Abstract
Aim
We set out to determine the potential contribution of community pharmacists to improve the transfer of care of patients from secondary to primary care settings.
Method
We systematically reviewed the literature on interventions that involved community pharmacy post-discharge. We considered all relevant studies, including both randomized and non-randomized controlled trials, irrespective of patient population. Our primary outcome was any impact on patient and medication outcomes, while the secondary outcome was to identify intervention characteristics that influenced all reported outcomes.
Results
We retrieved 14 studies that met our inclusion criteria. There were four studies reporting outcomes relating to the identification and rectification of medication errors that were significantly improved with community pharmacy involvement. Other patient outcomes such as medication adherence and clinical control were not unanimously positively or negatively influenced via the inclusion of community pharmacy in a transfer of care post-discharge intervention. Some inconsistencies in implementation and process evaluation of interventions were found across the reviewed studies. This limited the accuracy with which true impact could be considered.
Conclusions
There is evidence that interventions including a community pharmacist can improve drug related problems after discharge. However, impact on other outcomes is not consistent. Further studies are required which include process evaluations to describe fully the context of the intervention so as to determine better any influencing factors. Also applying more stringent controls and closer adherence to protocols in both intervention and control groups would allow clearer correlations to be made between the intervention and the outcomes.
Keywords: community pharmacist, community pharmacy, community pharmacy intervention, continuity of care, hospital care, transfer of care
Introduction
The transition of patients from primary to secondary care settings (and vice versa) is historically acknowledged as risky. Twenty percent of patients have been reported to experience adverse events within 3 weeks of discharge, 60% of which could have been ameliorated or avoided 1. Patients are exposed to errors, which can have a detrimental effect on their health, recovery and overall satisfaction with the healthcare system 2,3.
Patients are often departing from a confusing and hectic discharge environment, supplied with messages about medicines management, follow-up appointments and other post-discharge information. The process is vulnerable to misunderstanding and miscommunication, often leaving the patient, carers and families ill-prepared to manage their care appropriately during the transition home 3,4. Only 10% of elderly patients will be discharged on the same medication that they were admitted to hospital on 5. Sixty percent of patients will have three or more medicines changed during their hospital stay 6, 28–40% of medications are stopped within hospital and 45% of medicines prescribed at discharge are new 7.
Pharmacists can potentially play a key role in patient care, especially at these transitions 8. Indeed, the Royal Pharmaceutical Society of Great Britain (RPSGB) in 1992 advocated that hospital pharmacists should produce documentation for patients on discharge so as to assist with communication when they leave one healthcare setting and enter another 9. The restructuring of the NHS in England, with the introduction of Clinical Commissioning Groups (CCGs) and their support and encouragement for new health care providers, has re-emphasized the important role the pharmacist can play in these transitions 10. The working party responsible for the RPSGB report recommends fostering links within the community between pharmacists, clinicians, nurses, etc., so as to ensure patient needs are met when they move between healthcare settings 11. Community pharmacists can offer accessibility, expertise in therapeutics, face-to-face contact and skills in drug problems and adherence 10,12. A recent report from the Royal Pharmaceutical Society (RPS) (previously RPSGB), ‘Keeping patients safe when they transfer between care providers - getting the medicines right’ (June 2012), provides guidance on the medicine information that should accompany a patient from one care setting to another 13. Early adopter sites of this guidance piloted and trialled various interventions and services, many of which involved a role for community pharmacy. The adopting hospitals recognized the contribution of community pharmacies and begun referring patients for a medicines use review (MUR) or new medicines service (NMS) consultation post-discharge 14. Urban and colleagues 15 summarize that poor communication to community pharmacists at discharge can cause unintended medication discrepancies and hinder continuity. They further promote the provision of consistent and timely communication to community pharmacy post-discharge to ensure seamless transition and reduction in adverse issues.
Although much literature has been published on the positive input of hospital pharmacists on admission and during discharge 4,16,17, less is known about their community counterparts and the effects of their interventions on patient outcomes. The RPS report 13 which recommended the improvement of communication during patient transfer and the increasing recognition and evidence of the clinical skills of community pharmacists, should lead to an increase in more clinical services being provided and commissioned within the community.
Some studies have restricted the interventions of interest to medicines reconciliation or medication review, and have limited the population to, for example, those suffering from heart failure 18. Others have investigated the potential of post-discharge community pharmacy interventions to improve continuity of care 2,8,19–22.
We therefore aimed to evaluate and quantify systematically the effects of community pharmacy interventions on all potential outcomes of patients of all demographics and conditions discharged from hospital and considered to be at a point of transition.
Methods
Searching
The Cochrane Collaboration glossary of terms and the University of York guidelines for the conduct of systematic reviews and search strategies were consulted to frame the search. The included key points of research reporting, as specific in the Consolidated Standards of Reporting Trials (CONSORT) guidelines 23 for the publication of research describing RCTs, was utilized to assess the clarity of reporting in the included studies. Our search strategy identified research on interventions made or contributed by community pharmacies after hospital discharge. The following electronic databases were searched to identify evidence: MEDLINE, EMBASE, CINAHL, NHS EED, Cochrane Controlled Trials Register, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE) and Web of Science. Trial registers and websites of funding organizations were searched for ongoing studies. On-line search of The Pharmaceutical Journal as well as hand searches of relevant conference abstracts (such as the RPS conferences) were conducted. Hand searches through reference lists of key articles were also undertaken and relevant information on unpublished and in-progress research from key experts in the field was requested and included.
Key words and synonyms used in the electronic search to frame the setting or aim of healthcare provision included continuity of care, continuous care, continuum of care, seamless care, barriers to care and ongoing care. Word derivatives for interventionists included pharmacist, pharmacy, pharmacies, community pharmacy, pharmacy services and pharmacy practice. The search filters used were randomized controlled trials, controlled clinical trials, random allocation, single-blind method, clinical trials, crossover trials and placebos.
Study selection
All titles retrieved via literature search were reviewed by one of the authors (HN) for relevance. Two of the authors (HN and ZN) then independently assessed the abstracts of these papers against the inclusion criteria. The studies were delineated by their reported population/patient, intervention, control and outcomes and included (i) the population of patients who were identified as post-discharge, (ii) intervention involved a community pharmacist or member of the community pharmacy team, (iii) intervention focused on continuity of care, transfer of care or follow-up care, (iv) intervention occurred post-discharge from a hospital setting, (v) controlled trials that were randomized or non-randomized and (vi) all reported outcomes were of interest. Papers were not excluded on the basis of language, country of origin or publication date. Full papers from those abstracts that were considered relevant were requested and assessed independently by the two authors for their suitability for inclusion and differences resolved by discussion with reference to a third reviewer (AT) if necessary.
Validity assessment
Validity assessment was guided by criteria recommended by Cochrane for assessing methodological quality 24. Blinding of the assessors was not considered specifically relevant to the end points of the studies. We therefore critiqued studies for potential influence of bias or confounding factors impacting on reported outcomes. We compared baseline characteristics of groups, and reported whether the studies described the clear and transparent flow of patients and why, if any, losses or drop-outs occurred. We also clearly defined primary and secondary outcomes, and provided a sample size calculation. We reported whether >80% of patients were retained in the trial, as well as any training that the pharmacists received or resources they required for the intervention. Two authors (HN and ZN) independently carried out this analysis and any discrepancies were resolved through discussion with the third author (AT). The appraisal will be used for descriptive purposes and will also highlight variations between studies.
Data abstraction
Data were extracted on a piloted data extraction form adapted from an established Cochrane version. Two authors (HN and ZN) extracted data independently and checked for agreement or discrepancies. The third author (AT) was consulted for additional review where appropriate. Data included type of participants, intervention details, outcomes and trial quality characteristics.
Study characteristics
Classification of interventions
Interventions had to be delivered by a community pharmacist or member of the community pharmacy team. Singular or multiple interventions made by other healthcare professionals only (excluding community pharmacies post-discharge) were excluded. All forms of intervention made post-didcharge from a hospital setting were considered. All populations of patients were considered irrespective of their age, clinical condition or diagnosis, etc. All subsequent outcomes from interventions were considered including ‘soft’, e.g., patient satisfaction, medication adherence and ‘hard’, e.g., clinical test results, mortality. Studies were categorized by type of patient population, intervention components, funding/resources required for intervention and intervention preference compared with control. Interventions were classified according to the system reported and utilized by Hesselink and colleagues 1. Contributing elements and examples of activities are described in Table1.
Table 1.
Elements of an intervention, and example activities, that can influence the safety and quality of transitions between care settings 1
| Influential element | Description | Examples of activities to improve this element |
|---|---|---|
| Information | The quality of information that is exchanged between care settings in terms of completeness, accuracy and clarity. | Medication reconciliation by a hospital pharmacist, study pharmacist, liaison pharmacist or community pharmacist; electronic templates as the main method of information sharing; database-generated discharge summaries comprising structured formats to organize information; clinical decision support, alerts for pending results and online reference information. |
| Coordination of care | The quality of assessment, planning, and organization of follow-up services and needs. | Organizing post-discharge services or follow-up; a discharge planning protocol; early assessment of follow-up needs and resources; general practitioner input into discharge planning; post-discharge check for follow-up needs, adjustments and arrangements. |
| Communication | The quality of exchanging information in terms of personal and direct contact, accessibility, and timeliness. | The use of a liaison pharmacist or nurse; use of a fax or e-mail to transmit discharge summaries, plans and other relevant information in a timely manner; electronic notifications to inform GPs about patient hospital visits and available discharge information; telephone outreach from hospital to home care to notify the primary contact for follow-up consultation; face-to-face meetings in community or hospital; case conferences by telephone. |
Outcomes
Due to heterogeneity in outcomes measured, all outcomes have been considered and reported. Outcome data were extracted at the study's pre-specified last follow-up point. Formal pooling for meta-analysis was not possible due to the diversity of outcomes and scales employed, but informal pooling highlights were studies showed a significant positive, non-significant positive, negative or no effect.
Results
Search results and study characteristics
A total of 1528 titles were identified from our literature search, which yielded 144 potentially relevant studies. Further assessment of the abstracts of these studies and hand searches led to a total of 14 controlled trials identified that fit the inclusion criteria for the review. Figure1 describes the steps involved in the search and selection process.
Figure 1.
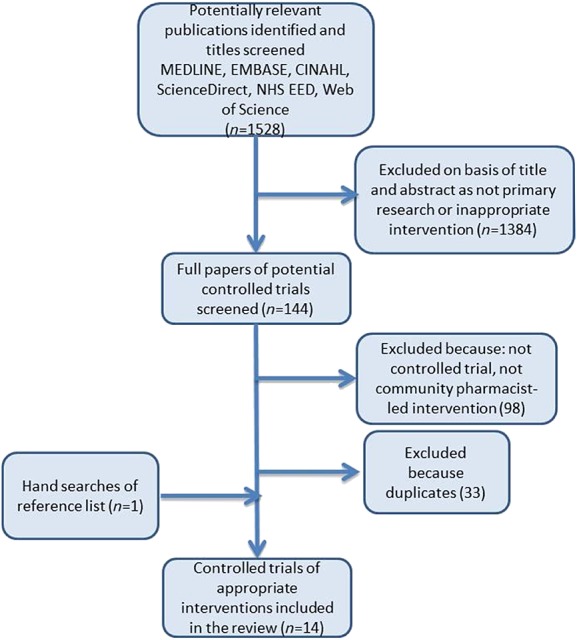
Flowchart describing study selection and excluded studies
Studies are described by their nature, location and patient cohort, which were included as shown in Table2.
Table 2.
Study characteristics
| Study [Reference] | Type of study | Location of study | Patient cohort included |
|---|---|---|---|
| Duggan et al. 19 | Randomized controlled trial | London, UK | Patients aged between 16 and 79 years old, showing no psychiatric illness or alcohol abuse |
| Stafford et al. 25,26 | Prospective, non-randomized controlled cohort study | Australia | Patients aged over 18 years old discharged with either newly initiated warfarin or continuing pre-admission therapy, with an indication necessitating at least 3 months of therapy |
| Nazareth et al. 27 | Randomized controlled trial | London, UK | Patients aged over 75 years old discharged on four or more medications |
| Bellone et al. 28 | Retrospective electronic record review | Texas, USA | Patients aged between 18 and 65 years old who were on at least three prescription medication |
| Beachesne et al. 29 | Randomized controlled trial | Montreal, Canada | Patients admitted to the emergency department for a respiratory disorder or hospitalised at the respiratory unit |
| Hugtenberg et al. 30 | Controlled intervention study | Amsterdam, the Netherlands | Patients discharged on five or more medications, and showed no signs of mental illness |
| Hugtenberg et al. 31 | Randomized controlled trial | the Netherlands | Patients aged over 60 years old discharged on five or more medications |
| Holland et al. 32 | Randomized controlled trial | Norfolk and Suffolk, UK | Patients aged over 80 years old discharged on two or more medications |
| Holland et al. 33 | Randomized controlled trial (economic evaluation) | UK | Patients diagnosed with heart failure and discharged on two or more medications |
| Pacini et al. 34 | |||
| Gurjal et al. 35 | Randomized controlled trial | Queensland, Australia | Patients admitted to the coronary care unit, cardiology ward or general medical wards with a diagnosis of ST-elevated MI or non-ST-elevated MI |
| Calvert et al. 36 | Randomized controlled trial | North Carolina, USA | Patients with coronary artery disease discharged on aspirin, β-adrenoceptor blocker and a statin |
| Crotty et al. 37 | Randomized controlled trial | South Australia | Patients with a life expectancy of ≥ 1 month and being discharged for the first time from hospital to a long term residential care facility |
Study validity
Six of the studies reported some statistical differences in baseline characteristics between their intervention and control groups 25,26,28,30,37. The majority (n = 10) of studies clearly described the patient flow and where and why losses or drop-out occurred 19,25–27,29,32,33,35–37. In most of these studies, the retention rate was ≥ 80%. However, one study did not include a flow diagram 34, but made reference to the previously reported trial 33, and another trial was reported as an abstract so lacked much of the required information for quality assessment 31. Sample size calculations were reported in 64% of studies (n = 9/14) 25–29,34–37, but only half achieved their required quota 25,26,34,35. All studies clearly defined their primary and/or secondary outcomes, and stipulated as and when specific resources or funding were utilized in the implementation of the interventions.
Interventions
Table3 outlines the key characteristics of the individual interventions. This includes the year the study was published, the healthcare professional involved (e.g. hospital pharmacist), and the classification of the intervention (e.g. information, coordination, communication). Two of the Australian studies reported on interventions delivered by a pharmacist belonging to the Home Medicine Review (HMR) programme 25,26. This programme provided governmental remuneration for appropriately accredited pharmacists, who are generally based in community pharmacies, to carry out home visits to review medication and provide education and counselling. In these studies, the pharmacists had to undertake additional training in the area of warfarin therapeutics and patient education. In another group of related studies, pharmacists with a postgraduate qualification or recent continued professional development in therapeutics also had to receive additional training in heart failure, drugs used, exercise, diet, smoking cessation and communication skills 32–34. Two studies ensured pharmacists received training on the use of the intervention protocol 27,36 and two studies involved reimbursement for pharmacists participating in the trial 35,36. One study in USA only considered pharmacists providing services within Community Care health centres, which specifically provide services to the medically underserved 37. The remaining Australian study made use of a transition pharmacist (TP) who coordinated the medication communication transfer to primary care, community pharmacy and GP 37. Only four trials reported utilizing existing pharmacist roles, with no further training or funding deemed necessary 19,29–31. The majority of the interventions (n = 9) involved the community pharmacist making a home visit 25–28,30,32–34,37, one involved telephone communication 36, one was face-to-face interaction in the community pharmacy 35 and three were unclear to the authors as they were not described in detail 19,29,31. These different forms of communication and interaction were performed in order to carry out a follow-up interview to provide education, counselling, check adherence and medication issues, remove inappropriate/excess medications and provide information on laboratory monitoring. Both primary and secondary outcomes measured were diverse, often with different measuring scales and in the majority of cases showing little agreement amongst studies (Table2). Of the 10 studies whose interventions involved increased information sharing between providers, improved coordination of care and improved communication, seven showed some statistically significantly positive outcomes.
Table 3.
Intervention characteristics
| Study [Reference] | Intervention characteristics | Key players | Classification of intervention | ||
|---|---|---|---|---|---|
| Information | Coordination | Communication | |||
| Duggan et al. 19 | Patients were given a letter on discharge that documented their prescribed medication and were asked to give this to their CP. The CP would compare the discharged medication list with the medications subsequently prescribed by the GP and report any discrepancies. | Hospital pharmacy, CP, GP | ✓ | ✓ | ✓ |
| Stafford et al. 25,26 | Prior to hospital discharge, the HMR referral process was initiated. At discharge all community-based healthcare providers received summary of patient's inpatient warfarin therapy in addition to usual discharge summary. Two to three home visits by CP, the first within 8–10 days post-discharge. Visits involved medication review, INR monitoring and targeted warfarin education, referrals to GP where necessary. | Hospital pharmacy, CP, GP | ✓ | ✓ | ✓ |
| Nazareth et al. 27 | Hospital pharmacist assessed medication, rationalization of treatment, assessment of patient's ability to manage their medication, provision of drug information and liaison with carers and community professionals. Discharge plan was given to patient and CP and GP and any other relevant healthcare professional involved. CP made home visit 7–14 days post-discharge to check discrepancies with medication being taken and those prescribed. CP assessed patient's understanding of and adherence to regimen and intervened where appropriate. CPs arranged further visits at their own discretion. | Hospital pharmacist, CP, GP, other healthcare professionals | ✓ | ✓ | ✓ |
| Bellone et al. 28 | A CP visit within 60 days post-discharge that could have included a number of interventions: discontinuation/initiation of drug therapy, dosage adjustments, medication counselling, adherence counselling and laboratory monitoring. | CP | |||
| Beachesne et al. 29 | Medication history carried out by clinical pharmacist and communicated information with CP. On discharge clinical pharmacist counsels on discharge medication and completes a discharge plan including admission diagnosis, comorbidities, allergies/drug intolerances, medications pre-admission, changes made and contact details of hospital pharmacist. In intervention group additionally a list of three DRPs with proposed actions to resolve them were included and CP was phoned and faxed information. | Hospital pharmacy, CP | ✓ | ✓ | ✓ |
| Hugtenberg et al. 30 | Usual care included fax of discharge prescription to CP, also newly prescribed drugs are accompanied with personalized letters of information, and patients receive additional oral information. The intervention added in a check by CP of drugs pre- and post-hospitalization and differences were recorded and subsequent intervention made as a consequence. Other interventions included taking a medication passport, producing a daily medication scheme, sending these to patient and GP, synchronizing discharge and concomitant medication on time, interviewing patient and checking home drug supplies. Interventions were not standardized. | Hospital pharmacy, CP, GP | ✓ | ✓ | ✓ |
| Hugtenberg et al. 31 | CP performed a medication review at discharge, after 3, 6 and 9 months post-discharge. | CP, pharmacy technicians and not clear who else | ✓ | ||
| Holland et al. 32 | Discharge letter sent to CP. CP arranged home visits to assess patient's ability to self-medicate and drug adherence. Educated patient and proxies, removed out of date drugs, reported ADR or interactions to GP, and reported need for compliance aid to local pharmacy. One follow-up visit at 6–8 weeks post-discharge to review and reinforce original advice. | Hospital, CP, GP | ✓ | ✓ | ✓ |
| Holland et al. 33 | CP made home visit within 2 weeks post-discharge to provide patient medication education and lifestyle advice. Patients also completed a sign and symptom monitoring diary card. Recommendations were fed back to GP. One follow-up visit at 6–8 weeks postdischarge to review and reinforce original advice. |
CP | ✓ | ✓ | |
| Pacini et al. 34 | |||||
| Gurjal et al. 35 | CP reviewed patient monthly to assess if medication was being collected and record any DRP. At 3 and 6 months post-discharge longer discussions were tailored to medication beliefs informed by researcher. CP funded | Researcher, CP | |||
| Calvert et al. 36 | At discharge standardized adherence counselling from study pharmacist and medication review. Provided a pocket medication card, a list of tips for remembering to take medications and pillbox. Fax of medication, and barriers to adherence sent to CP. Study pharmacist called patient 1–2 weeks post-discharge to confirm collection of medication. CP verified adherence immediately and 6, 12, 18 and 24 weeks post-discharge. When medication stopped or missed or any intervention was sent to study pharmacist and/or GP. CP funded. | Study pharmacist, CP. | ✓ | ✓ | ✓ |
| Crotty et al. 37 | On hospital discharge to long term facility, physician and CP faxed a medication transfer summary compiled by transition pharmacist (TP). TP organized medication review by CP within 10–14 days post-discharge, and a case conference involving him/her, the CP, physician and nurse at the facility with 14–28 days post-discharge. | TP, CP, physician, nurse | ✓ | ✓ | ✓ |
Outcomes
Only the outcomes that resulted from identification and rectification of drug reported problems received unanimous statistically significant positive effects with the intervention(s) and were seen in more than one study (n = 4). Each of these studies described interventions that focus specifically on identifying and rectifying drug related problems. Three of them 19,29,30 included sufficient detail to demonstrate that the interventions included activities from all key influential elements to improve safety and quality of transferring between settings (Table3). In these studies, there was also a reported increase in the information transferred from hospital to pharmacy to facilitate the checking and monitoring of drug histories and discrepancies. This in turn helped increase the likelihood of meetings being arranged between community pharmacists and the respective patients to discuss medication issues. There was also increased communication between the care settings to organize a follow-up visit or review as the patient makes the transition. The remaining study reported significant improvements in drug related outcomes 31, possibly due to the increased coordination of pharmacist-led medication reviews. Table4 provides further details on the outcomes reported for each study and the positive, negative and lack of statistically significant results. Of the key primary outcomes, such as hospital readmissions, mortality, patient medication adherence and the wider outcomes of quality of life and patient satisfaction, there was either no significant difference awarded with the intervention or little agreement between trials. As a consequence, these studies do not collectively evidence that the implemented interventions achieved any other statistically significant successful outcome in the intervention arm compared with the control. The factor of reimbursement or additional pharmacist support (either via a liaison pharmacist or specialist training in the intervention) did not contribute to improved trial outcomes across the studies. We found that no population group characteristic was associated with significantly improved trial outcomes. However, six of the nine interventions that incorporated the three recognized characteristics of information sharing, coordination of care and communication did demonstrate some outcomes classified as statistically significant, which suggests the importance of these particular factors in the design and delivery of interventions to improve transfer of care.
Table 4.
The types of outcomes and statistical significance of effects by studied interventions.((+) Outcome with statistically significant effect in favour of the intervention, (−) outcome with statistically significant effect in favour of the control, and (+∏) outcome with statistically significant effect in favour of a subgroup in the intervention arm)
| Study [Reference] | Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital use | Identification (number and type) of DRP | Rectification (number and type) of DRP | Medication adherence | Patient knowledge | Patient satisfaction | Death | Quality of life | Quality of prescribing | Clinical adverse effects | Condition control | Economic evaluation | |
| Duggan et al. 19 | ✓(+) | |||||||||||
| Stafford et al. 25 | ✓ | ✓ | ✓ | ✓(+) | ✓ | |||||||
| Stafford et al. 26 | ✓(+) | |||||||||||
| Nazareth et al. 27 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Bellone et al. 28 | ✓(+) | |||||||||||
| Beachesne et al. 29 | ✓(+) | ✓(+) | ✓ | |||||||||
| Hugtenberg et al. 30 | ✓(+) | ✓(+) | ✓(+) | ✓ | ✓ | |||||||
| Hugtenberg et al. 31 | ✓(+) | |||||||||||
| Holland et al. 32 | ✓(−) | ✓ | ✓ | |||||||||
| Holland et al. 33 | ✓(−) | ✓ | ✓ | ✓ | ✓ | |||||||
| Pacini et al. 34 | ✓ | |||||||||||
| Gurjal et al. 35 | ✓ | |||||||||||
| Calvert et al. 36 | ✓(+∏) | |||||||||||
| Crotty et al. 37 | ✓(+) | ✓(+) | ✓ | ✓(+∏) | ||||||||
| Total | 6 | 4 | 2 | 6 | 2 | 4 | 5 | 3 | 1 | 2 | 2 | 2 |
Discussion
Main study findings
This work has indicated there is a role to be played by community pharmacists in improving the transfer of care for post-discharged patients. The particular outcome that has been demonstrated to be most successfully achieved is that of identification and rectification of drug related problems. The interventions in these particular studies were clearly designed to impact a focused outcome, e.g. drug related problems and protocols were appropriately structured and adhered to. However, due to the design and implementation of many of the remaining studies, the full potential of the interventions may not have been fully appreciated. Most authors described the limitations to their studies (Table5) and provided an opportunity for those planning a future intervention to reflect upon the design and delivery of their interventions, the evaluation methodology, data collection and analysis so as to avoid such impingements on possible future outcomes.
Table 5.
Specific limitations of the included studies that may have influenced observed outcomes
| Study [Reference] | Recognized limitations of the study |
|---|---|
| Duggan et al. 19 | Non-English speaking patients were excluded from the study which may have caused a reduction in the magnitude of the impact of the intervention, since language difficulties may have increased the difference between the study arms. |
| A consensus methodology was used to classify the clinical significance of unintentional discrepancies by the viewpoint of one panel which may not have allowed exploration of breadth of judgement. | |
| A lack of sufficient information about each individual to define discrepancies more accurately as having a possible adverse effect on the patient may have resulted in an underestimation of the number of serious discrepancies. | |
| Stafford et al. 25 | Reporting of drug related problems may have been lower than expected since potentially hazardous warfarin drug interactions may have not necessitated attention if, for example, regular monitoring was taking place or that the combination had been taken for a period of time with no adverse effects. In some cases some drug combinations are clinically justified, e.g. warfarin and an antiplatelet in a patient with atrial fibrillation. |
| Data were analyzed from reports conducted by a small number of accredited pharmacists and therefore may not represent a wider roll out of the intervention. | |
| Identification of drug related problems was only possible from what was reported in the Home Medicines Review. | |
| Drug related problems that were resolved without the requirement for prescriber involvement may not have been documented. | |
| Some pharmacists may have used clinical decision support systems within the reviews and reports and this was not recorded. These support systems may have aided drug interaction identification. | |
| Short term medications, such as antibiotics, were excluded from the drug interaction list despite their potential to interact with warfarin. | |
| Stafford et al. 26 | The methodology did not allow for assessment of warfarin knowledge in the population assigned to the usual care arm at 2–3 days post-discharge. It was decided that if a visit had taken place at this point it may have led to patients being motivated to self-educate and lead to confounding of study results. Therefore improvement in knowledge in the intervention group cannot be unequivocally explained by the home-based warfarin-education. |
| The sample size was small and restricted in geographical area from which patients were included. | |
| The OAK test for assessing patient knowledge possesses some inherent limitations such as requiring seventh grade reading level. | |
| Improvement in patient knowledge was short-lived with regression within three months. Patients may have confounded results with familiarity with the OAK test or the higher rate of loss to follow-up in the intervention group may have meant that ‘non-responders’ may have exhibited differences in warfarin knowledge | |
| Nazareth et al. 27 | Baseline adherence was already high within the study population leaving little room for improvement. Data relating to patient knowledge and adherence was limited since information was collected only from those without cognitive impairment. |
| The delivery of the intervention was not standardized or identical amongst the study population. | |
| Bellone et al. 28 | This was a small retrospective review based one patients only admitted to one acute care facility and does not account for admissions or readmissions to other facilities. |
| External provider interventions were not considered that could have contributed to positive outcomes regarding hospital readmissions. | |
| Baseline characteristics of the populations in the study arms differed significantly. | |
| Specific information relating to specific disease, classes of medications and reasons for referrals was not collected to identify where pharmacists could make a more significant impact. | |
| Specific descriptions of the intervention performed by pharmacists were absent and may again have demonstrated important elements of an intervention. | |
| Number of adverse events was not collected to investigate correlation to readmissions. | |
| Beachesne et al. 29 | This was a small pilot study and included only a short period of time to follow-up. |
| Patients in the intervention group were aware that their CP had a list of unresolved drug related problems that required interventions which may have motivated pharmacists to intervene more frequently. | |
| Adherence was measured using a non-validated simple tool that tends to overestimate adherence. Also adherence was assessed at 6 weeks post-discharge which is considered a short period of time to accurately measure. | |
| Both intervention and control group patients received written discharge plans which could have explained high concordance in both groups. | |
| Hugtenberg et al. 30 | Participating pharmacists were not randomized. Those deciding to participate in the intervention group may have differed from the control group. |
| Changes made to drug therapy by a pharmacist have not been checked to assess for an improvement in the appropriateness of drugs dispensed. | |
| Reasons for drug changes were not collected. | |
| Information on rehospitalizations and drug related problems were not recorded. | |
| Not all intervention activities were fully implemented by the pharmacists. | |
| The medication review carried out by pharmacists was unstructured, and pharmacists were not specifically trained for this task. | |
| Hugtenberg et al. 31 | Insufficient detail provided to assess limitations accurately. |
| Interviews by pharmacy technicians in both control and intervention populations may have increased awareness of patients to potential drug related problems to query or raise with their CP. | |
| Holland et al. 32 | The intervention may have helped patients understand their conditions better. Patients may have been more aware of warning signs earlier and increased help seeking behaviour to result in more primary care use. |
| The intervention may have resulted in better adherence to medications and indirectly precipitated iatrogenic illness that may previously have been avoided. | |
| The home visit with increased contact time with the patient may have increased complexity of care resulting in increased anxiety, confusion or dependence. | |
| Otherwise the trial had high internal validity. | |
| Holland et al. 33 | The intervention may have been too late in the course of the disease to bring about behaviour change. |
| The pharmacists were not specialists in the disease, i.e. heart failure, and had limited knowledge in specific care, e.g. titrating drugs such as low dose β-adrenoceptor blockers. | |
| Pacini et al. 34 | |
| Gurjal et al. 35 | The intervention to improve adherence in an ‘adherence feedback’ loop did not include or identify reasons for non-adherence to allow pharmacists to tackle these specific issues. |
| Therefore the intervention was not tailored specifically to each patient's needs. | |
| The sample included patients who had experienced an MI, focussing specifically on non-adherent patients within this population may have yielded different results. | |
| The control group were followed up every month by the same CP and more of this group attended a cardiac rehabilitation programme. These programmes are recognized to improve adherence and contact with the same CP may have reinforced positive behaviour, both of which reducing the possibility of difference to be detected between the intervention and control group. | |
| Calvert et al. 36 | The use of two methods to assess medication adherence demonstrated discrepancies in findings. Prescription refill data as a measure of adherence gave findings that were substantially lower than that determined by patient self-report of use. |
| The study failed to recruit sufficient patients and therefore resulted in a lower power to detect difference between study arms. | |
| Follow-up interviews were not available for all patients with prescription refill records hence physician-directed discontinuations of medications were not noted and may have resulted in an overestimation of non-adherence. | |
| Crotty et al. 37 | The small sample size may have resulted in a lower power to detect differences in secondary outcomes. |
| The use of MAI may have introduced limitation s in assessing appropriate prescribing in older patients. Patients with high MAI in hospital or long term care facilities are assumed to be more vulnerable to adverse outcomes. The assessment would have been improved if supplemented by those of a panel of expert clinicians. |
The evidence here also suggests a need for randomized controlled trials that have a more stringent outline for the control rather than comparison with uncontrolled ‘usual care’. Caution should be heeded to regulate and account for activities that can take place in the control group that have characteristics similar to the intervention and can impact on subsequent outcomes reducing potential differences between the groups. Protocol violations also need to be minimized to ensure standardized delivery of the intervention and allow for subsequent accurate evaluation of outcomes. Thompson & Schoenfeld 38 deliberated over the use of usual care as a comparator to an intervention group. They recognized the need to acknowledge that usual care, in the absence of randomized controlled trials, is the safety standard. However, they then highlighted that the unstandardized nature of this comparator group runs the risk of merging with the intervention during the trial and reducing differences between groups. Meaningful difference then becomes harder to deduce. They discussed how the use of usual care in a two-armed randomized controlled trial is appropriate for drug and devices and for non-pharmacological interventions that lie well outside of usual care practices. Adhering to these principles improves the investigation and deduction of findings regarding impact through the minimisation of confounding factors. The observed inconsistencies in practice in these particular studies make usual care difficult to understand and describe, therefore limiting its value as a comparator arm in the trial. Thompson & Schoenfeld suggested the use of a strict protocol and computer-aided decision support to improve both usual care and intervention group, but also rationalize that this itself might hinder the natural process, adaptation and change of usual care 38. In light of this intricate debate, when dealing with complex interventions, we should consider the possible trial of two or more versions of a particular intervention. These versions may differ in one key component, which if absent, does not impinge on the coherency of the intervention but may allow identification of specific elements that impact upon efficacy and quality. Another consideration was raised by Gurjal and colleagues 35, where it was deduced that their medication adherence intervention was not tailored enough to measure improvements in individual patient outcomes. Potentially interventions cannot be ‘broadly’ protocolled but must be adapted at an individual level and outcomes measured equally on a specific basis. Furthermore, Holland and colleagues 33 hypothesized that the negative effect of their intervention, i.e. increased primary care use, may have been due to their intervention being too late in a disease course to evoke a change in behaviour. This further supports the argument that interventions should be carefully designed, tailored and delivered to respond to specific population needs.
Strengths and weaknesses of the study
Our search strategy included key databases and was supplemented by reviewing the references of relevant studies, review articles and conducting a citation search of identified studies. Where insufficient information was included in studies, authors were contacted. Our inclusion criteria were wide enough to capture any intervention made by a community pharmacist at the primary-secondary care interface and did not exclude on the specifics of the populations, the interventions or outcomes reported. It became clear from our early literature search that the role of pharmacists, not specifically community pharmacists, is one that offers much potential to improve the transfer of patient care. Many studies reported on interventions solely performed by clinical pharmacists, hospital-based pharmacists or a liaison pharmacist who was not necessarily based in the community, all of whom were excluded in this review. This offers another perspective to investigate the particular characteristics, location and profile of a pharmacist that is a prerequisite of a ‘successful’ intervention. Our main focus was to concentrate on evaluating controlled studies only. We recognize this as a rigorous method for determining whether a cause-effect relation exists between intervention and outcome. However, in our systematic review, it was clear that much research exists of a qualitative and uncontrolled nature which could highlight some valuable lessons in the design and implementation of the interventions. The Medical Research Council has described that an evaluation of a complex intervention, which these transfer of care interventions can generally be considered to be, must include the investigation of how the intervention works. A more descriptive analysis of the context would facilitate the identification of the key active ingredients of an intervention, allowing for a better understanding of the causal mechanisms 39. Hence, a process evaluation should complement an evaluation of effectiveness of any complex intervention.
Unfortunately, due to the heterogeneous nature of the patient populations tested, the baseline risk and opportunity to impact on outcomes may have differed amongst trials, as patients may have been in receipt of varying types of care provision from other sources within the healthcare system. This was keenly referred to in the intervention evaluations reported by Stafford and colleagues, where ‘warfarinized’ patients, even in the control group received a contact visit with a GP 8 days after discharge that could have shared components of monitoring, counselling or medicines management information similar to that of the pharmacist intervention 25,26. Bellone and colleagues also listed one of their limitations as not considering other provider interventions taking place at the same time that could also possibly contribute to the hospital readmission rates 28. Also the variation in patient groups between studies, including age, comorbidities, etc., may have made them more or less vulnerable to poor outcomes, therefore affecting the impact potential of the evaluated intervention. For example, the randomization of patients between the control and intervention group in one study resulted in significantly more White patients on fewer medications and fewer diseases designated to the control group. All of these factors reflect a population that would be less likely to be rehospitalized 28. Unstandardized delivery of the interventions, violations in protocols across providing pharmacies, and between individual pharmacists and even between patients from the same pharmacists, may have veiled beneficial effects in certain situations.
As reported in a previous review 1 that focused on any interventions made by any primary care providers at hospital discharge, we found that the intensity in intervention (number of interactions with the community pharmacist) did not appear to correlate directly with the effectiveness of the intervention. This reflects upon the complexity of factors to consider in the design and implementation of a ‘successful’ intervention. The evaluation of the qualitative and uncontrolled studies may shed further light onto the context and the interplay of patient, pharmacist and non-pharmacist issues and in turn the design of future interventions.
Findings in comparison with other studies
This is the only review we are aware of that focuses on interventions involving community pharmacists made to improve the continuity of patient care post-discharge from hospital. Unfortunately, before now there has been little pooled evidence around community pharmacist-led interventions. The findings do agree in essence with related evaluations of interventions in the transfer of patients between primary and secondary care. A recent systematic review of patient handovers from secondary to primary care at discharge by Hesselink and colleagues 1 describes that most interventions focused on the sharing of discharge information, facilitation of continuity of care, and direct and timely communication between healthcare providers. The authors also deduced that no singular intervention was evidenced to guarantee positive effects on specific outcome measures. There was an acknowledgement that their review, in common with this review, evaluated complex interventions, including the number of interactions between components, the unstandardized delivery and receipt of interventions, the variability in targeting of the interventions, the number and diversity of outcomes and the degree of flexibility or tailoring of intervention components. It therefore becomes very problematic to isolate the fundamental role of any player or characteristic of that intervention 40. This remains an issue despite the majority of the studies being classed as clear or very clear in their assessment for clarity of reporting against the CONSORT statement. Another review that specifically looked at medication reviews in older patients as an intervention to reduce hospital readmissions, reported how variations in the delivery of care and patient selection hindered the ability to recommend consistent benefit from such interventions 41. Okumura and colleagues 42 also concluded that the poor description of the counselling interventions evaluated in their review weakened their critique and subsequent evidence to support patient counselling as a robust intervention to improve patient outcomes. They advocated that clinical pharmacy services should adopt a systematic tool, e.g. DEPICT: Descriptive Elements of Pharmacist Interventions Characterization Tool 40, to allow better understanding of the service and its components to ensure reproducibility and standardization of delivery. Also, if a process evaluation is nested in a trial it can be used to assess fidelity and quality of implementation, clarify causal mechanisms and identify contextual factors associated with variation in outcomes 39.
Conclusion
This review provides evidence to support the role of community pharmacists in identifying and rectifying medication errors post-discharge, as part of interventions aiming to improve the transfer of care. However, insufficient data and flawed study design and implementation mean that further impact on patient outcomes cannot be deduced. To demonstrate consistent benefit more studies are required which are stricter in their intervention and usual care arms. Clear delineation will facilitate causal relationships to be better explored. Studies should also include process evaluations as standard so that contextual factors can be accounted for. These research modifications will improve the evidence base to inform future interventions and potentially describe the facilitative accompanying environment required to improve continuity of care successfully.
Our findings are important at a time when many community pharmacies in the UK are responding to the recent RPS guidance to improve transfer of care. Until now MURs and NMS are services accessed by discharged patients, despite the lack of empirical robust data to support their potential in improving continuity of care. Although medicine related outcomes have here been evidenced, community pharmacy has yet to provide convincing verification of the impact on a range of economic, clinical and humanistic outcomes. If other interventions, excluding community pharmacy, are able to demonstrate robustly such collective effects on the continuity of care, the clinical qualities and role of community pharmacy in patient care will not be fully realized and possibly ignored. It is important that we recognize how more work needs to be done in this important area.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- Hesselink G, Schoonhoven L, Barach P, Spijker A, Gademan P, Kalkman C, Liefers J, Vernooij-Dassen M, Wollersheim H. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012;157:417–28. doi: 10.7326/0003-4819-157-6-201209180-00006. [DOI] [PubMed] [Google Scholar]
- Braund R, Coulter CV, Bodington AJ, Giles LM, Greig AM, Heaslip LJ, Marshall BJ. Drug related problems identified by community pharmacists on hospital discharge prescriptions in New Zealand. Int J Clin Pharm. 2014;36:498–502. doi: 10.1007/s11096-014-9935-8. [DOI] [PubMed] [Google Scholar]
- Angley M, Ponniah AP, Spurling LK, Sheridan L, Colley D, Nooney VB, Bong X, Padhye V, Shakib S. Feasibility and timeliness of alternatives to post-discharge Home Medicines Reviews for high-risk patients. J Pharm Pract Res. 2011;41:27–32. [Google Scholar]
- Vuong T, Marriott JL, Kong DCM, Siderov J. Implementation of a community liaison pharmacy service: a randomised controlled trial. Int J Pharm Pract. 2008;16:127–35. [Google Scholar]
- Mansur N, Weiss A, Beloosesky Y. Relationship of in-hospital medication modifications of elderly patients to postdischarge medications, adherence, and mortality. Ann Pharmacother. 2008;42:783–9. doi: 10.1345/aph.1L070. [DOI] [PubMed] [Google Scholar]
- Himmel W, Kochen MM, Soms U, Hummers-Pradier E. Drug changes at the interface between primary and secondary care. Int J Clin Pharmacol Ther. 2004;42:103–9. doi: 10.5414/cpp42103. [DOI] [PubMed] [Google Scholar]
- Thompson-Moore N, Liebl MG. Health care system vulnerabilities: understanding the root causes of patient harm. Am J Health Syst Pharm. 2012;69:431–6. doi: 10.2146/ajhp110299. [DOI] [PubMed] [Google Scholar]
- Dvorak SR, McCoy RA, Voss GD. Continuity of care from acute to ambulatory care setting. Am J Health Syst Pharm. 1998;55:2500–4. doi: 10.1093/ajhp/55.23.2500. [DOI] [PubMed] [Google Scholar]
- Royal Pharmaceutical Society (RPS) Policy statement: pharmaceutical aspects of community care. Pharmaceut J. 1992;248:541–4. [Google Scholar]
- Ellitt GR, Engblom E, Aslani P, Westerlund T, Chen TF. Drug related problems after discharge from an Australian teaching hospital. Pharm World Sci. 2010;32:622–30. doi: 10.1007/s11096-010-9406-9. [DOI] [PubMed] [Google Scholar]
- Munday A, Kelly B, Forrester JWE, Timoney A, McGovern E. Do general practitioners and community pharmacists want information on the reasons for drug therapy changes implemented by secondary care? Br J Gen Pract. 1997;47:563–6. [PMC free article] [PubMed] [Google Scholar]
- Ponniah A, Anderson B, Shakib S, Doecke CJ, Angley M. Pharmacists' role in the post-discharge management of patients with heart failure: a literature review. J Clin Pharm Ther. 2007;32:343–52. doi: 10.1111/j.1365-2710.2007.00827.x. [DOI] [PubMed] [Google Scholar]
- Royal Pharmaceutical Society (RPS) 2011. Keeping patients safe when they transfer between care providers - getting the medicines right. Good practice guidance for healthcare professionals.. Available at http://www.rpharms.com (last accessed 13 November 2014)
- Royal Pharmaceutical Society (RPS) 2012. Keeping patients safe when they transfer between care providers - getting the medicines right. Individual reports from the Early Adopter Sites. Available at http://www.rpharms.com (last accessed 13 November 2014)
- Urban R, Paloumpi E, Rana N, Morgan J. Communicating medication changes to community pharmacy post-discharge: the good, the bad, and the improvements. Int J Clin Pharm. 2013;35:813–20. doi: 10.1007/s11096-013-9813-9. [DOI] [PubMed] [Google Scholar]
- Hatoum HT, Catizone C, Hutchinson RA, Purohit A. An eleven-year review of the pharmacy literature: documentation of the value and acceptance of clinical pharmacy. Drug Intell Clin Pharm. 1986;20:33–48. doi: 10.1177/106002808602000105. [DOI] [PubMed] [Google Scholar]
- Hawes EM, Maxwell WD, White SF, Mangun J, Lin FC. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transitions. J Prim Care Community Health. 2014;5:14–8. doi: 10.1177/2150131913502489. [DOI] [PubMed] [Google Scholar]
- Ponniah A, Shakib S, Doecke CJ, Boyce M, Angley M. Post-discharge medication reviews for patients with heart failure: a pilot study. Pharm World Sci. 2008;30:810–5. doi: 10.1007/s11096-008-9230-7. [DOI] [PubMed] [Google Scholar]
- Duggan C, Feldman R, Hough J, Bates IAN. Reducing adverse prescribing discrepancies following hospital discharge. Int J Pharm Pract. 1998;6:77–82. [Google Scholar]
- Geurts MME, van der Flier M, de Vries-Bots AMB, Brink-van der Wal TIC, de Gier JJ. Medication reconciliation to solve discrepancies in discharge documents after discharge from the hospital. Int J Clin Pharm. 2013;35:600–7. doi: 10.1007/s11096-013-9776-x. [DOI] [PubMed] [Google Scholar]
- Booij AD, de Boer WO, Kokenberg M, Tromp T. Interventions in seamless care. Pharm World Sci. 2003;25:41–2. doi: 10.1023/a:1023249031578. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Mast MR, Nijpels G, Elders PJ, Dekker JM, Hugtenburg JG. Identification of drug-related problems of elderly patients discharged from hospital. Patient Prefer Adherence. 2014;8:155–65. doi: 10.2147/PPA.S48357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Getzsche PC, Lang T. The revised CONSORT statement for reporting randomised trials: explanation and elaboration. Ann Intern Med. 2001;134:663–94. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- Higgins PT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, West Sussex: Wiley-Blackwell; 2009. eds. Available at http://cochrane-handbook.org [updated September 2009] [Google Scholar]
- Stafford L, Peterson GM, Bereznicki LRE, Jackson SL, van Tienen EC, Angley MT, Bajorek BV, McLachlan AJ, Mullan JR, Misan GM, Gaetani L. Clinical outcomes of a collaborative, home-based postdischarge warfarin management service. Ann Pharmacother. 2011;45:325–34. doi: 10.1345/aph.1P617. [DOI] [PubMed] [Google Scholar]
- Stafford L, van Tienen EC, Bereznicki LR, Peterson GM. The benefits of pharmacist-delivered warfarin education in the home. Int J Pharm Pract. 2012;20:384–9. doi: 10.1111/j.2042-7174.2012.00217.x. [DOI] [PubMed] [Google Scholar]
- Nazareth I, Burton A, Shulman S, Smith P, Haines A, Timberall H. A pharmacy discharge plan for hospitalized elderly patients - a randomized controlled trial. Age Aging. 2001;30:33–40. doi: 10.1093/ageing/30.1.33. [DOI] [PubMed] [Google Scholar]
- Bellone JM, Barner JC, Lopez DA. Postdischarge interventions by pharmacists and impact on hospital readmission rates. J Am Pharm Assoc. 2012;52:358–62. doi: 10.1331/JAPhA.2012.10172. [DOI] [PubMed] [Google Scholar]
- Beauchesne M-F, Nenciu LM, Dinh T-H, Tasse M, Fillion A, Labrecque M, Blais L. Active communication of a pharmacy discharge plan for patients with respiratory diseases: a pilot study. J Pharm Tech. 2007;23:67–74. [Google Scholar]
- Hugtenburg JG, Borgsteede SD, Beckeringh JJ. Medication review and patient counselling at discharge from the hospital by community pharmacists. Pharm World Sci. 2009;31:630–7. doi: 10.1007/s11096-009-9314-z. [DOI] [PubMed] [Google Scholar]
- Hugtenburg J, Ahmad A, Dekker JM, Kostense PJ, Nijpels G. Medication review in elderly patients discharged from hospital substantially decreased drug related problems. Diabetologia. 2012;55:S412. doi: 10.1001/archinternmed.2012.2816. [DOI] [PubMed] [Google Scholar]
- Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, Christou M, Evans D, Hand C. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ. 2005;330:293–5. doi: 10.1136/bmj.38338.674583.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R, Brooksby L, Lenaghan E, Ashton K, Hay L, Smith R, Shepstone L, Lipp A, Howe A, Hall R, Harvey I. Effectiveness of visits from community pharmacists for patients with heart failure: HeartMed randomised controlled trial. BMJ. 2007;334:1098–101. doi: 10.1136/bmj.39164.568183.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini M, Smith RD, Wilson ECF, Holland R. Home-based medication review in older people - is it cost effective? Pharmacoeconomics. 2007;25:171–80. doi: 10.2165/00019053-200725020-00008. [DOI] [PubMed] [Google Scholar]
- Gujral G, Winckel K, Nissen LM, Cottrell WN. Impact of community pharmacist intervention discussing patients' beliefs to improve medication adherence. Int Clin Pharm. 2014;36:1048–58. doi: 10.1007/s11096-014-9993-y. [DOI] [PubMed] [Google Scholar]
- Calvert SB, Kramer JM, Anstrom KJ, Kaltenbach LA, Stafford JA, LaPointe NMA. Patient-focused intervention to improve long-term adherence to evidence-based medications: a randomized trial. Am Heart J. 2012;163:657–65. doi: 10.1016/j.ahj.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother. 2004;2:257–64. doi: 10.1016/j.amjopharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Thompson BT, Schoenfeld D. Usual care as the congtrol group in clinical trials of nonpharmacologic interventions. Proc Am Thorac Soc. 2007;4:577–82. doi: 10.1513/pats.200706-072JK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council (MRC) 2006. Developing and evaluating complex interventions: new guidance. Available at http://www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/ (last accessed 15 November 2014)
- Correr CJ, Melchiors AC, de Souza TT, Rotta I, Salgado TM, Fernandez-Llimos F. A tool to characterize the components of pharmacist interventions in clinical pharmacy services: the DEPICT project. Ann Pharmacother. 2013;47:946–52. doi: 10.1345/aph.1S006. [DOI] [PubMed] [Google Scholar]
- Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol. 2008;65:303–16. doi: 10.1111/j.1365-2125.2007.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura LM, Rotta I, Correr CJ. Assessment of pharmacist-led patient counseling in randomized controlled trials: a systematic review. Int J Clin Pharm. 2014;36:882–91. doi: 10.1007/s11096-014-9982-1. [DOI] [PubMed] [Google Scholar]


