Abstract
One in five American children grows up in poverty. Childhood poverty has far-reaching adverse impacts on cognitive, social and emotional development. Altered development of neurocircuits, subserving emotion regulation, is one possible pathway for childhood poverty’s ill effects. Children exposed to poverty were followed into young adulthood and then studied using functional brain imaging with an implicit emotion regulation task focused. Implicit emotion regulation involved attention shifting and appraisal components. Early poverty reduced left dorsolateral prefrontal cortex recruitment in the context of emotional regulation. Furthermore, this emotion regulation associated brain activation mediated the effects of poverty on adult task performance. Moreover, childhood poverty also predicted enhanced insula and reduced hippocampal activation, following exposure to acute stress. These results demonstrate that childhood poverty can alter adult emotion regulation neurocircuitry, revealing specific brain mechanisms that may underlie long-term effects of social inequalities on health. The role of poverty-related emotion regulatory neurocircuitry appears to be particularly salient during stressful conditions.
Keywords: childhood poverty, emotion regulation, adulthood, task performance
Introduction
One in five American children spend all or part of their lives in poverty (United States Census Bureau, 2011), and accumulating data suggest that childhood poverty has far-reaching adverse impacts on cognitive, social and emotional development (Noble et al., 2007; Hackman and Farah, 2009; Hackman et al., 2010). Although the precise mechanisms through which childhood poverty affects health and development are not clear, recent work implicates functional and structural changes in the brain circuits responsible for emotional regulation as one plausible mechanism (Kim et al., 2013). Emotion regulation is a dynamic process that modifies responses to emotionally arousing conditions. Emotion regulation involves allocation of attentional resources and cognitive strategies, which could be implicit or explicit (Gross, 1999; Gross and Thompson, 2007). Given that one’s ability to regulate emotion is a critical factor for maintaining good mental (Brassen et al., 2012; Reiss, 2013) and physical health (Kubzansky et al., 2011), diminished ability to respond adaptively to emotionally charged situations may be an important factor underlying social inequalities in mental and physical health. However, direct evidence for these links is sparse, and until recently, this has not been studied prospectively. Recently, we have reported that childhood poverty is associated with diminished explicit emotional regulation ability (Kim et al., 2013), wherein adults were required to actively reappraise emotionally upsetting stimuli to dampen their negative affective responses. How childhood poverty affects the development of more implicit emotion regulation strategies has not been studied. Furthermore, the direct functional implication of altered emotional modulation/regulation circuitry among low-relative to middle-income experience has not been tested experimentally.
Similarly to explicit strategies like ‘distancing’ or ‘reappraisal’, implicit emotion regulation strategies lead to decreased emotional responses to emotionally evocative stimuli (Hariri et al., 2000, 2003; Lieberman et al., 2007, 2011). Implicit emotion regulation strategies also modify activation of brain regions including the amygdala and insula that are involved in emotion regulation (Kohn et al., 2013). Unlike explicit emotion regulation protocols, wherein subjects are instructed to engage in specific strategies to modify their emotions, implicit emotion regulation reflect more typical, naturalistic responses that are often used when people are confronted with strong emotional situations. These might include slowing down and ‘thinking about it’ (appraising situation) or ‘focusing on what can be done’ (shifting attention to specific tasks). In this sense, one might also expect that these more ‘natural’ strategies for coping with emotion are learned earlier and thus might be affected by early childhood experiences of poverty given the plethora of psychosocial (e.g. insensitive and harsh parenting) and physical (e.g. substandard housing) stressors accompanying childhood poverty (Evans, 2004). Explicit emotion regulation strategies may also develop later in life because they rely upon developed cognitive abstraction skills like shifting between two alternative interpretations to the same complex stimuli (e.g. modulate your emotion by imagining that the people crying while leaving a church/temple are expressing joy rather than sadness). To test these hypotheses, we designed an implicit emotional regulation study for adult subjects raised in poverty and in middle-income families during their childhood.
Numerous studies have also shown that childhood poverty predicts subsequent deficits in cognitive processes including language, reading, math, as well as indicators of educational attainment such as high school graduation or college matriculation (Duncan and Brooks-Gunn, 1997; Duncan and Murnane, 2011). If emotional regulation is required during performance of cognitive task (e.g. regulating emotional responses during difficult test or managing emotional responses to surprising novel stimuli), diminished emotional regulation could contribute to a diminished task performance. To examine potential functional significance of hypothesized differences in emotional regulation, we assessed task performance in our study by assessing the accuracy on a face and space identification task, while the subject was implicitly processing and regulating emotional responses elicited by emotional facial expressions.
Moreover, effective regulation of emotional responses is likely more critical at the time of increased stress (Lazarus and Folkman, 1984; Raio et al., 2013); however, the effects of stress on emotional regulation and underlying neurocircuitry have not been formally tested. Furthermore, childhood poverty is associated with elevated levels of chronic stress, including heightened blood pressure, basal cortisol and catecholamines (e.g. epinephrine) levels, contributing to higher allostatic load (Evans et al., 2012). At the neurocircuitry level, childhood poverty is implicated in altering function of key limbic and cortical regions involved in emotional regulation like amygdala, hippocampus and prefrontal cortex (Hackman and Farah, 2009; Luby et al., 2013). These same regions are known to be sensitive to stress levels and stress hormones (Dedovic et al., 2009; Belujon and Grace, 2011; Schwabe, 2013) and have been implicated both in stress response and emotional response/regulation (Drevets et al., 2008; Shin and Handwerger, 2009; Godsil et al., 2013). These lines of evidence raise the possibility that childhood poverty effects on emotional regulation may be particularly pronounced during elevated stress.
To this end, we conducted an fMRI study with a standard, implicit emotion regulation protocol both before and after the induction of psychological stress. We also examined whether this differential recruitment of brain circuits account for expected differences in task performance among adults from poor backgrounds. We predicted that whether childhood poverty negatively affected the development of emotion modulation mechanisms, then we should see diminished recruitment of brain regions implicated in emotional regulation including the dorsolateral prefrontal cortex (DLPFC), dorsal medial prefrontal cortex (dmPFC) and hippocampus (Erk et al., 2010), in association with diminished performance on cognitive identification task. Similarly, during emotion induction/processing, we expected enhanced recruitment of ‘emotion generating’ regions such as the amygdala and insula (Habel et al., 2007; Bruce et al., 2012; Kohn et al., 2013). We predicted that these hypothesized differences will be further pronounced following stress induction.
Methods
Participants
Participants were drawn from individuals participating in a prospective longitudinal study on the effects of childhood poverty (Evans and Schamberg, 2009; Evans and Kim, 2010, 2012). Half of the sample spent much of their childhood at or below the poverty line [low socioeconomic status (Low-SES)] and the other half had never been poor based on the household’s income-to-needs ratio (Mid-SES) (Kim et al., 2013). A total of 54 subjects, who were right-handed, healthy, un-medicated and without psychiatric conditions, were recruited and consented to participate. Recruitment details are presented in the Supplementary (also see Kim et al., 2013). Prior to participation, the participants provided written informed consent as approved by the local Institutional Review Board. Of 54 subjects, 49 subjects completed 2 days of experiments and had complete fMRI data (2 subjects failed to complete scans and 3 subjects had missing fMRI data due to scanner problems). Thus, data from 23 subjects with history of childhood poverty (13 men and 10 women aged 24.3 ± 1.2 years) and 26 subjects who grew up in middle-income households (14 men and 12 women, aged 23.1 ± 1.2 years) were analyzed. Demographic characteristics of our subjects are presented in Table 1.
Table 1.
Characteristics of participants analyzed in this study
| Mid-SES (n = 26) | Low-SES (n = 23) | ||
|---|---|---|---|
| Demographics | |||
| Age at MRI (mean ± s.d.) | 23.1 ± 1.2 | 24.3 ± 1.2 | |
| Female/Male | 12/14 | 10/13 | |
| Ethnicity (white/mixed) | 26/0 | 20/3 | |
| Unemployed | 25% | 23% | |
| Married or cohabitating | 89% | 43% | |
| Poverty related variables | |||
| Income-to-needs ratioa at age 9 | 2.67 ± 0.78 | 0.76 ± 0.37 | |
| Income-to-needs ratio at age 22 | 4.48 ± 3.46 | 1.76 ± 1.23 | |
| Maternal high school dropout | 0% | 8% | |
| Maternal college graduate | 44% | 8% | |
| Single mother | 30% | 46% | |
| Childhood stress | |||
| Childhood alostatic load (age 9–17) | 1.09 ± 0.81 | 1.51 ± 0.86 | |
Note: Income-to-needs ratio = 1 = US poverty line
Experimental paradigm
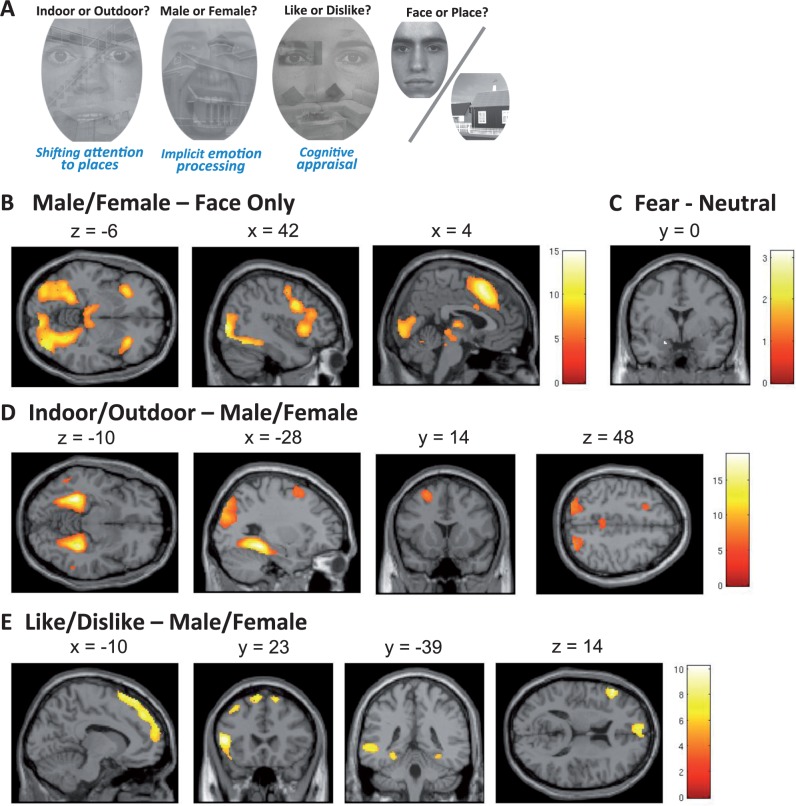
To examine implicit emotion regulation, we selected the shifted-attention emotion appraisal task (SEAT), originally developed by Anderson et al. (2003) and modified by us (Klumpp et al., 2011; Sripada et al., 2013a,b). This task activates networks involved in emotion processing and implicit emotion regulation by appraisal and attention shift (Fusar-Poli et al., 2009; Kohn et al., 2013). For our task stimuli, we’ve used 40 gray-scaled compound pictures depicting facial pictures displaying one of two emotions (fearful or neutral) (Ekman and Friesen, 1976; Gur et al., 2002). In half of the pictures, faces are superimposed onto backgrounds of an indoor scene and the other half onto an outdoor scene. There are additionally 10 gray-scaled non-compound pictures of neutral faces (face only) and 10 gray-scaled non-compound pictures of an indoor or outdoor scene (place only). The faces and places in non-compound pictures are different from those used in the face-place compound pictures. Each face-place compound picture was presented three times (1.5 s/presentation) during the experiment in random order. While viewing a compound picture, the subject was cued to press a button to indicate (i) whether the face is of a male or female (Male/Female), or (ii) whether the subject liked or disliked the face (Like/Dislike), or (iii) whether the background was an indoor or outdoor scene (Indoor/Outdoor). An example of the compound pictures as well as the task cue is depicted in Figure 1A. As demonstrated in prior work with the SEAT protocol, these tasks engage psychological processes of (i) implicit emotional processing (Male/Female), (ii) cognitive appraisal (Like/Dislike) and (iii) shifting attention (Indoor/Outdoor), and subserved the basis conditions for emotion regulation processing contrasts (defined in fMRI Data Analysis section later). Briefly, focusing on the facial features (‘Male/Female’ task) requires implicit processing of facial expressions, i.e. emotional processing (Fusar-Poli et al., 2009), while focusing on facial features and assessing ‘likability’ (‘Like/Dislike’ task) engages cognitive appraisal mechanism that had been shown to automatically modulate emotional responses (Hariri et al., 2000, 2003; Lieberman et al., 2007). Similarly, focusing on the building component of the picture (‘Indoor/Outdoor’ task) shifts attention to a non-emotional component of the same compound stimulus, thus modulating an overall emotional response. Each non-compound picture (face only or place only) was presented two times and the subject was cued to press a button to indicate whether the picture was a face or a place (Face/Place). The task cue (e.g. Indoor/Outdoor, Male/Female, Like/Dislike or Face/Place) was presented for 750 ms followed by a 250-ms blank screen before all stimuli presentation. A fixation cross was presented during the inter-trial interval (jittered duration 3–8 s). Conditions were presented at random order. The stimuli were presented using E-Prime (Psychological Software Tools, Pittsburgh, PA), via magnetic resonance compatible liquid crystal display goggles (Nordic Neuro Labs, Milwaukee, WI). Participant responses were recorded by a button glove attached to the subject’s hand and linked to the E-Prime system. Prior to experimental trials, subjects completed a practice session with images not used in the experiment. As both the implicit emotion processing (Male/Female) and shifting attention (Indoor/Outdoor) tasks have correct answers associated with them, we used the subject’s accuracy in identifying the faces as male or female and the scenes as indoor/outdoor) as an index of task performance.
Fig. 1.
Task instruction, stimuli and brain activation associated with each contrast. The task instruction (Indoor/Outdoor, Male/Female, Like/Dislike or Face/Place) is presented before subsequent stimuli presentation, and all the conditions are randomized. The left hemisphere is on the top in the axial slices and on the left in the coronal slices.
Stress manipulation and cortisol measurement
Two days of experiments were scheduled using a counterbalanced, within-subjects design. Subjects underwent the trier social stress test (TSST) before fMRI session on 1 of 2 days in counterbalanced order. The TSST is a well-validated stress induction procedure that reliably activates hypothalamo–pituitary–adrenal stress response, which combines a job application speech with a cognitively challenging mental arithmetic task performed in front of an ‘expert’ audience, and structured to create high demand/low feedback characteristics (Kirschbaum et al., 1993). On each experimental day, saliva samples for cortisol were collected from subjects at the same time during the day: immediately before, immediately after, 15 min after and 1.5 h after TSST completion (or Control test). Cortisol was assayed using a commercially available Coat-a-Count radioimmunoassay kits from Diagnostic Products Corporation (Los Angeles, CA). For further details see Supplementary.
Acquisition of MRI data
All scanning was performed using a Philips 3.0 Tesla Achieva X-series MRI (Philips Medical Systems, Andover, MA). A total of 600 T2*-weighted echo planar gradient-recall echo volumes (echo time = 25 ms, repetition time = 2000 ms, 64 × 64 matrix, flip angle = 90°, field of view = 22 cm, 42 contiguous 3 mm axial slices per volume), were acquired in four runs (150 volumes/run). Five additional volumes were acquired at the beginning of each run to allow for equilibration of the MRI signal and were subsequently discarded. A high-resolution T1-weighted structural image (1 × 1 × 1 mm voxel size) was also obtained for anatomic normalization.
Data analysis
MRI data were analyzed using the statistical parametric mapping software package, SPM8 (Welcome Department of Cognitive Neurology, London, UK). After, slice timing correction, functional volumes were realigned to correct for head motion, spatially normalized to a standard template based upon the Montreal Neurological Institute (MNI) reference brain, and spatially smoothed using an 8-mm FWHM Gaussian kernel. Statistical analyses were carried out using the general linear model. Six regressors for composite pictures (3 types of tasks and 2 types of faces) and 2 regressors for non-composite pictures (face only and place only) were modeled with canonical hemodynamic response functions. The realignment parameters were also included in the model.
To isolate brain circuits related to the different emotion regulation processes discussed, i.e. (i) emotion regulation by shifting attention and (ii) emotion regulation by cognitive appraisal, we created the following three specific contrasts in the first-level analysis for each participant: (i) Male/Female—Faces Only (only neutral faces are included in Faces Only set), (ii) Indoor/Outdoor—Male/Female and (iii) Like/Dislike—Male/Female. Then, for each first-level contrast, second-level analyses were performed according to the following: (i) to identify brain regions associated with two types of emotional regulation we’ve applied one sample t-tests for subtracted first-level contrasts (i.e. Like/Dislike—Male/Female and Indoor/Outdoor—Male/Female) averaging over two experimental sessions (Stressed and Non-stressed) from all subjects, (ii) as we are mainly interested in the main effect of childhood poverty, and the interaction of childhood poverty and stress during emotion regulation, we conducted the following two analyses. First, we used childhood poverty (childhood income to need) as a continuous measure with OLS regression in the two emotion regulation contrasts. We used childhood income to need as a continuous regressor, as well as compared categorically poverty vs non-poverty groups both to see whether (i) there is a relationship between brain activation and childhood poverty and (ii) the childhood poverty group as a whole differs from the non-poverty group. Second, to examine the effect of experimental stress and identify interactions between stress and childhood poverty, Stress and Non-Stress conditions were contrasted for each of the two emotion regulation contrasts, and the results were compared for poverty and non-poverty groups (Low- vs Mid-SES), and regressed with continuous income-to-need ratio. While our main focus was to examine whether stress induction interacted differentially with childhood poverty, for all analyses, we examined childhood poverty as a continuous variable as well as a categorical variable.
The statistical threshold of P < 0.05 familywise error (FWE) corrected for the whole brain was used, except for a priori hypothesized regions corrected for small volume [search volume is a priori region of interest (ROI) masks]. These a priori ROI included the bilateral amygdala, hippocampus and insula, as these regions are typically of interest in emotion, emotion regulation and stress response (Rauch et al., 2006; Wager et al., 2008), and DLPFC/IFG region as we have reported in our previous publication (Kim et al., 2013) . These ROI masks were created in MNI space using the WFU Pick atlas software (Maldjian et al., 2003) with its associated automated AAL atlas (Tzourio-Mazoyer et al., 2002).
Multiple regression and mediation analysis with bootstrapping tests (Shrout and Bolger, 2002) were analyzed using MATLAB (The Mathworks, Natick, MA). For further details see Supplementary.
Results
Behavioral results and recognition accuracy
Our subjects identified compound pictures at ∼ 70–80% accuracy (i.e. above chance level but without displaying ceiling effects); however, lower income participants were less accurate in identifying both gender of the face and background location relative to non-poverty subjects. There was a significant positive correlation between the childhood income-to-needs ratio and the accuracy of Male/Female and Indoor/Outdoor identification (r = 0.417, P = 0.003). Mean levels of accuracy were MPoverty = 73.6 ± 1.9% vs MNon-poverty = 79.5 ± 1.0%, [t(47) = 2.84, P = 0.007], for the poverty and middle-income groups, respectively. There was no significant poverty effect on the less demanding task, i.e. identifying the non-compound pictures (P = 0.269, MPoverty = 96.4 ± 2.5%; MNon-poverty = 99.0 ± 0.3%).
Salivary cortisol levels
The TSST caused a significant increase in salivary cortisol levels immediately after, and 15 min after TSST (immediately prior to the fMRI scanning) when compared both with the same-day baseline, and in comparison to corresponding cortisol measures on the control day (P < 0.001, and P < 0.001 for 0 and 15 min time points). There were no main effects or interaction effects of childhood poverty on cortisol response, demonstrating effective and comparable physiologic stress response in both groups. The TSST and control conditions were counterbalanced and no order effects were observed. Cortisol levels between two groups with different time periods are displayed in Supplementary Figure S1.
fMRI results
Effects of tasks
Three conditions of the SEAT task were used to activate networks of regions supporting (i) implicit emotion processing, (ii) emotional regulation by shifting attention and (iii) emotional regulation by cognitive appraisal (Klumpp et al., 2011; Sripada et al., 2013a,b). We first confirmed the overall effects of tasks across all subjects, across two experimental sessions, in Table 2 (unless otherwise specified, all findings reported are whole brain, cluster-level threshold of P < 0.05 FWE—corrected). Consistent with the effects of implicit emotion processing (Fusar-Poli et al., 2009), the Male/Female—Faces Only (neutral) contrast revealed significant activations in the emotional processing regions and salience network, including insula, dmPFC/dorsal anterior cingulate cortex (dACC) and fusiform gyrus. Amygdala activation was significant in Fearful — Neutral contrast (a priori defined ROI, SVC correction) across conditions (Figure 1A, Table 2). We then used the Male/Female condition (implicit emotion processing), as a ‘baseline’ to examine the effects of emotion regulation through shifting attention (Indoor/Outdoor) and cognitive appraisal (Like/Dislike). Emotion regulation by shifting attention (Indoor/Outdoor—Male/Female) activated peaks in the parahippocampus and attention control regions like DLPFC (Figure 1B, Table 2). Emotion regulation by cognitive appraisal (Like/Dislike—Male/Female contrast) activated the broad area of mPFC, regions in the attentional control network including the left inferior frontal gyrus/DLPFC, left middle temporal gyrus/angular gyrus and bilateral parahippocampus/hippocampus areas (Figure 1C, Table 2). In sum, these results fully replicate previously reported findings (Klumpp et al., 2011; Sripada et al., 2013a,b), verifying activation of emotional response and emotion regulating networks by the SEAT protocol.
Table 2.
Brain activation associated with each contrast and effects of poverty
| Region | Side | Z | kE | x | Y | z |
|---|---|---|---|---|---|---|
| Emotional processing—male/female—face only contrast | ||||||
| dmPFC/dACC (BA6/8/9/32) | L/R | >8 | 3560 | −4 | 16 | 50 |
| Inferior/middle frontal gyrus/precentral gyrus/anterior insula | L | >8 | 2571 | −30 | 26 | 2 |
| R | >8 | 2612 | 34 | 24 | 6 | |
| Fusiform | L | >8 | 614 | −40 | −61 | −12 |
| R | >8 | 935 | 31 | −68 | −10 | |
| Thalamus | L/R | >8 | 1139 | −10 | −16 | 10 |
| Calcarine/occipital/lingual | L/R | >8 | 7290 | 36 | −88 | 8 |
| Middle frontal gyrus (BA10) | L | 5.85 | 59 | −32 | 52 | 18 |
| Cingulate gyrus (BA24) | L/R | 5.61 | 53 | 6 | 10 | 30 |
| Fear—neutral | ||||||
| Amygdala | L | 2.99 | 5a | −18 | 0 | −18 |
| Modulation by attention: indoor/outdoor—male/female contrast | ||||||
| Parahippocampus/hippocampus/fusiform | L | >8 | 1402 | −28 | −40 | −12 |
| R | >8 | 1256 | 32 | −38 | −12 | |
| Occipital/parietal/angular/precuneus gyrus | L | >8 | 1573 | −34 | −86 | 28 |
| R | >8 | 1243 | 44 | −78 | 30 | |
| Cuneus/lingual/precuneus/calcarine/PCC | L/R | 7.76 | 1674 | 20 | −54 | 18 |
| Middle/superior frontal gyrus (BA6/8) | L | 6.06 | 303 | −26 | 14 | 56 |
| PCC | L/R | 5.72 | 346 | 8 | −36 | 42 |
| Inferior/middle temoral gyrus | L | 5.25 | 45 | −56 | −56 | −8 |
| R | 5.05 | 17 | 60 | −48 | −8 | |
| Modulation by appraisal: like/dislike—male/female contrast | ||||||
| Inferior frontal gyrus (BA45/46/47) | L | 7.38 | 749 | −50 | 28 | 8 |
| mPFC (BA10/8/6)/DLPFC (BA9) | L | 6.6 | 1569 | −8 | 34 | 58 |
| Middle temporal/inferior parietal/angular gyrus (BA39/40) | L | 6.31 | 691 | −44 | −58 | 28 |
| Middle temporal gyrus (BA21/22) | L | 6.3 | 401 | −54 | −38 | 0 |
| dmPFC (BA6/8) | R | 6.23 | 102 | 14 | 30 | 60 |
| Middle frontal gyrus (BA8) | L | 5.94 | 226 | −38 | 18 | 52 |
| Parahippocampus/fusiform gyrus | L | 5.61 | 174 | −28 | −42 | −10 |
| R | 5.09 | 55 | 32 | −38 | −12 | |
| Superior frontal gyrus (BA9) | R | 5.3 | 59 | 16 | 58 | 32 |
| Effects of childhood poverty (group comparison & regression income-to-need age 9) | ||||||
| During cognitive appraisal | L | 4.24 | 240 | −56 | 12 | 36 |
| Childhood poverty as continuous variable | ||||||
| DLPFC (BA9) (positive correlation with income) | ||||||
| Childhood poverty as categorical variable | ||||||
| No areas | ||||||
| In interaction with stress induction (stress > non-stress) | ||||||
| Childhood poverty as continuous variable | ||||||
| No areas | ||||||
| Childhood poverty as categorical variable | ||||||
| During appraisal | ||||||
| Hippocampus (non-poverty > poverty) | L | 3.86 | 157a | −24 | −32 | −8 |
| During emotion processing | ||||||
| Insula (poverty > non-poverty) | L | 3.55 | 2a | −36 | −4 | −2 |
Note: BA, Brodmann area; L, Left; R, Right; Z, Z value of the peak activation within the cluster; kE, cluster size (voxels);
a Indicates corrected with a priori mask; Coordinates for the peak voxel are listed as MNI coordinates.
Childhood poverty
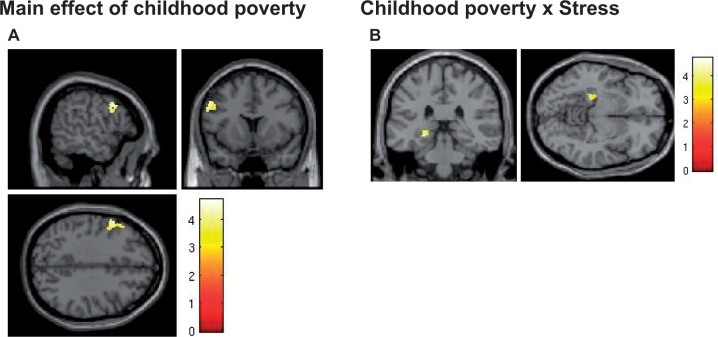
Next, we examined correlates of childhood poverty using childhood income-to-needs ratio as a continuous variable in regression analyses. To examine the effects of acute stress on emotional regulation, the same analyses were performed using the subtraction (Stressed—Non-stressed) contrast. The results are summarized in Table 2. Regression of the income-to-needs ratio at age 9, during emotion regulation by cognitive appraisal (Like/Dislike—Male/Female contrast), revealed a significant peak in the left DLPFC (lDLPFC)/IFG region (BA9) (MNI −56, 12, 36; 240 voxels; Z = 4.24; P = 0.045 whole-brain FWE corrected) (Figure 2A). Of note, we have recently reported that childhood poverty predicts recruitment of the similar region (x, y, z = –40, 12, 28) during effortful volitional regulation task (Kim et al., 2013). To control for the possibility that differential brain activations were contributed specifically by brain responses to ‘incorrect’ trials, we repeated our analyses including only the correct trials. The results were unaffected and reported peaks remained significant. We also directly compared brain responses to correct vs incorrect trials and found no differences in activation patterns to two types of trials.
Fig. 2.
Effects of childhood poverty on brain activation. (A) Left DLPFC (BA9) activation during appraisal (Like/Dislike—Male/Female) was positively correlated with childhood income. (B) Stress differentially affected the left hippocampus activation during appraisal between groups. (ROI SVC correction, P < 0.05).
Effects of stress
Childhood poverty was associated with higher activation, after stress exposure, in the insula, but not in the amygdala, during implicit emotion processing. Regression analysis using income to need at age 9 revealed significant negative correlation in the left insula after stress induction (MNI −34, −4, −2; Z = 3.55; 117 voxels under P < 0.001 uncorrected threshold, 2 voxels survived after P < 0.05 FWE SVC corrected). Categorical group analysis revealed no between-group significant differences in the region. In contrast, during emotion regulation by cognitive appraisal, the effect of the poverty was observed in the opposite direction in the left hippocampus. Hippocampus activation decreased more after stress in the poverty group compared with the non-poverty group (MNI −24, −32, −8; Z = 3.86; 157 voxels; P = 0.029 SVC corrected) (Figure 2B). Regression of income-to-need ratio did not identify significant voxels in the hippocampus.
Childhood poverty, brain activation and task performance
The childhood income-to-needs ratio at age 9 (r = 0.417, P = 0.003) and lDLPFC activation during emotion regulation by cognitive appraisal (r = 0.477, P = 0.001), both predicted recognition accuracy. To examine whether childhood poverty effects on recognition accuracy in adulthood were mediated by the lDLPFC recruitment, we conducted a statistical mediation analysis (Preacher and Hayes, 2008). The positive association between childhood income and adult recognition accuracy was mediated by lDLPFC activation [indirect effect: 1.34 (95% CI: −0.70 to 3.38)] (Figure 3). As a partial check on spuriousness, we reversed the order of inclusion of the outcome variable (accuracy) and the mediator (lDLPFC activation). Recognition accuracy did not mediate the relation between childhood poverty and lDLPFC activation, suggesting that Childhood poverty → lDLPFC → Task performance is a tenable model for how childhood might influence adult task performance.
Fig. 3.
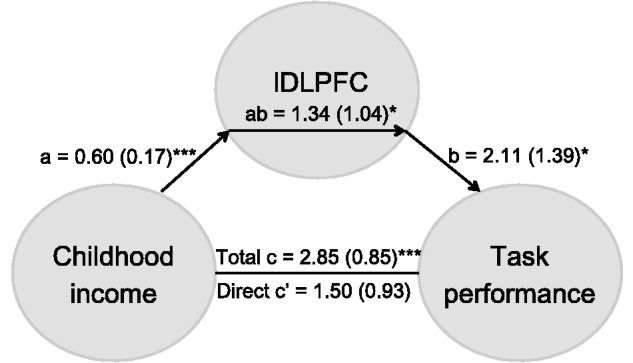
Mediation model and analysis for the association between childhood income, task performance, and lDLPFC activation. Path coefficients are shown next to arrows indicating each link in the analysis, with standard errors in parentheses. *** P < 0.001, * P < 0.05.
To examine whether our findings reflect experiences of early childhood poverty or manifest concurrent adult income status, we repeated the earlier analyses with a statistical control for adult income levels or family income levels at later waves (ages 13 and 17). None of the reported results changed after statistically controlling for adult income level. Furthermore, adult income levels were unrelated to emotion regulation, task performance or fMRI, suggesting that early poverty is linked to brain and behavior in adulthood, independently of subsequent financial status.
Discussion
The primary aim of this study was to examine whether childhood poverty predicts implicit emotion regulation circuitry in adults. Dysfunctional emotion regulation could help explain some of the ubiquitous social inequalities in human health and achievement. To our knowledge, this is the first prospective investigation of the effects of childhood poverty on the recruitment of implicit emotional regulation neurocircuitry and to consider this under both stress and non-stress conditions. Our findings replicate and extend prior work showing that childhood poverty with this same sample interferes with adult’s ability to use volitional reappraisal to alter their emotional responses to aversive scenarios (Kim et al., 2013). In this article, we extend this work in two important ways. One, we show that an emotion regulation task that does not require the explicit use of an effortful strategy, such as reappraisal, is also sensitive to childhood poverty. Two, we show that when this happens under stress, the influences of childhood poverty are more pronounced. We also investigated functional significance of this differential recruitment by examining accuracy of correct gender identification during implicit processing of emotional signals. We show that childhood poverty predicts both diminished recruitment of lDLPFC during an implicit emotional modulation task, and lower accuracy on recognition task of the same stimuli, in adulthood. Furthermore, the positive association between childhood income and adult performance was mediated by the lDLPFC activation. These effects of childhood poverty remained significant when adult income levels were included in the analyses.
The SEAT fMRI task robustly activated brain networks known to be involved in emotional response and implicit emotional regulation including insula, amygdala, hippocampus, dACC/dmPFC, dorsal and ventral LPFC and parahippocampal regions, replicating prior findings in other samples (Sripada et al., 2013b). Previous studies show that the cognitive appraisal task used in our study, while different from volitional regulation tasks like ‘reappraisal’ or ‘distancing’, activates emotion regulation circuitry (Liberzon et al., 2000; Phan et al., 2004), and similarly dampens emotional reactions (Lieberman et al., 2011) to aversive stimuli. Use of the SEAT task in this study also allowed us to examine whether childhood poverty is linked to implicit, involuntary emotion regulation, i.e. emotion regulation by shifting attention (Indoor/outdoor—Male/Female contrast). The development of implicit emotion regulation strategies, such as shifting attention, also happened earlier in life while encountering stressful situations. These processes are not dependent on complex semantic processing that requires shifting between two alternating cognitive schemas, in contrast to explicit emotion regulation strategies. Taken together with recently reported data (Kim et al., 2013), the current findings suggest that childhood poverty predicts recruitment of the lDLPFC during implicit emotion regulating tasks in adults, but not during shifting of attention, potentially linking it to more complex cognitive processes like introspection, reflection and semantic labeling, required both for implicit appraisal and explicit reappraisal. Furthermore, all of the effects of childhood poverty remained significant when adult income levels were included in the analyses, suggesting that early experiences of disadvantage irrespective of later economic conditions appear to contribute to the development of emotion regulation abilities as well as their neurocognitive signature.
As hypothesized, additional differences in brain activations as a function of childhood poverty were also uncovered in relation to stress. These include increased insula response during implicit emotional processing and decreased hippocampal recruitment during cognitive appraisal. Of interest, these only manifested following experimental stress. Indeed, both hippocampus and insula are amongst the prime candidates for this type of stress effects. Hippocampus and insula are responsive to circulating cortisol levels and play key roles in hypothalamo–pituitary–adrenal axis (‘stress axis’) response and regulation (Liberzon et al., 2007; King et al., 2009; McEwen and Gianaros, 2011). Acute stress affected hippocampal and insula recruitment in the two groups differentially, even though both groups had comparably elevated salivary cortisol levels. This confirms our prediction that comparable levels of stress lead to preferential recruitment of emotion generation circuitry (insula), as opposed to emotion regulation circuitry (hippocampus), in adults with a history of childhood poverty. This, coupled with a more general decrease in the recruitment of emotion regulating regions, suggests that individuals who grew up in poverty might find it more challenging to face highly stressful situations that require regulation of emotional responses. It is important to note that we found no significant between-group differences in salivary cortisol responses to TSST. The link between cortisol reactivity to TSST and SES is indeed complex, with some studies suggesting that lower SES leads to enhanced TSST response (Souza-Talarico et al., 2014), other studies suggesting that this link might be moderated by factors like race and gender (Hackman et al., 2012) and still other suggesting that these factors are independent of each other (Pilgrim et al., 2010). Relevant to current findings, it is important to note that differential brain responses in childhood poverty group can be present even when magnitude of systemic cortisol response to stress does not differ.
A critical limitation of the present and other work on SES and health is reliance on non-experimental data. Short of random assignment to income levels, scientists must rely on observational study designs (see Liberzon et al., 2007 for a thoughtful discussion of causality) in health inequalities research. Although we have shown that early childhood poverty in a prospective design is linked to adult behavioral and brain functioning, independently of endogenous variables like gender, maternal education and mental health, these data remain correlational and should not be interpreted as causal evidence. One alternative explanation of many poverty and health findings is genetics. However, we believe it is highly unlikely that genetics can fully account for our data. First, within one generation, shifts in family income status cause dramatic changes in children’s health and achievement (Adler et al., 2012), and genetic changes could not occur that fast. Second, when low-SES children are adopted, they show dramatic gains in intelligence (see Nisbett, 2009 for a review) as well as improvements in physical health (Osler et al., 2006). Furthermore, twin studies indicate a substantially greater environmental vs genetic contribution in relation to poverty on developmental outcomes (Caspi et al., 2000). Moreover, the few studies taking advantage of random assignment of varying levels of exposure to income (Costello et al., 2010; Ludwig et al., 2011, 2012; Dahl and Lochner, 2012) demonstrate income effects on child development. Genetics is likely relevant to susceptibility to health and achievement inequalities, but it seems unlikely that genetics alone could account for the large and ubiquitous effects of SES on health and achievement.
It is also important to consider whether differential motivation, task engagement or effort could account for our findings. We have examined accuracy on the items depicting faces and places only. These items were interspersed randomly within stimuli set and were analyzed separately. Both groups had comparable accuracy on these items, with no significant differences due to poverty. Furthermore, examination of the RTs suggests that poverty group subjects did not have shorter RTs and did not exhibit time accuracy trade offs, which might have indicated less effort or task engagement. Subject’s current poverty levels also did not predict differential performance, making it less likely that childhood, but not current, poverty would predict differential engagement/effort. Finally, our post-experimental debriefs revealed no differences in subjective response. While not definitive, these data suggest that differential effort/task engagement cannot account for the childhood poverty effects on performance.
One critical question is how does childhood poverty affect the brain? Nutritional status or access to health care or insurance coverage are unlikely pathways for these effects given that health inequalities occur in wealthy countries and in many countries such as EU members, Canada, Costa Rica that have highly developed welfare safety nets including good universal health care (Adler et al., 2012). One plausible, explanatory variable for the developmental sequelae of childhood poverty is parenting. As a group, low-SES parents provide less cognitive stimulation (e.g. language, reading aloud, books) and tend to interact with their children in a less sensitive manner (see reviews by Bradley and Corwyn, 2002; Grant et al., 2003; Conger and Donnellan, 2007). Both of these pathways, particularly the latter, could also influence the development of emotion regulation capacities, yet the precise neurobiological mechanisms involved are unknown. Another plausible mechanism is elevated chronic stress accompanying childhood poverty (Evans and Kim, 2010; McEwen and Gianaros, 2010). Increased chronic stress marked by elevated allostatic load (McEwen and Gianaros, 2011; Evans and Kim, 2012) has been linked to long-term plasticity in both structure and function of the brain (Shonkoff and Garner, 2012). Interestingly, these effects might be especially pronounced, when tested under stress, because increased stress during development might be ‘resetting’ stress responsivity later in life (Goldstein and McEwen, 2002). This is consistent with our findings that exposure to experimental stress further unmasked differences in brain activations between the poverty and non-poverty groups. Interestingly, we observed differential effects of stress in our groups although the magnitude of stress response (as reflected by plasma cortisol levels) did not differ significantly between the groups. This suggests that sensitivity to stress hormones, or other adaptations at the target sites in the brain, might be mediating differential stress effects rather than systemic cortisol levels.
Sadly, up to 20% of American children currently grow up in poverty (United States Census Bureau, 2011). The physical and psychological costs of childhood poverty are among the most daunting challenges facing American society (Knudsen et al., 2006). With the advent of new scientific tools and application of rigorous prospective epidemiological designs, we stand at the threshold of unlocking the specific neurocircuitry underlying these well-documented, negative sequelae of childhood poverty. Our findings suggest that these negative sequelae are mediated, at least in part, by specific brain mechanisms involved in involuntary modulation of emotional responses. Our findings also suggest that altered brain functions associated with childhood poverty become more pronounced during stress exposure. Perhaps elevated levels of chronic stress associated with poverty may play a role in this process.
Supplementary Material
Acknowledgements
The authors thank Erika Blackburn, Sarah Garfinkel and Robert Varney for assistance with data collection.
Funding
This study was supported by National Institutes of Health Grant RC2MD004767, the William T. Grant Foundation, the John D. and Catherine T. MacArthur Foundation Network on Socioeconomic Status and Health, the Robert Wood Johnson Foundation and grant from the University of Michigan Injury Center by the Centers for Disease Control & Prevention Award Number U49/CE002099.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of Interest. None declared.
REFERENCES
- Adler N., Bush N.R., Pantell M.S. (2012). Rigor, vigor, and the study of health disparities. Proceedings of the National Academy of Sciences of the United States of America, 109(Suppl 2), 17154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.K., Christoff K., Panitz D., De Rosa E., Gabrieli J.D. (2003). Neural correlates of the automatic processing of threat facial signals. The Journal of Neuroscience, 23(13), 5627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P., Grace A.A. (2011). Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Annals of the New York Academy of Sciences, 1216, 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F. (2002). Socioeconomic status and child development. Annual Review of Psychology, 53, 371–99. [DOI] [PubMed] [Google Scholar]
- Brassen S., Gamer M., Peters J., Gluth S., Buchel C. (2012). Don't look back in anger! Responsiveness to missed chances in successful and nonsuccessful aging. Science, 336(6081), 612–4. [DOI] [PubMed] [Google Scholar]
- Bruce S.E., Buchholz K.R., Brown W.J., Yan L., Durbin A., Sheline Y.I. (2012). Altered emotional interference processing in the amygdala and insula in women with post-traumatic stress disorder. Neuroimage Clinical, 2, 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Taylor A., Moffitt T.E., Plomin R. (2000). Neighborhood deprivation affects children's mental health: environmental risks identified in a genetic design. Psychological Science, 11(4), 338–42. [DOI] [PubMed] [Google Scholar]
- Conger R.D., Donnellan M.B. (2007). ‘An interactionist perspective on the socioeconomic context of human development'. Annual Review of Psychology, 58, 175–99. [DOI] [PubMed] [Google Scholar]
- Costello E.J., Erkanli A., Copeland W., Angold A. (2010). Association of family income supplements in adolescence with development of psychiatric and substance use disorders in adulthood among an American Indian population. Journal of the American Medical Association, 303(19), 1954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G.B., Lochner L. (2012). The impact of family income on child achievement: evidence from the earned income tax credit. American Economic Review, 102(5), 1927–56. [Google Scholar]
- Dedovic K., Duchesne A., Andrews J., Engert V., Pruessner J.C. (2009). The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage, 47(3), 864–71. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Furey M.L. (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function, 213(1–2), 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G.J., Brooks-Gunn J., editors. (1997). Consequences of Growing Up Poor . New York: Russell Sage Foundation. [Google Scholar]
- Duncan G.J., Mruname R.J., editors. (2011). Whither Opportunity . New York: Russell Sage Foundation. [Google Scholar]
- Ekman P., Friesen W.V. (1976). Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Erk S., Mikschl A., Stier S., et al. (2010). Acute and sustained effects of cognitive emotion regulation in major depression. The Journal of Neuroscience, 30(47), 15726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W. (2004). The environment of childhood poverty. The American Psychologist , 59(2), 77–92. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Chen E., Miller G.E., Seeman T.E. (2012). How poverty gets under the skin: a life course perspective. In: Maholmes V., King R., editors. The Oxford Handbook of Poverty and Child Development. New York: Oxford University Press. [Google Scholar]
- Evans G.W., Kim P. (2010). Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Annals of the New York Academy of Sciences, 1186, 174–89. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Kim P. (2012). Childhood poverty and young adults' allostatic load: the mediating role of childhood cumulative risk exposure. Psychological Science, 23(9), 979–83. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Schamberg M.A. (2009). Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences of the United States of America, 106(16), 6545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., et al. (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience, 34(6), 418–32. [PMC free article] [PubMed] [Google Scholar]
- Godsil B.P., Kiss J.P., Spedding M., Jay T.M. (2013). The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? European Neuropsychopharmacology, 23(10), 1165–81. [DOI] [PubMed] [Google Scholar]
- Goldstein D.S., McEwen B. (2002). Allostasis, homeostats, and the nature of stress. Stress, 5(1), 55–8. [DOI] [PubMed] [Google Scholar]
- Grant K.E., Compas B.E., Stuhlmacher A.F., Thurm A.E., McMahon S.D., Halpert J.A. (2003). Stressors and child and adolescent psychopathology: moving from markers to mechanisms of risk. Psychological Bulletin, 129(3), 447–66. [DOI] [PubMed] [Google Scholar]
- Gross J.J. (1999). Emotion regulation: past, present, future. Cognition & Emotion, 13(5), 551–73. [Google Scholar]
- Gross J.J., Thompson R.A. (2007). Emotion regulation: conceptual foundations. Handbook of Emotion Regulation, 3, 24. [Google Scholar]
- Gur R.C., Schroeder L., Turner T., et al. (2002). Brain activation during facial emotion processing. Neuroimage, 16(3 Pt 1), 651–62. [DOI] [PubMed] [Google Scholar]
- Habel U., Windischberger C., Derntl B., Robinson S., Kryspin-Exner I., Gur R.C., Moser E. (2007). Amygdala activation and facial expressions: explicit emotion discrimination versus implicit emotion processing. Neuropsychologia, 45(10), 2369–77. [DOI] [PubMed] [Google Scholar]
- Hackman D.A., Betancourt L.M., Brodsky N.L., Hurt H., Farah M.J. (2012). Neighborhood disadvantage and adolescent stress reactivity. Frontiers in Human Neuroscience, 6, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J. (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13(2), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. (2010). Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11(9), 651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Bookheimer S.Y., Mazziotta J.C. (2000). Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport, 11(1), 43–8. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Fera F., Weinberger D.R. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry, 53(6), 494–501. [DOI] [PubMed] [Google Scholar]
- Kim P., Evans G.W., Angstadt M., et al. (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences of the United States of America, 110(46), 18442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A.P., Abelson J.L., Britton J.C., Phan K.L., Taylor S.F., Liberzon I. (2009). Medial prefrontal cortex and right insula activity predict plasma ACTH response to trauma recall. Neuroimage, 47(3), 872–80. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. (1993). The ‘Trier Social Stress Test'–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Klumpp H., Ho S.S., Taylor S.F., Phan K.L., Abelson J.L., Liberzon I. (2011). Trait anxiety modulates anterior cingulate activation to threat interference. Depression and Anxiety, 28(3), 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen E.I., Heckman J.J., Cameron J.L., Shonkoff J.P. (2006). Economic, neurobiological, and behavioral perspectives on building America's future workforce. Proceedings of the National Academy of Sciences of the United States of America, 103(27), 10155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. (2014). Neural network of cognitive emotion regulation - an ALE meta-analysis and MACM analysis. Neuroimage, 87:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky L.D., Park N., Peterson C., Vokonas P., Sparrow D. (2011). Healthy psychological functioning and incident coronary heart disease: the importance of self-regulation. Archives of General psychiatry, 68(4), 400–8. [DOI] [PubMed] [Google Scholar]
- Lazarus R.S., Folkman S. (1984). Stress, Appraisal, and Coping . NY: Springer. [Google Scholar]
- Liberzon I., King A.P., Britton J.C., Phan K.L., Abelson J.L., Taylor S.F. (2007). Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. The American Journal of Psychiatry, 164(8), 1250–8. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Taylor S.F., Fig L.M., Decker L.R., Koeppe R.A., Minoshima S. (2000). Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology, 23(5), 508–16. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Eisenberger N.I., Crockett M.J., Tom S.M., Pfeifer J.H., Way B.M. (2007). Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science, 18(5), 421–8. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Inagaki T.K., Tabibnia G., Crockett M.J. (2011). Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion, 11(3), 468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J., Belden A., Botteron K., et al. (2013). The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. Journal of the American Medical Association Pediatrics, 167(12), 1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J., Duncan G.J., Gennetian L.A., et al. (2012). Neighborhood effects on the long-term well-being of low-income adults. Science, 337(6101), 1505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J., Sanbonmatsu L., Gennetian L., et al. (2011). Neighborhoods, obesity, and diabetes–a randomized social experiment. The New England Journal of Medicine, 365(16), 1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. (2010). Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Gianaros P.J. (2011). Stress- and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett R.E. (2009). Intelligence and How to Get It: Why Schools and Cultures Count. New York: W.W. Norton & Co. [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. (2007). Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science, 10(4), 464–80. [DOI] [PubMed] [Google Scholar]
- Osler M., Petersen L., Prescott E., Teasdale T.W., Sorensen T.I. (2006). Genetic and environmental influences on the relation between parental social class and mortality. International Journal of Epidemiology, 35(5), 1272–7. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Taylor S.F., Welsh R.C., Ho S.H., Britton J.C., Liberzon I. (2004). Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage, 21(2), 768–80. [DOI] [PubMed] [Google Scholar]
- Pilgrim K., Marin M.F., Lupien S.J. (2010). Attentional orienting toward social stress stimuli predicts increased cortisol responsivity to psychosocial stress irrespective of the early socioeconomic status. Psychoneuroendocrinology, 35(4), 588–95. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–91. [DOI] [PubMed] [Google Scholar]
- Raio C.M., Orederu T.A., Palazzolo L., Shurick A.A., Phelps E.A. (2013). Cognitive emotion regulation fails the stress test. Proceedings of the National Academy of Sciences of the United States of America, 110(37), 15139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S.L., Shin L.M., Phelps E.A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biological Psychiatry, 60(4), 376–82. [DOI] [PubMed] [Google Scholar]
- Reiss F. (2013). Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Social Science & Medicine, 90, 24–31. [DOI] [PubMed] [Google Scholar]
- Schwabe L. (2013). Stress and the engagement of multiple memory systems: integration of animal and human studies. Hippocampus, 23(11), 1035–43. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Handwerger K. (2009). Is posttraumatic stress disorder a stress-induced fear circuitry disorder? Journal of Traumatic Stress, 22(5), 409–15. [DOI] [PubMed] [Google Scholar]
- Shonkoff J.P., Garner A.S. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–46. [DOI] [PubMed] [Google Scholar]
- Shrout P.E., Bolger N. (2002). Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods, 7(4), 422–45. [PubMed] [Google Scholar]
- Souza-Talarico J.N., Plusquellec P., Lupien S.J., Fiocco A., Suchecki D. (2014). Cross-country differences in basal and stress-induced cortisol secretion in older adults. PLoS One, 9(8), e105968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., Marx C.E., King A.P., Rajaram N., Garfinkel S.N., Abelson J.L., Liberzon I. (2013a). DHEA Enhances Emotion Regulation Neurocircuits and Modulates Memory for Emotional Stimuli. Neuropsychopharmacology, 38, 1798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., Marx C.E., King A.P., Rampton J.C., Ho S.S., Liberzon I. (2013b). Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biological Psychiatry, 73(11), 1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. (2011). Income, Poverty, and Health Insurance in the United States Current Population Reports, P60-243, Washington DC. [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.