Abstract
Cigarette smoking is an economically and epidemiologically expensive public health concern. Most adult smokers become addicted during adolescence, rendering it a crucial period for prevention and intervention. Although litigation claims have delayed implementation, graphic warning labels proposed by the U.S. Food and Drug Administration (FDA) may be a promising way to achieve this goal. We aimed to determine the efficacy of the labels in reducing in-scanner craving and to characterize the neurobiological responses in adolescent and adult smokers and non-smokers. While undergoing functional magnetic resonance imaging, thirty-nine 13- to 18-year-old adolescent and forty-one 25- to 30-year-old adult smokers and non-smokers rated their desire to smoke when presented with emotionally graphic warning labels and comparison non-graphic labels. Compared with adult smokers, adolescent smokers exhibited greater craving reduction in response to the warning labels. Although smokers evinced overall blunted recruitment of insula and dorsolateral prefrontal cortex (DLPFC) relative to non-smokers, an effect that was stronger in adolescent smokers, parametrically increasing activation of these regions was associated with greater craving reduction. Functional connectivity analyses suggest that greater DLPFC regulation of limbic regions predicted cigarette craving. These data underscore a prominent role of frontoinsular circuitry in predicting the efficacy of FDA graphic warning labels in craving reduction in adult and adolescent smokers.
Keywords: adolescent smoking, craving, FDA warning labels, functional magnetic resonance imaging, frontoinsular circuitry
INTRODUCTION
Smoking is the most preventable cause of disease and death worldwide (World Health Organization, 2011). Eighty percent of adult smokers become addicted to tobacco by age 18, and people who do not start smoking in adolescence are unlikely ever to do so (U.S. Food and Drug Administration, 2010). Therefore, targeting adolescents as a means to prevent smoking initiation is crucial to addressing this expensive public health concern (Backinger et al., 2003).
Several countries have implemented graphic, pictorial warnings on cigarette packaging in an effort to reduce smoking. Initial outcomes are promising, showing reduced smoking initiation rates, increased awareness of health consequences, negative reactions to smoking cues and increased cessation rates (Hammond, 2011; Partos et al., 2013). In 2010, the U.S. Food and Drug Administration (2010) (FDA) released 36 graphic warning labels depicting potential negative outcomes of smoking to appear on tobacco products (Hammond et al., 2013). These graphic warnings included images of blackened lungs, a cancer patient and decaying teeth among other emotionally aversive images. Litigation claims have delayed implementation in the U.S., but preliminary surveys examining their efficacy among young or potential smokers are encouraging: graphic warning labels on cigarette packaging increased the perceived dangers of smoking as well as reduced the social appeal of cigarette smoking (Peters et al., 2007; McCool et al., 2012; CDC, 2013; Pepper et al., 2013). In adults, neuroimaging work has shown that frontoparietal regions, including the dorsomedial prefrontal cortex, inferior frontal gyrus and parietal lobe, integrate the content of persuasive antismoking campaigns and predict decreased smoking a month later (Wang et al., 2013). What remains unknown is how effective these warning labels will be at reducing smoking ‘in-the-moment’ when adolescents crave a cigarette. Our first goal was to address this question.
The second goal was to examine neural responses to the proposed warning labels in both established adult smokers and in adolescent early smokers to determine the neural mechanisms that might mediate effective craving reduction. This question was motivated by (i) the importance of determining whether the proposed warning labels are effective in the most vulnerable smoking population and (ii) the hypothesis that the neural systems that make adolescents susceptible to cigarette-promoting ads might also be uniquely responsive to those that aim to reduce smoking.
Adolescent smoking onset has consistently been associated with exposure to tobacco advertisement (Emery et al., 1999; Lovato et al., 2003; Wills et al., 2007), suggesting that adolescents are particularly susceptible to cigarette ads (Upadhyaya et al., 2006). One explanation for this phenomenon is that the adolescent brain undergoes dynamic changes in frontolimbic circuitry (Galván, 2013), which has been previously implicated in craving and addiction (Goldstein and Volkow, 2011). Prefrontal cortical (PFC) regions [e.g. dorsolateral prefrontal cortex (DLPFC)] show protracted development that continues well into the late adolescent years and early 20s (Sowell et al., 2003). Previous research from our lab suggests that relative to non-smoking counterparts, young smokers differentially recruit the PFC during risky decision making (Galvan et al., 2013) and that decreased DLPFC recruitment is associated with heavier nicotine dependence (Galván et al., 2011). Limbic structures, including the striatum and amygdala, involved in reward processing and addiction (Volkow et al., 2012) also exhibit changes during adolescence that elicit reward-seeking and emotional hyperreactivity (Galván et al., 2006). Collectively, these data suggest that neural systems important in mediating craving are particularly vulnerable during adolescence (Eaton, 2012).
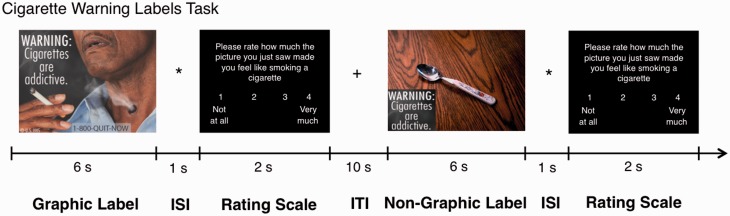
This study investigates neural responses to the proposed FDA graphic warning labels in adolescent and young adult smokers and non-smokers. While undergoing functional magnetic resonance imaging (fMRI), participants reported in-scanner craving following presentation of FDA warning labels, which showed either a graphic image conveying the dangers of smoking or a control (non-graphic) image that only presented the text warning without the graphic image (Figure 1). We predicted that warning labels showing graphic images vs non-graphic images would elicit reduced craving in smokers, with little effect on non-smokers. Consistent with previous literature, we also hypothesized that smokers would show greater neural activation to the graphic labels in regions implicated in emotion processing [e.g. amygdala (Due et al., 2002; David et al., 2005)], craving [e.g. ventral striatum (Goldstein and Volkow, 2011) and insula (Naqvi et al., 2007)] and craving suppression [e.g. DLPFC (Kober et al., 2010)] and that this effect would be exaggerated in adolescent smokers (Galván et al., 2011, 2013).
Fig. 1.
Schematic of cigarette warning labels task, including an example of a graphic label and non-graphic label and the rating scale in which participants reported their desire to smoke on a Likert scale from 1 (not at all) to 4 (very much). ISI, Interstimulus interval; ITI, Intertrial interval.
MATERIALS AND METHODS
Participants
Eighty English-speaking, right-handed non-smokers and smokers (n = 39 adolescents, ages 13–18, 26 female; and n = 41 young adults, ages 25–30, 24 female) were recruited via advertisements in the Los Angeles community and through Craigslist. Participants were screened by a detailed telephone interview to determine study eligibility. Exclusion criteria included current use of psychoactive medications, recent drug/alcohol use, pregnancy or metal in the body (e.g. braces, permanent retainers). Additionally, participants who reported a diagnosis of a neurological, developmental or psychiatric disorder or other substance use (except marijuana) during telephone screening were excluded. Participants who reported regular marijuana use were instructed to abstain from the substance for a minimum of ≥24 h before test days, which was verified with a urinary drug screen; visits were rescheduled if the urine drug screening tested positive for marijuana use. Significant differences in age, ethnicity, IQ, cigarettes per day, Fagerstrom scores, smoking duration and reported alcohol use and a trend toward differences in sex and socieoeconomic status (SES; measured by maternal education) are noted in Table 1.
Table 1.
Characteristics of research participants
| Group | Adolescent Smokers (n = 19) | Adolescent Non-smokers (n = 20) | Adult Smokers (n = 21) | Adult Non-smokers (n = 20) |
|---|---|---|---|---|
| Sex (M/F) a | 14/5* | 8/12 | 10/11 | 7/13* |
| Age | 17.47 (0.70) (range: 13–18) | 16.05 (1.28) (range: 13–18) | 27.19 (1.66) (range: 25–30) | 27.5 (1.7) (range: 25–30) |
| Ethnicityb | ||||
| Caucasian | 10.53% (n = 2) | 25% (n = 5) | 33.33% (n = 7) | 20.99% (n = 3) |
| African American | 15.79% (n = 3)*+ | 0% (n = 0)* | 38.1% (n = 8)+# | 17.28% (n = 3)# |
| Hispanic/Latino | 15.79% (n = 3)*+ | 50% (n = 10)* | 19.05% (n = 4)+# | 28.40% (n = 5)# |
| Asian American | 42.11% (n = 8) | 10% (n = 2) | 0% (n = 0) | 14.81% (n = 2) |
| Other | 15.79% (n = 3) | 15% (n = 3) | 9.52% (n = 2) | 18.52% (n = 7) |
| IQc | 105.89 (10.81)+ | 108 (13.45) | 104.43 (14.18)* | 116.9 (15.84)*+ |
| Maternal educationd | ||||
| Did not finish HS | 5.26% (n = 1) | 5% (n = 1) | 4.76% (n = 1) | 10% (n = 2) |
| HS diploma | 52.63% (n = 10) | 20% (n = 4) | 47.62 (n = 10) | 25% (n = 5) |
| GED | 0% (n = 0) | 10% (n = 2) | 0% (n = 0) | 0% (n = 0) |
| AA degree | 21.05% (n = 4) | 15% (n = 3) | 14.29% (n = 3) | 15% (n = 3) |
| BA degree | 0% (n = 0) | 15% (n = 3) | 4.76% (n = 1) | 15% (n = 3) |
| Masters degree | 15.79% (n = 3) | 20% (n = 4) | 23.81% (n = 5) | 20% (n = 4) |
| Other | 5.26% (n = 1) | 15% (n = 3) | 4.76% (n = 1) | 15% (n = 3) |
| Cigarettes/daye | 5.92 (5.09)*# | 0* | 10.05 (5.49)+# | 0+ |
| Fagerstrom scoree | 2.16 (2.12)* (range: 0–8) | 0* | 3.24 (2.10)+ (range: 0–8) | 0+ |
| Fagerstrom score (revised scale)e | 1.21 (1.08)* (range: 0–6) | 0* | 1.52 (1.25)+ (range: 0–6) | 0+ |
| Smoking duration (in months)f | 16.74 (15.35)* (range: 3 months–5.5 years) | 0 | 71.48 (49.56)* (range: 1 –15 years) | 0 |
| Last smoked cigarette (hours) before scang | 25.69 (59.32)* | 0 | 7.41 (8.47)* | 0 |
| Last reported alcohol consumption (hours)h | 108 (83.14) (47.37% reported any use) | 246 (317.41) (20% reported use) | 39.2 (21.98) (23.81% reported use) | 89.3 (75.92) (45% reported use) |
| Smokerlyzer breath CO level [intake session]i | 1.74 (.99)*# (6–7 ppm) | 1 (0)* (0–6 ppm) | 2.86 (1.28)+# (10–11 ppm) | 1.06 (.024)+ (0–6 ppm) |
| Smokerlyzer break CO average level [scan session]i | 1.94 (1.09)*# (6–7 ppm) | 1 (0)* (0–6 ppm) | 2.86 (1.24)+# (10–11 ppm) | 1.05 (0.23)+ (0–6 ppm) |
| NicAlert cotinine average level [intake session]j | 2.75 (2.49)*# (∼ 82–200 ng/ml) | 0.71 (0.77)* (∼ 0–30 ng/ml) | 4.27 (1.64)+# (∼ 500–1000 ng/ml) | 0.61 (0.61)+ (∼ 0–30 ng/ml) |
| NicAlert cotinine average level [scan session]j | 4.33 (2.29)* (∼ 300–500 ng/ml) | 0.56 (0.51)* (∼ 0–30 ng/ml) | 4.61 (1.65)+ (∼ 500–1000 ng/ml) | 0.59 (0.62)+ (∼ 0–30 ng/ml) |
aA marginal trend toward significance was observed for differences in gender between adolescent smokers and adult non-smokers (P = 0.07).
bSignificant differences were observed for ethnicity (African-American and Hispanic/Latino) within the adolescent and adult groups and between adolescent and adult smokers.
cSignificant differences were observed for IQ between adult smokers and non-smokers (P = 0.02); there was also a marginal trend toward significance between adolescent smokers and adult non-smokers (P = 0.07).
dA marginal trend toward significance was observed for differences in maternal education between smokers and non-smokers (P = 0.08).
eSignificant differences were observed for cigarettes/day and dependence between adolescent and adult smokers and non-smokers (P < 0.000); adolescent and adult smokers (P = 0.01) were significantly different for cigarettes/day only.
fSignificant differences were observed for smoking duration between adolescent and adult smokers and non-smokers (P < 0.001).
gSignificant differences were observed for hours since last smoked cigarette before scan visit between adolescent and adult smokers (P < 0.01).
hThere were significant differences among groups in percentage who reported any alcohol use.
iThe Smokerlyzer measures CO in the breath as parts per million (ppm); it provides 7 levels of reading with each level representing a range of ppm [level 1 = 0–6 ppm (non-smoker); level 7 ≥ 51 ppm (heavy smoker)]. Significant differences were observed between adolescent smokers and non-smokers (intake: P = 0.03; scan: P = 0.01), adult smokers and non-smokers (intake: P < 0.000; scan: P < 0.000) and adolescent and adult smokers (intake: P < 0.000; scan: P = 0.01).
jThe NicAlert Test measures urinary cotinine concentration in (ng/ml); it provides seven levels of reading with each level representing a range of ng/ml [level 0 = 0–10 ng/ml (non-smoker); level 6 ≥ 1000 ng/ml (heavy smoker)]. Significant differences were observed between adolescent smokers and non-smokers (intake: P = 0.005; scan: P = 0.001) and adult smokers and non-smokers (intake: P < 0.000; scan: P < 0.000).
*,#,+Denotes significant group differences (see footnotes above for details).
At the initial visit, adult participants provided informed consent and participants under the age of 18 provided assent as required by the University of California, Los Angeles Institutional Review Board. Participants received monetary compensation for participation. Participants were classified as non-smokers (<5 cigarettes in lifetime) or smokers (daily smoking ≥6 months; ≥5 cigarettes/day). We recognize that smoking five cigarettes/day is rather low for a definition of ‘smoker,’ but this amount is consistent with previous studies in the adolescent literature on the smoking frequency in youth (Ernst et al., 2009; Galván et al., 2011, 2013). Smoking status was verified by carbon monoxide (CO > 6 ppm) in breath (Smokerlyzer, Bedfont Scientific, Kent, UK) and qualifying urinary cotinine levels (NicAlert test strips, Nymox Pharmaceutical Corp., Hasbrouck Heights, NJ) at both sessions (Table 1). Baseline smoking behavior was characterized by recording daily smoking consumption and administering the Fagerstrom Test for Nicotine Dependence (FTND; (Fagerstrom and Schneider, 1989) to assess nicotine dependence. To control for age differences on smoking experience, we also calculated a revised FTND score that excluded statements that may be less relevant for adolescents (e.g. ‘How soon after you wake up do you smoke your first cigarette?’) and found no significant differences from the original measure (Table 1). Abstinence from substance use (except nicotine for smokers) was confirmed by urine drug screening on test days (Instant-View Multi-Panel 12-Test Drug Screen. ALFA Scientific Designs Inc., Poway, CA). In addition, the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) was administered to estimate IQ, which was greater than 84 (M = 108.79, s.d. = 14.33) in all participants. Finally, participants were acclimated to the MRI with a mock scanner.
Cigarette warning labels task
Within 1 week of the initial visit, participants underwent fMRI scanning. Before scanning, they provided information about time since last cigarette and time since last alcohol consumption (Table 1). They also provided a pre-task craving rating (‘how much do you feel like smoking a cigarette?’) on a Likert scale of 1–4 (1 = not at all; 4 = very much) (Table 2). Over two fMRI runs, participants viewed two types of interspersed warning labels. The first type were nine of the FDA warning labels previously approved for print on tobacco products in 2010 (Brody et al., 2007; U.S. Food and Drug Administration, 2010) (Figure 1; Supplementary Material). They also viewed nine ‘non-graphic’ labels (Supplementary Material) that contained the same warning text without the graphic images or any smoking/cigarette paraphernalia as a control; to control for visuospatial effects, the non-graphic images contained non-smoking related images (such as a spoon). The non-graphic images were created in our laboratory and were matched to the graphic images in terms of spatial resolution, text length and color presentation. Inclusion of these non-graphic images allowed us to isolate the effects of the emotionally evocative FDA warning labels. The order of the label types was randomized. After each 6-s label presentation, participants provided a craving rating on a Likert scale of 1–4 (scale same as earlier) (2 s) and response times were recorded. Each trial was followed by a jittered intertrial interval (ITI) (mean duration = 10 s). Immediately after the scan, subjects completed the urge to smoke (UTS; Jarvik et al., 2000) scale (1–7: 1 = definitely not; 7 = definitely) to assess cigarette craving. Because they were on different scales, pre-scan craving and UTS ratings were standardized (z-scored) for analyses.
Table 2.
Cigarette warning labels fMRI task craving ratings
| Craving rating | Adolescent Smokers (n = 19) | Adolescent Non-smokers (n = 20) | Adult Smokers (n = 21) | Adult Non-smokers (n = 20) |
|---|---|---|---|---|
| Pre-scan | ||||
| Original (range: 1–4) | 2.39 (0.7)* | 1.11 (0.24)* | 2.43 (0.87)+ | 1.08 (0.19)+ |
| z-scored | 0.74 (0.8)* | −0.73 (0.27)* | 0.79 (1)+ | −0.76 (0.21)+ |
| Warning label (range: 1–4) | ||||
| Non-graphic | 1.36 (0.4)* | 1 (0)* | 1.5 (0.69)+ | 1.02 (0.05)+ |
| Graphic | 1.01 (0.17)* | 1 (0)* | 1.56 (0.52)+ | 1.01 (0.03)+ |
| Post-scan UTS | ||||
| Original (range: 1–7) | 3.82 (1.98)* | 1.02 (0.05)* | 4.47 (1.86)+ | 1.02 (0.07)+ |
| z-scored | 0.66 (0.9)* | −0.79 (0.04)* | 0.91 (0.82)+ | −0.79 (0.08)+ |
There were significant differences between smokers and non-smokers for pre-task craving (original: P < 0.000; z-scored: P < 0.000), craving ratings to non-graphic FDA labels (P = 0.003) and graphic FDA labels (P = 0.04) and UTS ratings following the scan (original: P < 0.000; z-scored: P < 0.000).
*Denotes significant differences within the adolescent group.
+ Denotes significant differences within the adult group.
Behavioral data analysis
Three-way analyses of variance (ANOVA) [2 (label type) × 2 (age group) × 2 (smoking group)] statistical analyses were conducted in IBM Statistical Package of the Social Science. First, we determined the effects of smoking group in response to the graphic vs non-graphic labels (n = 80) while controlling for demographic covariates, including SES, ethnicity and gender. To further examine the effects of label type and age group while accounting for differences in smoking behavior, we then conducted secondary analyses controlling for SES, ethnicity, gender, hours since last cigarette, cigarettes per day, smoking duration, Fagerstrom dependence, hours since alcohol consumption, pre-scan craving and IQ in smokers only (n = 40).
MRI data acquisition, preprocessing and analysis
Functional images were acquired on a three Tesla Siemens Trio MRI scanner using a low-resolution gradient-echo, T2*-weighted echo-planar image (EPI) sequence (TR = 2 s, TE = 30 ms, flip angle: 90°, 271 volumes, 34 slices, slice thickness 4 mm, 1420 Hz/Px). Imaging data were preprocessed and analyzed using the FMRIB Software Library (FSL) 4.1.6 toolbox (www.fmrib.ox.ac.uk/fsl). First, all images were skull-stripped using FSL Brain Extraction Tool (BET). Second, image time-courses were realigned to compensate for small head movements (Jenkinson et al., 2002). All data reported are from scans that exhibited ≤3 mm in translational movement; one additional participant was excluded due to excess motion (>3 mm motion). There were no significant differences in translational movement between adolescents (M = 0.3, s.d. = 0.3) and adults (M = 0.27, s.d. = 0.36). Third, data were smoothed using a 5-mm full width at half maximum (FWHM) Gaussian kernel to increase the signal-to-noise ratio and filtered in the temporal domain using a non-linear high-pass filter (60-s cutoff). Finally, individual participant data were aligned into standard Montreal Neurological Institute (MNI) space using a three-step registration process. EPI images were first registered to the T2*-weighted Matched Bandwidth (1420 Hz/Px), then to the high-resolution structural magnetization-prepared rapid-acquisition gradient-echo (MPRAGE) scan, and into standard MNI space using linear registration with FMRIB’s Linear Image Registration Tool (FLIRT) for group comparisons and correlation analyses.
The FSL FEAT package was used for statistical analysis. A general linear model analysis was conducted, controlling for the covariates listed earlier. Events (non-graphic and graphic labels) were modeled at label presentation (epoch = 6 s) and convolved with a double gamma haemodynamic response function (HRF) in FSL. The rating period was modeled as a nuisance variable. The rest periods and jittered ITIs were not explicitly modeled and therefore served as an implicit baseline. Six motion parameters were modeled as regressors of no interest. At the first level for each run for each participant, four primary contrasts of interest were generated: graphic > baseline, non-graphic > baseline, graphic > non-graphic and non-graphic > graphic. We focused on activation during presentation of graphic and non-graphic labels. A second-level, fixed effects analysis combined two runs of the label type for each participant. Z statistic images were thresholded using clusters determined by z = 2.3 and a cluster significance threshold of P < 0.05 at the individual subject level. Finally, at the group level, a priori region of interest analyses were conducted using the FMRIB local analysis of mixed effects (FLAME) 1 module in FSL (Beckmann et al., 2003) in anatomically defined bilateral amygdala, striatum, insula and DLPFC. Z (Gaussianised T) statistic images were corrected using a cluster-based using Gaussian Random Field family wise error correction (Poline et al., 1997), with a cluster-forming threshold of z > 2.3 and corrected extent threshold of P < 0.05. As part of our preprocessing, we also identified parameter estimates in our functional data that were beyond two standard deviations of the mean and excluded these outliers from statistical analyses (i.e. degrees of freedom may vary in certain analyses).
PPI analysis
We examined functional connectivity (FC) during the graphic vs non-graphic labels contrast using psychophysiological interaction (PPI) analyses (Friston et al., 1997) in FSL. Using a seed-based approach, we used a 5 mm anatomical sphere from the Harvard-Oxford probabilistic atlas to define three seed regions for the PPI analyses: bilateral amygdala, bilateral insula and bilateral ventral striatum. Each seed region was transformed into the functional space of each participant using FSL FLIRT before a deconvolved time-series was extracted. Following the standard PPIplus modeling procedure (McLaren et al., 2012), 11 regressors were included in the first-level design matrix: (i) PPI for graphic − non-graphic labels, (ii) PPI for graphic + non-graphic labels, (iii) task regressor for graphic − non-graphic labels, (iv) task regressor for graphic + non-graphic labels, (v) seed region time course and (vi) six motion parameters as regressors of no interest. The first-level PPIs were then entered into a group-level regression analysis to investigate differences by age and smoking groups.
To further investigate the relationship between FC and behavioral measures, Pearson’s correlations were performed between beta weights of connectivity and behavioral self-reports in all participants and smokers separately.
RESULTS
Behavioral results
A 2 (label type) × 2 (age group) × 2 (smoking group) ANOVA revealed a significant main effect of label type [F(1, 71) = 23.95, P < 0.000], a significant main effect of smoking group [F(1, 71) = 23.67, P < 0.000] and a significant label type × smoking group interaction [F(1, 71) = 22.06, P < 0.000] on craving ratings. Relative to adolescent non-smokers, adolescent smokers showed higher craving ratings to non-graphic labels [t(37) = 3.99, P < 0.000] and to graphic labels [t(37) = 3.34, P = 0.002]. Relative to adult non-smokers, adult smokers also showed higher craving ratings to non-graphic labels [t(38) = 3.16, P = 0.003] and to graphic labels [t(38) = 2.17, P = 0.04]. Means are reported in Table 2.
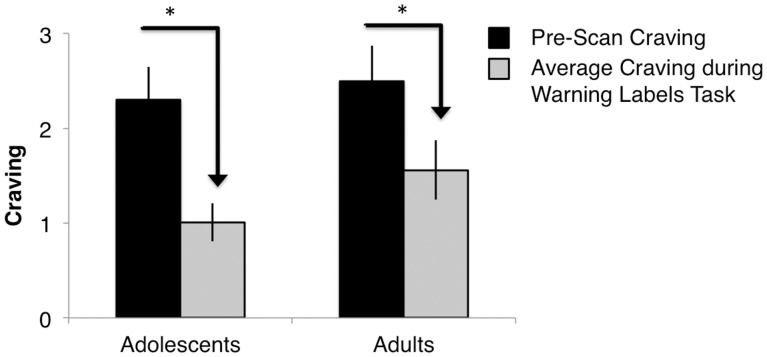
A univariate ANOVA revealed a main effect of smoking group [F(1,71) = 102.11, P < 0.000] (Msmokers = 0.76, s.d. = 0.9; Mnon-smokers = −0.75, s.d. = 0.24) and no interactions with age group on pre-scan cigarette craving (Table 2). There was also a significant main effect of smoking group on post-scan craving [F(1, 71) = 135.21, P < 0.000], such that smokers (M = 0.79, s.d. = 0.86) reported higher craving following the labels task than non-smokers. (M = −0.79, s.d. = 0.06). Adolescent and adult smokers showed higher pre-scan craving [adolescents: t(37) = 7.74, P< 0.000; adults: t(38) = 6.75, P< 0.000] and higher post-scan craving [adolescents: t(37) = 7.24, P< 0.000; adults: t(38) = 9.28, P< 0.000] relative to adolescent and adult non-smokers. Means are reported in Table 2. There were no interactions with age group on pre- or post-scan craving. To determine whether FDA labels were effective at reducing craving ratings, we conducted a 2 (age group) × 2 (smoking group) ANOVA. The craving rating was a difference score calculated by subtracting pre-scan craving (before presentation of FDA labels) from mean average craving during the labels task. There was a significant main effect for smoking group [F(1, 71) = 11.39, P < 0.001] and interaction with age such that there was greater reduction in craving in smokers vs non-smokers during presentation of the FDA labels. There was also a smoking group × age group interaction [F(1,71) = 5.03, P = 0.03], such that adolescents reported a greater reduction from baseline craving when viewing the graphic labels than adults [tadolescents(38) = 5.56, P < 0.000; tadults(39) = 4.51, P < 0.000] (Figure 2).
Fig. 2.
Smokers reported reduced average craving during FDA warning labels task compared with pre-scan craving; this effect was stronger in adolescent smokers.
Smokers only
To examine age effects in smokers, secondary analyses were conducted on smokers only. After controlling for all smoking-related covariates, the three-way ANOVA revealed a significant main effect of label type [F(1, 13) = 7.3, P = 0.02], revealing lower craving in response to the graphic (M = 1.1, s.d. = 0.11) vs non-graphic (M = 1.41, s.d. = 0.45) labels. There were no effects with age.
fMRI results
Neural activation in the ROIs, amygdala, striatum, insula and DLPFC, was examined using a series of univariate ANOVAs for the contrasts of interest. The graphic > baseline contrast revealed significant activation in the bilateral amygdala (20, −4, −14, z = 4.06; −20, −4, −14, z = 4.80), right putamen (22, 6, 6, z = 3.94), bilateral caudate (8, 4, 10, z = 3.56; −6, 4, 8, z = 2.92), bilateral insula (30, 26, 0, z = 4.59; −32, 22, 0, z = 3.28) and bilateral DLPFC (30, 34, −14, z = 5.45; −12, 54, 42, z = 4.52). The non-graphic > baseline contrast revealed significant activation in the bilateral amygdala (14, −12, −14, z = 3.09; −32, −2, −26, z = 2.56), bilateral putamen (32, −14, −6, z = −3.64; −26, −6, 2, z = 3.73), right insula (32, 24,−2, z = 4.48) and bilateral DLPFC (56, 34, 18, z = 5.44; −42, 44, 18, z = 4.48).
All participants
Main effect of label type
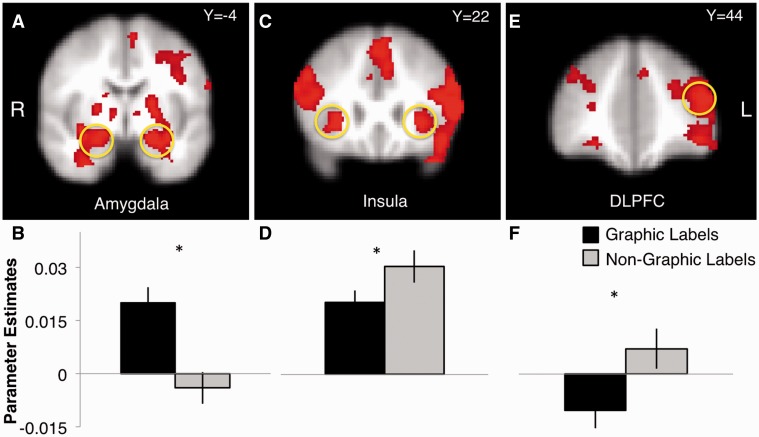
A significant main effect of label type in the bilateral amygdala (Figure 3A) showed greater activation to the graphic (Mright = 0.02, s.d. = 0.04; Mleft = 0.02, s.d. = 0.04) vs non-graphic labels (M = 0.004, s.d. = 0.06; M = −0.004, s.d. = 0.04) [Fright(1, 74) = 7.93, P = 0.006; Fleft(1, 74) = 15.92, P < 0.000] (Figure 3B). There was a significant main effect in the bilateral insula (Figure 3C), such that non-graphic labels (Mright = 0.03, s.d. = 0.04; Mleft = 0.04, s.d. = 0.05) elicited greater activation than graphic labels (Mright = 0.02, s.d. = 0.03; Mleft = 0.02, s.d. = 0.03), [Fright(1, 76) = 5.73, P = 0.02; Fleft(1, 76) = 9.22, P = 0.003] (Figure 3D). There was a significant main effect on left DLPFC activation (Figure 3E), such that non-graphic labels (M = 0.007, s.d. = 0.05) elicited greater activation than graphic labels (M =−0.01, s.d. = 0.05) [F(1,76) = 7.87, P = 0.006] (Figure 3F).
Fig. 3.
Main effect of label type in amygdala (A, B), insula (C, D) and DLPFC (E, F).
Main effect of age group
There were no significant main effects of age group in the ROIs.
Main effect of smoking group
In response to graphic > non-graphic labels, there was a significant main effect of smoking group in the right amygdala, such that smokers (M = 0.003, s.d. = 0.09) exhibited blunted activation relative to non-smokers (M = 0.04, s.d. = 0.05), [F(1, 76) = 3.83, P = 0.05]. There was a significant main effect of smoking group in the bilateral insula, such that smokers (Mright = −0.02, s.d. = 0.05; Mleft = −0.03, s.d. = 0.07) showed blunted activation relative to non-smokers (Mright = 0.0002, s.d. = 0.03; Mleft = −0.002, s.d. = 0.03) [Fright(1, 76) = 7.3, P = 0.008; Fleft(1, 76) = 6.97, P = 0.01]. A significant main effect of smoking group also revealed a similar pattern in DLPFC activation, with decreased recruitment in smokers (Mright = −0.02, s.d. = 0.07; Mleft = −0.04, s.d. = 0.07) relative to non-smokers (Mright = 0.003, s.d. = 0.02; Mleft = −0.001, s.d. = 0.04) [Fright(1, 76) = 5.37, P = 0.02; Fleft(1,76) = 7.68, P = 0.007].
Interaction effects
There was a label type × smoking group interaction in the right putamen, such that right putamen activation to the non-graphic vs graphic labels was greater in smokers (M = 0.02, s.d. = 0.05) relative to non-smokers (M = 0.01, s.d. = 0.03), [F(1, 76) = 4.01, P = 0.05].
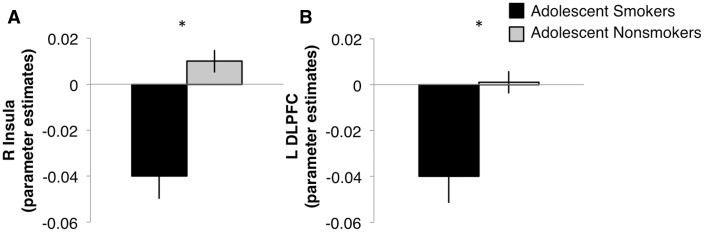
A significant label type × smoking group interaction revealed greater bilateral insula activation to the non-graphic vs graphic labels [Fright(1,76) = 5.89, P = 0.02; Fleft(1, 76) = 5.89, P = 0.02] in smokers (Mright = 0.04, s.d. = 0.05; Mleft = 0.06, s.d. = 0.06) than non-smokers (Mright = 0.02, s.d. = 0.03; Mleft = 0.02, s.d. = 0.03). In response to the graphic > non-graphic labels, there was greater bilateral insula activation exhibited by adolescent non-smokers (Mright = 0.01, s.d. = 0.03; Mleft = 0.003, s.d. = 0.02) compared with adolescent smokers (Mright =−0.04, s.d. = 0.06; Mleft = −00.03, s.d. = 0.07), [tright(37) = −2.86, P = 0.007; tleft(37) = −2.08, P = 0.04] (Figure 4A). In response to non-graphic > baseline labels, adolescent smokers (Mright = 0.05, s.d. = 0.065; Mleft = 0.06, s.d. = 0.06) showed greater bilateral insula activation than adolescent non-smokers (Mright = 0.01, s.d. = 0.03; Mleft = 0.01, s.d. = 0.03), [tright(37) = 2.89, P = 0.006; tleft(37) = 3.05, P = 0.004]. For adult smokers (M = 0.05, s.d. = 0.05) vs non-smokers (M = 0.02, s.d. = 0.03), this effect in response to the non-graphic > baseline labels was found in the left insula only [t(39) = 2.63, P = 0.01]. There was a label type × smoking group × age group interaction in the right insula [F(1, 76) = 5.11, P = 0.03], where greater insula activation in response to non-graphic vs graphic labels was exhibited by adolescent and adult smokers, with no differences among non-smokers for either age group.
Fig. 4.
Adolescent smokers exhibited blunted activation to graphic labels in the A) right insula and B) left DLPFC regions compared with adolescent non-smokers.
There was a label type × smoking group interaction in the bilateral DLPFC, such that there was greater activation to the non-graphic vs graphic labels, [Fright(1, 76) = 4.9, P = 0.03; Fleft(1, 76) = 7.87, P = 0.006] in smokers (Mright = 0.03, s.d. = 0.06; Mleft = 0.03, s.d. = 0.06) than non-smokers (Mright = 0.02, s.d. = 0.03; Mleft = –0.02, s.d. = 0.03). In response to the graphic > non-graphic labels, adolescents smokers (Mright = −0.04, s.d. = 0.07; Mleft = −0.04, s.d. = 0.07) elicited less DLPFC recruitment than non-smokers (Mright = 0.001, s.d. = 0.03; Mleft = 0.001, s.d. = 0.03) [tright(37) = −2.4, P = 0.02; tleft(37) = −2.13, P = 0.04] (Figure 4B). In response to the non-graphic > baseline labels, there was greater left DLPFC activation in adolescent (M = 0.03, s.d. = 0.06) and adult (M = 0.03, s.d. = 0.05) smokers relative to adolescent (M = −0.03, s.d. = 0.02) and adult (M = −0.01, s.d. = 0.04) non-smokers [adolescents: t(37) = 4.06, P < 0.000; adults: t(39) = 2.71, P = 0.01].
A label type × smoking group × age group interaction [F(1, 75) = 3.97, P = 0.05] revealed greater caudate activation to the non-graphic > baseline labels for adolescent smokers, greater caudate activation to the graphic > baseline labels for adolescent non-smokers and no difference between adult smokers and non-smokers. In response to the graphic > non-graphic labels, adolescent non-smokers (M = 0.02, s.d. = 0.04) exhibited greater caudate activation compared with adolescent smokers (M = −0.02, s.d. = 0.06) [t(37) = −2.43, P = 0.02]; this effect was also observed in response to graphic > baseline labels (Msmoker = −0.01, s.d. = 0.05; Mnon-smoker = 0.03, s.d. = 0.05) [t(37) = −2.77, P = 0.01]. Among the non-smokers, adolescents (M = 0.02, s.d. = 0.04) showed greater caudate activation to graphic > non-graphic labels compared with adults (M = −0.002, s.d. = 0.03), [t(37) = −2.02, P = 0.05].
Smokers only
Main effects
There were no main effects of label type or age in the ROIs.
Interaction effects
Controlling for all smoking-related covariates, there was a significant label type × age group interaction in the right amygdala, such that there was greater amygdala activation to the graphic labels vs non-graphic labels in adult smokers (M = 0.02, s.d. = 0.04) relative to adolescent smokers (M = 0.01, s.d. = 0.04), [F(1, 16) = 11.17, P = 0.004]. In response to the non-graphic > graphic labels, there was a significant label type × age group interaction in the left putamen [F(1, 18) = 4.55, P = 0.05], where adolescent smokers (M = 0.04, s.d. = 0.06) exhibited greater activation than adult smokers (M = 0.002, s.d. = 0.03). No interaction effects were observed in the bilateral amygdala, bilateral putamen, right caudate, bilateral insula and bilateral DLPFC.
Correlations with self-reports
Across all participants, a linear regression established that in-scanner craving to the graphic labels significantly predicted post-scan craving, F(1, 75) = 25.42, P < 0.000. In response to the graphic > non-graphic labels, lower pre-scan craving reports were significantly correlated with greater activation in the right putamen [r(78) = −0.22, P = 0.05], right caudate [r(77) = −0.31, P = 0.007], bilateral insula [rright(79) = −0.28, P = 0.01; rleft(78) = −0.39, P < 0.000] and bilateral DLPFC [rright(79) = −0.3, P = 0.008; rleft(79) = −0.43, P < 0.000]. Following the scan, lower craving ratings were significantly associated with greater activation in the right caudate [r(78) = −0.23, P < 0.05], bilateral insula [rright(79) = −0.23, P = 0.04; rleft(79) = −0.25, P = 0.03] and bilateral DLPFC [rright(79) = −0.3, P = 0.007; rleft(79) = −0.33, P = 0.003]. Across all participants, a linear regression revealed a positive correlation between craving reduction and the right amygdala [r(79) = 0.27, P = 0.016, bilateral putamen [rright(79) = 0.28, P = 0.01; rleft(79)= 0.26, P= 0.02], right caudate [r(78) = 0.31, P = 0.007], bilateral insula [rright(79) = 0.35, P = 0.001; rleft(78)= 0.41, P< 0.000] and bilateral DLPFC [rright(79) = 0.33, P = 0.003; rleft(79)= 0.53, P< 0.000], such that greater activation in these regions was associated with greater craving reduction. Positive correlations between craving reduction and the right insula were driven by adolescents, while positive correlations between craving reduction and the left insula, left DLPFC, right caudate and left putamen were driven by adults (Table 3).
Table 3.
Regression between activation and craving reduction
| Brain region |
r (P) |
|
|---|---|---|
| Adolescents | Adults | |
| All participants | ||
| R insula | 0.38 (0.02) | 0.34 (0.03) |
| L insula | 0.4 (0.01) | 0.43 (0.006) |
| R DLPFC | 0.32 (0.04) | 0.34 (0.04) |
| L DLPFC | 0.44 (0.006) | 0.61 (0.000) |
| R caudate | 0.28 (0.09) | 0.35 (0.03) |
| L putamen | 0.15 (0.37) | 0.42 (0.007) |
| Smokers only | ||
| R insula | 0.48 (0.08) | 0.47 (0.04) |
| L insula | 0.62 (0.03) | 0.39 (0.07) |
| R DLPFC | 0.56 (0.05) | 0.23 (0.2) |
| L DLPFC | 0.52 (0.06) | 0.54 (0.02) |
| L putamen | 0.51 (0.07) | 0.62 (0.01) |
Among smokers, higher in-scanner craving to the graphic vs non-graphic labels was significantly correlated with greater left amygdala activation [r(35) = 0.46, P = 0.005], right caudate activation [r(35) = 0.46, P = 0.006] and left insula activation [r(36) = 0.39, P = 0.02]; these effects were driven by the adult smokers [adolescent smokers: r(17) = 0.28, P = 0.29; r(18) = 0.38, P = 0.12; r(18) = 0.29, P = 0.25; adult smokers: r(18) = 0.74, P < 0.000; r(17) = 0.71, P = 0.001; r(18) = 0.59, P = 0.01]. Removal of one outlier from each analyses did not change the results [correlations between in-scanner craving to the graphic labels and amygdala [r(34) = 0.46, P = 0.006], caudate [r(34) = 0.47, P = 0.005] and insula [r(35) = 0.37, P = 0.03] activation remains]. Smokers [r(39) = −0.4, P = 0.01] who reported lower craving ratings before the scan exhibited greater left putamen and left DLPFC activation, effects driven by adult smokers [adolescent smokers: rputamen(19) = −0.09, P = 0.71 and rDLPFC(19) = −0.29, P = 0.23; adult smokers: rputamen(19) = −0.45, P = 0.05 and rDLPFC(20) = −0.55, P = 0.01]. For smokers only, there was a positive correlation between craving reduction and the left putamen, bilateral insula and bilateral DLPFC (Table 3). There were no significant correlations between task activation in the ROIs and cigarettes per day or Fagerstrom dependence.
PPI connectivity results
PPI analyses were conducted in the graphic > non-graphic contrast with three a priori-defined seed regions: bilateral amygdala, bilateral insula and bilateral putamen. Voxel-based comparisons revealed FC between the three seed regions and several frontolimbic regions (see Table 4 for complete list of regions and below for specific effects).
Table 4.
Functional connectivity in response to graphic > non-graphic labels
| Seed | Anatomical region | Hemisphere | MNI coordinates (mm) |
z-stat | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Increased connectivity for graphic > non-graphic labels | ||||||
| Bilateral amygdala | dlPFC | R/L | 34 | 64 | 12 | 4.71 |
| −16 | 54 | 38 | 3.03 | |||
| mPFC | R/L | 12 | 52 | −6 | 2.57 | |
| −4 | 50 | −8 | 3.35 | |||
| Parietal lobe | L | −24 | −46 | 50 | 2.91 | |
| Putamen | R | 32 | −4 | 10 | 3.04 | |
| Insula | L | −40 | −4 | −14 | 2.93 | |
| NAcc | R/L | 6 | 16 | −2 | 2.86 | |
| −6 | 14 | −6 | 2.80 | |||
| Caudate | R/L | 6 | 16 | 0 | 2.95 | |
| −6 | 16 | 0 | 2.90 | |||
| Cingulate gyrus | R/L | 4 | 36 | −4 | 2.93 | |
| −8 | 38 | 0 | 2.71 | |||
| Paracingulate gyrus | R/L | 6 | 54 | −2 | 3.35 | |
| −4 | 46 | −4 | 3.19 | |||
| Precuneous cortex | R | 16 | −62 | 20 | 3.37 | |
| Occipital cortex | R/L | 36 | −70 | 28 | 4.39 | |
| −30 | −70 | 28 | 3.13 | |||
| Bilateral insula | dlPFC | R/L | 8 | 62 | 30 | 3.82 |
| −4 | 64 | 28 | 3.78 | |||
| mPFC | L | −12 | 40 | −10 | 2.98 | |
| Parietal lobe | L | −36 | −52 | 54 | 3.72 | |
| Amygdala | R/L | 24 | 0 | −14 | 2.92 | |
| −30 | −4 | −14 | 2.86 | |||
| Putamen | R/L | 20 | 6 | −12 | 3.77 | |
| −18 | 12 | −12 | 3.53 | |||
| NAcc | L | −12 | 12 | −12 | 4.18 | |
| Caudate | R/L | 12 | 24 | −4 | 2.51 | |
| −12 | 14 | −2 | 3.68 | |||
| Thalamus | L | −12 | −24 | 2 | 2.47 | |
| Occipital cortex | R | 22 | −78 | 32 | 3.44 | |
| Bilateral putamen | dlPFC | R/L | 12 | 68 | 22 | 3.92 |
| −4 | 64 | 28 | 4.21 | |||
| Amygdala | L | −30 | −2 | −14 | 2.32 | |
| Paracingulate gyrus | R | 6 | 54 | −2 | 2.55 | |
| Occipital cortex | R/L | 40 | −84 | 12 | 3.80 | |
| −36 | −80 | 12 | 3.52 | |||
| Decreased connectivity for graphic > non-graphic labels | ||||||
| Bilateral amygdala | Occipital cortex | R/L | 34 | −72 | 28 | 3.8 |
| −36 | −76 | 18 | 3.02 | |||
| Bilateral insula | Parietal lobe | L | −32 | −46 | 58 | 3.31 |
| Putamen | R | 28 | 0 | 14 | 2.79 | |
| Caudate | L | −12 | 18 | 14 | 2.82 | |
| Hippocampus | L | −20 | −14 | −26 | 3 | |
| Occipital cortex | R/L | 40 | −84 | 16 | 3.05 | |
| −42 | −78 | 16 | 3.18 | |||
| Bilateral putamen | dlPFC | L | −34 | 36 | 14 | 3.08 |
| Insula | R/L | 40 | 2 | 8 | 3.9 | |
| −32 | 22 | −6 | 2.68 | |||
| Cingulate gyrus | R | 2 | 28 | 14 | 2.36 | |
| Occipital cortex | R/L | 42 | −82 | 24 | 3.91 | |
| −34 | −82 | 20 | 3.59 | |||
P < 0.05 cluster corrected.
All participants
Main effect of smoking group
A univariate ANOVA revealed a main effect of smoking group, such that non-smokers exhibited greater positive FC between the bilateral amygdala and right DLPFC, right putamen and right nucleus accumbens (NAcc); between the bilateral insula and DLPFC; and between bilateral putamen and left DLPFC. Smokers exhibited greater positive FC than non-smokers between the bilateral insula and right amygdala. See Table 5 for statistical values.
Table 5.
Greater positive FC to graphic > non-graphic labels
| Seed |
M (s.d.) |
F/t | P | ||
|---|---|---|---|---|---|
| Brain region | Smokers (n = 40) | Non-smokers (n = 40) | |||
| Non-smokers > smokers | |||||
| Bilateral amygdala | |||||
| R DLPFC | All | −0.0001 (0.001) | 0.004 (0.001) | 10.17 | 0.002 |
| Adolescents | 0.0001 (0.001) | 0.001 (0.001) | −2.22 | 0.03 | |
| Adults | −0.0002 (0.0005) | 0.001 (0.001) | −2.08 | 0.04 | |
| R putamen | All | 0 (0.0002) | 0.0001 (0.0003) | 7.25 | 0.01 |
| Adolescents | NS | ||||
| Adults | −0.0001 (0.0002) | 0.0001 (0.0003) | −2.55 | 0.02 | |
| R NAcc | All | 0 (0.0005) | 0.0002 (0.0005) | 4.47 | 0.04 |
| Adolescents | NS | ||||
| Adults | −0.0002 (0.0004) | 0.0001 (0.0005) | −1.8 | 0.08 | |
| Bilateral insula | |||||
| R/L DLPFC | All | −0.0003 (0.001) | 0.0002 (0.001) | 14.54 | <0.000 |
| 0.0002 (0.001) | 0.0004 (0.001) | 7.2 | 0.009 | ||
| Adolescents | −0.0002 (0.001) | 0.001 (0.001) | −3.76 | 0.001 | |
| −0.0001 (0.001) | 0.001 (0.001) | −2.15 | 0.04 | ||
| Adults | NS | ||||
| Bilateral amygdala | |||||
| L DLPFC | All | −0.0002 (0.001) | 0.0003 (0.001) | 5.54 | 0.02 |
| Adolescents | 0.0001 (0.0008) | 0.001 (0.001) | 1.91 | 0.06 | |
| Adults | NS | ||||
| Smokers > non-smokers | |||||
| Bilateral insula | |||||
| R Amygdala | All | 0.0002 (0.001) | −0.0002 (0.0005) | 10.06 | 0.002 |
| Adolescents | 0.0001 (0.001) | −0.0002 (0.0005) | 1.82 | 0.08 | |
| Adults | 0.0003 (0.0004) | −0.0001 (0.0005) | 2.84 | 0.01 | |
NS, not significant.
Main effect of age group
A univariate ANOVA revealed a significant main effect of age group on positive FC between the bilateral amygdala and right DLPFC, right NAcc and right caudate; bilateral insula and right DLPFC; and bilateral putamen and bilateral DLPFC, with greater positive FC in adolescents compared with adults. There was a significant main effect of age group on negative FC between the bilateral insula and left parietal lobe. See Table 6 for statistical values.
Table 6.
Greater FC in adolescents vs adults to graphic > non-graphic labels
| Seed |
M (s.d.) |
F/t | P | ||
|---|---|---|---|---|---|
| Brain region | Adolescents (n = 39) | Adults (n = 41) | |||
| Positive FC | |||||
| Bilateral amygdala | |||||
| R DLPFC | All | 0.004 (0.001) | −0.0001 (0.001) | 8.79 | 0.004 |
| Non-smoker | 0.001 (0.001) | 0.0001 (0.001) | −2.37 | 0.02 | |
| Smoker | 0.0001 (0.001) | −0.0003 (0.001) | −1.81 | 0.08 | |
| R NAcc | All | 0.0002 (0.001) | 0 (0.0004) | 6.36 | 0.01 |
| Non-smoker | NS | ||||
| Smoker | 0.0001 (0.0005) | −0.0002 (0.0004) | −2.01 | 0.05 | |
| R caudate | All | 0.0002 (0.001) | −0.0001 (0.0004) | 6.37 | 0.01 |
| Non-smoker | 0.0003 (0.001) | 0 (0.0005) | −1.94 | 0.06 | |
| Smoker | NS | ||||
| Bilateral insula | |||||
| R DLPFC | All | 0.0002 (0.001) | −0.0002 (0.001) | 6.43 | 0.01 |
| Non-smoker | 0.001 (0.001) | 0 (0.001) | −2.76 | 0.01 | |
| Smoker | NS | ||||
| Bilateral putamen | |||||
| R/L DLPFC | All | 0.0003 (0.001) | −0.0002 (0.0001) | 8.79 | 0.004 |
| 0.0003 (0.001) | −0.0002 (0.001) | 8.98 | 0.004 | ||
| Non-smoker | 0.001 (0.001) | −0.0002 (0.001) | −3.04 | 0.004 | |
| 0.001 (0.001) | −0.0001 (0.001) | −2.42 | 0.02 | ||
| Smoker | NS | ||||
| 0.0001 (0.001) | −0.0004 (0.001) | −1.91 | 0.06 | ||
| Negative FC | |||||
| Bilateral insula | |||||
| L parietal | All | 0 (0.0003) | −0.0001 (0.0003) | 4.92 | 0.03 |
| Non-smoker | 0.0001 (0.0003) | −0.0001 (0.0003) | −2.02 | 0.05 | |
| Smoker | NS | ||||
NS, not significant.
Interaction effects
A univariate ANOVA revealed a smoking group × age group interaction on positive FC between the bilateral amygdala and left parietal lobe, between the bilateral insula and left mPFC and between the bilateral insula and left caudate, such that adolescent smokers exhibited greater FC than adult smokers. See Table 7 for statistical values.
Table 7.
Interaction effects of smoking group and age on FC to graphic > non-graphic labels
| Seed |
M (s.d.) |
F/t | P | ||
|---|---|---|---|---|---|
| Brain region | Smokers (n = 40) | Non-smokers (n = 40) | |||
| Positive FC | |||||
| Bilateral insula | |||||
| L parietal | All | 0.0001 (0.0003) | 0.0001 (0.0003) | 5.62 | 0.02 |
| Adolescent | 0.0001 (0.0003) | 0 (0.0002) | NS | NS | |
| Adult | 0.0001 (0.0002) | 0.0002 (0.0004) | NS | NS | |
| Bilateral amygdala | |||||
| L MPFC | All | 0.0002 (0.001) | 0.0002 (0.001) | 4.29 | 0.04 |
| Adolescent | 0.0001 (0.0005) | 0.0003 (0.001) | NS | NS | |
| Adult | 0.0004 (0.001) | 0 (0.001) | NS | NS | |
| L caudate | All | 0.0001 (0.0004) | 0 (0.0003) | 8.44 | 0.005 |
| Adolescent | −0.0001 (0.0003)* | 0.0001 (0.0003) | −1.94 | 0.06 | |
| Adult | 0.0002 (0.0003)* | −0.0001 (0.0005) | 2.21 | 0.03 | |
| Negative FC | |||||
| Bilateral putamen | |||||
| R insula | All | 0.0001 (0.0004) | 0 (0.0003) | 6.19 | 0.02 |
| Adolescent | −0.0001 (0.0004)+ | 0 (0.0003) | NS | NS | |
| Adult | 0.0003 (0.0003)+ | 0 (0.0004) | 2.68 | 0.01 | |
NS, not significant.
*t = 2.8, P = 0.01.
+t = 2.94, P = 0.01.
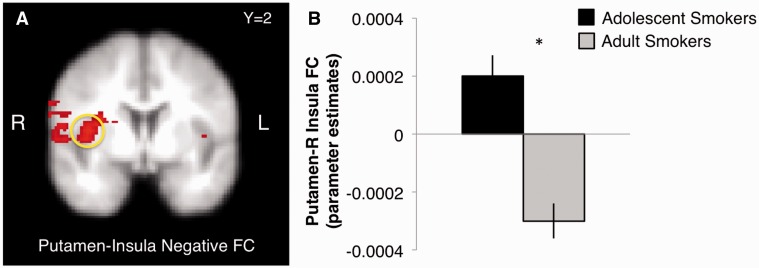
Smokers only
Main effect of age group
After controlling for all smoking-related covariates in smokers, there was a significant main effect of age group on positive FC between the bilateral insula and right putamen [F(1, 17) = 4.79, P = 0.04], such that adult smokers (M = 0.0002, s.d. = 0.0003) exhibited greater positive FC compared with adolescent smokers (M =−0.0001, s.d. = 0.0004). A significant main effect of age revealed that adult smokers (M = 0.0002, s.d. = 0.0004) showed greater positive FC between the bilateral insula and left caudate than adolescent smokers (M = −.0001, s.d. = 0.0003), [F(1, 18) = 5.63, P = 0.03]. There was also a main effect of age on negative FC between the bilateral putamen and right insula [F(1, 18) = 8.07, P = 0.01], an effect driven by the adult smokers (M = 0.0003, s.d. = 0.0003) vs adolescent smokers (M = − 0.0002, s.d. = 0.0004) (Figure 5).
Fig. 5.
Main effect of age group on negative FC between bilateral putamen and insula in response to graphic > non-graphic contrast.
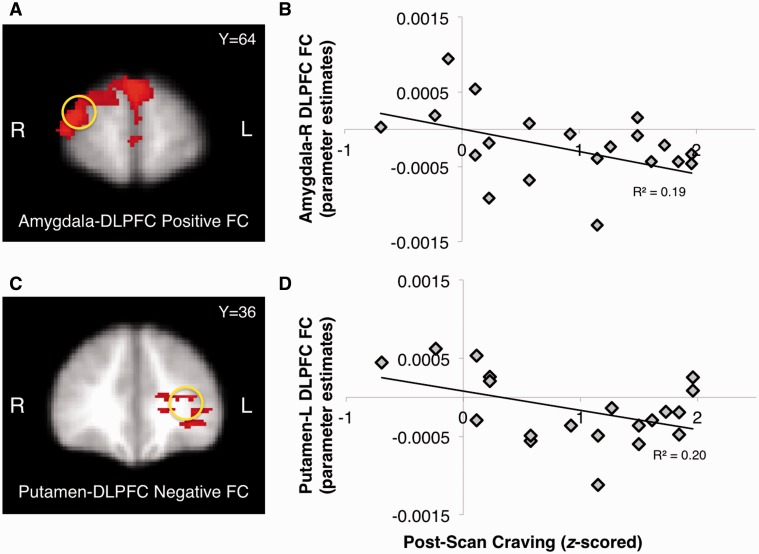
Correlations with self-reports
Among adult smokers only, higher craving following the scan was negatively correlated with FC between the bilateral amygdala and right DLPFC [r(21) = −0.44, P = 0.05] (Figure 6A and B); this was not observed in adolescent smokers. Adult smokers also showed higher post-scan craving with an increase in negative FC between the bilateral putamen and left DLPFC [r(20) = 0.45, P = 0.05] (Figure 6C and D).
Fig. 6.
FC between amygdala-DLPFC (A, B) and putamen-DLPFC (C, D) in response to graphic > non-graphic contrast was negatively correlated with post-scan craving in adult smokers.
DISCUSSION
The goal of this study was to determine whether the proposed FDA cigarette warning labels are effective at reducing in-scanner craving in adolescent smokers. A second goal was to characterize the neurobiological responses to the cigarette warning labels to assess the mechanism of their potential efficacy. Our interest in examining these previously unexplored questions in adolescents was inspired by extant epidemiological data showing the need to eliminate smoking and nicotine dependence early in a smoker’s experience, before casual use advances to dependence (Backinger et al., 2003). These data suggest that the FDA warning labels are effective at reducing cigarette craving in both adult and adolescent smokers but that the effect is stronger in adolescents. The data also reveal that the graphic labels elicit a robust network of frontolimbic regions. Overall, smokers elicited a blunted response in the insula and DLFPC compared with non-smokers during exposure to graphic labels, an effect that was strongest in adolescent smokers vs non-smokers. In the amygdala, there were developmental differences such that adult smokers elicited greater recruitment of the amygdala than adolescent smokers.
These findings suggest that the addition of graphic, emotionally aversive, images to health warning labels reduces craving above and beyond text-only warning labels without graphic images. Interestingly, this effect was greater in adolescent vs adult smokers. Such graphic imagery in television advertisements has also been found to be effective at communicating the deleterious harms of tobacco use in smokers and is easily adaptable in low- to middle-income countries (Wakefield et al., 2013). Indeed, previous reports highlight the emotional impact of graphic health warning depictions (Berg et al., 2011; Cameron et al., 2013) and subsequent craving (Loeber et al., 2011). Self-reported craving to smoking cues is predictive of subsequent cessation, with greater craving reports indicative of increased difficulty to initiate quitting and relapse (Waters et al., 2004). Relatedly, a cessation trial using ecological momentary assessments found that participants who reported less craving 1 year following their quit attempts were the most successful at tobacco abstinence (Schlam et al., 2012).
The cigarette warning labels elicited neural activation in frontolimbic regions. Compared with non-graphic labels, graphic warning labels elicited greater amygdala activation, illustrating the emotionally evocative nature of the graphic labels. Given the role of the amygdala in responding to arousing, emotional and aversive information (Phelps and LeDoux, 2005), these data suggest that it may play a role in reducing craving in young smokers. Downregulation of craving has been previously associated with changes in amygdala activation in adult smokers (Kober et al., 2010). Additionally, a recent study showed that amygdala activation to antismoking messages in adult smokers trying to quit was predictive of post-intervention cessation outcomes (Jasinska et al., 2012). Given the dynamic changes in the amgydala during adolescence (Ernst and Fudge, 2009), the current amygdala findings point to an intriguing possibility: that exploiting the adolescent sensitivity to negatively arousing information may be an effective way to reduce or prevent cigarette smoking. Importantly, this amgydala sensitivity to the warning labels was as robust in the non-smoking adolescents as it was in the adolescent smokers, suggesting that targeting the limbic system with arousing warnings before the onset of smoking may help reduce smoking initiation.
Engagement of the insula and DLPFC differentiated smokers from non-smokers. Smokers, particularly adolescent smokers, evinced blunted activation of these regions in response to the graphic labels. Given the role of the insula in translating interoceptive (visceral) states to emotional responses (Craig, 2002), integrating visceral states, emotional saliency and cognitive output (Goldstein et al.2009) and in emotional awareness (Critchley et al.2004), one interpretation of the smoker’s blunted response in this study is that they were less viscerally reactive to the graphic FDA labels, thereby making the labels less effective at discouraging smoking and cigarette craving. Indeed, we found evidence for a parametric relationship between insula activation and craving reduction, whereby individuals with greater activation reported significantly less craving after seeing the graphic labels than before seeing them. Another interpretation is that because of the insula’s role in cigarette addiction (Naqvi et al., 2007), which is often associated with cue-induced motivation for nicotine (Forget et al., 2010; Pushparaj et al., 2013), dampened response might be evidence of decreased motivation and craving for cigarettes following presentation of the graphic labels.
Activation in the DLPFC differentiated smokers from non-smokers and adolescent from adult smokers in response to the graphic labels. Smokers of both age groups showed dampened DLPFC recruitment than non-smokers, and similar to the association in the insula, this activation was associated with craving reduction. Individuals with greater DLPFC activation reported greater craving reduction, underscoring the important role of the DLPFC in mediating craving, as demonstrated previously. Kober et al. (2010) reported that downregulation of craving was associated with increased recruitment of DLPFC, along with dorsomedial PFC and ventrolateral PFC, in cigarette smokers (Kober et al., 2010), an effect that appears to underlie cognitive strategies that help diminish craving by focusing on the long-term consequences (e.g. I may get lung cancer) of smoking (Kober et al., 2009). In this study, smokers with greater DLPFC activation reported lower craving ratings, a finding that is consonant with our previous research showing that decreased DLPFC recruitment is associated with heavier nicotine dependence (Galván et al., 2011).
FC analyses revealed age effects in the relationship between regulatory regions and emotionally responsive regions (e.g. bilateral amygdala and right DLPFC) that are consonant with previous findings showing a developmental shift from positive to negative connectivity in human frontolimbic circuitry (Gee et al. 2013); these data suggest that improved regulatory control over limbic regions is subserved by dynamic changes in the connectivity between these systems. Consistent with our hypothesis, craving was associated with strength of connectivity between frontolimbic (DLPFC and amygdala) and frontostriatal (DLPFC and striatum) regions; adult smokers who reported greater craving following the scan evinced stronger negative connectivity between the DLPFC and subcortical regions. We interpret these results as evidence for greater regulatory strength of the prefrontal cortex over limbic activation during greater craving (Kober et al. 2009). One possible explanation for the observation that this relationship was only significant in adults is that the protracted development of FC between frontal and limbic regions (Gabard-Durnam et al. 2014) may have precluded effective engagement of DLPFC regulation over limbic regions in response to the graphic images.
The strengths of this study include the novelty, potential public health and policy implications and the large sample size of adolescent smokers who do not present with comorbid substance use, a challenging population to recruit. Nonetheless, a few limitations should be noted. First, we did not impose abstinence in our participants so there may have been variability in time since previous cigarette before the scan. However, we wanted to avoid nicotine withdrawal in the heaviest smokers because it can influence task performance (Azizian et al., 2009). Second, the non-graphic labels were not matched for the presence of faces and contain only non-cigarette images and verbal warnings, which may elicit activation based on the irrelevant images rather than on the verbal warnings common to both label types. Finally, our sample included sociodemographic differences between groups in terms of sex, socioeconomic status and smoking history. Consistent with previous literature, higher tobacco use is found among males vs females (Hu et al., 2006; King et al., 2012). Gender norms, especially among ethnic minorities, may explain these key sex differences, as smoking among women is usually socially unacceptable (An et al., 2008; Choi et al., 2008). In addition, higher smoking prevalence is also found among those with lower maternal education (Siapush et al., 2006; Hayatbakhsh et al., 2013) and among ethnic minorities (Caraballo et al., 2008). Furthermore, higher daily cigarette use and Fagerstrom dependence levels in adult vs adolescent smokers are likely attributed to a longer smoking history (Chassin et al., 1996; Hu et al., 2006). However, Ernst et al. (2009) similarly reported an average consumption of 2–5 cigarettes/day in a large sample of 75 adolescent smokers between 12–16 years (Ernst et al., 2009); our own work also previously reported a similar average cigarette consumption in youth (Galván et al., 2011; Galvan et al., 2013). We also acknowledge expected differences between adolescent and adult smokers in Fagerstrom scores due to different constraints on smoking behaviors (e.g. adolescents are unlikely to have the opportunity to smoke ad libitum because of parental monitoring); however, there was no equivalent measure of nicotine dependence for youth populations to implement in this study.
CONCLUSIONS
Although the Family Smoking Prevention and Tobacco Control Act requiring the new FDA warning labels has been challenged, this study provides new evidence in support of the efficacy of the proposed U.S. health warning labels on cigarettes and characterizes neural mechanisms that may underlie this effect in the U.S. sample. Importantly, our data suggest that emotional systems are responsive to the graphic nature of labels and that adolescent smokers and non-smokers are particularly responsive, as indexed by craving reduction and associated neural correlates, to the proposed labels.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Acknowledgments
The authors thank participants and helpful comments from members of the Galván Lab. This work was supported by grant from the California Tobacco-Related Disease Research Program [19KT-0026 to A.G.].
REFERENCES
- An N, Cochran S, Mays V, McCarthy W. Influence of American acculturation on cigarette smoking behaviors among Asian American subpopulations in California. Nicotine and Tobacco Research. 2008;10:579–87. doi: 10.1080/14622200801979126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizian A, Monterosso J, ONeill JO, London ED. Magnetic resonance imaging studies of cigarette smoking. Handbook of Experimental Pharmacology. 2009;192:113–43. doi: 10.1007/978-3-540-69248-5_5. [DOI] [PubMed] [Google Scholar]
- Backinger C, Fagan P, Matthews E, Grana R. Adolescent and young adult tobacco prevention and cessation: current status and future directions. Tobacco Control. 2003;12:46–53. doi: 10.1136/tc.12.suppl_4.iv46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C, Jenkinson M, Smith S. General multilevel linear modeling for group analysis in fMRI. Neuroimage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Berg C, Thrasher J, Westmaas J, Buchanan T, Pinsker E, Ahluwalia J. College student reactions to health warning labels: sociodemographic and psychosocial factors related to perceived effectiveness of different approaches. Preventive Medicine. 2011;53:427–30. doi: 10.1016/j.ypmed.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A, Mandelkern M, Olmstead R, et al. Neural substrates of resisting craving during cigarette cue exposure. Biological Psychiatry. 2007;62(6):642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L, Pepper J, Brewer N. Responses of young adults to graphic warning labels for cigarette packages. Tobacco Control. 2013;24:e14–22. doi: 10.1136/tobaccocontrol-2012-050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo R, Yee S, Gfroerer J, Mirza S. Adult tobacco use among racial and ethnic groups living in the United States, 2002-2005. Preventing Chronic Disease. 2008;5(3):A78. [PMC free article] [PubMed] [Google Scholar]
- CDC Antismoking messages and intention to quit – 17 countries, 2008-2011. MMWR Morbidity and Mortality Weekly Report. 2013;62:417–22. [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Presson C, Rose J, Sherman S. The natural history of cigarette smoking from adolescence to adulthood: demographic predictors of continuity and change. Health Psychology. 1996;15:478–84. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Choi S, Rankin S, Stewart A, Oka R. Effects of acculturation on smoking behavior in Asian Americans: a meta-analysis. Journal of Cardiovascular Nursing. 2008;23:67–73. doi: 10.1097/01.JCN.0000305057.96247.f2. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- David S, Munafo M, Johansen-Berg H, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:488–94. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due D, Huettel SA, Hall W, Rubin D. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–60. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, et al. Youth risk behavior surveillance -United States, 2011. Morbidity and Mortality Weekly Report. 2012;61(4):1–168. [PubMed] [Google Scholar]
- Emery S, Choi W, Pierce J. The social costs of tobacco advertising and promotions. Nicotine Tobacco Research. 1999;1(Suppl. 2):S83–91. doi: 10.1080/14622299050011871. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge J. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience Biobehavioral Reviews. 2009;33:367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Luckenbaugh D, Moolchan E, Temple V, Jenness J, Ke K. Decision-making and facial emotion recognition as predictors of substance-use initiation among adolescents. Addictive Behaviors. 2009;35:286–9. doi: 10.1016/j.addbeh.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K, Schneider N. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. Journal of Behavioral Medicine. 1989;12:159–82. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biological Psychiatry. 2010;68:265–71. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, et al. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;15:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Schonberg T, Mumford J, Kohno M, Poldrack RA, London ED. Greater risk sensitivity of dorsolateral prefrontal cortex in young smokers than in nonsmokers. Psychopharmacology. 2013;229:345–55. doi: 10.1007/s00213-013-3113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A. The teenage brain: sensitivity to rewards. Current Directions in Psychological Science. 2013;22:88–93. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Hare T, Parra C, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Poldrack RA, Baker C, McGlennen K, London ED. Neural correlates of response inhibition and cigarette smoking in late adolesence. Neuropsychopharmacology. 2011;36:970–8. doi: 10.1038/npp.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience. 2013;33(10):4584–93. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Volkow N. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews Neuroscience. 2011;12:652–69. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, et al. The neurocircuitry of impaired insight in drug addiction. Trends in Cognitive Sciences. 2009;13(9):372–80. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D. Health warning messages on tobacco products: a review. Tobacco Control. 2011;20:327–37. doi: 10.1136/tc.2010.037630. [DOI] [PubMed] [Google Scholar]
- Hammond D, Reid J, Driezen P, Boudreau C. Pictorial health warnings on cigarette packs in the United States: an experimental evaluation of the proposed FDA warnings. Nicotine Tobacco Research. 2013;1(Suppl. 2):93–102. doi: 10.1093/ntr/nts094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatbakhsh R, Mamun A, Williams G, OCallaghan M, Najman J. Early childhood predictors of early onset of smoking: a birth prospective study. Addictive Behaviors. 2013;38:2513–9. doi: 10.1016/j.addbeh.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Hu M-C, Davies M, Kandel D. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. American Journal of Public Health. 2006;96(2):299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik M, Madsen D, Olmstead R, Iwamoto-Schaap P, Elins J, Benowitz N. Nicotine blood levels and subjective craving for cigarettes. Pharmacology, Biochemistry and Behavior. 2000;66:553–8. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Jasinska A, Chua H, Ho S, Polk T, Rozek L, Strecher V. Amygdala response to smoking-cessation messages mediates the effects of serotonin transporter gene variation on quitting. Neuroimage. 2012;61:766–73. doi: 10.1016/j.neuroimage.2011.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- King B, Dube S, Tynan M. Current tobacco use among adults in the United States: findings from the National Adult Tobacco Survey. American Journal of Public Health. 2012;102:e93–100. doi: 10.2105/AJPH.2012.301002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Kross E, Mischel W, Hart C, Ochsner K. Regulation of craving by cognitive strategies in cigarette smokers. Drug and Alcohol Dependence. 2009;106:52–5. doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross E, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences. 2010;107:14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Vollstadt-Klein S, Wilden S, et al. The effect of pictorial warnings on cigarette packages on attentional bias of smokers. Pharmacology Biochemistry and Behavior. 2011;98:292–8. doi: 10.1016/j.pbb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Lovato C, Linn G, Best A. Impact of tobacco advertising and promotion on increasing adolescent smoking behaviors. Cochrane Databse System Reviews. 2003;4:CD003439. doi: 10.1002/14651858.CD003439. [DOI] [PubMed] [Google Scholar]
- McCool J, Webb L, Cameron L, Hoek J. Graphic warning labels on plain cigarette packs: will they make a difference to adolescents? Social Science & Medicine. 2012;74:1269–73. doi: 10.1016/j.socscimed.2011.12.043. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalied form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partos T, Borland R, Yong H, Thrasher J, Hammond D. Cigarette packet warning labels can prevent relapse: findings from the International Tobacco Control 4-Country policy evaluation cohort study. Tobacco Control. 2013;22:e43–50. doi: 10.1136/tobaccocontrol-2011-050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper J, Cameron L, Reiter P, McRee A-L, Brewer NT. Non-smoking male adolescents reactions to cigarette warnings. PLoS One. 2013;8:e65533. doi: 10.1371/journal.pone.0065533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E, Romer D, Slovic P, et al. The impact and acceptability of Canadian-style cigarette warning labels among U.S. smokers and nonsmokers. Nicotine and Tobacco Research. 2007;9:473–81. doi: 10.1080/14622200701239639. [DOI] [PubMed] [Google Scholar]
- Phelps E, LeDoux J. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Poline J-B, Worsley KJ, Evans A, Friston K. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Pushparaj A, Hamani C, Yu W, et al. Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology. 2013;38:690–8. doi: 10.1038/npp.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam T, Piper M, Cook J, Fiore M, Baker T. Life 1 year after a quit attempt: real-time reports of quitters and continuing smokers. Annals of Behavioral Medicine. 2012;44:309–19. doi: 10.1007/s12160-012-9399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapush M, McNeill A, Hammond D, Fong G. Socioeconomic and country variations in knowledge of health risks of tobacco smoking and toxic constituents of smoke: results from the 2002 International Tobacco Control (ITC) Four Country Survey. Tobacco Control. 2006;15:iii65–70. doi: 10.1136/tc.2005.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E, Peterson B, Thompson P, Welcome S, Henkenius A, Toga A. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Upadhyaya H, Drobes D, Wang W. Reactivity to in vivo smoking cues in older adolescent cigarette smokers. Nicotine and Tobacco Research. 2006;8:135–40. doi: 10.1080/14622200500432112. [DOI] [PubMed] [Google Scholar]
- U. S. Food and Drug Administration. (2010). Family Smoking Prevention and Tobacco Control Act: Cigarette Health Warnings. Retrieved from: http://www.fda.gov/TobaccoProducts/Labeling/Labeling/CigaretteWarningLabels/default.htm (accessed 1 March 2014) [Google Scholar]
- Volkow N, Wang G, Fowler F, Tomasi D. Addiction circuitry in the human brain. Annual Review of Pharmacology and Toxicology. 2012;52:321–36. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield MA, Hayes L, Durkin S, Borland R. Introduction effects of the Australian plain packaging policy on adult smokers: a cross-sectional study. BMJ Open. 2013;3(7):e003175. doi: 10.1136/bmjopen-2013-003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Ruparel K, Loughead J, et al. Content matters: neuroimaging investigation of brain and behavioral impact of televised anti-tobacco public service announcements. Journal of Neuroscience. 2013;33:7420–7. doi: 10.1523/JNEUROSCI.3840-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A, Shiffman S, Sayette M, Paty J, Gwaltney C, Balabanis M. Cue-provoked craving and nicotine replacement therapy in smoking cessation. Journal of Consulting and Clinical Psychology. 2004;72:1136–43. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- Wills T, Sargent J, Stoolmiller M, Gibbons F, Worth K, Dal Cin S. Movie exposure to smoking cues and adolescent smoking onset: a test for mediation through peer affiliations. Health Psychology. 2007;26:769–76. doi: 10.1037/0278-6133.26.6.769. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2011). . WHO Report on the Global Tobacco Epidemic, 2011: warning about the dangers of tobacco. 2011 Geneva, Switzerland: WHO Press. Retrieved from: http://www.who.int/tobacco/global_report/2011/en/ (accessed 1 March 2014) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.