Abstract
Arthritis is the commonest cause of disabling chronic pain, and both osteoarthritis (OA) and rheumatoid arthritis (RA) remain major burdens on both individuals and society. Peripheral release of calcitonin gene-related peptide (CGRP) contributes to the vasodilation of acute neurogenic inflammation. Contributions of CGRP to the pain and inflammation of chronic arthritis, however, are only recently being elucidated. Animal models of arthritis are revealing the molecular and pathophysiological events that accompany and lead to progression of both arthritis and pain. Peripheral actions of CGRP in the joint might contribute to both inflammation and joint afferent sensitization. CGRP and its specific receptors are expressed in joint afferents and up-regulated following arthritis induction. Peripheral CGRP release results in activation of synovial vascular cells, through which acute vasodilatation is followed by endothelial cell proliferation and angiogenesis, key features of chronic inflammation. Local administration of CGRP to the knee also increases mechanosensitivity of joint afferents, mimicking peripheral sensitization seen in arthritic joints. Increased mechanosensitivity in OA knees and pain behaviour can be reduced by peripherally acting CGRP receptor antagonists. Effects of CGRP pathway blockade on arthritic joint afferents, but not in normal joints, suggest contributions to sensitization rather than normal joint nociception. CGRP therefore might make key contributions to the transition from normal to persistent synovitis, and the progression from nociception to sensitization. Targeting CGRP or its receptors within joint tissues to prevent these undesirable transitions during early arthritis, or suppress them in established disease, might prevent persistent inflammation and relieve arthritis pain.
Keywords: Calcitonin gene-related peptide, inflammation, joints, pain
Introduction
Arthritis is the commonest cause of disabling chronic pain, and both osteoarthritis (OA) and rheumatoid arthritis (RA) remain major burdens on both individuals and society. In the UK alone, 7.5 million working days are lost per year due to musculoskeletal conditions. OA and RA together cost the UK economy £30.7 billion per year (2008) 1. OA and RA are each painful, disabling conditions that affect synovial joints. OA is the commonest form of arthritis, affecting half of people over 50 years of age, and almost everyone aged over 70 years will have OA of one or more joints. RA affects approximately 2% of Western populations, and although it can begin at any age, peak onset is currently in the fifth decade. OA and RA are each characterized by pain, stiffness, joint swelling, tenderness and deformity, and loss of function. Both diseases are associated with inflammation of the joint lining (synovitis) and damage to articular cartilage. Whereas OA and RA are distinct pathological entities, with predominant new bone formation in OA, and specific and systemic immune responses in RA, both require treatment with analgesics, and joint damage due to RA can ultimately lead to the development of secondary OA. Furthermore, both diseases affect people of similar age, and approximately half of people at presentation with early RA also have radiographic evidence of OA in either their hands or feet.
Arthritis pain results from a combination of changes within the joint with sensitization of peripheral and central sensory pathways 2,3. Structural, biomechanical, cellular and biochemical changes within the joint each may contribute to increased nociceptive inputs. Synovitis 4 and subchondral bone marrow changes 5 detected by magnetic resonance imaging (MRI) have been particularly associated with pain in patients with OA or RA, and treatments that suppress inflammation 6 or reduce subchondral bone turnover 7 are finding potential as analgesic strategies. Experimental evidence indicates that joint-innervating nociceptors are sensitized in patients with OA, and that this peripheral sensitization makes an important contribution to pain severity 8. Furthermore, there is increasing evidence that central sensitization also contributes to arthritis pain, both in man 9 and in animal models 10. Inflammation or continuous nociceptive input from the joint might drive sensitization. Nerve growth factor (NGF) is upregulated during synovitis 11,12 and causes peripheral nerve sensitization, without per se inducing nociception 13. Superior clinical efficacy of NGF blockers compared with traditional analgesics such as non-steroidal anti-inflammatory agents and opioids validates peripheral sensitization as a key target in the development of new analgesics in OA 14,15. Congenital insensitivity to pain results in clinically important tissue damage (e.g. ulceration of extremities) and sometimes death in childhood 16. It therefore is clinically attractive to target pathological sensitization, while leaving intact a normal level of nociceptive signalling that protects the organism against injury and is critical for survival.
The sensory neuropeptide calcitonin gene-related peptide (CGRP) influences peripheral sensitization and inflammation, and evidence implicates a role for CGRP in the pain and inflammation of chronic arthritis. In this article we will review the evidence for a role of CGRP in arthritis. Small molecule CGRP receptor antagonists and antibodies that block CGRP or its receptors have recently been developed with clinical efficacy against migraine pain. The recent initiation of trials in OA makes review of evidence of contributions from CGRP to arthritis pain extremely timely.
Models of arthritis pain: strengths and limitations
OA is problematic across a range of species, and is recognized in people, and in veterinary practice in particular in dogs, horses and pigs. Spontaneous OA is observed in guinea pigs, although researchers more commonly study chemically or surgically induced models of OA in rats or mice, in which disease develops rapidly from a defined time of onset (Table1) 26. RA is restricted to humans, although immune-mediated arthritis can be induced in rodents, for example by immunization in association with Freund's complete adjuvant 26. Serum from K/BxN mice can be used to induce transient inflammatory arthritis in a wide range of mouse strains 27. Type II collagen is a major component of articular cartilage, and collagen-induced arthritis can be induced in susceptible strains of rats and mice by immunization with native heterologous type II collagen emulsified in Freund's incomplete or complete adjuvant, respectively 28. Rodent models of inflammation or inflammatory pain, commonly focus on the skin, following chemical (e.g. carrageenan) or immune (e.g. Freunds' complete adjuvant) induction in the footpad 29. Although inflammation is not restricted to the skin in these models and can be detected in the joints of the injected hind paw 30, the relevance of cutaneous pain mechanisms to human arthritis remains uncertain.
Table 1.
Summary and comparison of some of the most commonly used animal models of OA
| Model | Species | Initiating stimulus | Pain behaviour | Disease onset | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| Dunkin Hartley guinea pig | Guinea pig | Spontaneous | ? | 10–20 weeks | Clinical relevance since disease onset is insidious and similar to human OA | Delayed and variable onset and incidence, identification of suitable controls is problematic. Disease bilateral, so standard pain tests not suitable | 17 |
| STR/ort mouse | Mouse | Spontaneous | ? | 12–16 weeks | 18,19 | ||
| ACLT | Dog, rat | Surgical | x | 2–3 weeks | Useful for modelling injury induced OA, develops less rapidly than MIA model, DMM model amenable to genetic manipulation | Considerable variations between studies regarding model and sham surgeries, relatively technically difficult and time consuming | 20–22 |
| MNX | Rat | Surgical | √ | 1–2 weeks | 48,23 | ||
| DMM | Mouse | Surgical | √ | 4–8 weeks | 24 | ||
| MIA | Rat, mouse | Chemical | √ | <1 week | Pain behaviour and peripheral/central sensitization partially characterized, rapid and consistent onset of OA-like pathology, amenable to pharmacological studies (preferred model of pharmaceutical industry) | Extensive and rapidly developing pathology, raises questions over relevance to human OA | 44,45,63,25 |
None of these animal models reflects all aspects of human arthritis, although each may mimic key features of arthritis pathology and pain mechanisms. Cutaneous innervation differs from that of the synovium, particularly by the presence of specialist sensory terminals, an abundance of myelinated nerve fibres and the presence of non-peptidergic unmyelinated sensory nerves in skin 31. Neuropeptide pathways, including CGRP, differ between rodents and man. For example there may be more abundant vascular CGRP binding in guinea pig and porcine compared with human synovium 32. Human OA is usually of spontaneous onset, with progression over years rather than weeks, and only a small proportion of patients describe injury sufficient to cause internal derangement of the knee prior to OA onset. Human RA is a persistent, polyarticular disease, whereas rodent models of immune-mediated arthritis are typically self-limiting.
Rodent OA and inflammatory arthritis models have largely been developed to study pathology in the articular cartilage or immune-mediated joint damage, respectively. Chemically induced OA, following intra-articular injection of the chondrocyte toxin mono-iodoacetate (MIA), and surgically induced OA following transection of the medial meniscus (MNX) in rats, each results in pain behaviour, cartilage damage, osteophyte formation and mild synovitis, each key features of human OA. In recent years, methods have been developed to explore pain behaviour and functional sensory changes in arthritis models 33,34. Weight-bearing asymmetry and reduced paw withdrawal thresholds to punctate mechanical stimulation suggest a combination of peripheral and central sensitization in animal models of knee arthritis, comparable with the pain on standing and widespread reduced pain detection thresholds to mechanical stimulation reported in people with arthritis 35. Expression of CGRP and its receptor components is broadly comparable between rodents and humans, both in dorsal root ganglion (DRG) neurons 36,37 and in joint tissues 38–40. Furthermore, NGF blockade reduced pain behaviour in rodent models of OA pain 26,41 and also in clinical trials 14,15,42. Increasingly, therefore, it seems that these models might also reflect pain mechanisms which contribute to human arthritis pain, and they display translational validity for development of novel pharmacological agents that might relieve the burden of arthritis pain in the future.
Rodent arthritis models permit more detailed characterization of pain mechanisms than would be possible in humans. MIA- or MNX-induced OA in rats is each associated with increased afferent fibre sensitivity 43. Electrophysiological evidence of peripheral sensitization of articular C and A fibre nociceptors parallels the development of pain behaviours such as weightbearing asymmetry 44. This increased sensitivity is characterized by reduced mechanical thresholds of Aδ-fibres to punctate stimulation of the joint capsule, and increased magnitude of evoked responses to suprathreshold stimulation. Furthermore, C fibre afferents from osteoarthritic knees display increased spontaneous activity 43. Spinal nociceptive reflexes evoked by punctate stimulation of the ipsilateral hindpaw and recorded from the hind limb muscle biceps femoris demonstrate reduced thresholds for activation in MIA-treated rats 45. Together these findings indicate a combination of both peripheral and central sensitization that follow the development of arthritis.
Structural change in the arthritic joint: inflammation and angiogenesis
Synovitis and cartilage damage are inter-related. Synovitis is the main drive to joint damage in RA and effective immunosuppression reduces progression of joint damage. In OA also, synovitis is associated with more severe structural damage in cross sectional studies 46 and with more rapid progression of radiographic damage in prospective studies 47. In animal models of OA, synovitis inhibition not only reduces pain behaviour, but also retards structural progression 48.
Synovitis is also associated with pain, both in RA and in OA. Clinical efficacy (albeit only partial) of cyclo-oxygenase inhibitors and glucocorticosteroids in both diseases suggests involvement of prostanoids in chronic arthritis pain, and efficacy of TNF-alpha blockade in RA suggests potential contributions from other mediators. The precise molecular entities that mediate the chronic pain that is associated with joint inflammation remain to be determined, although upregulation of NGF 12 and clinical efficacy of NGF blockade in painful OA 14,15,42 suggest a central role for neurotrophins and peptidergic nerves.
Angiogenesis is a characteristic feature of chronic inflammation and, both in OA and in RA, affects several joint tissues, including synovium, osteochondral junction and meniscus 49. In synovium, angiogenesis occurs concurrently with vascular regression, resulting in vascular redistribution away from the synovial lining 50. New blood vessels also grow into non-calcified articular cartilage and inner two-thirds of the knee meniscus, structures which in the normal joint are avascular 51,52. The possible influences of CGRP on angiogenesis, inflammation and sensitization are illustrated in Figure1.
Figure 1.
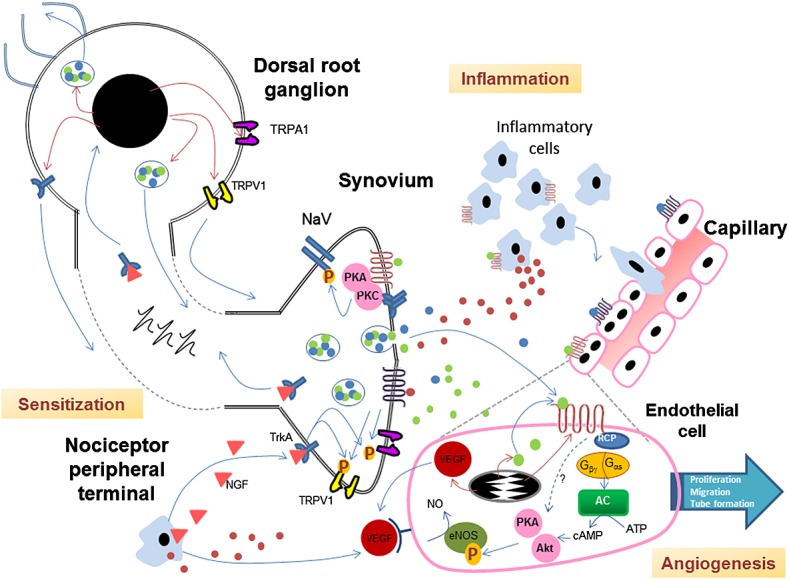
Interactions between sensitization, inflammation and angiogenesis mediated by CGRP
Structural change in the arthritic joint: innervation and CGRP pathway components
Several tissues are richly innervated in normal joints 53, including synovium, ligament and tendon insertions 54, subchondral bone 51,55, periosteum 56 and the outer one-third of the meniscus 52. Immunoreactivities for substance P and CGRP are localized to sensory nerves in both human and rat synovia 57,58 and three-quarters of knee afferents in rat DRGs express CGRP 59. CGRP expression 60,61 and release 62,63 are increased in afferents from joints affected by OA in rats and mice. Increased expression is associated with increases in the number of medium and large DRG cells which express CGRP 61. However, decreases in CGRP mRNA expression have also been reported in DRGs from rodents with OA, suggesting that regulation of CGRP expression may be dependent on experimental conditions or OA stage 64. Sensory nerve growth accompanies blood vessel growth in a range of tissues 65. Accompanying angiogenesis in the arthritic joint, CGRP-immunoreactive sensory nerve terminals grow into channels that penetrate articular cartilage 51 and along neovasculature in the inner two thirds of the knee meniscus 52. With the progression of arthritis, therefore, joint structures that are normally not innervated might become sources of pain.
The density of nerve fibres within the synovium might vary during arthritis, with focal rarefaction of nerves due to synovial hyperplasia and, possibly, retraction of nerve terminals from a hostile, inflammatory environment 39,57. Thus sensory nerves might appear to be depleted from inflamed synovium 39,57. By contrast, synovial CGRP-immunoreactive nerve fibre density might appear greater in OA patients with pain as compared with asymptomatic controls 66, particularly in joint compartments displaying increased sensitivity 67. Associations between nerve terminal density and sensitivity have been observed in human skin 68, and changes in nerve terminal densities in joint tissues might therefore also be associated with changes in sensitivity. Arthritis pain often displays neuropathic qualities 69, indicating that joint sensitivity might also result from altered peripheral nerve function accompanying damage to peripheral nerve terminals. However, investigation of ATF3 immunoreactivity, a marker of nerve damage, indicated that arthritis in rats was not associated with significant damage to the central components of joint afferents 70.
The CGRP receptor is a heterodimer consisting of the calcitonin receptor-like receptor (CRLR) and receptor activity modifying protein-1 (RAMP-1). RAMP-1 confers specificity for CGRP 71, whereas association of CRLR with RAMPs 2 or 3 confers specificity for adrenomedullins 72. Human and rat synovia express CRLR and RAMP-1 38,73 and binding sites for CGRP 74. CGRP receptors are expressed within the synovium by endothelial and immune cells 75. Within dorsal root ganglia, CGRP receptors are expressed exclusively on CGRP-positive neurons 76, indicating that CGRP-containing sensory nerve terminals within the knee are likely to also bear CGRP receptors. CRLR is up-regulated in medium diameter cell bodies of afferents from rat knees affected by MIA-induced OA 43.
CGRP functions as a component of molecular pathways that also include other neuropeptides such as substance P, the transient receptor potential (TRP) receptors, such as ankyrin repeat 1 (TRPA1) and vanilloid 1 (TRPV1) ion channels, and tropomyosin receptor kinase-A (TrkA) receptors for NGF 77 (Figure1). Activity in CGRP pathways is increased during arthritis. Substance P is co-localized with CGRP in secretory granules within peripheral sensory nerves and NGF, acting through TrkA, leads to increased CGRP and substance P expression and axonal transport to peripheral nerve terminals 78. As with CGRP, substance P 79 and TRPV1 expressions are up-regulated in sensory nerves during arthritis 45. TRPV1 or TRPA1 activation results in co-release of CGRP and substance P from sensory nerve terminals. NGF is up-regulated in RA 11 and OA synovium and in the latter, at least, associated with increased pain 12. NGF stimulates TRPV1 phosphorylation and increases neuronal sensitivity to TRPV1 and TRPA1 ligands 80. TRPA1 inhibitors or genetic deficiency have also been associated with reduced pain behaviour and/or inflammation in mice following intra-articular urate injection 81, intraplantar complete Freund's adjuvant injection 82,83 or in rats with OA 62,84. CGRP might mediate effects of TRPV1 or TRPA1 activation on pain behaviour, inflammation or vasodilation 62. Peptidergic sensory nerves might also act in concert with other neuronal cell types, including articular sympathetic fibres 49. In tissues such as skin, non-peptidergic, GDNF-dependent sensory fibres expressing TRPC3 might also contribute to pain transmission 85, although non-peptidergic fibres might not be present in deeper articular structures 31.
Neurogenic acute inflammation
Acute inflammation in the skin displays classic features of redness, swelling, increased temperature and pain, corresponding to the pathological processes of vasodilation, plasma extravasation and neuronal sensitization. Acute inflammation may be viewed as a protective response, which normally resolves to leave a tissue that is identical to that which preceded the insult. Capsaicin, the pungent component in chilli peppers 86 can be used to stimulate the release of neuropeptides from sensory nerves, leading to neurogenic inflammation. Joint swelling indicates acute synovitis 24 h after intra-articular capsaicin injection 38.
Peripheral release of CGRP has long been recognized to contribute to the vasodilation of acute neurogenic inflammation 87. CGRP induces vasodilation in the joint 74 and CGRP-induced vasodilation can be enhanced by substance P 88. Tachykinins, rather than CGRP, are predominantly responsible for the increased vascular permeability of neurogenic inflammation, although increased blood flow following vasodilation by CGRP might also increase plasma extravasation when vascular permeability is already increased. For example, CGRP can potentiate IL-1α-induced oedema formation in the skin 89, and also potentiates substance P-induced oedema in the synovium 90. CGRP injection alone into rat knees does not, however, induce sufficient oedema to be detected as increased joint swelling 38. Anti-inflammatory neuropeptides, such as opioid peptides, galanin, vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide and somatostatin, are also released from activated sensory nerve terminals and modulate inflammatory processes 91–93.
Neurogenic angiogenesis
Whereas the contributions of neuropeptides to acute neurogenic inflammation are now well established, their relevance to chronic inflammation is only recently being elucidated. Distinguishing features of chronic, as opposed to acute inflammation include recruitment and activation of macrophages, cellular proliferation and angiogenesis. Repair normally follows chronic inflammation, leading to a functional tissue that might differ in its cellular or matrix composition from that which preceded the insult. Chronic inflammation is often associated with the generation of specific immune responses, and with concurrent tissue damage and repair, as opposed to the sequential progress from insult to resolution that is observed during acute inflammation. Synovial infiltration by inflammatory cells, including macrophages, is observed following intra-articular capsaicin injection 94. CGRP might contribute to this immune cell recruitment and can moderate pro-inflammatory mediator release that, in turn, can further sensitize joint afferents 90. However, synovial macrophage infiltration was not significantly reduced by the CGRP1 receptor antagonist BIBN4096BS following intra-articular capsaicin injection, suggesting that other factors might also be important 38.
Angiogenesis is also a key component in the transition from acute to persistent inflammation, concomitant with the shift from resolution to repair. Persistent angiogenesis might further augment inflammation, through increased recruitment of inflammatory cells 95. Trophic effects of sensory nerves have been well described in the vasculature, both during embryogenesis 96 and during chronic inflammation 97. Intra-articular injection of capsaicin in rats results in increased endothelial cell proliferation in the synovium 38. This neurogenic angiogenesis reflects the potential of nerves to initiate blood vessel growth, through which the sensory nervous system contributes to innate defence systems, detecting threat and initiating repair following tissue injury. In return, as blood vessels grow, sensory nerves extend axons along them, and innervation of neovasculature is an essential component of vascular maturation and the stabilization of the otherwise immature neovascular bed 65. It is not surprising, therefore, to find that angiogenesis and nerve growth are closely integrated process. Factors that stimulate nerve growth (e.g. NGF) or vascular growth (e.g. vascular endothelial growth factor), reciprocally also stimulate vascular and nerve growth, respectively 49. Neuropeptides, such as substance P 98 and CGRP 99 stimulate angiogenesis. Indeed, neuropeptide up-regulation might partly mediate the in vivo potential of NGF to induce angiogenesis.
CGRP increases proliferation 100, migration 100 and tube formation 101 by vascular endothelial cells in vitro 102 and enhances sponge-induced angiogenesis in vivo 101. Furthermore, implanted tumour growth and tumour-associated angiogenesis were decreased in CGRP knockout mice compared with wild-type mice 101, and the CGRP receptor antagonist CGRP8-37, inhibited tumour-associated angiogenesis and growth in mice 101. Angiogenic effects of CGRP in vitro might be mediated by AMP-activated protein kinase (AMPK) 99, Akt 103 and endothelial nitric oxide synthase phosphorylation 99, as well as by up-regulation of vascular endothelial growth factor and its receptors 104 (Figure1). Furthermore, CGRP might lead to up-regulation of its own gene and of CGRP1 receptors by endothelial cells, thereby participating in a positive feedback loop that further increases blood vessel growth 104. Indeed, potential expression of CGRP by endothelial cells suggests an autocrine, as well as neurogenic role, and expression might be further directly up-regulated by other factors traditionally thought to act primarily on sensory neurones, including TRPV1 agonists 105.
Intra-articular injection of CGRP induced dose-dependent endothelial proliferation in the synovium of rat knees, and this angiogenic effect was blocked by the non-peptide CGRP receptor antagonist BIBN4096BS, but not by its inactive enantiomer 38. Inhibition by BIBN4096BS, and lack of angiogenic effects of adrenomedullin even at 8 nmol dosing in this model, suggest mediation of CGRP-induced angiogenesis by CGRPR1. However, adrenomedullin was expressed in rat and human synovia, and can induce angiogenesis under other experimental conditions 106. More detailed characterization of possible contributions from endogenous adrenomedullin to synovial inflammation would be required to confirm exclusivity of these effects to CGRP. BIBN4096BS also inhibited synovial angiogenesis induced by intra-articular injection of capsaicin, indicating the potential of endogenous CGRP release to stimulate blood vessel growth during neurogenic angiogenesis 38. CGRP and substance P act synergistically to increase endothelial cell proliferation 107, and a combination of NK1 and CGRP receptor antagonists abolished the increased synovial endothelial cell proliferation that followed intra-articular capsaicin injection 38. Neurogenic angiogenesis extends the long recognized potential of sensory nerves to generate acute inflammation, and might be one of the switches that leads to the persistence of synovitis 108. Although a contribution of neurogenic angiogenesis to synovitis and pain in humans remains unproven, CGRP blockade would be unlikely to exacerbate synovitis and anti-inflammatory effects might synergize with reduced sensitization to improve arthritis pain.
Sensory nerves do not act in isolation and it has been suggested that a balance between sensory and sympathetic nerve function might regulate synovitis 109. Specifically, sympathetic neurotransmitters might act directly on monocytes to suppress inflammation and facilitate tissue repair, balancing pro-inflammatory effects of sensory neuropeptides. Selective retraction of sympathetic fibres 109, combined with increased proportions of joint afferents that express CGRP, might contribute to the maintenance of inflammation.
CGRP and the bone/cartilage unit
Articular cartilage and subchondral bone provide strength, movement and resilience to synovial joints. Each has a low resting metabolic activity and a high matrix content, although, in arthritis, matrix turnover is increased through the combined action of chondrocytes, osteoblasts and osteoclasts. Chondrocytes respond to CGRP by increased cAMP production 110, which might inhibit hypertrophic differentiation, although normal articular cartilage is not innervated and the biological relevance of this observation awaits confirmation. CGRP is also anabolic for bone, inhibiting osteoclast differentiation 111 and facilitating osteoblast activity 112. Reduction of osteoblast apoptosis might be mediated by the catenin/wnt pathway 113. CGRPα deficient mice, induced by genetic modification 112 or by depletion of capsaicin-sensitive nerves 114, display an osteopenic bone phenotype, consistent with a contribution of endogenous CGRP to normal bone homeostasis. The potential relevance of these findings to human arthritis remains uncertain, but recent unexpected findings of rapidly progressive OA after NGF blockade 15 emphasize the need to consider effects on joint structure when testing analgesic effects of treatments that target the sensory nervous system. In a mouse model, sensory denervation was associated with structural exacerbation of knee OA 115, as might be seen in neuropathic arthropathy in man, although the contribution of CGRP to this observation remains uncertain (Table2).
Table 2.
Summary of the contribution of CGRP to angiogenesis, inflammation and pain
| Site of action | Effect | Tissue/model system | Species | Model | Reference |
|---|---|---|---|---|---|
| Vasculature | Vasodilation | Synovium | Rat | Naive | 74 |
| Potentiation of IL-1α-induced oedema | Skin | Rabbit | Naive | 89 | |
| Potentiation of substance P-induced oedema | Synovium | Rat | Naive | 90 | |
| Endothelial cell proliferation | Synovium | Rat | Naïve, capsaicin induced synovitis | 49 | |
| HUVECs cells in vitro | Human | Normal | 100 | ||
| Angiogenesis | Tumour | Mouse | Lewis lung carcinoma | 101 | |
| Bone/cartilage | Inhibition of hypertrophic differentiation | Cultured chondrocytes | Rat | Naive | 110 |
| Inhibition of osteoclast differentiation | Bone marrow cultures | Mouse | Naive | 111 | |
| Facilitation of osteoblast activity | Bone | Mouse | Naive | 112 | |
| Nociceptive pathways | Sensory neuron sensitization | Skin and joint in vivo | Rat | Naïve, MIA, MNX | 43,116 |
| Sensory neurones in vitro | Rat | Naive | 117 | ||
| Contribution to pain behaviour | Whole animal behaviour | Rat | Naïve, MIA, meniscal tear | 62,116,118,119 |
CGRP and peripheral sensitization
Several lines of evidence implicate roles for CGRP in the peripheral mechanisms of OA pain 60,61,66,118,120,121. CGRP sensitizes cutaneous nociceptors to noxious stimuli in vivo 116 and sensitizes sensory neurons in vitro 76,117. However, peripheral administration of a single dose of CGRP in the rat exhibits low potency in inducing acute hyperalgesia, but can lead to sensitization to CGRP itself or other inflammatory mediators following repeated administration 116, suggesting that under conditions of sustained CGRP release sensitization may become evident. In non-arthritic knees, CGRP-induced peripheral sensitization was characterized by increased response frequencies of joint-innervating nociceptive afferents to punctate stimulation of the joint capsule, and by reduced threshold mechanical force needed to evoke nociceptor firing 43. MIA-induced OA was also associated with peripheral sensitization, and the reduced thresholds observed after CGRP injection were similar to those observed in arthritic knees. Local CGRP injection further increased mechanically evoked response frequencies, above those frequencies observed in afferents from osteoarthritic knees without CGRP injection. The proportion of afferents that were sensitized by CGRP increased from 50% in normal knees, to 90% in osteoarthritic knees and this response was associated with an increase in the number of back-labelled knee afferents expressing the G-protein coupled receptor component CLR 43.
CGRP might sensitize peripheral sensory nerves by phosphorylation of tetrodotoxin-resistant (TTX-R) voltage-gated sodium channels 76,117,122 (NaV) via intracellular pathways involving PKA and C (Figure1). CGRP lowers the threshold for action potential generation 123 and enhances TTX-R NaV currents in DRG neurons in vitro 117, consistent with the in vivo reduction of joint nociceptor mechanical thresholds demonstrated in rat knees 43. Intra-articular injection of CGRP also increases phosphorylated forms of the MAP kinases p38 and ERK in sensory ganglia, which might additionally contribute to nociceptor sensitization 124. CGRP receptors are thought to be localized to sensory neurones expressing CGRP, raising the possibility of an autoreceptor role. MAP kinase phosphorylation is associated with augmented evoked release of CGRP 124, and therefore CGRP released by sensory neurones may act in an autocrine fashion to increase CGRP release further, thereby creating a potential positive feedback loop that might further increase sensitization and neurogenic inflammation.
Weightbearing asymmetry is associated with increased expression of CGRP 60,61 and its receptor components in knee innervating afferents 43, and with increased CGRP release from primary afferents after OA induction in rats by intra-articular injection of MIA 62. Furthermore, ablation of CGRP-expressing sensory terminals in knees prevents the development of MIA-induced weightbearing asymmetry 118. The involvement of CGRP in MIA-induced pain behaviour is further implicated by systemic administration of the peripherally restricted, non-peptide CGRP antagonist BIBN4096BS 120, and a humanized antibody directed against CGRP, LY2951742 62,119 each reducing weight-bearing asymmetry in rats with OA. LY2951742 demonstrated analgesic efficacy in the meniscal tear model for longer than 60 days following only a single injection, consistent with the long half-life of a monoclonal antibody. The analgesic effects of CGRP neutralization were thought to involve a prostaglandin independent mechanism which could have important implications for the treatment of pain in NSAID-insensitive patients. Local injection of the CGRP receptor antagonist CGRP8-37 significantly increased sensory nerve thresholds and reduced firing rates evoked by punctate stimulation of osteoarthritic rat knees, despite having no effect on afferents from non-arthritic knees 119. This contribution of CGRP to peripheral sensitization was dependent on the presence of OA, rather than on the chemical or surgical model of OA induction. Specificity for pathological pain responses associated with sensitization, while not affecting normal, potentially protective nociceptive pain, is a desirable characteristic of novel analgesics.
Similar increased contributions of TRPV1 45 and NGF 48 to afferent sensitivity and pain behaviour were observed following OA induction. Each, as with CGRP, was associated with increased expression of TRPV1 or the high affinity NGF receptor TrkA, respectively. As with CGRP receptor antagonists, local administration of TRPV1 inhibitors can reverse the increases in evoked response rates and decreases in mechanical thresholds of rat knee nociceptors in MIA-induced OA, as well as attenuate MIA-induced weightbearing asymmetry. Increased sensitivity of OA knees to weightbearing asymmetry induced by intra-articular NGF injection is inhibited by prior treatment with the non-selective cyclo-oxygenase inhibitor indomethacin, suggesting that inflammation may contribute to the development of this augmented pain state, either directly or through continuous nociceptive input 45. NGF blockade has been particularly successful at reducing OA pain in randomized controlled trials, but clinical development has been delayed by unexpected adverse events 125. Rare incidences of rapidly progressive OA were reported, some of which led to total joint replacement 49. This effect was not associated with analgesic potency, and might possibly reflect direct actions of NGF on non-neuronal cells. CGRP might act downstream of NGF, but further research is required to determine whether CGRP blockade can replicate the analgesic benefits of NGF blockade, whilst avoiding unwanted effects.
Central sensitization also contributes to arthritis pain, as suggested by widespread reductions in pressure pain thresholds 33, blunting of conditioned pain modulation 8 and alterations in central pain processing 9,45,64. CGRP knockout mice do not display secondary hyperalgesia after developing knee inflammation 99. Antibodies to CGRP are anti-inflammatory 126 and also reduced central sensitization in non-articular pain models 127 and, in the absence of CNS penetration, this is presumably mediated by peripheral CGRP blockade. Further research is needed to determine whether reduction of peripheral sensitization by CGRP blockade also reduces central sensitization associated with arthritis pain. Following the onset of joint pathology, pain progresses from primary nociception through peripheral and central sensitization, often leading to augmented pain states that have commonly been associated with chronic arthritis 35,128. The potential of peripherally directed treatments to prevent this pain progression offers hope that the current impact of arthritis pain can be avoided for future generations.
Clinical translation
Although a number of small molecule CGRP antagonists have shown efficacy against pain in migraine trials, off-target hepatoxicity has hampered further development for migraine and other therapeutic indications that might benefit from CGRP neutralization. As a result, recent attention has been given to the development of monoclonal antibodies targeting the CGRP pathway 129. There are three anti-CGRP and one CGRP receptor antibodies currently in clinical development. The three anti-CGRP antibodies in development are LY2951742 (Eli Lilly and Co.), ALD-403 (Alder Biopharmaceuticals) and TEV-48125 (Teva Pharmaceuticals). The CGRP receptor antibody is AMG334 (Amgen). Since these antibodies do not cross the blood–brain barrier these are thought to produce their analgesic effects by acting on peripheral targets. These monoclonal antibodies exhibit high level target specificity and long terminal half-lives and all are currently at phase IIb of clinical development 129. Proof-of-efficacy has been obtained in migraine and, to date, no safety concerns have been reported including any relevant cardiovascular related side effects. Long acting analgesics such as monoclonal antibodies have the particular potential to prevent, rather than merely treat, clinical pain, and initial studies in migraine have provided evidence that CGRP blockade might reduce the frequency of migraine attacks, as well as reducing their severity. Ely Lilly have commenced phase II clinical trials of their monoclonal antibody to CGRP in patients with OA 130. As such, these monoclonal antibodies are well placed to bring patient benefit in the near future.
Conclusions
At the level of the joint, CGRP acts on its receptor expressed on resident cells within the synovium (e.g. endothelial cells), on peripheral terminals of sensory neurons and on inflammatory cells 75,76. Actions of CGRP at these cellular targets are thought to influence angiogenesis, inflammation and peripheral sensitization, suggesting an important contribution to the pain and inflammation of chronic arthritis 65. Angiogenic effects of CGRP receptor activation on endothelial cells is thought to be mediated by adenylate cyclase activation and protein kinases 99, resulting in proliferation, migration and tube formation 100. An additional vascular effect of CGRP expected to promote inflammation is vasodilation 74, which is likely to augment plasma extravasation induced by substance P and other inflammatory mediators. Putative indirect mechanisms of peripheral sensitization driven by CGRP include the release of pro-inflammatory mediators from macrophages 131 which activate and/or sensitize sensory neurons 132. A direct mechanism might involve activation of sensory nerve fibres that express CGRP receptors and signalling via PKA and C, thereby further increasing sensitization through phosphorylation of tetrodotoxin-resistant NaV. Given these effects, CGRP might contribute to synovitis and arthritis pain, although further research is required to determine the specific contributions of CGRP to OA or RA pain, and the potential of CGRP blockade or receptor antagonists to improve patient outcomes. CGRP might make key contributions during the transition from normal to persistent synovitis, and during the progression from nociception to sensitization. Intervening to prevent these undesirable transitions in joint pathophysiology during early disease has the potential to prevent persistent inflammation and progressive pain. If antibodies to CGRP can replicate the improvements in arthritis pain observed with NGF blockers, without concomitant adverse events, the burden of arthritis could be substantially relieved.
CGRP, substance P, TrkA, TRPV1 and TRPA1 are synthesized in dorsal root ganglia and transported to peripheral terminals of primary afferents. TRPV1 or TRPA1 activation stimulates co-release of CGRP and substance P, which, in turn, act on G-protein coupled receptors (GPCR), both on resident cells within the synovium (e.g. endothelial cells), and on peripheral terminals of sensory neurons. CGRP induces release of pro-inflammatory mediators from inflammatory cells such as macrophages which further sensitize sensory neurons. NGF released from inflammatory cells binds TrkA and is retrogradely transported to the dorsal root ganglion, where it might induce increased CGRP expression. CGRP stimulates vasodilation, augmenting plasma extravasation induced by substance P and other inflammatory mediators. CGRP also activates adenylate cyclase (AC) and protein kinases (PK), and up-regulates VEGF expression in endothelial cells thereby stimulating angiogenesis. Endothelial cells might also express CGRP, resulting in a positive feedback loop, and CGRP acts on receptors on sensory nerve terminals thereby further increasing sensitization.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Financial support
PM is supported by Arthritis Research UK, grant number 18769.
References
- The economic costs of arthritis for the UK economy [Internet]. Oxford Economics. 2010.
- Walsh DA, McWilliams DF. Mechanisms, impact and management of pain in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:581–92. doi: 10.1038/nrrheum.2014.64. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Little CB, McDougall JJ. A commentary on modelling osteoarthritis pain in small animals. Osteoarthritis Cartilage. 2013;21:1316–26. doi: 10.1016/j.joca.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber U, Ostergaard M, Lambert RG, Maksymowych WP. The impact of MRI on the clinical management of inflammatory arthritides. Skeletal Radiol. 2011;40:1153–73. doi: 10.1007/s00256-011-1204-5. [DOI] [PubMed] [Google Scholar]
- McQueen FM. A vital clue to deciphering bone pathology: MRI bone oedema in rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 2007;66:1549–52. doi: 10.1136/ard.2007.082875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, Uthman I, Khy V, Tremblay JL, Bertrand C, Pelletier JP. Safety and efficacy of long-term intra-articular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–7. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- Laslett LL, Dore DA, Quinn SJ, Boon P, Ryan E, Winzenberg TM, Jones G. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis. 2012;71:1322–8. doi: 10.1136/annrheumdis-2011-200970. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–81. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, Tracey I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–34. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, Turner JM, Hathway GJ, Bennett AJ, Walsh DA, Kendall DA, Lichtman A, Chapman V. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PLoS One. 2013;8:e80440. doi: 10.1371/journal.pone.0080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloe L, Manni L, Sebastiani G, Tuveri MA. Nerve growth factor in the synovia of patients with rheumatoid arthritis: correlation with TNF-alpha and IL-1 beta and possible functional significance. Clin Exp Rheumatol. 1999;17:632–3. [PubMed] [Google Scholar]
- Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 2014;66:3018–27. doi: 10.1002/art.38778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363:1521–31. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiseo PJ, Kivitz AJ, Ervin JE, Ren H, Mellis SJ. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain. 2014;155:1245–52. doi: 10.1016/j.pain.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Nagasako EM, Oaklander AL, Dworkin RH. Congenital insensitivity to pain: an update. Pain. 2003;101:213–9. doi: 10.1016/S0304-3959(02)00482-7. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Andruski B, Schuelert N, Hallgrimsson B, Matyas JR. Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley guinea pigs. Pain. 2009;141(3):222–32. doi: 10.1016/j.pain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Gaffen JD, Gleave SJ, Crossman MV, Bayliss MT, Mason RM. Articular cartilage proteoglycans in osteoarthritic STR/Ort mice. Osteoarthritis Cartilage. 1995;3:95–104. doi: 10.1016/s1063-4584(05)80042-1. [DOI] [PubMed] [Google Scholar]
- Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage. 2001;9:85–91. doi: 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, Rodan GA, Duong le T. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–43. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Ferland CE, Laverty S, Beaudry F, Vachon P. Gait analysis and pain response of two rodent models of osteoarthritis. Pharmacol Biochem Behav. 2011;97:603–10. doi: 10.1016/j.pbb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Ashraf S, Mapp PI, Walsh DA. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011;63:2700–10. doi: 10.1002/art.30422. [DOI] [PubMed] [Google Scholar]
- Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, Malfait AM. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc Natl Acad Sci USA. 2012;109:20602–7. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Burston JJ, Hathway GJ, Woodhams SG, Pearson RG, Bennett AJ, Kendall DA, Scammell BE, Chapman V. The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol Pain. 2011;7:88. doi: 10.1186/1744-8069-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent TL, Williams RO, Maciewicz R, Silman A, Garside P. Arthritis Research UKamwg. Mapping pathogenesis of arthritis through small animal models. Rheumatology (Oxford) 2012;51:1931–41. doi: 10.1093/rheumatology/kes035. [DOI] [PubMed] [Google Scholar]
- Monach PA, Mathis D, Benoist C. The K/BxN arthritis model. Curr Protoc Immunol. doi: 10.1002/0471142735.im1522s81. 2008; Chapter 15:Unit 15 22. [DOI] [PubMed] [Google Scholar]
- Di Paola R, Cuzzocrea S. Predictivity and sensitivity of animal models of arthritis. Autoimmun Rev. 2008;8:73–5. doi: 10.1016/j.autrev.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol. 2003;225:115–21. doi: 10.1385/1-59259-374-7:115. [DOI] [PubMed] [Google Scholar]
- Almarestani L, Fitzcharles MA, Bennett GJ, Ribeiro-da-Silva A. Imaging studies in Freund's complete adjuvant model of regional polyarthritis, a model suitable for the study of pain mechanisms, in the rat. Arthritis Rheum. 2011;63:1573–81. doi: 10.1002/art.30303. [DOI] [PubMed] [Google Scholar]
- Ivanavicius SP, Blake DR, Chessell IP, Mapp PI. Isolectin B4 binding neurons are not present in the rat knee joint. Neuroscience. 2004;128:555–60. doi: 10.1016/j.neuroscience.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Wharton J, Blake DR, Polak JM. Species and tissue specificity of vasoactive regulatory peptides. Int J Tissue React. 1993;15:109–24. [PubMed] [Google Scholar]
- Suokas AK, Sagar DR, Mapp PI, Chapman V, Walsh DA. Design, study quality and evidence of analgesic efficacy in studies of drugs in models of OA pain: a systematic review and a meta-analysis. Osteoarthritis Cartilage. 2014;22:1207–23. doi: 10.1016/j.joca.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Sagar DR, Suokas AK, Kelly S, Walsh DA, Chapman V. Mechanisms of nociception in models of osteoarthritic pain. In: Graven-Nielsen T, Arendt-Nielsen L, editors. Musculosketal Pain basic mechanisms and implication. Wolters Kluwer, Washington: IASP Press; 2014. . Chapter 18. In:. ISBN 978-0-931092-23-7. [Google Scholar]
- Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, Arendt-Nielsen L, Zhang W. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20:1075–85. doi: 10.1016/j.joca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience. 2003;120:677–94. doi: 10.1016/s0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnett NW, Grady EF. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol. 2005;490:239–55. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- Mapp PI, McWilliams DF, Turley MJ, Hargin E, Walsh DA. A role for the sensory neuropeptide calcitonin gene-related peptide in endothelial cell proliferation in vivo. Br J Pharmacol. 2012;166:1261–71. doi: 10.1111/j.1476-5381.2012.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buma P, Verschuren C, Versleyen D, Van der Kraan P, Oestreicher AB. Calcitonin gene-related peptide, substance P and GAP-43/B-50 immunoreactivity in the normal and arthrotic knee joint of the mouse. Histochemistry. 1992;98:327–39. doi: 10.1007/BF00270017. [DOI] [PubMed] [Google Scholar]
- Imai S, Tokunaga Y, Maeda T, Kikkawa M, Hukuda S. Calcitonin gene-related peptide, substance P, and tyrosine hydroxylase-immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J Orthop Res. 1997;15:133–40. doi: 10.1002/jor.1100150120. [DOI] [PubMed] [Google Scholar]
- McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, Dawbarn D, Vincent TL, Inglis JJ. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149:386–92. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Sanga P, Katz N, Polverejan E, Wang S, Kelly KM, Haeussler J, Thipphawong J. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain. 2013;154:1910–9. doi: 10.1016/j.pain.2013.05.051. [DOI] [PubMed] [Google Scholar]
- Bullock CM, Wookey P, Bennett A, Mobasheri A, Dickerson I, Kelly S. Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol. 2014;66:2188–200. doi: 10.1002/art.38656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Dunham JP, Murray F, Read S, Donaldson LF, Lawson SN. Spontaneous firing in C-fibers and increased mechanical sensitivity in A-fibers of knee joint-associated mechanoreceptive primary afferent neurones during MIA-induced osteoarthritis in the rat. Osteoarthritis Cartilage. 2012;20:305–13. doi: 10.1016/j.joca.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Kelly S, Chapman RJ, Woodhams S, Sagar DR, Turner J, Burston JJ, Bullock C, Paton K, Huang J, Wong A, McWilliams DF, Okine BN, Barrett DA, Hathway GJ, Walsh DA, Chapman V. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis. 2015;74:252–9. doi: 10.1136/annrheumdis-2013-203413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Yousef A, McWilliams DF, Hill R, Hargin E, Wilson D. Evaluation of a Photographic Chondropathy Score (PCS) for pathological samples in a study of inflammation in tibiofemoral osteoarthritis. Osteoarthritis Cartilage. 2009;17:304–12. doi: 10.1016/j.joca.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Wenham CY, Conaghan PG. The role of synovitis in osteoarthritis. Ther Adv Musculoskelet Dis. 2010;2:349–59. doi: 10.1177/1759720X10378373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S, Mapp PI, Burston J, Bennett AJ, Chapman V, Walsh DA. Augmented pain behavioural responses to intra-articular injection of nerve growth factor in two animal models of osteoarthritis. Ann Rheum Dis. 2014;73:1710–8. doi: 10.1136/annrheumdis-2013-203416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–8. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- Stevens CR, Blake DR, Merry P, Revell PA, Levick JR. A comparative study by morphometry of the microvasculature in normal and rheumatoid synovium. Arthritis Rheum. 1991;34:1508–13. doi: 10.1002/art.1780341206. [DOI] [PubMed] [Google Scholar]
- Suri S, Gill SE. Massena de Camin S, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66:1423–8. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, Walsh DA. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann Rheum Dis. 2011;70:523–9. doi: 10.1136/ard.2010.137844. [DOI] [PubMed] [Google Scholar]
- Mapp PI. Innervation of the synovium. Ann Rheum Dis. 1995;54:398–403. doi: 10.1136/ard.54.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta A, Corradini C, Verdoia C, Miani A, Castano S, Castano P. The nervous structures of anterior cruciate ligament of human knee, healthy and lesioned, studied with confocal scanning laser microscopy. Ital J Anat Embryol. 2004;109:167–76. [PubMed] [Google Scholar]
- Reimann I, Christensen SB. A histological demonstration of nerves in subchondral bone. Acta Orthop Scand. 1977;48:345–52. doi: 10.3109/17453677708992006. [DOI] [PubMed] [Google Scholar]
- Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264:469–80. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Kidd BL, Gibson SJ, Terry JM, Revell PA, Ibrahim NB, Blake DR, Polak JM. Substance P, calcitonin gene-related peptide and C-flanking peptide of neuropeptide Y-immunoreactive fibres are present in normal synovium but depleted in patients with rheumatoid arthritis. Neuroscience. 1990;37:143–53. doi: 10.1016/0306-4522(90)90199-e. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Walsh DA, Garrett NE, Kidd BL, Cruwys SC, Polak JM, Blake DR. Effect of three animal models of inflammation on nerve fibres in the synovium. Ann Rheum Dis. 1994;53:240–6. doi: 10.1136/ard.53.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton PC, Wilson AW, Bountra C, Chessell IP, Day NC. Changes in dorsal root ganglion CGRP expression in a chronic inflammatory model of the rat knee joint: differential modulation by rofecoxib and paracetamol. Eur J Pain. 2007;11:283–9. doi: 10.1016/j.ejpain.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005;388:75–80. doi: 10.1016/j.neulet.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Ferreira-Gomes J, Adaes S, Sarkander J, Castro-Lopes JM. Phenotypic alterations of neurons that innervate osteoarthritic joints in rats. Arthritis Rheum. 2010;62:3677–85. doi: 10.1002/art.27713. [DOI] [PubMed] [Google Scholar]
- Puttfarcken PS, Han P, Joshi SK, Neelands TR, Gauvin DM, Baker SJ, Lewis LG, Bianchi BR, Mikusa JP, Koenig JR, Perner RJ, Kort ME, Honore P, Faltynek CR, Kym PR, Reilly RM. A-995662 [(R)-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydronaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain. 2010;150:319–26. doi: 10.1016/j.pain.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Ogbonna AC, Clark AK, Gentry C, Hobbs C, Malcangio M. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur J Pain. 2013;17:514–26. doi: 10.1002/j.1532-2149.2012.00223.x. [DOI] [PubMed] [Google Scholar]
- Im HJ, Kim JS, Li X, Kotwal N, Sumner DR, van Wijnen AJ, Davis FJ, Yan D, Levine B, Henry JL, Desevre J, Kroin JS. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010;62:2995–3005. doi: 10.1002/art.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Hu DE, Mapp PI, Polak JM, Blake DR, Fan TP. Innervation and neurokinin receptors during angiogenesis in the rat sponge granuloma. Histochem J. 1996;28:759–69. doi: 10.1007/BF02272149. [DOI] [PubMed] [Google Scholar]
- Saxler G, Loer F, Skumavc M, Pfortner J, Hanesch U. Localization of SP- and CGRP-immunopositive nerve fibers in the hip joint of patients with painful osteoarthritis and of patients with painless failed total hip arthroplasties. Eur J Pain. 2007;11:67–74. doi: 10.1016/j.ejpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Saito T, Koshino T. Distribution of neuropeptides in synovium of the knee with osteoarthritis. Clin Orthop Relat Res. 2000;376:172–82. doi: 10.1097/00003086-200007000-00024. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci. 1999;11:1698–704. doi: 10.1046/j.1460-9568.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Hochman JR, Davis AM, Elkayam J, Gagliese L, Hawker GA. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1236–42. doi: 10.1016/j.joca.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Ivanavicius SP, Ball AD, Heapy CG, Westwood FR, Murray F, Read SJ. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterisation. Pain. 2007;128:272–82. doi: 10.1016/j.pain.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Hay DL, Conner AC, Howitt SG, Takhshid MA, Simms J, Mahmoud K, Poyner DR. The pharmacology of CGRP-responsive receptors in cultured and transfected cells. Peptides. 2004;25:2019–26. doi: 10.1016/j.peptides.2004.06.007. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Uzan B, Ea HK, Launay JM, Garel JM, Champy R, Cressent M, Liote F. A critical role for adrenomedullin-calcitonin receptor-like receptor in regulating rheumatoid fibroblast-like synoviocyte apoptosis. J Immunol. 2006;176:5548–58. doi: 10.4049/jimmunol.176.9.5548. [DOI] [PubMed] [Google Scholar]
- McMurdo L, Lockhart JC, Ferrell WR. Modulation of synovial blood flow by the calcitonin gene-related peptide (CGRP) receptor antagonist, CGRP(8-37) Br J Pharmacol. 1997;121:1075–80. doi: 10.1038/sj.bjp.0701237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assas BM, Pennock JI, Miyan JA. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front Neurosci. 2014;8:23. doi: 10.3389/fnins.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segond von Banchet G, Pastor A, Biskup C, Schlegel C, Benndorf K, Schaible HG. Localization of functional calcitonin gene-related peptide binding sites in a subpopulation of cultured dorsal root ganglion neurons. Neuroscience. 2002;110:131–45. doi: 10.1016/s0306-4522(01)00547-4. [DOI] [PubMed] [Google Scholar]
- Castaneda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, Ghilardi JR, Mantyh PW. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience. 2011;178:196–207. doi: 10.1016/j.neuroscience.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362–4. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Garrett NE, Kidd BL, Cruwys SC, Tomlinson DR. Changes in preprotachykinin mRNA expression and substance P levels in dorsal root ganglia of monoarthritic rats: comparison with changes in synovial substance P levels. Brain Res. 1995;675:203–7. doi: 10.1016/0006-8993(95)00066-y. [DOI] [PubMed] [Google Scholar]
- Malin S, Molliver D, Christianson JA, Schwartz ES, Cornuet P, Albers KM, Davis BM. TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci. 2011;31:10516–28. doi: 10.1523/JNEUROSCI.2992-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan G, Hoffmeister C, Rossato MF, Oliveira SM, Silva MA, Silva CR, Fusi C, Tonello R, Minocci D, Guerra GP, Materazzi S, Nassini R, Geppetti P, Ferreira J. TRPA1 receptor stimulation by hydrogen peroxide is critical to trigger hyperalgesia and inflammation in a model of acute gout. Free Radic Biol Med. 2014;72:200–9. doi: 10.1016/j.freeradbiomed.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Garrison SR, Stucky CL. Contribution of transient receptor potential ankyrin 1 to chronic pain in aged mice with complete Freund's adjuvant-induced arthritis. Arthritis Rheumatol. 2014;66:2380–90. doi: 10.1002/art.38724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes ES, Russell FA, Spina D, McDougall JJ, Graepel R, Gentry C, Staniland AA, Mountford DM, Keeble JE, Malcangio M, Bevan S, Brain SD. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor alpha-induced inflammatory hyperalgesia and Freund's complete adjuvant-induced monarthritis. Arthritis Rheum. 2011;63:819–29. doi: 10.1002/art.30150. [DOI] [PubMed] [Google Scholar]
- Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, Segreti JA, Han P, Zhang XF, Niforatos W, Bianchi BR, Baker SJ, Zhong C, Simler GH, McDonald HA, Schmidt RG, McGaraughty SP, Chu KL, Faltynek CR, Kort ME, Reilly RM, Kym PR. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–72. doi: 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- Elg S, Marmigere F, Mattsson JP, Ernfors P. Cellular subtype distribution and developmental regulation of TRPC channel members in the mouse dorsal root ganglion. J Comp Neurol. 2007;503:35–46. doi: 10.1002/cne.21351. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology. 1997;37:133–52. doi: 10.1016/s0162-3109(97)00055-6. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Buckley TL, Brain SD, Collins PD, Williams TJ. Inflammatory edema induced by interactions between IL-1 and the neuropeptide calcitonin gene-related peptide. J Immunol. 1991;146:3424–30. [PubMed] [Google Scholar]
- Cruwys SC, Kidd BL, Mapp PI, Walsh DA, Blake DR. The effects of calcitonin gene-related peptide on formation of intra-articular oedema by inflammatory mediators. Br J Pharmacol. 1992;107:116–9. doi: 10.1111/j.1476-5381.1992.tb14472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Ganea D. Anti-inflammatory neuropeptides: a new class of endogenous immunoregulatory agents. Brain Behav Immun. 2008;22:1146–51. doi: 10.1016/j.bbi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof AK, Gluck L, Gajda M, Lupp A, Brauer R, Schaible HG, Schulz S. Differential antiinflammatory and antinociceptive effects of the somatostatin analogs octreotide and pasireotide in a mouse model of immune-mediated arthritis. Arthritis Rheum. 2011;63:2352–62. doi: 10.1002/art.30410. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Szabo A, Nemeth J, Jakab B, Pinter E, Banvolgyi A, Kereskai L, Keri G, Szolcsanyi J. Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund's adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004;50:1677–85. doi: 10.1002/art.20184. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Kerslake S, Brain SD, Blake DR, Cambridge H. The effect of intra-articular capsaicin on nerve fibres within the synovium of the rat knee joint. J Chem Neuroanat. 1996;10:11–8. doi: 10.1016/0891-0618(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Wade M, Mapp PI, Blake DR. Focally regulated endothelial proliferation and cell death in human synovium. Am J Pathol. 1998;152:691–702. [PMC free article] [PubMed] [Google Scholar]
- Vogel KS. Development of trophic interactions in the vertebrate peripheral nervous system. Mol Neurobiol. 1993;7:363–82. doi: 10.1007/BF02769183. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457–65. [PubMed] [Google Scholar]
- Seegers HC, Hood VC, Kidd BL, Cruwys SC, Walsh DA. Enhancement of angiogenesis by endogenous substance P release and neurokinin-1 receptors during neurogenic inflammation. J Pharmacol Exp Ther. 2003;306:8–12. doi: 10.1124/jpet.103.050013. [DOI] [PubMed] [Google Scholar]
- Zheng S, Li W, Xu M, Bai X, Zhou Z, Han J, Shyy JY, Wang X. Calcitonin gene-related peptide promotes angiogenesis via AMP-activated protein kinase. Am J Physiol Cell Physiol. 2010;299:C1485–92. doi: 10.1152/ajpcell.00173.2010. [DOI] [PubMed] [Google Scholar]
- Haegerstrand A, Dalsgaard CJ, Jonzon B, Larsson O, Nilsson J. Calcitonin gene-related peptide stimulates proliferation of human endothelial cells. Proc Natl Acad Sci U S A. 1990;87:3299–303. doi: 10.1073/pnas.87.9.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M, Suzuki T, Hosono K, Hayashi I, Hashiba S, Onuma Y, Amano H, Kurihara Y, Kurihara H, Okamoto H, Hoka S, Majima M. Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc Natl Acad Sci U S A. 2008;105:13550–5. doi: 10.1073/pnas.0800767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YL, Reddy DM, Green KE, Chauhan MS, Wang HQ, Nagamani M, Hankins GD, Yallampalli C. Calcitonin gene-related peptide (CALCA) is a proangiogenic growth factor in the human placental development. Biol Reprod. 2007;76:892–9. doi: 10.1095/biolreprod.106.059089. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, Blucher N, Fox SB, Bicknell R, Smith DM, Rees MC. Adrenomedullin and CGRP interact with endogenous calcitonin-receptor-like receptor in endothelial cells and induce its desensitisation by different mechanisms. J Cell Sci. 2006;119:910–22. doi: 10.1242/jcs.02783. [DOI] [PubMed] [Google Scholar]
- Tuo Y, Guo X, Zhang X, Wang Z, Zhou J, Xia L, Zhang Y, Wen J, Jin D. The biological effects and mechanisms of calcitonin gene-related peptide on human endothelial cell. J Recept Signal Transduct Res. 2013;33:114–23. doi: 10.3109/10799893.2013.770528. [DOI] [PubMed] [Google Scholar]
- Luo D, Zhang YW, Peng WJ, Peng J, Chen QQ, Li D, Deng HW, Li YJ. Transient receptor potential vanilloid 1-mediated expression and secretion of endothelial cell-derived calcitonin gene-related peptide. Regul Pept. 2008;150:66–72. doi: 10.1016/j.regpep.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Itoh H, Sawada N, Fukunaga Y, Sone M, Yamahara K, Yurugi T, Nakao K. Adrenomedullin promotes proliferation and migration of cultured endothelial cells. Hypertens Res. 2003;26(Suppl):S93–8. doi: 10.1291/hypres.26.s93. [DOI] [PubMed] [Google Scholar]
- Hu DE, Hiley CR, Smither RL, Gresham GA, Fan TP. Correlation of 133Xe clearance, blood flow and histology in the rat sponge model for angiogenesis. Further studies with angiogenic modifiers. Lab Invest. 1995;72:601–10. [PubMed] [Google Scholar]
- Ashraf S, Mapp PI, Walsh DA. Angiogenesis and the persistence of inflammation in a rat model of proliferative synovitis. Arthritis Rheum. 2010;62:1890–8. doi: 10.1002/art.27462. [DOI] [PubMed] [Google Scholar]
- Lehner B, Koeck FX, Capellino S, Schubert TE, Hofbauer R, Straub RH. Preponderance of sensory versus sympathetic nerve fibers and increased cellularity in the infrapatellar fat pad in anterior knee pain patients after primary arthroplasty. J Orthop Res. 2008;26:342–50. doi: 10.1002/jor.20498. [DOI] [PubMed] [Google Scholar]
- Edoff K, Hildebrand C. Neuropeptide effects on rat chondrocytes and perichondrial cells in vitro. Neuropeptides. 2003;37:316–8. doi: 10.1016/j.npep.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Bava U, Kamona SA, Cooper GJ, Reid IR. Effects of calcitonin, amylin, and calcitonin gene-related peptide on osteoclast development. Bone. 2001;29:162–8. doi: 10.1016/s8756-3282(01)00494-x. [DOI] [PubMed] [Google Scholar]
- Schinke T, Liese S, Priemel M, Haberland M, Schilling AF, Catala-Lehnen P, Blicharski D, Rueger JM, Gagel RF, Emeson RB, Amling M. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J Bone Miner Res. 2004;19:2049–56. doi: 10.1359/JBMR.040915. [DOI] [PubMed] [Google Scholar]
- Mrak E, Guidobono F, Moro G, Fraschini G, Rubinacci A, Villa I. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by beta-catenin stabilization. J Cell Physiol. 2010;225:701–8. doi: 10.1002/jcp.22266. [DOI] [PubMed] [Google Scholar]
- Offley SC, Guo TZ, Wei T, Clark JD, Vogel H, Lindsey DP, Jacobs CR, Yao W, Lane NE, Kingery WS. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res. 2005;20:257–67. doi: 10.1359/JBMR.041108. [DOI] [PubMed] [Google Scholar]
- Salo PT, Seeratten RA, Erwin WM, Bray RC. Evidence for a neuropathic contribution to the development of spontaneous knee osteoarthrosis in a mouse model. Acta Orthop Scand. 2002;73:77–84. doi: 10.1080/000164702317281459. [DOI] [PubMed] [Google Scholar]
- Nakamura-Craig M, Gill BK. Effect of neurokinin A, substance P and calcitonin gene related peptide in peripheral hyperalgesia in the rat paw. Neurosci Lett. 1991;124:49–51. doi: 10.1016/0304-3940(91)90819-f. [DOI] [PubMed] [Google Scholar]
- Natura G, von Banchet GS, Schaible HG. Calcitonin gene-related peptide enhances TTX-resistant sodium currents in cultured dorsal root ganglion neurons from adult rats. Pain. 2005;116:194–204. doi: 10.1016/j.pain.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Kalff KM, El Mouedden M, van Egmond J, Veening J, Joosten L, Scheffer GJ, Meert T, Vissers K. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol. 2010;641:108–13. doi: 10.1016/j.ejphar.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Collins EC, Darling RJ, Allan BW, Leung D, Conner EM, Nelson J, Gaynor B, Xu J, Wang XF, Lynch RA, Li B, McCarty D, Oskins JL, Lin C, Johnson KW, Chambers MG. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage. 2014;22:578–85. doi: 10.1016/j.joca.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Hirsch S, Corradini L, Just S, Arndt K, Doods H. The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. Pain. 2013;154:700–7. doi: 10.1016/j.pain.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Ishida K, Kawamata T, Tanaka S, Shindo T, Kawamata M. Calcitonin gene-related peptide is involved in inflammatory pain but not in postoperative pain. Anesthesiology. 2014;121:1068–79. doi: 10.1097/ALN.0000000000000364. [DOI] [PubMed] [Google Scholar]
- Ryu PD, Gerber G, Murase K, Randic M. Calcitonin gene-related peptide enhances calcium current of rat dorsal root ganglion neurons and spinal excitatory synaptic transmission. Neurosci Lett. 1988;89:305–12. doi: 10.1016/0304-3940(88)90544-7. [DOI] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–55. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel MF, Lane NE. Control of arthritis pain with anti-nerve-growth factor: risk and benefit. Curr Rheumatol Rep. 2012;14:583–8. doi: 10.1007/s11926-012-0289-8. [DOI] [PubMed] [Google Scholar]
- Bowler KE, Worsley MA, Broad L, Sher E, Benschop R, Johnson K, Yates JM, Robinson PP, Boissonade FM. Evidence for anti-inflammatory and putative analgesic effects of a monoclonal antibody to calcitonin gene-related peptide. Neuroscience. 2013;228:271–82. doi: 10.1016/j.neuroscience.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Storer RJ, Supronsinchai W, Srikiatkhachorn A. Animal models of chronic migraine. Curr Pain Headache Rep. 2015;19:467. doi: 10.1007/s11916-014-0467-7. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Hauser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia - I: examination of rates and predictors in patients with rheumatoid arthritis (RA) Pain. 2011;152:291–9. doi: 10.1016/j.pain.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Walter S, Rapoport AM. Therapeutic antibodies against CGRP or its receptor. Br J Clin Pharmacol. 2015 doi: 10.1111/bcp.12591. . doi: 10.111/bcp12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2014. A study of LY2951742 in participants with mild to moderate osteoarthritis knee pain [Internet]. Clinical Trials.gov identifier: NCT0219292190 (last accessed 7 May 2015)
- Yaraee R, Ebtekar M, Ahmadiani A, Sabahi F. Neuropeptides (SP and CGRP) augment pro-inflammatory cytokine production in HSV-infected macrophages. Int Immunopharmacol. 2003;3:1883–7. doi: 10.1016/S1567-5769(03)00201-7. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


