Abstract
Immunotherapy for cancer has been a focus 50 years ago. At the time, this treatment was developed prior to cloning of the cytokines, no knowledge of regulatory T-cells, and very little information that mesenchymal stem cells (MSCs) (originally colony forming unit-fibroblasts [CFU-F]) could be licensed by the inflammatory microenvironment to suppress an immune response. Given the information available at that time, mononuclear cells from the peripheral blood were activated ex vivo and then replaced in the patients with tumor. The intent was to harness these activated immune cells to target the cancer cells. These studies did not lead to long-term responses because the activated cells when reinfused into the patients were an advantage to the resident MSCs, which can home the tumor and then become suppressive in the presence of the immune cells. The immune suppression caused by MSCs would also expand regulatory T-cells, resulting instead in tumor protection. As time progressed, these different fields converged into a new approach to use immunotherapy for cancer. This article discusses these approaches and also reviews chimeric antigen receptor in the context of future treatments for solid tumors, including breast cancer.
Keywords: CAR T-cell, mesenchymal stem cell, T-cells, cancer stem cells
Introduction
Breast cancer continues to be a major hurdle, with one of eight women predicted to be diagnosed with breast cancer and with an estimated 230,000 cases this year.1 Breast cancer is traditionally treated with a combination of chemotherapy and surgery, with or without hormonal therapy depending on the stage and receptor expression. However, the search for other innovative therapies continues. After decades of failed trials and research, it appears that manipulating and harnessing the immune system’s antitumor qualities is beginning to show promise for various tumors, particularly melanoma.2,3 As immunomodulation and immunotherapy is further studied with the information extrapolated to different tumors, the benefit for breast cancer has shown some compelling evidence, most recently presented by Nanda et al, at the 2014 San Antonio Breast Cancer Symposium on program death (PD-1) inhibitor, pembrolizumab (MK-3475), for triple-negative breast cancer. The outcome of this trial indicated that the application of immunotherapy for breast cancer requires more research for comparable outcome as for melanoma. This review discusses the novel approach for different immunotherapies in malignancy, with an emphasis on breast cancer.
Introduction to Immunotherapy
The human immune system has captured the interest, curiosity, and imagination of scientists for many years. The ability of the immune system to recognize all that is foreign for clearance while recognizing all that is self embodies the central dogma of immunotherapy. Mechanisms are in place to hold the immune system in check to avoid autoimmunity. On the other hand, what the immune system recognizes as “foreign” versus “self” colors a spectrum of foreign attack to autoimmunity. Bacteria are recognized as foreign due to vast differences from human being. In contrast, cancer cells that may be the result of a single gene deletion or mutations may not present much differently to the immune system than a normal cell. At the heart of immunomodulation is a balancing act between the immune system’s recognition of a cancer cell and the avoidance of attacking self, which could lead to autoimmunity.
Immunotherapy was first practiced in the 19th century. At that time, the investigators were most likely unaware that a new field has begun. At that time, Coley observed a bacterial infection overlaying a neck mass, which resulted in resolution of the mass.4 It is probably unlikely that Coley had the scientific insight that antigen cross-reactivity between bacteria and tumor maybe the cause that incited an immune response that unlocked the antitumorigenic potential. He nonetheless began to inject the bacteria (eventually called Coley’s toxins) into tumors. The limited results, in combination with the inability to explain this phenomenon, spawned a reluctant attitude from the scientific community to accept the findings. More so, promising results from chemotherapy and radiation came to fruition, and immunotherapy fell into the shadows of its therapeutic counterparts.
Championed by Dr. Farber and Dr. Hoentz, chemotherapy and radiation soon became the forefront of cancer therapy, and eventually the standard of care for many malignancies. Immunotherapy, on the other hand, continued to hold the interest by a group of scientists, thereby maintaining the field. In 1957, Burnet offered the explanation that antigenic differences between normal healthy cells and tumor cells allowed for immune recognition and subsequent eradication of the latter.5 Decades later, further evidence of antitumor effects of the immune system materialized as various researchers demonstrated a positive correlation between higher numbers of tumor-infiltrating lymphocytes (TILs) and positive prognosis.6–9 Subsequently, the correlation of a faulty immune system and its role in malignancy came to light as tumor incidence was found to be more prevalent in those with immunosuppression even if it were to be partitioned into non-AIDS defining malignancies.10–14 Additional evidence of the antitumor effect of TILs and T-cell effect includes the manipulation of IL-2, a T-cell growth factor. In the setting of various malignancies, IL-2 activation of T-cells demonstrated regression of invasive metastatic disease in melanoma and renal cancers.15,16 These findings suggested a positive correlation between an intact and functioning, and perhaps augmented, immune system and tumor cell death. IL-2 therapy supported the idea that augmenting the potential of an immune system, from its baseline level at diagnosis, may tip the scale in favor of antitumorigenic qualities and in fact is still used today at several institutions. Other mechanisms that are intrinsic to the tumor cell itself have become a major focal point. Self-attack, thwarting T-cell checkpoint mechanisms (PD-1 ligand [PD-1L] expression) known to exist on normal cells but recently described on tumor cells became a major area of study for a variety of tumors after extensive and significant results in melanoma. Finally, the tumor microenvironment itself can display characteristics that support the tumor for further growth and proliferation with impeding the immune system’s antitumor effects. Interaction between the tumor and the immune system can then be broken up into mechanisms intrinsic to tumor cells, immune cell attack, and tumor microenvironment. However, each cannot be addressed individually without consideration of the other.
Immune Check Point Inhibitors
There are several ongoing investigations on immunotherapies, but currently, perhaps the most promising results have been linked to PD-1 and Cytotoxic T-lymphcyte-associated protein 4 (CTLA-4) agents most extensively tried in patients with melanoma and renal cell carcinoma.17,18 Briefly, PD-1L and other similar ligands are found in peripheral tissues and tumor, which interact with T-cells to inhibit an immune response. Thus, PD-1 and its ligand has become an attractive avenue for antitumor therapy.19,20 A similar mechanism with therapeutic promise exists with the immune regulatory T-cell molecule, CTLA-4.21 This has spawned the development of immunomodulatory agents for many cancers, including, but not limited to, non-small cell lung carcinoma22 and pancreatic cancer.23 Most relevant to this review, PD-1 has also been studied in breast cancer and the data indicated that its expression is particularly associated with more aggressive phenotypes.24,25 In addition, a recent report indicated that breast cancer correlated with poor prognosis in the setting of PD-1L expression.26 Nanda et al, at the 2014 San Antonio Breast Cancer Symposium, reported on a Phase Ib trial (MK-3475) with pembrolizumab, a humanized IgG4 PD-1 antibody. The results showed an overall response rate of 18% and a six-month progression-free survival of 23.3%. Finally, even though PD-1 and CTLA-4 agents have shown the most promise, efforts are underway to study PD-1 coinhibitory receptors that further suppress other T-cell subsets necessary for intact anti-immune functions of the immune system.27 At this time, it is yet to be determined if these coinhibitors could be used in conjunction with CTLA-4 or PD-1 agents and if the outcome would be synergistic. In this vein, PD-1, CTLA-4, and other immune checkpoint blockade agents may only be a piece of the puzzle in optimizing the antitumor qualities of the immune system in this setting. Furthermore, the cancer cell itself is only one target for an effective and potent immune response and the tumor microenvironment must also be addressed. Regardless, PD-1 checkpoint inhibitors continue to be investigated in a variety of tumors, including, but not limited to, solid and liquid tumors with over 100 clinical trials currently listed on clinicaltrials.gov, with 9 pertaining to breast cancer.
In line with the theme of modern antitumor therapeutics, studies on the microenvironment have also been under intense investigation. The tumor microenvironment has been studied as an avenue of intervention due to its nurturing effects for tumor cell survival, proliferation, and eventual progression. However, the role of tumor stromal cells to protect the cancer cell from immune attack has become an important area of interest. The infiltration of TILs has traditionally been viewed as an antitumor phenomenon and can perhaps be highlighted by recent studies, including a Phase II study demonstrating the robust prognostic factor of TILs in triple-negative breast cancer.28–31 Conversely, the expression of PD-1 by immune cells is associated with low TILs and a poorer prognosis in renal cell carcinoma.32,33
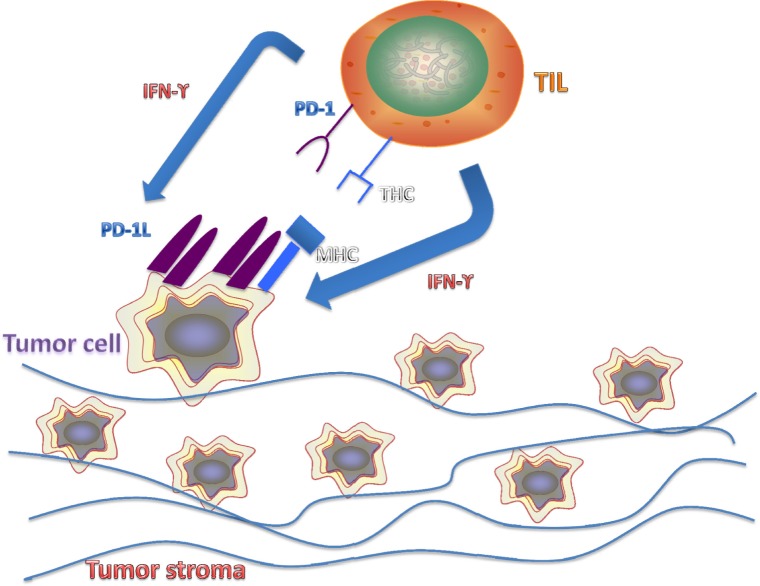
As the experimental evidence increases, the data suggest a more potent antitumor effect with higher levels of TIL and lower levels of PD-1. In some cases, there are contradicting evidences between TIL and PD-1 relationship. For example, T-cell-derived IFN-γ has been shown to upregulate PD-1L expression on tumor cells.34,35 The somewhat contradicting and most likely complicated relationship between TILs and PD-1L expression requires much more research in order to elucidate the possible crosstalk between the two (Fig. 1).
Figure 1.
Paradoxical relationship between antitumor effect of TILs and upregulation of PD-1L in tumor cells. TILs, though shown to have a positive correlation in antitumor qualities of the immune system, have also been paradoxically demonstrated to upregulate PD-1L expression on tumor cell through IFN-γ release, a mechanism for tumor immune evasion.
The issue of tumor heterogeneity as it relates to chemotherapy has been extensively studied. A similar concern has shown little attention when designing immune therapy. While PD-1L agents have shown promise for treating tumors, studies to stratify the affected cells should be a major concern moving forward. Specifically, tumor heterogeneity may reveal particular subsets of cells that may be resistant to the immunotherapy. This might be similar to cancer stem cells that resist chemotherapy. Recent studies demonstrated that, in particular, breast cancer cell lines, such as those with a triple-negative hormone phenotype, show different expressions of PD-1L. This suggested that perhaps a subset of breast cancer cells may show increased invasiveness with high PD-1L to evade immune surveillance.36 The expression of PD-1L, unsurprisingly, has also been identified with the potential as a prognostic marker.3 However, PD-1L as a biomarker may not be efficient because despite undetected in tumors, the patients responded to the inhibitor.37 The heterogeneity of PD-1L expression could perhaps demonstrate that PD-1 agents may only be part of the solution. If so, this would require additional agents to complement tumor cell killing. The combined use of PD-1 and CTLA-4 inhibitors showed promising outcome for melanoma treatment.38 This has recently been confirmed in the Phase III checkmate 067 study.39
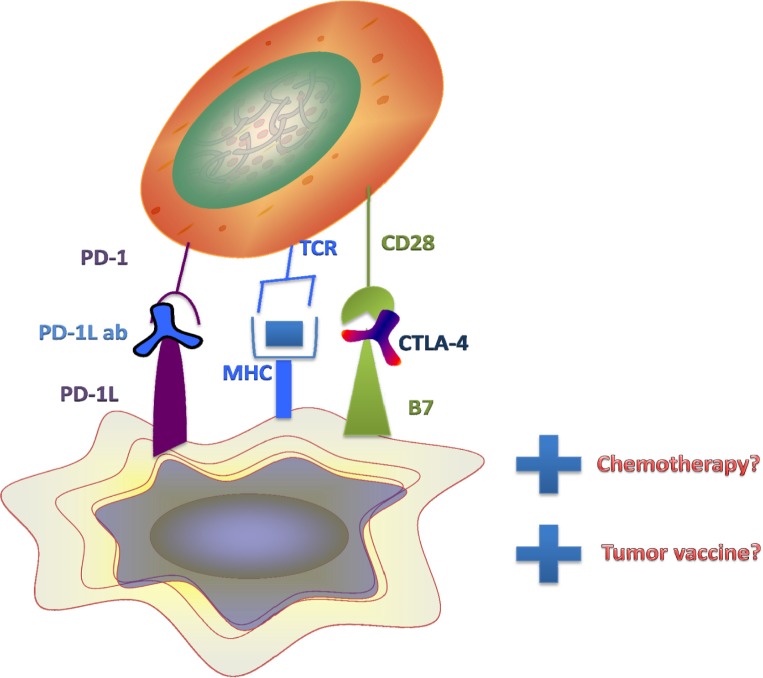
Further investigations are needed to determine the optimal doses for combined use of immunotherapeutic agents. On the other hand, efficient anticancer treatment may require the use of chemotherapy with an immunomodulating agent. It is possible that the research might indicate that multiple agents within both categories should be combined, in addition to tumor vaccine, partly to differentiate tumor cells into cells that will undergo death through senescence (Fig. 2). These decisions are important because combination of immune modulating agents has been linked to serious side effects.40,41
Figure 2.
PD-1 and CD28 on T-cells interact with PD-1L and B7 on tumor cells and suppress T-cell activity. PD-1 and CTLA-4 agents interfere with this interaction. Further combinatorial effects of chemotherapy by targeted mechanisms in the setting of immune cell attack may augment tumor cell killing. Tumor vaccine therapy may be a further way to block intrinsic processing and presentation of immune modulating receptors, including PD-1L and B7, found on the tumor cell surface.
The ability to ascertain the characteristics of “superresponders” and to scrutinize those of nonresponders may hold a key to future combinatorial regimens. The selection criteria to enroll patients for immune checkpoint inhibitors would depend on the tumor as well as the individual immune profile. Regarding tumor heterogeneity, tumor-initiating cells, otherwise known as cancer stem cells, may represent a different population of cells that make up the bulk of the tumor. Cells that are positive for stem cell markers, such as Oct4, Msi1, and nanog, may have a different immune modulatory signature than cells comprising the tumor bulk. Regulatory influences on immune checkpoint inhibitors propose another avenue for therapeutic potential.
Microenvironment
The tumor microenvironment harbors many pro-tumorigenic and immunosuppressive qualities that are consistent with protecting the tumor cells from the immune system, thereby thwarting the antitumor mechanisms to support tumor cell survival, proliferation, and eventual metastasis. The tumor microenvironment itself forms a hostile environment for immune cells at the tumor site, although there are many existing barriers to inhibit successful tumor infiltration. In a normal setting, lymphocytes home toward the site of tumor but require a number of events and conditions for success. Trafficking into the tumor site is one of the first and most important mechanisms to dissect when evaluating the relationship between TILs and the tumor microenvironment. Chemokines for CXCR3/CXCR5, one of the major chemokine receptors for TILs, facilitate tumor infiltration with increased expression.42 However, not all tumors, including breast cancer, express chemokines at a level to facilitate tumor infiltration.43,44 There are other conditions that may also hinder TIL, including decreased expression of attachment factors such as ICAM-1 and -2 and VCAM-1 seen in several types of malignancy.45 Finally, the tumor site itself shows anti-immunogenic properties.
The tumor environment comprises several factors. A detailed discussion of these factors is beyond the scope of this review but is important to understand how tumors interact with the microenvironment. Among cells within the microenvironment are myeloid-derived suppressor cells, regulatory T-cells (Treg), and tumor-associated macrophages (TAMs). These cells have gained much attention and have shown to contribute to an environment that supports tumor growth.46–48 The roles of these different infiltrating cells must be considered when developing antitumor modalities, particularly cell-based therapies that require engineered cells that home to the tumor. In this sense, agents that propagate cell-based immunotherapies must be considered in combination with other agents that may circumvent antitumor mechanisms. In this vein, there are questions if cell-based therapies can be used in conjunction with agents shown to decrease adhesion molecules to ensure TIL infiltration and PD-1L agents to activate cell cytotoxicity. Considering the advent of various avenues of antitumorigenic intervention, research studies could show a convergence of the different cellular mechanisms with antitumor properties.
Adoptive Cell Therapy—Chimeric Antigen Receptor T-cells
Adoptive T-cell transfer, a term first developed in the 1950s,49 laid the groundwork for the potent effects of tumor-infused lymphocytes that would serve as a key precursor to current investigations into CAR T-cells.50,51 CAR T-cells are hybrid receptors that contain both an extracellular antigen recognizing domain and an intracellular domain that activate signaling cascades. While the native T-cell receptor (TCR)-CD3 complex is composed of six different components (α, β, γ, δ, ε, ζ), the ζ chain, in particular, is capable of downstream signaling. Eshhar et al first demonstrated that the single-chain of an Fv antibody molecule was fused to the γ chain of the Fc receptor or to the ζ of the CD3 complex resulting in T-cells that exhibited both antibody type specificity and subsequent IL-2 cytotoxic cell signaling.52 As time has passed, this modality has evolved significantly, notably with regard to the costimulatory domains. T-cell activation by the TCR alone, however, does not illicit antitumor effects in addition to cytokine production, proliferation, and persistence. This quandary had provoked researchers to investigate costimulatory domains.
The costimulatory domains have borne the classifications of CAR T-cells: first-, second-, and third-generation CARs. While there are a number of costimulatory domains, including, but not limited to, Inducible T-cell co-stimulator (ICOS), CD28, 4-1BB, and OX40, CD28 seems to be the most studied and most effective.53–55 This concept, more importantly, has been demonstrated in a clinical trial with CD19-CAR-modified T-cells that were administered with non-Hodgkin’s B-cell lymphomas.56 The original single costimulatory domain in CAR T-cells has been coined as first-generation CARs. Second-generation CARs include those with two costimulatory domains engineered in the intracellular space, and third-generation CARs carry multiple intracellular costimulatory domains (ie, CD28, 4-1BB) but yet show positive or promising results.15,57
There are several characteristics of CAR T-cells that offer a particular advantage when compared with other cell-based immunotherapies. These target-binding sites display an affinity much higher than TCRs, but most important are MHC independent, lending itself away from tumor escape secondary to MHC loss variants. This may prove to be a specific advantage over NK cell therapy, which depends on the recognition of MHC receptors. Another particular advantage of CAR T-cells includes the ability to cross the blood–brain barrier.54 This characteristic may prove to be invaluable in treating malignancies that may involve or have metastasized to the central nervous system. However, side effects involving central nervous system must also be taken into consideration. Uniquely, infusing manipulated T-cells could circumvent the toxic side effects of chemotherapy, and while killing efficiency is not as robust initially in this cell-based immunotherapy, it holds promise for a lasting antitumor response reflected in the development of memory T-cells.57
A particular concern of CAR T-cells is the concept of “on-target, off-tumor toxicity.” This can otherwise be described as the ability of CAR to recognize self-antigens that display similar affinity to target tumor antigens. This is a well-recognized phenomenon and has attributed to a number of different toxicities and side effects. While this is currently a major concern, it remains to be seen whether CAR development will evolve to create specific antibodies that mitigate “on-target, off-tumor toxicity”.
Clinical side effects, however, must also be addressed. Perhaps the most well-documented side effect include cytokine release syndrome, which is driven by a number of cytokines, including IFN-γ, TNF-α, and IL-2,58,59 and, most importantly, IL-6.54 Patients who suffer from cytokine release syndrome display fever as high as 104°F, myalgias, nausea, and anorexia with complications that may include hemodynamic or respiratory instability requiring admissions to the intensive care unit with supportive care and steroid administration.58 On the other hand, significant progress has been made with the anti-IL-6 receptor monoclonal antibody, toclizumab previously studied in glucocorticoid-resistant graft-versus-host disease (GvHD).60 There is a need for further investigation of CAR T-cells; cytokine release syndrome also needs to be studied further and addressed.
As CAR T-cells are engineered further, a particular approach to build CAR T-cells involves the sustained response through replenishment. Specifically, CAR T-cells are traditionally engineered through viral gene transfer to peripheral T-cells.61 On the other hand, CAR-engineered hematopoietic stem cells may provide a continuous source of targeted antitumor effects that would sustain remissions. Further evidence has shown that the transfer to less mature stem cell-like cells offers increased persistence and replenishing capabilities in vivo.
CAR T-cells display some persistent antitumor effects in a way that could prove to be longstanding. At the same time, the introduction of CAR genes through lentivirus (or other retroviruses, etc.) has been achieved in T-cells. An interesting concept that would induce longstanding antitumor qualities would be the introduction of these genes into hematopoietic stem cells. Furthermore, as the most primitive line of blood cells, these effects could transcend multiple blood lines, including, but not limited to, NK cells. One particular limitation of this idea is the tendency of hematopoietic stem cells to differentiate if removed from their bone marrow niche. This obstacle may be circumvented through delivery directly into the bone marrow, as opposed to extracting HSCs and then reinfusing, but identifying a vehicle that can enter the bone marrow and transfer DNA may prove to be a major challenge.
To date, the most significant positive outcome with CAR T-cells have been reported for leukemia. However, a similar positive outcome for solid tumors, such as breast cancer, needs to overcome several barriers. This is particularly a challenge because solid tumors do not have common markers as for the leukemias. A recent study investigated the use of CAR T-cells in the murine model of breast cancer and the results showed modest improvement.62 The study design, however, brings to light a major problem that needs to be addressed before CAR therapy is to be considered a standard of care over chemotherapy. The observation that multiple administrations of CAR T-cells needed to be infused into the mice for a durable response does not contribute to the idea that this does not follow the dynamics of chemotherapy. This brings up an interesting point on questions whether or not the use of naive memory-type CAR T-cells would be more efficient than the effector approach currently being developed. This approach would ensure that the CAR T-cells are not exhausted or undergo activation-induced cell death.63 Currently, scientists at the University of Pennsylvania have been studying the utility of CAR T-cells in breast cancer patients (NCT01837602). CAR T-cells as a therapeutic modality in solid tumors could prove to be a targeted approach with long-lasting effects.
The future of antitumor agents will most likely include cell-based therapies, in addition to chemotherapy and possibly in combination. In addition to the direct cytotoxic activity, killing of T-cells, there is also evidence with regard to the disruptive effect that TILs have on the stromal environment of tumors.64 This approach could be an efficient mechanism of CAR T-cells for solid tumors. Since only a small number of TILs are able to infiltrate the tumor site, CAR T-cells may need to be targeted to these cells.45 Another possible concern with cell-based therapies is immunoediting of tumor antigens. As tumor cells are destroyed by CAR T-cells, which will at some point be formed from memory cells, they are indeed directed against the tumor antigen that was originally engineered to recognize. In this setting, immunomodulation may prove to be a problem as antigenic shift may cause tumor cells to evolve in a way to create new tumor antigens that may not be recognized by the original CAR T-cells. Tumor evasion, previously described as an evasion mechanism in chemotherapy, may become a dilemma in cell-based therapies.
The use of CAR T-cells has shown promise with hematological malignancies. However, it is unlikely that CAR T-cell treatment will replace chemotherapy. Safety studies using Her2 CAR T-cells for solid tumors have shown promise.65,66 Going forward, experimental treatment will be required to determine how the CAR T-cell approach will be combined with chemotherapy for solid tumors, such as breast cancer.
The advent of hierarchy in the differentiation of memory T-cells from memory T-stem cell could lead to a continuous supply of CAR T-cells. Alternatively, CAR T-cells could have also been provided in a sustained manner by engineering hematopoietic stem cells with CAR.67
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) remain an elusive but potentially powerful protumor cells mostly due to their ability to be immune suppressors. MSCs are multipotent progenitor cells originally reported in the bone marrow stroma. Given their role in immunosuppression and inflammation, interest in elucidating what factors dictate its polarity is growing. Understanding the role of immunosuppression of MSCs in the microenvironment, thwarting an antitumor immune attack, in addition to understanding the pro-inflammatory role of MSCs, may unlock a potentially powerful mechanism for antitumor therapeutics. As previously mentioned, the tumor microenvironment has become a major focal point of understanding tumor cell survival, including those cells that act as a defense mechanism against TILs.
MSCs have been demonstrated to home to the tumor microenvironment and subsequently differentiate into cells that support the cancer niche, including, but not limited to, tumor-associated myofibroblasts, cancer-associated fibroblasts.68–72 Under the influence of TNF-α and lymphotoxin-α1β2, MSCs have also been shown to promote microenvironment immunosuppression and tumor stroma genesis as lymphoma cell differentiation into stroma cells.73
Further comprehension of this process may demonstrate avenues of intervention preventing MSC homing and contribution to the microenvironment, yet manipulating this intrinsic homing mechanism may prove to be of significant therapeutic benefit. Due to the homing ability of MSCs to tumors, these stem cells have been engineered as drug delivery system. The cytokine, IFN-β, delivered with MSCs, has been shown to promote a variety of antitumor effects, including differentiation and apoptosis.74,75 The hope of delivery of such cytokines or other small molecule delivery to the tumor site may prove to be a valuable and safe modality of tumor intervention.
Tumor microenvironment immunosuppression has been a major focal point of this report as well as other schools of thought. This property of MSCs can be an advantage in other areas of tumor treatments. Specifically, the immunosuppressive property of MSC can be an invaluable function in circumstances of GvHD in bone marrow transplantation (BMT).
Interestingly, MSC-based therapies have shown promise particularly in the setting of acute GvHD but have been less promising in chronic GvHD.76–79 Perhaps the most delirious pitfall of MSC therapy for GvHD is the potential for relapse and the loss of graft-versus-tumor effect, as seen in a study of leukemia.80 The promising potential of MSC therapy in GvHD, in addition to positive results thus far, warrants further research for MSCs for the potential to be a significant option for patients with GvHD status post-BMT. Currently, there are over 30 trials found on clinicaltrials.gov on the role of MSCs in GvHD in the setting of BMT.
Tumor-associated Macrophages
In the bone marrow, monocytes are derived from myeloid progenitor cells. Monocytes leave the bone marrow and enter peripheral blood, where they circulate throughout systemic circulation to support inflammation as well as ensure cellular homeostasis.81 Monocytes are recruited toward tissues where they mature into macrophages (MΦ).82 Depending on the soluble factors released from the microenvironment, they can differentiate from monocytes into either pro-inflammatory (M1 type MΦ) or anti-inflammatory (M2 type MΦ).83 During tissue inflammation, M1 MΦ are activated and release pro-inflammatory cytokines to combat microbial infections. To subside the inflammatory response, M2 MΦ are activated and release anti-inflammatory cytokines to aid in tissue remodeling.84
During tumor growth and metastasis, TAMs infiltrate the tumor where they use the immunosuppressive properties to protect tumor cells and support tumor remodeling and metastasis.85 Once inside the tumor, TAMs can migrate toward the hypoxic region of the tumor, releasing various cytokines and growth factors forming blood vessels and epithelial cells.86 Like other cells, such as MSCs, TAMs can contribute to the tumor cell microenvironment and further support tumor cell survival.
Immune surveillance is the body’s defense mechanism for protection against malignant tumor formation; however, some cancers can evade the immune system in similar ways as foreign pathogens.87 During numerous cell cycle events within a tumor, mutations can result in tumor evasion. For example, loss of Human leukocyte antigen-1 (HLA 1) molecules has been shown, during multiple cellular divisions in tumors.88 The tumor microenvironment can influence MF polarization by releasing particular cytokines that causes reprograming of the infiltrating MΦ to differentiate into TAMs, resulting in immune tolerance and thereby supporting tumor progression.89,90
Macrophage as Immunotherapeutic Agent
As discussed above for MSCs, the utilization of MΦ as drug delivery agents to the tumor is a new and innovative area of therapeutics. Since MΦs can enter tumors and migrate toward the hypoxic region of the tumor,91 the usage of MΦ, engineered to produce cytokines, antibodies, or other molecules, can be a method to cause tumor regression. Liposomes encapsulated MΦ containing doxorubicin as biocarrier molecules was used to deliver chemotherapeutic agents to A549 non-small cell lung cancer cells.92 Time release of packaged material, specific location of drug toward the tumor, and a decrease of immune tolerance provide advantages to using MΦ as biocarrier for future therapeutic options to treat cancer.
Conclusion
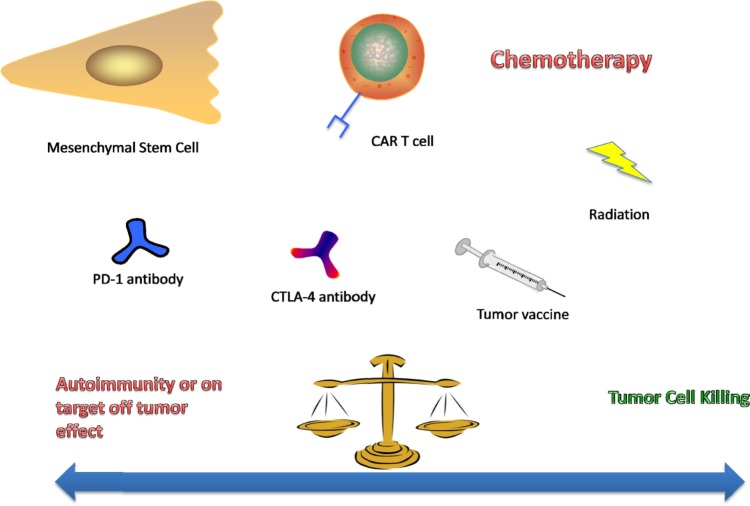
The silver bullet of antitumorigenic cell-based therapies or a single immune modulating agent is a highly sought after, yet perhaps the limitations of a single approach may be circumvented with the introduction of a second or even third intervention. While the results of immunomodulating agents or CAR T-cells have been promising, perhaps the full optimization of this therapy has yet to be harnessed. In light of further understanding of the immunosuppressive characteristics of the tumor microenvironment and its stroma, perhaps agents that target those mechanisms in conjunction with cell-based therapies would demonstrate better results than either agent alone. Agents against multiple targets in a pathway have shown good results in a number of tumors and advocate combinatory use of multiple agents in treating a single patient, yet autoimmunity must remain a major concern when implementing these therapies (Fig. 3). In the era where cell-based therapies are beginning to be considered as a success, this form of treatment could be considered as the standard for antitumorigenic regimens. Perhaps tumor therapeutics is entering a time where cell-based therapies will only be part of a regimen that is needed to optimize the efficacy of nonbiologics. It may be better to fight a war with a choreographed army than a “single bullet.” To reiterate what was discussed above, although we promote combinatorial treatment among cell-based treatment and other drugs, there are side effects to such approaches.
Figure 3.
As the choice of antitumor therapeutics continues to grow, the consideration of combining multiple agents is inevitable. Gaining an appropriate antitumor effect without significant side effects of provoking autoimmunity in the patient will be a major consideration in the clinic and early phases of clinical trials.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 386 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived the concepts: GRN, NDW, MB, PR. Evaluated the literature: GRN, NDW, MB, PR. Wrote the first draft of the manuscript: GRN, NDW, MB, PR. Agree with manuscript results and conclusions GRN, NDW, MB, PR. Jointly developed the structure and arguments for the paper: GRN, NDW, MB, PR. Made critical revisions and approved final version: GRN, NDW, MB, PR. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.American Cancer Society . Cancer Facts and Figures. American Cancer Society; OR Atlanta, Georgia: 2015. 2015. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/ [Google Scholar]
- 2.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases 1893. Clin Orthop Relat Res. 1991;262:3–11. [PubMed] [Google Scholar]
- 5.Burnet M. Cancer; a biological approach. I. The processes of control. Br Med J. 1957;1:779–786. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 7.Kim ST, Jeong H, Woo OH, et al. Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am J Clin Oncol. 2013;36:224–231. doi: 10.1097/COC.0b013e3182467d90. [DOI] [PubMed] [Google Scholar]
- 8.Kmiecik J, Poli A, Brons NH, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol. 2013;264:71–83. doi: 10.1016/j.jneuroim.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Piersma SJ, Jordanova ES, van Poelgeest MI, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 10.Clifford GM, Rickenbach M, Polesel J, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 11.Frisch M, Biggar RJ, Engels EA, Goedert JJ, AIDS-Cancer Match Registry Study Group, editors. Association of cancer with AIDS-related immunosuppression in adults. J Am Med Assoc. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 12.Grulich AE, Li Y, McDonald A, Correll PK, Law MG, Kaldor JM. Rates of non-AIDS-defining cancers in people with HIV infection before and after AIDS diagnosis. AIDS. 2002;16:1155–1161. doi: 10.1097/00002030-200205240-00009. [DOI] [PubMed] [Google Scholar]
- 13.Herida M, Mary-Krause M, Kaphan R, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21:3447–3453. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 14.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 15.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 17.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer—response. Clin Cancer Res. 2013;19:5542. doi: 10.1158/1078-0432.CCR-13-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 20.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 22.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 23.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 24.Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965–2970. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 25.Sun S, Fei X, Mao Y, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother. 2014;63:395–406. doi: 10.1007/s00262-014-1519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flies DB, Han X, Higuchi T, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4(+) T cell-mediated immunity. J Clin Invest. 2014;124:1966–1975. doi: 10.1172/JCI74589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014;148:467–476. doi: 10.1007/s10549-014-3185-2. [DOI] [PubMed] [Google Scholar]
- 30.Issa-Nummer Y, Loibl S, von Minckwitz G, Denkert C. Tumor-infiltrating lymphocytes in breast cancer: a new predictor for responses to therapy. Oncoimmunology. 2014;3:e27926. doi: 10.4161/onci.27926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 32.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 34.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 35.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 36.Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014;9:e88557. doi: 10.1371/journal.pone.0088557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Errico A. Melanoma: CheckMate 067—frontline nivolumab improves PFS alone or in combination with ipilimumab. Nat Rev Clin Oncol. 2015;12:435. doi: 10.1038/nrclinonc.2015.112. [DOI] [PubMed] [Google Scholar]
- 40.Kroemer G, Galluzzi L. Combinatorial immunotherapy with checkpoint blockers solves the problem of metastatic melanoma-An exclamation sign with a question mark. Oncoimmunology. 2015;4:e1058037. doi: 10.1080/2162402X.2015.1058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahzari M, Liu D, Arnaout A, Lochnan H. Immune checkpoint inhibitor therapy associated hypophysitis. Clin Med Insights Endocrinol Diabetes. 2015;8:21–28. doi: 10.4137/CMED.S22469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bedognetti D, Spivey TL, Zhao Y, et al. CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive therapy and interleukin-2. Br J Cancer. 2013;109:2412–2423. doi: 10.1038/bjc.2013.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulligan AM, Raitman I, Feeley L, et al. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin Cancer Res. 2013;19:335–346. doi: 10.1158/1078-0432.CCR-11-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. 2013;3:231. doi: 10.3389/fonc.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bianchi G, Borgonovo G, Pistoia V, Raffaghello L. Immunosuppressive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cells. Histol Histopathol. 2011;26:693–702. doi: 10.14670/HH-26.941. [DOI] [PubMed] [Google Scholar]
- 47.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schouppe E, De Baetselier P, Van Ginderachter JA, Sarukhan A. Instruction of myeloid cells by the tumor microenvironment: open questions on the dynamics and plasticity of different tumor-associated myeloid cell populations. Oncoimmunology. 2012;1:1135–1145. doi: 10.4161/onci.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billingham RE, Brent L, Medawar PB. Quantitative studies on tissue transplantation immunity. II. The origin, strength and duration of actively and adoptively acquired immunity. Proc R Soc Lond B Biol Sci. 1954;143:58–80. doi: 10.1098/rspb.1954.0054. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg SA. Adoptive immunotherapy of cancer using lymphokine activated killer cells and recombinant interleukin-2. Important Adv Oncol. 1986:55–96. [PubMed] [Google Scholar]
- 51.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 52.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Jensen M, Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 56.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–668. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Huu D, Matsushita T, Jin G, et al. IL-6 blockade attenuates the development of murine sclerodermatous chronic graft-versus-host disease. J Invest Dermatol. 2012;132:2752–2761. doi: 10.1038/jid.2012.226. [DOI] [PubMed] [Google Scholar]
- 61.Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans—immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323:570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 62.Globerson-Levin A, Waks T, Eshhar Z. Elimination of progressive mammary cancer by repeated administrations of chimeric antigen receptor-modified T cells. Mol Ther. 2014;22:1029–1038. doi: 10.1038/mt.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klebanoff CA, Yu Z, Hwang LN, Palmer DC, Gattinoni L, Restifo NP. Programming tumor-reactive effector memory CD8+ T cells in vitro obviates the requirement for in vivo vaccination. Blood. 2009;114:1776–1783. doi: 10.1182/blood-2008-12-192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schietinger A, Arina A, Liu RB, et al. Longitudinal confocal microscopy imaging of solid tumor destruction following adoptive T cell transfer. Oncoimmunology. 2013(2):226677. doi: 10.4161/onci.26677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whilding LM, Maher J. ErbB-targeted CAR T-cell immunotherapy of cancer. Immunotherapy. 2015;7:229–241. doi: 10.2217/imt.14.120. [DOI] [PubMed] [Google Scholar]
- 67.Gschweng E, De OS, Kohn DB. Hematopoietic stem cells for cancer immunotherapy. Immunol Rev. 2014;257:237–249. doi: 10.1111/imr.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Direkze NC, Hodivala-Dilke K, Jeffery R, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 69.Ishii G, Sangai T, Oda T, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232–240. doi: 10.1016/s0006-291x(03)01544-4. [DOI] [PubMed] [Google Scholar]
- 70.Kidd S, Spaeth E, Watson K, et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7:e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra PJ, Mishra PJ, Humeniuk R, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ame-Thomas P, Maby-El Hajjami H, Monvoisin C, et al. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109:693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- 74.Dong Z, Greene G, Pettaway C, et al. Suppression of angiogenesis, tumorigenicity, and metastasis by human prostate cancer cells engineered to produce interferon-beta. Cancer Res. 1999;59:872–879. [PubMed] [Google Scholar]
- 75.Qin XQ, Runkel L, Deck C, DeDios C, Barsoum J. Interferon-beta induces S phase accumulation selectively in human transformed cells. J Interferon Cytokine Res. 1997;17:355–367. doi: 10.1089/jir.1997.17.355. [DOI] [PubMed] [Google Scholar]
- 76.Fang B, Song YP, Liao LM, Han Q, Zhao RC. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;38:389–390. doi: 10.1038/sj.bmt.1705457. [DOI] [PubMed] [Google Scholar]
- 77.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 78.Lucchini G, Introna M, Dander E, et al. Platelet-lysate-expanded mesenchymal stromal cells as a salvage therapy for severe resistant graft-versus-host disease in a pediatric population. Biol Blood Marrow Transplant. 2010;16:1293–1301. doi: 10.1016/j.bbmt.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Muller I, Kordowich S, Holzwarth C, et al. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 80.Ning H, Yang F, Jiang M, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia. 2008;22:593–599. doi: 10.1038/sj.leu.2405090. [DOI] [PubMed] [Google Scholar]
- 81.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang J, Zhang L, Yu C, Yang X-F, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takashiba S, Van Dyke TE, Amar S, Murayama Y, Soskolne AW, Shapira L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kB. Infect Immun. 1999;67:5573–5578. doi: 10.1128/iai.67.11.5573-5578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 86.Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796:11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Perales M-A, Blachere NE, Engelhorn ME, et al. Strategies to overcome immune ignorance and tolerance. Semin Cancer Biol. 2002;12:63–71. doi: 10.1006/scbi.2001.0397. [DOI] [PubMed] [Google Scholar]
- 88.Khanna R. Tumour surveillance: missing peptides and MHC molecules. Immunol Cell Biol. 1998;76:20–26. doi: 10.1046/j.1440-1711.1998.00717.x. [DOI] [PubMed] [Google Scholar]
- 89.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216–223. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Choi J, Kim H-Y, Ju EJ, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33:4195–4203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]