Abstract
The efficacy of calcitonin gene-related peptide (receptor) (CGRP-(R)) blocking therapeutics in the treatment of acute migraine headache provided proof-of-concept for the involvement of CGRP in the pathophysiology of this disorder. One of the major hurdles for the development of any class of drugs, including CGRP blocking therapeutics, is the early clinical development process during which toxic and inefficacious compounds need to be eliminated as early as possible in order to focus on the most promising molecules. At this stage, human models providing proof of target engagement, combined with safety and tolerability studies, are extremely valuable in focusing on those therapeutics that have the highest engagement from the lowest exposure. They guide the go/no-go decision making, establish confidence in the candidate molecule by de-risking toxicity and safety issues and thereby speed up the early clinical development.
In this review the focus is on the so called ‘capsaicin model’ as a typical example of a target engagement biomarker used as a human model for the development of CGRP blocking therapeutics. By applying capsaicin onto the skin, TRPV1 channels are activated and a CGRP-mediated increase in dermal blood flow can be quantified with laser Doppler perfusion imaging. Effective CGRP blocking therapeutics in turn, display blockade of this response. The translation of this biomarker model from animals to humans is discussed as well as the limitations of the assay in predicting the efficacy of anti-migraine drugs.
Keywords: capsaicin model, CGRP blocking therapeutics, drug efficacy, migraine, target engagement biomarker, TRPV1
Introduction
Migraine is a chronic, incapacitating neurovascular disorder. According to the World Health Organization, 324 million people worldwide suffer from migraine 1. It is characterized by attacks of moderate to severe headache and autonomic nervous system dysfunction. In approximately one-third of migraine patients, the headache attacks are accompanied by an aura involving neurological symptoms such as transient visual, sensory, language or motor disturbances 2.
For many years the aetiology of migraine has been under investigation. The current general consensus is unified in the so called ‘trigeminovascular theory’. This theory argues that the trigeminovascular system (TGVS) is the main anatomic substrate for the pathophysiology of migraine. The TGVS encompasses the meninges and blood vessels as pain sensitive intracranial structures as well as the major afferent pathways (i.e. the trigeminal nerve) for transmitting pain to the central nervous system 3,4. Activation of the TGVS results in cranial vasodilatation mediated by the release of vasoactive neuropeptides, including calcitonin gene-related peptide (CGRP) 5. It is primarily located in small, unmyelinated sensory C fibres and myelinated Aδ fibres in the periphery. CGRP is usually found in nerves that are closely associated with other peptides in C fibres, in particular tachykinins, substance P and neurokinin A 6.
CGRP is a 37 amino acid neuropeptide which is widely distributed in the central and peripheral nervous system 7–9. It binds to the CGRP receptor, which is known as a calcitonin-like receptor (CLR) and belongs to the family of G-protein-coupled receptors (GPCRs) 10. The CGRP receptor is located on target cells in the surrounding tissue such as mast cells, immune cells and vascular smooth muscle cells (VSMCs) 11,12. By interaction with VSMCs, CGRP is known to be a very potent vasodilator. The resulting response, in peripheral tissues, is mainly characterized by local redness and warmth (secondary to vasodilatation), limited swelling and allodynia (i.e. hypersensitivity to heat and touch secondary to alterations in the excitability of primary sensory neurons). Collectively, these changes are referred to as ‘neurogenic inflammation’, because of the inflammatory symptoms resulting from the release of substances from the afferent fibres of primary sensory neurons 13,14.
The role of vasodilatation and neurogenic inflammation in migraine is supported by the fact that triptans, which are currently the most effective class of acute anti-migraine drugs, cause relative selective cranial vasoconstriction by 5-HT1B activation on VSMCs while they also inhibit presynaptic neuropeptide release by 5-HT1D activation 15–18. The release of CGRP from afferent fibres is considered pivotal in the pathogenesis of migraine. This is supported by the observation that intravenous infusions of CGRP induce dilatation of the middle meningeal artery and induce migraine-like headache in migraine patients 19,20. The involvement of CGRP was first confirmed by small molecule CGRP-R antagonists including olcegepant (i.e. BIBN4096BS), telcagepant (i.e. MK-0974) and MK-3207 which showed clear efficacy in phase II and/or phase III clinical trials in the treatment of acute migraine. Later on, the humanized monoclonal antibodies (mAbs) of Alder Biopharmaceuticals (ALD-403) and Eli Lily and Co. (LY2951742), which target the CGRP ligand itself, showed positive proof-of-concept (PoC) study results with a single dose (Phase 2a). Currently, these antibodies are in Phase 2b (dose-ranging study) of clinical development. The same accounts for AMG 334 (a fully human mAb targeting the CGRP receptor) and TEV-48125 (fully humanized antibody targeting the CGRP ligand) 21–24. Further on in this review, we will refer to these drugs collectively as CGRP blocking therapeutics irrespective of the fact whether they target the CGRP receptor or CGRP as ligand.
The capsaicin model for neurogenic inflammation
One way of investigating the role of CGRP in the pathophysiology of migraine and evaluating target engagement of CGRP blocking therapeutics, is by making use of what is commonly referred to as the ‘capsaicin model’.
Capsaicin is the pungent ingredient in hot chilli peppers activating the transient receptor potential vanilloid type 1 receptor (TRPV1). The TRPV1 receptor belongs to the TRP cation channel subfamily and is expressed on a subpopulation of primary sensory neurons consisting of Aδ and C fibre nociceptors. Although several putative endogenous ligands of the TRPV1 receptor have been identified, their physiological and pathophysiological effects in the sensory nervous system remain unclear. In contrast, the effect of binding of the exogenous ligand capsaicin with the TRPV1 receptor is well known to provoke the release of a number of bioactive substances including CGRP (Figure1) 25,26. Activation of the TRPV1 receptor leads to the non-selective influx of cations, nerve fibre depolarization and the subsequent release of neuropeptides by exocytosis. If induced in peripheral tissues, such as the skin, the resulting vasodilation can be clearly observed. Over time, a repetitive release can lead to depletion of neuropeptides and desensitization of sensory nerves. Indeed, long term treatment with capsaicin in order to deplete the sensory neurogenic component has been exploited in a beneficial manner to treat hyperalgesia and pain conditions in humans, for example neuropathic pain after a herpes zoster infection 27. Apart from chemical stimuli, also physical stimuli such as low pH and heat can activate the TRPV1 receptor and induce the release of CGRP 28,29.
Figure 1.
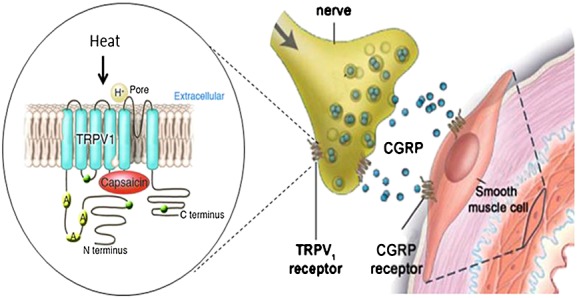
Activation of the TRPV1 receptor by capsaicin causes CGRP release. Figure1 is adapted, with permission, from Hershey et al. 34
Capsaicin-induced vasodilatation is blocked with the TRPV1 antagonist capsazepine as well as by CGRP blocking therapeutics 30. This confirms that vasodilatation, induced by capsaicin, is mediated by the release of CGRP. TRPV1 activation might play a major role in the release of CGRP from trigeminal nerves 31. TRPV1 receptors are found on neurons in the trigeminal and dorsal root ganglia 32. The peripheral terminals of capsaicin-sensitive neurons are sites of release for various pro-inflammatory neuropeptides, including CGRP. Hence, it has been suggested that activation of TRPV1 possibly initiates a migraine attack by causing CGRP release.
Investigating CGRP and CGRP blocking therapeutics with the capsaicin model
The complexity of migraine as a disease, the lack of translational models and the increasing drug development costs hamper the development of successful anti-migraine drugs. In order to tackle these challenges, the use of biomarkers to evaluate the potential of new targets at the earliest possible clinical stage of drug development has gained more interest. With the use of biomarkers one attempts to demonstrate target engagement at an early stage in order to obtain confidence in a drug candidate and to facilitate the further clinical drug development process.
Already in 1985, Helme et al. studied the dermal neurogenic inflammation that occurs after the topical application of capsaicin on the human skin. They assessed both the size of the flare response, induced by an increase in blood flow, and allodynia 14. Several years later, Brain et al. investigated the nature of the vasodilatating mediator(s) involved in this response. Capsaicin topically applied to the mouse ear induced significant increases in blood flow and oedema. Blood flow was assessed by laser Doppler flowmetry and oedema formation by 125I-albumin accumulation. The CGRP-R antagonist CGRP8–37, a CGRP fragment, abolished the increased blood flow to capsaicin in wild-type mice, which indicates that the capsaicin-induced increased blood flow involves activation of, and possible interactions with, CGRP receptors 33. Hereafter, Hershey et al. 34 developed a non-invasive pharmacodynamic capsaicin model in monkeys (Figure2A). Topical application of capsaicin was utilized to induce the release of endogenous CGRP and a vasodilatatory response which can be measured using laser Doppler imaging. Using the potent and selective CGRP antagonist compound 3, which is an analogue of the well-characterized compound BIBN4096BS, they demonstrated 62% inhibition in rat with 300 µg kg−1 i.v. and complete inhibition with only 30 µg kg−1 i.v.in the rhesus monkey. At the doses studied, compound 3 was equally effective on both the acute and prophylactic inhibition of CGRP-mediated vasodilation. This was the first non-invasive model in non-human primates that allowed rapid evaluation of CGRP-R antagonist activity against endogenous CGRP 34.
Figure 2.
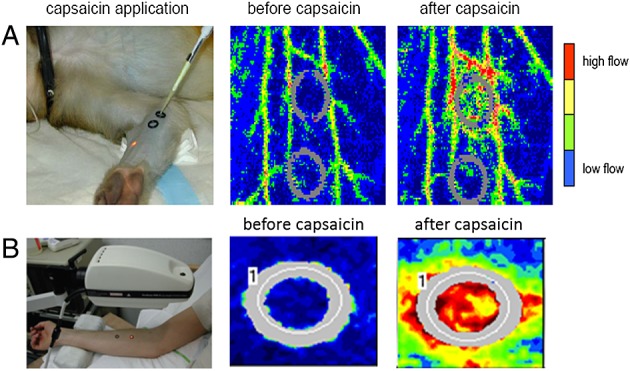
Non-invasive preclinical capsaicin model in primates (A) vs. humans (B). Capsaicin application clearly induces an increase in dermal blood flow, measured with laser Doppler imaging. Figure2 is adapted, with permission, from (A) Hershey et al. 34 and (B) Sinclair et al. 38
The capsaicin-induced dermal blood flow (CIDBF) model was translated into a human in vivo pharmacodynamic model by de Hoon et al. (Figures2B and 3) 35,36. The human CIDBF model involves the topical application of capsaicin on the human forearm, which induces CGRP release and thereby vasodilatation and increased dermal blood flow (DBF). This DBF is measured using laser Doppler scanning techniques. First, a capsaicin dose producing a robust and reproducible dermal blood flow response was established. Second, the influence of the forearm location on the capsaicin response was assessed. Third, the within-subject arm-to-arm and period-to-period reproducibility of the CIDBF model were confirmed (Table1 and Figure3). Finally, it was shown that there was no within-subject diurnal variation in the capsaicin response 35.
Figure 3.
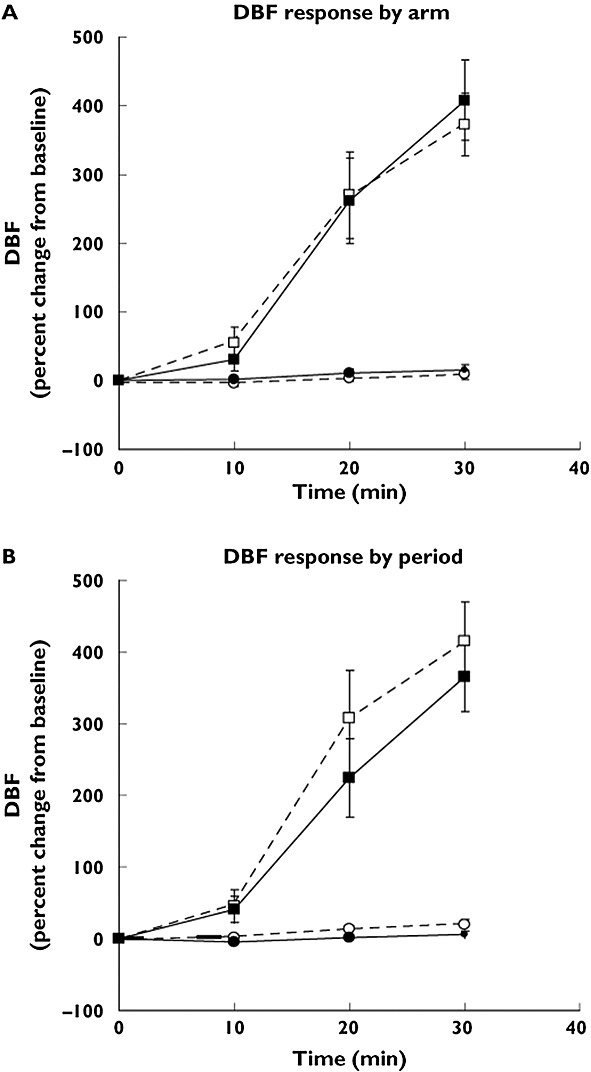
Dermal blood flow (DBF) response by arm and by period. (A) DBF response (percent change from baseline) after application of placebo and 1000 mg capsaicin. The mean of the DBF responses at the capsaicin or placebo application sites during both study periods was calculated. Number of subjects = 1, number of observations = 44. D, dominant arm; ND, non-dominant arm. Data are mean of observations and 90% confidence interval (CI). Placebo D (white circle); placebo ND (black circle); 1000 mg capsaicin D (white square); 1000 mg capsaicin ND (black square). (B) DBF response (percent change from baseline) after application of placebo and 1000 mg capsaicin. The mean of the DBF responses at the capsaicin or placebo application sites in both arms was calculated. Number of subjects = 1, number of observations = 44. P1, period 1; P2, period 2. Data are mean of observations and 90% of CI. Placebo P1 (white circle); placebo P2 (black circle); 1000 mg capsaicin P1 (white square); 1000 mg capsaicin P2 (black square). Figure3 is adapted, with permission, from Van der Schueren et al. 35
Table 1.
Test–retest reproducibility of CIDBF response and sample size calculations. Number of subjects = 11, number of observations = 44, the mean dermal blood flow (DBF) response in the two proximal rings was used in the test–retest analysis. Test–retest period-to-period and arm-to-arm reproducibility data for DBF response expressed as percent change from baseline at 30 min (t30) and as the area under the curve of the percent change from baseline (AUC(0,30 min)). RC repeatability coefficient; WCV within-subject coefficient of variation; CCC concordance correlation coefficient; 95% CI 95% two-sided confidence interval. Table1 is adapted, with permission, from Van der Schueren et al. 35
| DBF response | Test–retest reproducibility | Mean difference (95% CI) | RC | CCC | Sample size 20% shift | Sample size 50% shift |
|---|---|---|---|---|---|---|
| t30 (%) | Period-to-period | 49 (−19.6, 118.0) | 307 | 0.40 | 34 | 7 |
| Arm-to-arm | 36 (−85.4, 14.4) | 223 | 0.68 | 19 | 5 | |
| AUC(0,30 min) (% min−1) | Period-to-period | 1119 (−239, 2476) | 6058 | 0.33 | 76 | 14 |
| Arm-to-arm | 139 (−340, 619) | 2140 | 0.91 | 11 | 4 |
Capsaicin application is well tolerated and provokes only a local flare and stinging sensation which disappears within 2–6 h after application. A gradual increase in the DBF after both 300 µg and 1000 µg capsaicin application was quantified over a period of 30 to 45 min after capsaicin application. The lower 100 µg dose of capsaicin failed to increase DBF significantly. The most proximal sites of the human forearm skin showed the most reproducible and robust responses to capsaicin 35. Subsequently, the involvement of NO, CGRP, substance P (SP) and prostaglandins as mediators in the CIDBF model was tested. Capsaicin was topically applied on the forearm and the antagonist CGRP8–37 was administered intra-arterially via the brachial artery. CGRP8–37 significantly decreased the capsaicin-induced DBF increase, in contrast to indomethacin (cyclo-oxygenase inhibitor), L-NMMA (non-selective NOS inhibitor) and aprepitant (NK1-receptor antagonist) which did not affect the DBF response to capsaicin. These findings identified CGRP as the key mediator in CIDBF in humans. Consequently, these studies confirmed that CIDBF could be used as a pharmacodynamic model to assess target engagement of the CGRP receptor or ‘scavenging’ of CGRP by CGRP blocking therapeutics in vivo in early clinical trials 36.
In a subsequent study, long term repeatability of the model was further investigated in healthy male volunteers. No desensitization occurred after weekly applications for 4 weeks in healthy male volunteers 37. This opened the door for using the CIDBF model to evaluate CGRP blocking therapeutics in the early development of mAbs targeting CGRP as a ligand or its receptor. Indeed, because of the long half-life of these biologicals, long term reproducibility needed to be confirmed.
Because migraine prevalence is higher in women compared with men, during phase II clinical trials female migraineurs will also be included, Vermeersch et al. 37 went on to investigate the influence of female hormones during the menstrual cycle on the CIDBF response. In healthy women, the DBF response to capsaicin is increased during menstruation compared with the last week of the secretory phase of the menstrual cycle. This could be the result of (1) increased neuronal/TRPV1 sensitivity to capsaicin, (2) increased release of CGRP or (3) increased sensitivity to CGRP during the menstruation period. These results clearly indicate that the CIDBF response is influenced by female hormone changes during the menstrual cycle. In women suffering from migraine, the CIDBF response was consistently high, irrespective of the menstruation period compared with non-migraineurs. No difference was found between healthy men and men with migraine. This supports the general hypothesis that migraine headache is associated with female hormonal changes and primarily affects females 37. Taken together, these data give a potential explanation for the higher frequency of migraine in women compared with men and its relationship to menstruation.
The capsaicin model has not only been used to investigate the physiopathology of migraine but has mostly been used as a target engagement biomarker for several small molecule CGRP-R antagonists including telcagepant (MK-0974) 38,39 and MK-3207 40,41 as well as mAbs binding CGRP as a ligand (e.g. LY2951742 42,43, ALD403 44,45 and TEV-48125, formerly known as PF-04427429 or LBR-101 46) or the CGRP receptor (e.g. AMG 334).
Sinclair et al. investigated whether MK-0974, the first orally bioavailable CGRP antagonist, inhibited the capsaicin-induced increase in dermal microvascular blood flow in humans (Figure4) 38. MK-0974 caused a robust inhibition of CIDBF, 1 and 4 h after oral drug administration. Surprisingly, although MK-0974 has a high affinity for the CGRP receptor, relatively high plasma concentrations were required to reduce CGRP-mediated skin vasodilatation in healthy volunteers and alleviate headache in migraine patients. This is most likely due to the fact that access to peripheral CGRP receptors is restricted owing to high protein binding of the compound. However, it cannot be completely excluded that, in the case of a central mode of action being required for efficacy, restricted access to CGRP receptors located in the central nervous system, because of unfavourable brain penetration properties of the compound, might also have contributed 38. Unfortunately, the clinical development of telcagepant had to be terminated because of drug-induced hepatotoxicity 39. One might speculate that the high levels of drug required in order to achieve a sufficiently high free fraction of telcagepant, and consequently an efficacious anti-migraine dose, might have promoted hepatotoxicity liability.
Figure 4.
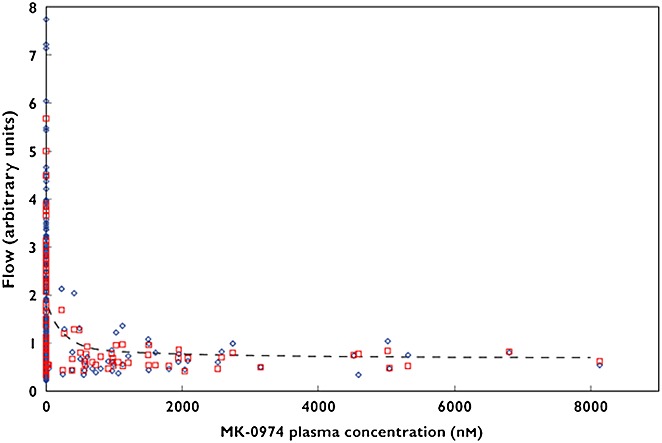
Capsaicin induced dermal blood flow (CIDBF) vs. telcagepant (MK-0974) plasma concentration. The blue diamonds show the measured DBF, red squares show the individual predicted values and the dashed lines indicate the population predicted means values. Figure4 is adapted, with permission, from Sinclair et al. 38
MK-3207 is a potent and orally bioavailable CGRP receptor antagonist of the human and rhesus monkey CGRP receptors in vitro. The in vivo potency was assessed in a rhesus monkey pharmacodynamic assay measuring capsaicin-induced changes in forearm DBF as well as in humans. MK-3207 produced a concentration-dependent inhibition of dermal vasodilation 40,41,47. In a randomized controlled trial in 574 migraine patients, MK3207 was shown to be well tolerated and effective in acute migraine treatment 22.
As migraine is a chronic disease, prophylactic treatment is an approach which can be considered to be complementary to acute treatment. Given the typically long half-life of mAbs, the development of CGRP (receptor) binding antibodies is an attractive alternative approach for migraine treatment. Recently, the first clinical phase II data with CGRP binding mAbs (i.e. LY2951742 42,43,48,49, ALD403 45,50 and TEV-48125 46) and CGRP-R binding monoclonal antibody AMG 334 51 have been completed and presented. During the development of these antibodies, as well as for previous CGRP-R targeting small molecules (i.e. telcagepant and olcegepant), the capsaicin challenge was used to prove target engagement. It was shown that the small molecule antagonists and AMG 334 targeting the CGRP-R were able to inhibit the capsaicin response by 90% or more whereas with mAbs targeting the CGRP ligand only partial inhibition was established 51. Although one might expect that the differences observed in the ability to inhibit the capsaicin response should translate into a difference in clinical efficacy, this has not been shown so far. All antibodies, regardless of their mode of action, showed a small, but significant reduction of 1 to 2 migraine days per months compared with placebo in phase II trials 48–52. In these trials, the 50% responder rate ranged from 46.5 to 61% for active treatment vs. 29.9% to 33% for placebo 48–53. Although these data show no difference in clinical efficacy between the receptor targeting antibody (AMG 334) and the ligand targeting antibodies (LY2951742, ALD403 and TEV-48125), it should be pointed out that the results of the different phase II trials are difficult to compare due to large differences in dosing ranging from 70 mg subcutaneous injection of AMG 334 up to 1000 mg 52 intravenously for ALD403 53. Therefore, phase III data need to be awaited to make a fair comparison between these compounds and to draw any firm conclusions.
Advantages and limitations of the capsaicin model
The capsaicin model is an in vivo pharmacodynamic model in animals and humans, which is non-invasive, technically uncomplicated and has a rapid and objective endpoint. This pharmacodynamic model allows repeated measurements which are adequately reproducible and repeatable over time. This model therefore facilitates early clinical evaluation of antagonists of mediators involved in neurogenic inflammation, including CGRP blocking therapeutics.
Like all pharmacodynamic biomarker models, this model also has its limitations. The capsaicin model remains a simulation of a naturally occurring pathophysiological process one wants to study. The effect of a drug on capsaicin-induced DBF can provide us with an index of anti-CGRP activity but is only indicative for its efficacy for inhibiting peripheral DBF. In that perspective, it proves peripheral target engagement but not necessarily guarantees therapeutic efficacy. Indeed, changes measured in the peripheral vasculature such as superficial dermal capillaries of the forearm, are not necessarily predictive of changes in the cranial circulation such as the trigeminovascular system. One way to overcome this limitation could be the application of capsaicin to the forehead skin 39.
Also, there is a possibility that therapeutic efficacy in the treatment of migraine, a CNS disorder, might require penetration in the central nervous system and drug concentrations needed for a peripheral inhibition of the response might not be representative for concentrations needed to achieve central target engagement. However, a strong argument in favour of our peripheral CIDBF model is the increasing evidence that central penetration to obtain anti-migraine efficacy is not necessarily needed. Indeed, recent PET data with telcagepant convincingly showed that, at therapeutic doses, no meaningful occupancy of CGRP-receptors was observed in the central nervous system 54. This is a very important observation supporting the use of the ‘peripheral’ CIDBF model as a relevant model to test potential CGRP blocking therapeutics for the treatment of migraine as a disorder of the CNS. In addition, this observation also supports the concept that, in order to relieve migraine, it is sufficient for CGRP blocking therapeutics to act peripherally and thus provides confidence in the development of mAbs as anti-migraine therapeutics despite their limited access to the brain.
Finally, when using the capsaicin model for testing target engagement of CGRP blocking therapeutics, it should be kept in mind that it is an indirect measurement, depending on TRPV1 receptor functionality. One way to avoid this is the direct application of CGRP. This can be achieved by topical application of CGRP in combination with iontophoresis 55, intravenous or intra-arterial infusion 20,56. Edvinsson et al. used laser Doppler flowmetry combined with iontophoresis of CGRP, acetylcholine and sodium nitroprusside in migraine patients and healthy controls 55. Olesen's group used intravenous CGRP infusions in an attempt to develop a headache model 20,57,58. These CGRP infusions caused migraine-like attacks in patients with migraine with aura whereas no migraine attacks were induced in patients with familial hemiplegic migraine. de Hoon et al. 56 used intra-arterial infusions of CGRP in the forearm blood flow (FBF) model. In this model bilateral venous occlusion plethysmography is used to measure changes in forearm perfusion induced by the intra-arterial infusion of vasoactive compounds into the brachial artery. By using this approach, the reproducibility of CGRP-induced vasodilatation of forearm blood flow was demonstrated, the vasodilatory mechanism of action of CGRP was further investigated in humans and potential differences between migraineurs and healthy subjects were explored. However, no differences between migraineurs and healthy volunteers were observed 59–61.
Conclusion
Development of the capsaicin-induced dermal blood flow model in humans has proven to be a very useful platform for evaluating target engagement and dose selection of CGRP blocking therapeutics in the early clinical development of anti-migraine drugs. The CIDBF sets an example that target engagement biomarkers should be more generally applied to improve the confidence in new compounds at the earliest possible stage of drug development. With the use of these biomarkers not only dose selection can be guided in early efficacy trials but also the go/no go decision making. By selecting the winners and killing the losers early, drug development costs and timelines can be significantly reduced.
Competing Interests
All authors have completed the Unified Competing Interest Form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work. JdH was principal investigator for clinical studies commissioned by Amgen Inc., Eli Lilly and Company and Merck Sharp & Dohme in the previous 3 years. There are no other relationships or activities that could appear to have influenced the submitted work.
References
- World Health Organization. The global burden of disease: 2004 update. Geneva: WHO; 2008. [Google Scholar]
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine - current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- Chan KY, Vermeersch S, de Hoon J, Villalon CM, Maassenvandenbrink A. Potential mechanisms of prospective antimigraine drugs: a focus on vascular (side) effects. Pharmacol Ther. 2011;129:332–51. doi: 10.1016/j.pharmthera.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Link AS, Kuris A, Edvinsson L. Treatment of migraine attacks based on the interaction with the trigemino-cerebrovascular system. J Headache Pain. 2008;9:5–12. doi: 10.1007/s10194-008-0011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 2009;124:309–23. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Franco-Cereceda A, Hua X, Hokfelt T, Fischer JA. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol. 1985;108:315–9. doi: 10.1016/0014-2999(85)90456-x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–35. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Brain SD, Cambridge H. Calcitonin gene-related peptide: vasoactive effects and potential therapeutic role. Gen Pharmacol. 1996;27:607–11. doi: 10.1016/0306-3623(95)00125-5. [DOI] [PubMed] [Google Scholar]
- Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–46. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Juaneda C, Dumont Y, Quirion R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol Sci. 2000;21:432–8. doi: 10.1016/s0165-6147(00)01555-8. [DOI] [PubMed] [Google Scholar]
- Dennis T, Fournier A, Cadieux A, Pomerleau F, Jolicoeur FB, St Pierre S, Quirion R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J Pharmacol Exp Ther. 1990;254:123–8. [PubMed] [Google Scholar]
- Wu D, Eberlein W, Rudolf K, Engel W, Hallermayer G, Doods H. Characterisation of calcitonin gene-related peptide receptors in rat atrium and vas deferens: evidence for a [Cys(Et)(2, 7)]hCGRP-preferring receptor. Eur J Pharmacol. 2000;400:313–9. doi: 10.1016/s0014-2999(00)00407-6. [DOI] [PubMed] [Google Scholar]
- Helme RD, McKernan S. Neurogenic flare responses following topical application of capsaicin in humans. Ann Neurol. 1985;18:505–9. doi: 10.1002/ana.410180414. [DOI] [PubMed] [Google Scholar]
- Stam AH, Haan J, van den Maagdenberg AM, Ferrari MD, Terwindt GM. Migraine and genetic and acquired vasculopathies. Cephalalgia. 2009;29:1006–17. doi: 10.1111/j.1468-2982.2009.01940.x. [DOI] [PubMed] [Google Scholar]
- Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med. 2011;13:e36. doi: 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–45. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Amin FM, Asghar MS, Hougaard A, Hansen AE, Larsen VA, de Koning PJ, Larsson HB, Olesen J, Ashina M. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 2013;12:454–61. doi: 10.1016/S1474-4422(13)70067-X. [DOI] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Kapijimpanga T, van der Geest RJ, van der Koning P, Larsson HB, Olesen J, Ashina M. Dilation by CGRP of middle meningeal artery and reversal by sumatriptan in normal volunteers. Neurology. 2010;75:1520–6. doi: 10.1212/WNL.0b013e3181f9626a. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179–86. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, Taraborelli D, Fan X, Assaid C, Lines C, Ho TW. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31:712–22. doi: 10.1177/0333102411398399. [DOI] [PubMed] [Google Scholar]
- Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, Lines CR, Rapoport AM. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–12. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Walter S, Rapoport AM. Therapeutic antibodies against CGRP or its receptor. Br J Clin Pharmacol. 2015;79:886–95. doi: 10.1111/bcp.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A. Neuropharmacological mechanisms of capsaicin and related substances. Biochem Pharmacol. 1992;44:611–5. doi: 10.1016/0006-2952(92)90393-w. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–68. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Szallasi A. Vanilloid receptor ligands: hopes and realities for the future. Drugs Aging. 2001;18:561–73. doi: 10.2165/00002512-200118080-00001. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Del Bianco E, Patacchini R, Santicioli P, Maggi CA, Tramontana M. Low pH-induced release of calcitonin gene-related peptide from capsaicin-sensitive sensory nerves: mechanism of action and biological response. Neuroscience. 1991;41:295–301. doi: 10.1016/0306-4522(91)90218-d. [DOI] [PubMed] [Google Scholar]
- Kessler F, Habelt C, Averbeck B, Reeh PW, Kress M. Heat-induced release of CGRP from isolated rat skin and effects of bradykinin and the protein kinase C activator PMA. Pain. 1999;83:289–95. doi: 10.1016/s0304-3959(99)00108-6. [DOI] [PubMed] [Google Scholar]
- Akerman S, Kaube H, Goadsby PJ. Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol. 2003;140:718–24. doi: 10.1038/sj.bjp.0705486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meents JE, Neeb L, Reuter U. TRPV1 in migraine pathophysiology. Trends Mol Med. 2010;16:153–9. doi: 10.1016/j.molmed.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res. 2001;890:184–8. doi: 10.1016/s0006-8993(00)03253-4. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Hershey JC, Corcoran HA, Baskin EP, Salvatore CA, Mosser S, Williams TM, Koblan KS, Hargreaves RJ, Kane SA. Investigation of the species selectivity of a nonpeptide CGRP receptor antagonist using a novel pharmacodynamic assay. Regul Pept. 2005;127:71–7. doi: 10.1016/j.regpep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Van der Schueren BJ, de Hoon JN, Vanmolkot FH, Van Hecken A, Depre M, Kane SA, De Lepeleire I, Sinclair SR. Reproducibility of the capsaicin-induced dermal blood flow response as assessed by laser Doppler perfusion imaging. Br J Clin Pharmacol. 2007;64:580–90. doi: 10.1111/j.1365-2125.2007.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Schueren BJ, Rogiers A, Vanmolkot FH, Van Hecken A, Depre M, Kane SA, De Lepeleire I, Sinclair SR, de Hoon JN. Calcitonin gene-related peptide8-37 antagonizes capsaicin-induced vasodilation in the skin: evaluation of a human in vivo pharmacodynamic model. J Pharmacol Exp Ther. 2008;325:248–55. doi: 10.1124/jpet.107.133868. [DOI] [PubMed] [Google Scholar]
- Vermeersch S, Frederiks P, Maassen VandenBrink A, de Hoon J. Capsaicin-induced CGRP-mediated vasodilatation of the human skin: influence of gender, female hormones and migraine. J Headache Pain. 2013;14 [Google Scholar]
- Sinclair SR, Kane SA, Van der Schueren BJ, Xiao A, Willson KJ, Boyle J, de Lepeleire I, Xu Y, Hickey L, Denney WS, Li CC, Palcza J, Vanmolkot FH, Depre M, Van Hecken A, Murphy MG, Ho TW, de Hoon JN. Inhibition of capsaicin-induced increase in dermal blood flow by the oral CGRP receptor antagonist, telcagepant (MK-0974) Br J Clin Pharmacol. 2010;69:15–22. doi: 10.1111/j.1365-2125.2009.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2014. MK0974 for Migraine Prophylaxis in Patients With Episodic Migraine (0974–049)(TERMINATED) - Full Text View - ClinicalTrials.gov.
- Salvatore CA, Moore EL, Calamari A, Cook JJ, Michener MS, O'Malley S, Miller PJ, Sur C, Williams DL, Jr, Zeng Z, Danziger A, Lynch JJ, Regan CP, Fay JF, Tang YS, Li CC, Pudvah NT, White RB, Bell IM, Gallicchio SN, Graham SL, Selnick HG, Vacca JP, Kane SA. Pharmacological properties of MK-3207, a potent and orally active calcitonin gene-related peptide receptor antagonist. J Pharmacol Exp Ther. 2010;333:152–60. doi: 10.1124/jpet.109.163816. [DOI] [PubMed] [Google Scholar]
- 2014. MK3207 for Treatment of Acute Migraines (3207–005) - Study Results - ClinicalTrials.gov.
- 2014. A Study of LY2951742 in Healthy Volunteers - Full Text View - ClinicalTrials.gov.
- 2014. A Study of LY2951742 in Participants With Migraine - Full Text View - ClinicalTrials.gov.
- 2014. Safety Tolerability and Pharmacokinetics of ALD403 - Full Text View - ClinicalTrials.gov.
- 2014. Safety, Efficacy and Pharmacokinetics of ALD403 - Full Text View - ClinicalTrials.gov.
- 2014. Acute Response Capsaicin Flare Study - Full Text View - ClinicalTrials.gov.
- Li CC, Vermeersch S, Denney WS, Kennedy WP, Palcza J, Gipson A, Han TH, Blanchard R, De Lepeleire I, Depre M, Murphy MG, Van Dyck K, de Hoon JN. Characterizing the PK/PD relationship for inhibition of capsaicin-induced dermal vasodilatation by MK-3207, an oral CGRP receptor antagonist. Br J Clin Pharmacol. 2015;79:831–7. doi: 10.1111/bcp.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby P, Dodick D, Martinez J, Ferguson M, Oakes T, Tanaka Y, Ni X, Zhang Q, Due M, Sklajarevski V. Onset of efficacy of LY2951742 in migraine prevention: post-hoc analysis of phase 2a, randomized, double-blind, placebo-controlled study data of a calcitonin gene-related peptide monoclonal antibody. Cephalalgia IHS 2015 abstracts supplement. 2015;35:55. (6S): (PO083) [Google Scholar]
- Dodick D, Goadsby P, Skljarevski V, Ferguson M, Oakes T, Tanaka Y, Ni X, Zhang Q, Due M, Martinez J. Sustained response outcomes from a phase 2a, randomized, double-blind, placebo-controlled study of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a post-hoc analysis. Cephalagia IHS 2015 abstracts supplement 2015. 2015;35:52. (6S): (PO075) [Google Scholar]
- Baker B, Smith J. A single dose, placebo-controlled, randomized, ascending dose study of ALD403, a humanized anti-calcitonin gene-related peptide monoclonal antibody administered IV or SC- pharmacokinetic and pharmacodynamic results. Cephalagia IHS 2015 abstracts supplement 2015. 2015;35:55. (6S): (PO082) [Google Scholar]
- Vu T, Ma P, Chen J, de Hoon J, van Hecken A, Yan L, Hamilton L, Vargas G. Pharmacokinetic/pharmacodynamic modeling of monoclonal antibody AMG 334 to characterize concentration relationship with capsaicin-induced increase in dermal blood flow in healthy subjects and migraine patients. Cephalagia IHS 2015 abstracts supplement 2015. 2015;35:47. (6S): (PO068) [Google Scholar]
- Lenz R, Silberstein S, Dodick D, Reuter U, Ashina M, Saper J, Cady R, Chon Y, Dietrich J, Sun H. Results of a randomized, double-blind, placebo-controlled, phase 2 study to evaluate the efficacy and safety of AMG 334 for the prevention of episodic migraine. Cephalagia IHS 2015 abstracts supplement. 2015;35:5. (6S): (OR02) [Google Scholar]
- Smith J, Dodick D, Goadsby P, Silberstein S, Lipton R, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R. Proof of concept clinical trial of ALD403, an anti-calcitonin gene-related peptide (CGRP) antibody in the prevention of migraine-6 month data. Cephalalgia IHS 2015 abstracts supplement. 2015;35:4. (6S): (OR01) [Google Scholar]
- Hostetler ED, Joshi AD, Sanabria-Bohorquez S, Fan H, Zeng Z, Purcell M, Gantert L, Riffel K, Williams M, O'Malley S, Miller P, Selnick HG, Gallicchio SN, Bell IM, Salvatore CA, Kane SA, Li CC, Hargreaves RJ, de Groot T, Bormans G, Van Hecken A, Derdelinckx I, de Hoon J, Reynders T, Declercq R, De Lepeleire I, Kennedy WP, Blanchard R, Marcantonio EE, Sur C, Cook JJ, Van Laere K, Evelhoch JL. In vivo quantification of calcitonin gene-related peptide receptor occupancy by telcagepant in rhesus monkey and human brain using the positron emission tomography tracer [11C]MK-4232. J Pharmacol Exp Ther. 2013;347:478–86. doi: 10.1124/jpet.113.206458. [DOI] [PubMed] [Google Scholar]
- Edvinsson ML, Edvinsson L. Comparison of CGRP and NO responses in the human peripheral microcirculation of migraine and control subjects. Cephalalgia. 2008;28:563–6. doi: 10.1111/j.1468-2982.2008.01558.x. [DOI] [PubMed] [Google Scholar]
- de Hoon JN, Pickkers P, Smits P, Struijker-Boudier HA, Van Bortel LM. Calcitonin gene-related peptide: exploring its vasodilating mechanism of action in humans. Clin Pharmacol Ther. 2003;73:312–21. doi: 10.1016/s0009-9236(03)00007-9. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Thomsen LL, Olesen J, Ashina M. Calcitonin gene-related peptide does not cause migraine attacks in patients with familial hemiplegic migraine. Headache. 2011;51:544–53. doi: 10.1111/j.1526-4610.2011.01861.x. [DOI] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- de Hoon JN, Smits P, Troost J, Struijker-Boudier HA, Van Bortel LM. Forearm vascular response to nitric oxide and calcitonin gene-related peptide: comparison between migraine patients and control subjects. Cephalalgia. 2006;26:56–63. doi: 10.1111/j.1468-2982.2005.00993.x. [DOI] [PubMed] [Google Scholar]
- Vanmolkot FH, de Hoon JN. Reproducibility of forearm vasodilator response to intra-arterial infusion of calcitonin gene-related peptide assessed by venous occlusion plethysmography. Br J Clin Pharmacol. 2005;59:387–97. doi: 10.1111/j.1365-2125.2005.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanmolkot FH, Van der Schueren BJ, de Hoon JN. Calcitonin gene-related peptide-induced vasodilation in the human forearm is antagonized by CGRP8-37: evaluation of a human in vivo pharmacodynamic model. Clin Pharmacol Ther. 2006;79:263–73. doi: 10.1016/j.clpt.2005.11.005. [DOI] [PubMed] [Google Scholar]


