passive movement. Current Opinion in Neurobiology. 2004;14(6):698–706. doi: 10.1016/j.conb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Decety J. Neural representations for action. Reviews in the Neurosciences. 1996;7(4):285–97. doi: 10.1515/revneuro.1996.7.4.285. [DOI] [PubMed] [Google Scholar]
- Dieguez S, Mercier MR, Newby N, Blanke O. Feeling numbness for someone else’s finger. Current Biology. 2009;19(24):R1108–9. doi: 10.1016/j.cub.2009.10.055. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH. The experimental induction of out-of-body experiences. Science. 2007;317(5841):1048. doi: 10.1126/science.1142175. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Holmes NP, Passingham RE. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. The Journal of Neuroscience. 2005;25(45):10564–73. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305(5685):875–7. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Evans N, Blanke O. Shared electrophysiology mechanisms of body ownership and motor imagery. Neuroimage. 2013;64:216–28. doi: 10.1016/j.neuroimage.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Jones EG. Thalamic input to areas 3a and 2 in monkeys. Journal of Neurophysiology. 1981;45(1):59–85. doi: 10.1152/jn.1981.45.1.59. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: implications for cognitive science. Trends in Cognitive Sciences. 2000;4(1):14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gardner EP. Somatosensory cortical mechanisms of feature detection in tactile and kinesthetic discrimination. Canadian Journal of Physiology and Pharmacology. 1988;66(4):439–54. doi: 10.1139/y88-074. [DOI] [PubMed] [Google Scholar]
- Gentile G, Guterstam A, Brozzoli C, Ehrsson HH. Disintegration of multisensory signals from the real hand reduces default limb self-attribution: an fMRI study. The Journal of Neuroscience. 2013;33(33):13350–66. doi: 10.1523/JNEUROSCI.1363-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage. 1999;10(1):63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage. 2000;11(6 Pt 1):684–96. doi: 10.1006/nimg.2000.0548. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage. 2001;14(3):617–31. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Ionta S, Heydrich L, Lenggenhager B, et al. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron. 2011;70(2):363–74. doi: 10.1016/j.neuron.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Tanaka M, Iriki A, Taoka M, Toda T. Processing of tactile and kinesthetic signals from bilateral sides of the body in the postcentral gyrus of awake monkeys. Behavioural Brain Research. 2002;135(1–2):185–90. doi: 10.1016/s0166-4328(02)00164-x. [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O. Converging patterns of finger representation and complex response properties of neurons in area-1 of the 1st somatosensory cortex of the conscious monkey. Experimental Brain Research. 1983;51(3):327–37. [Google Scholar]
- Iwamura Y, Taoka M, Iriki A. Bilateral activity and callosal connections in the somatosensory cortex. Neuroscientist. 2001;7(5):419–29. doi: 10.1177/107385840100700511. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The Neural and Behavioural Organization of Goal-Directed Movements. Oxford, UK: Clarendon Press; 1988. [Google Scholar]
- Jeannerod M. Being oneself. Journal of Physiology, Paris. 2007;101(4–6):161–8. doi: 10.1016/j.jphysparis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Nelson RJ, Sur M, Lin CS, Merzenich MM. Multiple representations of the body within the primary somatosensory cortex of primates. Science. 1979;204(4392):521–3. doi: 10.1126/science.107591. [DOI] [PubMed] [Google Scholar]
- Kammers MP, Verhagen L, Dijkerman HC, Hogendoorn H, De Vignemont F, Schutter DJ. Is this hand for real? Attenuation of the rubber hand illusion by transcranial magnetic stimulation over the inferior parietal lobule. Journal of Cognitive Neuroscience. 2009;21(7):1311–20. doi: 10.1162/jocn.2009.21095. [DOI] [PubMed] [Google Scholar]
- Kanayama N, Sato A, Ohira H. Crossmodal effect with rubber hand illusion and gamma-band activity. Psychophysiology. 2007;44(3):392–402. doi: 10.1111/j.1469-8986.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- Kanayama N, Sato A, Ohira H. The role of gamma band oscillations and synchrony on rubber hand illusion and crossmodal integration. Brain and Cognition. 2009;69(1):19–29. doi: 10.1016/j.bandc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Gould HJ, III, Cusick CG, Pons TP, Kaas JH. The relation of corpus callosum connections to architectonic fields and body surface maps in sensorimotor cortex of new and old world monkeys. The Journal of Comparative Neurology. 1983;219(4):384–419. doi: 10.1002/cne.902190403. [DOI] [PubMed] [Google Scholar]
- Kuehn E, Trampel R, Mueller K, Turner R, Schutz-Bosbach S. Judging roughness by sight—a 7-Tesla fMRI study on responsivity of the primary somatosensory cortex during observed touch of self and others. Human Brain Mapping. 2013;34(8):1882–95. doi: 10.1002/hbm.22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenggenhager B, Halje P, Blanke O. Alpha band oscillations correlate with illusory self-location induced by virtual reality. The European Journal of Neuroscience. 2011;33(10):1935–43. doi: 10.1111/j.1460-9568.2011.07647.x. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Tadi T, Metzinger T, Blanke O. Video ergo sum: manipulating bodily self-consciousness. Science. 2007;317(5841):1096–9. doi: 10.1126/science.1143439. [DOI] [PubMed] [Google Scholar]
- Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271–81. doi: 10.1016/j.neuroimage.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Martuzzi R, van der Zwaag W, Farthouat J, Gruetter R, Blanke O. Human finger somatotopy in areas 3b, 1, and 2: a 7T fMRI study using a natural stimulus. Human Brain Mapping. 2014;35(1):213–26. doi: 10.1002/hbm.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60(1):389–443. [Google Scholar]
- Petkova VI, Bjornsdotter M, Gentile G, Jonsson T, Li TQ, Ehrsson HH. From part- to whole-body ownership in the multisensory brain. Current Biology. 2011;21(13):1118–22. doi: 10.1016/j.cub.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Pozeg P, Rognini G, Salomon R, Blanke O. Crossing the hands increases illusory self-touch. PLoS One. 2014;9(4):e94008. doi: 10.1371/journal.pone.0094008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, Takaoka Y, Kawarasaki A, Shibutani H. Somatosensory properties of neurons in the superior parietal cortex (area 5) of the rhesus monkey. Brain Research. 1973;64:85–102. doi: 10.1016/0006-8993(73)90172-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Panchuelo RM, Besle J, Beckett A, Bowtell R, Schluppeck D, Francis S. Within-digit functional parcellation of Brodmann areas of the human primary somatosensory cortex using functional magnetic resonance imaging at 7 tesla. The Journal of Neuroscience. 2012;32(45):15815–22. doi: 10.1523/JNEUROSCI.2501-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Panchuelo RM, Francis S, Bowtell R, Schluppeck D. Mapping human somatosensory cortex in individual subjects with 7T functional MRI. Journal of Neurophysiology. 2010;103(5):2544–56. doi: 10.1152/jn.01017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Flor H, Heinze HJ, Rotte M. Dynamic modulation of the primary somatosensory cortex during seeing and feeling a touched hand. Neuroimage. 2006;29(2):587–92. doi: 10.1016/j.neuroimage.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Xu B, Flor H, Cohen LG. Effects of different viewing perspectives on somatosensory activations during observation of touch. Human Brain Mapping. 2009;30(9):2722–30. doi: 10.1002/hbm.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O, Stadler J, Zaitsev M. High resolution single-shot EPI at 7T. MAGMA. 2008;21(1–2):73–86. doi: 10.1007/s10334-007-0087-x. [DOI] [PubMed] [Google Scholar]
- Stringer EA, Chen LM, Friedman RM, Gatenby C, Gore JC. Differentiation of somatosensory cortices by high-resolution fMRI at 7 T. Neuroimage. 2011;54(2):1012–20. doi: 10.1016/j.neuroimage.2010.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tame L, Braun C, Lingnau A, et al. The contribution of primary and secondary somatosensory cortices to the representation of body parts and body sides: an fMRI adaptation study. Journal of Cognitive Neuroscience. 2012;24(12):2306–20. doi: 10.1162/jocn_a_00272. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cerebral Cortex. 2007a;17(10):2235–44. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Longo MR, Haggard P. Having a body versus moving your body: neural signatures of agency and body-ownership. Neuropsychologia. 2010;48(9):2740–9. doi: 10.1016/j.neuropsychologia.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Schutz-Bosbach S, Gallagher S. On agency and body-ownership: phenomenological and neurocognitive reflections. Consciousness and Cognition. 2007b;16(3):645–60. doi: 10.1016/j.concog.2007.05.012. [DOI] [PubMed] [Google Scholar]
- van den Bos E, Jeannerod M. Sense of body and sense of action both contribute to self-recognition. Cognition. 2002;85(2):177–87. doi: 10.1016/s0010-0277(02)00100-2. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. Journal of the American Medical Informatics Association. 2001;8(5):443–59. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel BL, Petrucelli LM, Werner G. Symmetry and connectivity in the map of the body surface in somatosensory area II of primates. Journal of Neurophysiology. 1969;32(2):170–83. doi: 10.1152/jn.1969.32.2.170. [DOI] [PubMed] [Google Scholar]
- Wolpert DM. Computational approaches to motor control. Trends in Cognitive Sciences. 1997;1(6):209–16. doi: 10.1016/S1364-6613(97)01070-X. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269(5232):1880–2. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Zeller D, Gross C, Bartsch A, Johansen-Berg H, Classen J. Ventral premotor cortex may be required for dynamic changes in the feeling of limb ownership: a lesion study. The Journal of Neuroscience. 2011;31(13):4852–7. doi: 10.1523/JNEUROSCI.5154-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.