Abstract
OBJECTIVE
This study measured serum and follicular fluid (FF) levels of biopterin, neopterin, vascular endothelial growth factor (VEGF), and macrophage colony-stimulating factor (M-CSF) in patients receiving mild ovarian stimulation for oocyte retrieval.
PATIENTS AND METHODS
Infertile patients who underwent ovarian stimulation were divided into the following: Group 1, no oocyte retrieval (n = 12), and Group 2, retrieval of more than four oocytes (n = 13). Median total gonadotropin dose in both groups was 150 IU. Biopterin and neopterin levels were measured using high-performance liquid chromatography. VEGF and M-CSF levels were measured by enzyme-linked immunosorbent assay.
RESULTS
Compared to Group 2, serum and FF levels of neopterin and VEGF and serum levels of M-CSF were significantly increased, and serum and FF levels of biopterin were significantly decreased in Group 1 (P < 0.05 each).
CONCLUSION
Biopterin and neopterin levels showed similar differences in FF and serum of patients with empty follicles. Decreased biopterin and increased neopterin in serum could predict poor oocyte retrieval.
Keywords: biopterin, neopterin, VEGF, M-CSF, ovarian folliculogenesis
Introduction
Ovarian folliculogenesis has been shown to be supported by nitric oxide (NO) production and vascular endothelial growth factor (VEGF).1 NO is synthesized from l-arginine by NO synthase (NOS) and plays a pivotal role in follicular angiogenesis.2 This process is thought to be initiated by VEGF signaling.3 Elevated VEGF levels in follicular fluid (FF) at the time of oocyte retrieval are reportedly associated with poor conception rates after in vitro fertilization (IVF) and gamete intrafallopian transfer.4 Similarly, levels of VEGF and NO in FF have been shown to correlate negatively with embryo quality as judged by morphology scoring from in vitro culture.5
NOS activity depends on the availability of cofactors such as tetrahydrobiopterin (BH4).6 Biosynthesis of BH4 starts from guanosine triphosphate (GTP), which is converted to 7,8-dihydroneopterin and then further converted to BH4 in the presence of 6-pyruvoyl tetrahydropterin synthase (PTPS). BH4 also serves as an essential cofactor for the hydroxylation of aromatic amino acids. In this process, BH4 is converted to dihydrobiopterin (BH2). Furthermore, BH2 is reduced to BH4 by dihydropteridine reductase. BH4 and BH2 are oxidized to biopterin and 7,8-dihydroneopterin is oxidized to neopterin, and these forms are detectable due to their fluorescence,7,8 although their roles in FF have not yet been investigated.
The aim of this study was to assess the concentrations of biopterin, neopterin, VEGF, and macrophage colony-stimulating factor (M-CSF) in the serum and FF of women attempting to become pregnant with IVF using the low-gonadotropin (mild stimulation) method.
Material and Methods
Between May and September 2013, a total of 147 infertile patients were treated with mild ovarian stimulation at Fujino Ladies Clinic, which has since changed its name to Nakamura Ladies Clinic.9,10 Indications for IVF included tubal occlusion (n = 3), endometriosis (n = 2), male factor (n = 2), and unexplained infertility (n = 18). Of these patients, those with an empty follicle were allocated to Group 1 (n = 13), while those from whom more than four oocytes (4–12 oocytes) were retrieved were allocated to Group 2 (n = 12).
For each individual from whom FF samples were available, only FF from a single cycle was analyzed. Records of all IVF procedures were reviewed to collect the following data: age, body mass index, duration of infertility, serum estradiol (E2) and luteinizing hormone (LH) (48 hours before oocyte retrieval), and total dose of gonadotropin. We punctured the follicle using a single-puncture method. Induction of ovulation was monitored by transvaginal sonography with a 5-MHz transvaginal probe. The dose of gonadotropin for stimulation was then adjusted according to serum E2 concentrations and the size of ovarian follicles as evaluated by ultrasonography. Clomifene citrate was administered on day 5 after the start of menstruation, and recombinant human menopausal gonadotropins (150–300 IU/day, Gonapure®; ASKA Pharmaceutical Co., Ltd.) or human menopausal gonadotropins (150–300 IU/day, HMG Ferring®; Ferring Pharmaceuticals or HMG Teizo®; ASKA Pharmaceutical Co., Ltd.) were also administered while monitoring ovarian follicles. FF of mature follicles (diameter >18 mm) was aspirated by the administration of gonadotropin-releasing hormone (GnRH)-agonist (Suprecur®; Sanofi) at 36 hours before oocyte retrieval. GnRH-agonist (Suprecur®) was administered as nasal drops (one drop per nostril). Follicle aspirates that were not clear or were contaminated with blood were excluded. All samples were immediately centrifuged at 400 × g for 10 minutes, and the supernatant was stored frozen at −70°C until assay. Collection of blood samples was performed by 48 hours before oocyte retrieval. Oocytes were cultured in Petri dishes in IVF20® (Vitrolife) at 37°C in a humidified 5% CO2/95% air environment. All patients provided written informed consent to participate in this study prior to enrollment. The research was approved by the ethics committee of Fujino Ladies Clinic, and conducted in accordance with the principles of the Declaration of Helsinki.
Determination of biopterin and neopterin concentrations was performed using high-performance liquid chromatography with fluorimetric detection.11,12 In brief, 10 μL of 30% w/v trichloroacetic acid solution was added to 100 μL of serum and FF, and this mixture was centrifuged. A 10-μL volume of the mixture was kept at room temperature for 60 minutes. Excess iodine in the solution was removed by adding 15 μL of 1% aqueous ascorbic acid. Biopterin and neopterin concentrations in the supernatant were then measured.
Serum and FF concentrations of VEGF were measured using enzyme-linked immunosorbent assay (ELISA; Quantikine Human VEGF Immunoassay; R&D Systems). This VEGF immunoassay is a solid-phase ELISA designed to measure the levels of VEGF-165 and contains Sf21-expressed, recombinant human VEGF-165 and antibodies raised against the recombinant protein. VEGF-121 and VEGF-165 are diffusible proteins secreted into the medium.13
M-CSF levels in serum and FF were measured using solid-phase ELISA (Quantikine M-CSF Immunoassay; R&D Systems). This assay uses the quantitative sandwich enzyme immunoassay technique.
E2 and LH concentrations in serum were measured by time-resolved fluoroimmunoassay (DELFIA hormone assay; PerkinElmer) and radioimmunoassay (DPC Immulite; Siemens Japan), respectively.
Statistical analysis
Statistical analysis was performed using SPSS version 20 software (IBM). Paired comparisons were analyzed using the unpaired Mann–Whitney U-test. Differences were analyzed according to the c2 test. Values of P < 0.05 were considered statistically significant, and all data were expressed as median and range.
Results
Patient characteristics are shown in Table 1. Regarding the reasons for infertility, no difference was seen between the groups (Table 2). Serum levels of E2 were significantly higher in Group 1 than in Group 2. The median number of oocytes retrieved was 5 (range, 4–12) in Group 2. Our success rate for egg retrieval (more than one egg) was 91.8% (135/147 cases).
Table 1.
Patient characteristics in this study (median, range).
| GROUP 1 (n = 12) | GROUP 2 (n = 13) | P | |
|---|---|---|---|
| Age (years) | 40 (30–44) | 39 (33–43) | NS |
| Body mass index (kg/m2) | 18.7 (17.7–23.3) | 18.6 (17.7–25.6) | NS |
| Duration of infertility (months) | 48 (36–204) | 72 (30–132) | NS |
| Estradiol (pg/ml) | 450 (193–660) | 915 (463–3000) | <0.001 |
| LH (pg/ml) | 7.9 (3.1–19.9) | 6.6 (4.4–13.2) | NS |
| Total gonadotropin dose (IU) | 150 (50–450) | 150 (0–450) | NS |
Notes: Serum estradiol and LH were measured 48 hours before oocyte retrieval. Group 1: unsuccessful oocyte retrieval. Group 2: retrieval of more than four oocytes.
Abbreviation: NS, not significant.
Table 2.
Reasons for infertility in both groups.
| GROUP 1 (n = 12) | GROUP 2 (n = 13) | TOTAL | |
|---|---|---|---|
| Tubal occlusion | 2 | 1 | 3 |
| Endometriosis | 1 | 1 | 2 |
| Male factor | 1 | 1 | 2 |
| Unexplained infertility | 8 | 10 | 18 |
Notes: Group 1: unsuccessful oocyte retrieval. Group 2: retrieval of more than four oocytes.
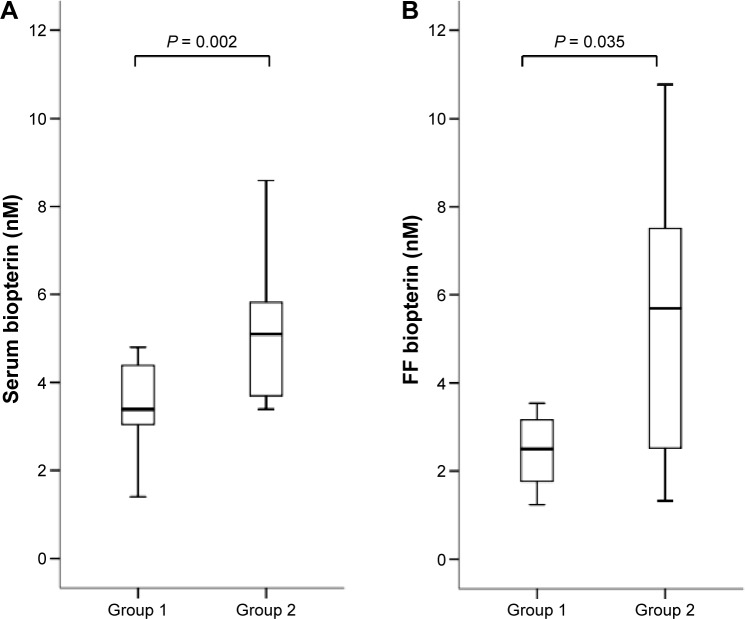
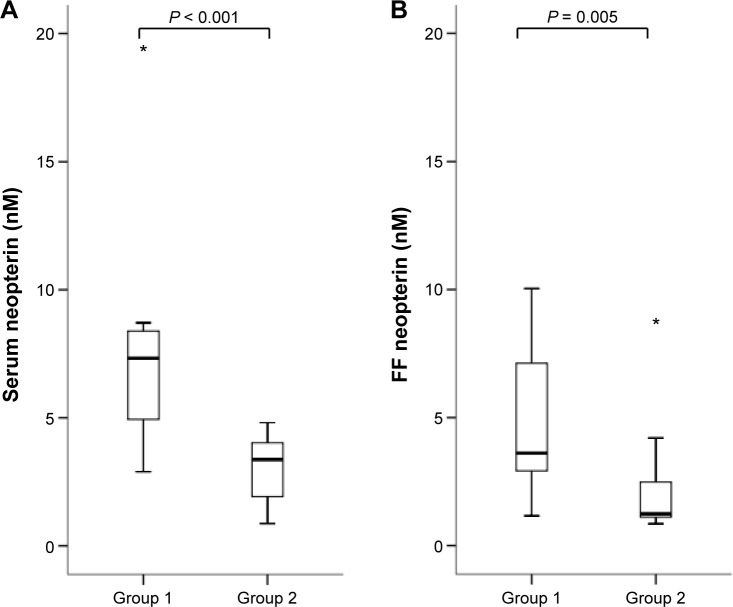
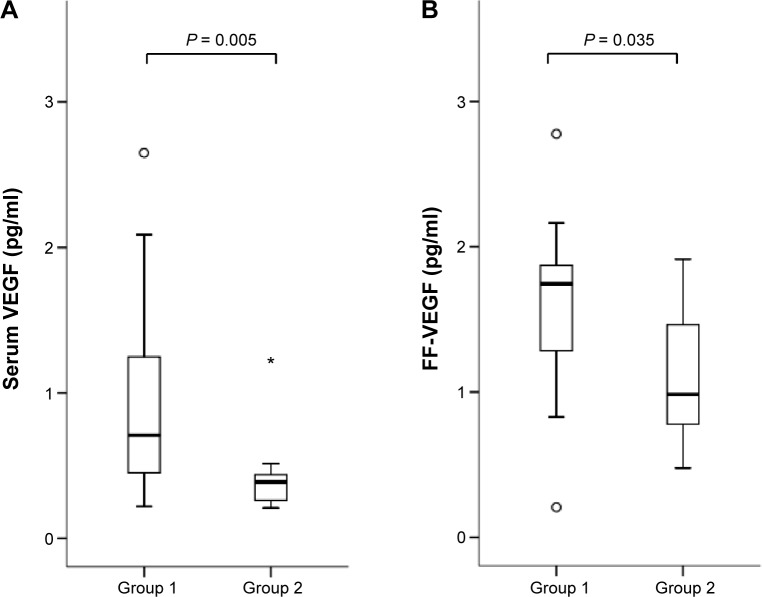
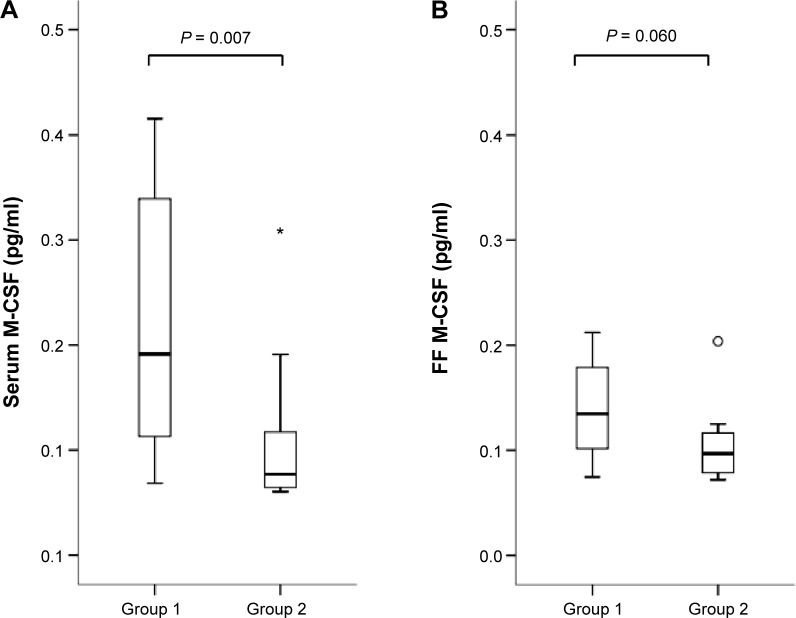
Serum and follicular concentrations in each group are shown in Figures 1–5. Biopterin levels from both serum and FF were significantly higher in Group 2 than in Group 1 (P = 0.002 in serum; P = 0.035 in FF). Conversely, neopterin and VEGF levels from both serum and FF and M-CSF levels from serum were significantly higher in Group 1 than in Group 2 (neopterin: P < 0.001 in serum and P = 0.005 in follicle; VEGF: P = 0.005 in serum and P = 0.035 in follicle; M-CSF: P = 0.007).
Figure 1.
Serum (A) and FF (B) biopterin levels in each group. Median (black bars), interquartile range (IQR; boxes), and values within 1.5 IQR (whiskers) are shown.
Figure 2.
Serum (A) and FF (B) neopterin levels in each group. Median (black bars), IQR (boxes), values within 1.5 IQR (whiskers), and values exceeding 3.0 IQR (asterisks) are shown.
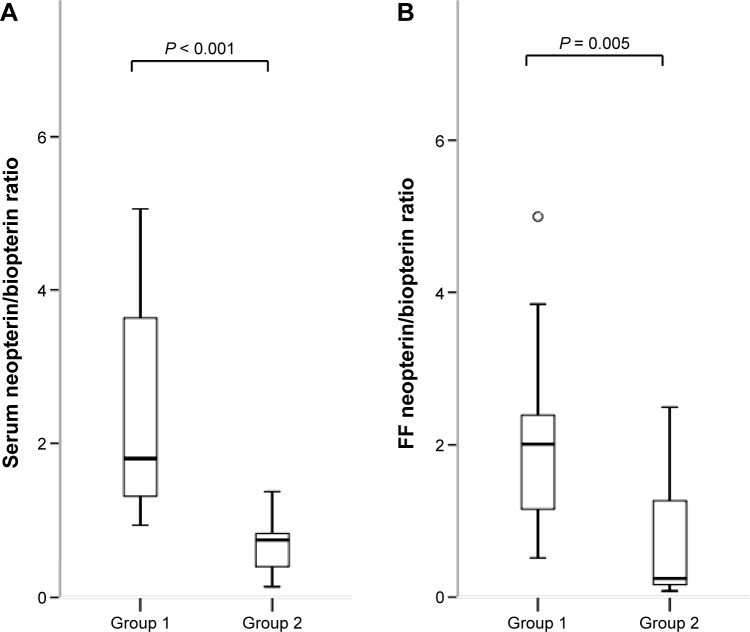
Figure 3.
Serum (A) and FF (B) neopterin/biopterin ratio in each group. Median (black bars), IQR (boxes), values within 1.5 IQR (whiskers), and values exceeding 1.5 IQR (circles) and 3.0 IQR (asterisks) are shown.
Figure 4.
Serum (A) and FF (B) VEGF levels in each group. Median (black bars), IQR (boxes), values within 1.5 IQR (whiskers), and values exceeding 1.5 IQR (circles) and 3.0 IQR (asterisks) are shown.
Figure 5.
Serum (A) and FF (B) M-CSF levels in each group. Median (black bars), IQR (boxes), values within 1.5 IQR (whiskers), and values exceeding 1.5 IQR (circles) and 3.0 IQR (asterisks) are shown.
Neopterin/biopterin ratio in serum and FF was significantly higher in Group 1 than in Group 2 (P < 0.001 in serum; P = 0.005 in FF).
Discussion
This study measured concentrations of neopterin, biopterin, VEGF, and M-CSF in the serum and FF of infertile patients who underwent ovarian stimulation and oocyte retrieval. Our results first confirmed the existence of biopterin and neopterin in FF and revealed significant differences in concentrations between poor and good responders.
Biopterin, as a product of BH4 metabolism, was significantly increased in both serum and FF of Group 2, and FF biopterin concentrations were similar to serum levels. BH4 is a key cofactor in NOS, and NO has been shown to play crucial roles in follicular growth and angiogenesis, in addition to oocyte competence.14 Moreover, Bevers et al reported that BH4 plays a determining role in the redox regulation of NOS-modulated endothelial responses.15 BH4 has also been shown to decrease oxidant stress and increase levels of NO, which acts to restore blood flow after ischemia in rats.16 In this sense, our finding of a significant increase in BH4 concentrations among good responders might be one explanation for follicular maturation.
Significantly increased neopterin levels were observed in both the serum and the FF of poor responders. In the synthetic pathway of BH4, the mechanism underlying increased neopterin levels together with decreased biopterin is thought to involve decreased activity of cofactors such as PTPS. However, PTPS activity was not investigated in the present study, and FF could potentially lack PTPS activity because of the lack of red blood cells.17 From another perspective, activated macrophages produce neopterin as a by-product of the guanosine triphosphate pathway, and elevated serum neopterin levels have been reported as an important biomarker of coronary and carotid atherosclerosis. Taken together with increased M-CSF levels in both serum and FF, abnormal cytokine responses in the serum of poor responders might influence neopterin levels in poor responders.18
Regarding ovarian angiogenesis, VEGF has been intensively investigated and a critical role has been identified in the regulation of follicular angiogenesis,1,19 follicle–luteal transition,20 control of luteal vascularization,21 blood flow, and luteal function.22 In clinical practice, poor responders to ovarian stimulation have been observed to show increased serum levels of VEGF,4 as indicated by our results. Grazul-Bilska et al23 reported that VEGF upregulates endothelial NOS expression in the ovine ovary, which would presumably mean that a higher demand for BH4 would be present in the process of oocyte retrieval.
Limitations to the present study included the relatively small number of patients and the fact that groups were defined by the number of oocytes retrieved. Although we identified clear differences between the groups, production of BH4 and neopterin within the follicle remains to be investigated, along with the synthetic pathways involved.
In conclusion, we have provided evidence of differences in serum and FF levels of biopterin and neopterin between poor responders and good responders at the time of oocyte retrieval. Decreased biopterin levels and increased neopterin levels in the serum of patients during ovarian stimulation appear to predict poor oocyte retrieval.
Acknowledgments
The authors thank all the patients and investigators involved in this study. This manuscript was kindly checked by a native English speaker, Matthew Morgan (FORTE, Tokyo, Japan).
Footnotes
ACADEMIC EDITOR: Yasuo Ito, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 480 words, excluding any confidential comments to the academic editor.
FUNDING: None of the authors received funding for this study.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: AH and DT. Analyzed the data: AH, DT, and HS. Wrote the first draft of the manuscript: AH, DT, TM, KO, YF, and NY. Contributed to the writing of the manuscript: AH, DT, and HS. Agree with manuscript results and conclusions: HS and MK. Jointly developed the structure and arguments for the paper: DT, HS, and MK. Made critical revisions and approved the final version: AH, DT, TM, HK, KO, YF, NY, HS, and MK. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Fraser HM, Duncan WC. Regulation and manipulation of angiogenesis in the ovary and endometrium. Reprod Fertil Dev. 2009;21(3):377–392. doi: 10.1071/rd08272. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 3.Jiang JY, Macchiarelli G, Tsang BK, Sato E. Capillary angiogenesis and degeneration in bovine ovarian antral follicles. Reproduction. 2003;125(2):211–223. [PubMed] [Google Scholar]
- 4.Friedman CI, Seifer DB, Kennard EA, Arbogast L, Alak B, Danforth DR. Elevated level of follicular fluid vascular endothelial growth factor is a marker of diminished pregnancy potential. Fertil Steril. 1998;70(5):836–839. doi: 10.1016/s0015-0282(98)00301-x. [DOI] [PubMed] [Google Scholar]
- 5.Barroso G, Barrionuevo M, Rao P, et al. Vascular endothelial growth factor, nitric oxide, and leptin follicular fluid levels correlate negatively with embryo quality in IVF patients. Fertil Steril. 1999;72(6):1024–1026. doi: 10.1016/s0015-0282(99)00442-2. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 7.Coppen A, Swade C, Jones SA, Armstrong RA, Blair JA, Leeming RJ. Depression and tetrahydrobiopterin: the folate connection. J Affect Disord. 1989;16(2–3):103–107. doi: 10.1016/0165-0327(89)90062-1. [DOI] [PubMed] [Google Scholar]
- 8.Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:1–16. [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraretti AP, Gianaroli L, Magli MC, Devroey P. Mild ovarian stimulation with clomiphene citrate launch is a realistic option for in vitro fertilization. Fertil Steril. 2015;104(2):333–338. doi: 10.1016/j.fertnstert.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Fauser BC, Devroey P, Yen SS, et al. Minimal ovarian stimulation for IVF: appraisal of potential benefits and drawbacks. Hum Reprod. 1999;14(11):2681–2686. doi: 10.1093/humrep/14.11.2681. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980;102(1):176–188. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- 12.Niederwieser A, Staudenmann W, Wetzel E. High-performance liquid chromatography with column switching for the analysis of biogenic amine metabolites and pterins. J Chromatogr. 1984;290:237–246. doi: 10.1016/s0021-9673(01)93579-4. [DOI] [PubMed] [Google Scholar]
- 13.Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266(18):11947–11954. [PubMed] [Google Scholar]
- 14.Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol. 2008;49(4–6):134–140. doi: 10.1016/j.vph.200806.008. Epub 2008 Jul 20. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erratum. Vascul Pharmacol. 2009;50(5–6):208. [Google Scholar]
- 15.Bevers LM, Braam B, Post JA, et al. Tetrahydrobiopterin, but not L-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension. 2006;47(1):87–94. doi: 10.1161/01.HYP.0000196735.85398.0e. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Tie G, Messina LM. Tetrahydrobiopterin, L-arginine and vitamin C act synergistically to decrease oxidant stress and increase nitric oxide that increases blood flow recovery after hindlimb ischemia in the rat. Mol Med. 2012;18:1221–1230. doi: 10.2119/molmed.2011.00103.revised. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shintaku H, Niederwieser A, Leimbacher W, Curtius HC. Tetrahydrobiopterin deficiency: assay for 6-pyruvoyl-tetrahydropterin synthase activity in erythrocytes, and detection of patients and heterozygous carriers. Eur J Pediatr. 1988;147(1):15–19. doi: 10.1007/BF00442604. [DOI] [PubMed] [Google Scholar]
- 18.Gieseg SP, Crone EM, Flavall EA, Amit Z. Potential to inhibit growth of atherosclerotic plaque development through modulation of macrophage neopterin/7,8-dihydroneopterin synthesis. Br J Pharmacol. 2008;153(4):627–635. doi: 10.1038/sj.bjp.0707408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138(6):869–881. doi: 10.1530/REP-09-0283. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds LP, Redmer DA. Growth and development of the corpus luteum. J Reprod Fertil Suppl. 1999;54:181–191. [PubMed] [Google Scholar]
- 21.Berisha B, Steffl M, Amselgruber W, Schams D. Changes in fibroblast growth factor 2 and its receptors in bovine follicles before and after GnRH application and after ovulation. Reproduction. 2006;131(2):319–329. doi: 10.1530/rep.1.00798. [DOI] [PubMed] [Google Scholar]
- 22.Robinson RS, Nicklin LT, Hammond AJ, Schams D, Hunter MG, Mann GE. Fibroblast growth factor 2 is more dynamic than vascular endothelial growth factor A during the follicle-luteal transition in the cow. Biol Reprod. 2007;77(1):28–36. doi: 10.1095/biolreprod.106.055434. [DOI] [PubMed] [Google Scholar]
- 23.Grazul-Bilska AT, Navanukraw C, Johnson ML, Arnold DA, Reynolds LP, Redmer DA. Expression of endothelial nitric oxide synthase in the ovine ovary throughout the estrous cycle. Reproduction. 2006;132(4):579–587. doi: 10.1530/REP-06-0009. [DOI] [PubMed] [Google Scholar]