Abstract
Aims
The aim was to evaluate the comprehension of participants of an improved informed consent document (ICD).
Method
This was a randomized controlled French multicentre study performed in real conditions. Participants were adult patients undergoing screening for enrolment in biomedical research studies, who agreed to answer a validated questionnaire evaluating objective and subjective comprehension scored from 0 (no comprehension) to 100 (excellent comprehension). Patients were provided either the original ICD or an ICD modified in terms of structure and readability. The primary end point was the score of objective comprehension. The secondary end-points were the enrolment rate in the clinical study and patient characteristics associated with the score of objective comprehension.
Results
Four hundred and eighty-one patients were included, 241 patients in the original ICD group and 240 patients in the modified ICD group. There was no difference between the two groups for the score of objective comprehension (original ICD 72.7 (95% CI 71.3, 74.1) vs. modified ICD 72.5 (95% CI 71.0, 74.0); P = 0.81). However, the rate of enrolment in the clinical study was lower in the group who received the modified ICD (64.4% (95% CI 58.3, 70.5)) than for the original ICD (73.0% (95% CI 67.4, 78.7)) (P = 0.042). Only female gender and high educational level were associated with a better objective comprehension.
Conclusions
Improving ICDs had no effect on participants’ understanding, whereas the rate of enrolment was lower in this group. In attempts at improving potential participants’ understanding of clinical research information, efforts and future trials should focus on other ways to improve comprehension.
Keywords: biomedical research, comprehension, informed consent, questionnaire
What is Already Known about this Subject
While patients participating in clinical trials perceive the amount of information as sufficient, their degree of understanding remains unsatisfactory.
It is unclear whether the use of an enhanced informed consent document improves patients’ understanding.
What this Study Adds
Improving informed consent documents in a large multicentre study had no effect on participants understanding but decreased the enrolment rate.
This was further supported by an updated systematic review. Consequently, to improve participants’ understanding in clinical research, efforts and future trials should focus on other ways to improve comprehension.
Introduction
Obtaining patients’ informed consent through an Institutional Review Board (IRB) approved informed consent document (ICD) is mandatory in biomedical research. In addition to its main objective of providing information to patients, when signed it is considered as a way to prove that participants agree to undergo the procedures required by the study. However, while patients participating in clinical trials perceive the amount of information as sufficient, their degree of understanding remains unsatisfactory 1. Whatever the language used, there is consistent evidence that ICDs are not easy to read. We previously showed that the readability of French ICDs was much lower than that of high school level texts 2 and was not improved following IRB reviews 3. Similarly, American IRBs often provide text for informed consent forms that falls short of their own readability standards 4 and readability was also low in other languages such as Spanish or German 5,6. In a qualitative analysis aimed at determining what should be improved, a Danish group concluded that poor presentation and specialized terminology were barriers to comprehension 7. Indeed, separating the information about the patients’ rights in a specific and separate booklet, led to a better comprehension 8. Accordingly, members of our group have developed French good practice recommendations 9,10 that include adding a schematic diagram of the study, a glossary, a summary and the use of short words and phrases. However, we showed in two randomized controlled simulated studies that while improved ICDs enhanced comprehension in healthy volunteers 11, it did not in patients with stroke, type 1 diabetes or sleep apnoea syndrome 12.
In 2004, Flory et al. showed in a systematic review that use of multimedia and of an enhanced ICD had only limited success in improving understanding. However, given that some of the studies were of poor quality, they concluded that further research was required 13. In 2013, a new systematic review was performed and suggested that enhanced consent forms and extended discussions were the most effective methods to improve participant understanding 14. However, the heterogeneity among studies was substantial. Indeed, only one of the seven ‘enhanced consent form’ studies performed in a real setting drew positive conclusions, while eight of the 15 studies in simulated conditions did. None of the studies enrolled more than 100 patients per group. Furthermore, to date most studies have focused on one to three clinical trials in a specific medical field, which makes conclusions difficult to apply across all clinical research settings. Therefore, our objective was to evaluate the effect of an improved ICD in a large multicentre study performed in real conditions encompassing a large number of different clinical trials. Patients’ understanding was the primary endpoint and enrolment rate a secondary end point.
Methods
Patients
Patients were recruited by six French clinical research centres: Clermont-Ferrand, Créteil, Grenoble, Lyon, Saint-Etienne and Toulouse. The inclusion criteria were patients aged 18 years and over without major cognitive disorders undergoing screening (first protocol visit) for potential enrolment in a biomedical research study, and who agreed to answer a comprehension questionnaire. The trial was registered at Clinicaltrials.gov NCT00908557.
Studies
The inclusion criteria for studies were phase IIb, III or IV, randomized, controlled, therapeutic studies for which French IRB (Comité de Protection des Personnes) approval was sought. A detailed list of the 18 single and multicentre studies and their medical fields is given in Table1.
Table 1.
List of studies, stratified by study centre
| Study centre | Medical field | Registration number(EUDRACT,IdRCB or NCT) | Referent ethics Committee/IRB | Informed consent document number of words(characters) | Number of subjects enrolled in LISYCOM | Number of subjects enrolled in the clinical study | |||
|---|---|---|---|---|---|---|---|---|---|
| Clinical trial | |||||||||
| Original ICD | Modified ICD | delta | Original ICD | Modified ICD | |||||
| Grenoble | 338 (70.4%) | 110/170 (65%) | 97/168 (58%) | ||||||
| BOSAS | Obstructive sleep apnoea | 2007-005333-11 | Sud-Est V | 2535 (13 928) | 2544 (13 551) | 9 (−377) | 2 (0.4%) | 1/1 (100%) | 1/1 (100%) |
| CICLO ET CEC | Heart surgery | 2008-005566-31 | Sud-Est V | 2321 (12 437) | 2374 (12 263) | 53 (−174) | 29 (6%) | 11/15 (73%) | 11/14 (78.6%) |
| EPO ET CEC | Heart surgery | NCT00273767 | Sud-Est V | 1415 (7901) | 1510 (8103) | 95 (202) | 5 (1%) | 3/3 (100%) | 2/2 (100%) |
| PREPIC-2 | Pulmonary embolism | NCT00457158 | Sud-Est I | 1367 (7251) | 1555 (8197) | 188 (946) | 2 (0.4%) | 1/1 (100%) | 1/1 (100%) |
| STATINFLASAS | Obstructive sleep apnoea | 2007-005286-35 | Sud-Est V | 2922 (15 995) | 2879 (14 922) | −43 (−1073) | 3 (0.6%) | 1/2 (50.0%) | 1/1 (100%) |
| STIMALGI | Fibromyalgia | 2010-A00865-34 | Sud-Est V | 1676 (8871) | 1696 (8836) | 20 (−35) | 29 (6%) | 8/14 (57%) | 10/15 (66.7%) |
| TELEDIAB-3 | Diabetes | 2011-A00852-39 | Sud-Est V | 1577 (8598) | 1711 (9127) | 134 (529) | 47 (9.8%) | 24/24 (100%) | 23/23 (100%) |
| TSE-TSE | Insomnia | 2011-A00761-40 | Sud-Est V | 2049 (10 674) | 1981 (10 003) | −68 (−671) | 221 (46%) | 61/110 (55%) | 48/111 (43%) |
| Lyon | 44 (9.2%) | 21/22 (95%) | 20/22 (91%) | ||||||
| IDA Adulte | Atopic dermatitis | 2007-007267-25 | Sud-Est II | 3182 (17 195) | 3256 (17 054) | 74 (−141) | 9 (1.8%) | 4/4 (100%) | 4/5 (80%) |
| RISAROS | Breast cancer | 2006-006943-29 | Sud-Est IV | 1549 (8228) | 1772 (9030) | 223 (802) | 22 (4.6%) | 11/12 (91.7%) | 9/10 (90%) |
| TELEDIAB-3 | Diabetes | 2011-A00852-39 | Sud-Est V | 1577 (8598) | 1711 (9127) | 134 (529) | 13 (2.7%) | 6/6 (100%) | 7/7 (100%) |
| Saint-Étienne | 57(11.9%) | 24/27 (89%) | 20/30 (67%) | ||||||
| VAC | Dermatology | 2010-A00371-38 | Sud-Est I | 1630 (8631) | 1779 (8157) | 149 (−474) | 7 (1.5%) | 4/4 (100%) | 2/3 (67%) |
| PREFACE | Atrial fibrillation | 2006-007032-10 | Sud-Est I | 1962 (10 775) | 2110 (11 329) | 148 (554) | 20 (4.1%) | 7/9 (78%) | 4/11 (36 %) |
| PREPIC-2 | Pulmonary embolism | NCT00457158 | Sud-Est I | 1367 (7251) | 1555 (8197) | 188 (946) | 30 (6.3%) | 13/14 (92%) | 14/16 (87%) |
| Toulouse | 21(4.4%) | 10/11 (91%) | 8/10 (80%) | ||||||
| AMANDYSK | Parkinson disease | 2006-006684-22 | Sud-Ouest et outre-Mer II | 2077 (11 328) | 2073 (11 114) | −4 (−214) | 8 (1.7%) | 4/4 (100%) | 3/4 (75%) |
| MSA-FLUOXETINE | Multi-system atrophy | 2007-004922-26 | Sud-Ouest et outre-Mer II | 1574 (8354) | 1736 (9049) | 162 (695) | 3 (0.6%) | 1/2 (50%) | 0/1 (0%) |
| NEURO-HD | Huntington's disease | 2006-006113-32 | Ile de France IX | 1026 (5695) | 1181 (6333) | 155 (638) | 8 (1.7%) | 4/4 (100%) | 4/4 (100%) |
| PREAMANDISK | Parkinson's disease | 2011-005201-75 | Ile de France IX | 2120 (11 673) | 2373 (12 268) | 253 (−595) | 2 (0.4%) | 1/1 (100%) | 1/1 (100%) |
| Clermont-Ferrand | 7(1.5%) | 4/4 (100%) | 3/3 (100%) | ||||||
| TRIVALVE | Heart surgery | 2011-A00485-36 | Sud-Est VI | 1685 (9593) | 1606 (8719) | −79 (−874) | 7 (1.5%) | 4/4 (100%) | 3/3 (100%) |
| Créteil | 13(2.7%) | 6/6 (100%) | 7/7 (100%) | ||||||
| NICOPARK-2 | Parkinson's disease | 2008-001338-13 | Ile de France IX | 1961 (11 167) | 2119 (11 345) | 158 (178) | 13 (2.7%) | 6 (100%) | 7/7 (100%) |
EUDRACT number European Union Drug Regulating Authorities Clinical Trials number; IDRCB French Health Authority biomedical research number. NCT Clinical Trials number.
Informed consent forms
For each study, the original ICDs (otherwise called informed consent forms, ICDs, or patient study information) provided by sponsors and investigators were modified by two members of the LISYCOM study team. We improved the lexicosyntactic readability mainly by reducing the length of words by using short synonyms and reducing the length of phrases. Some examples of modification are shown in Table2. We also applied the recommendations for good writing practice provided by Chassany et al. 9 consisting essentially in the addition of a glossary, a summary and a study diagram for each study modified ICD. The objective was to obtain a Flesch index for the modified ICDs greater than 43 or improved by 50% compared with the original text.
Table 2.
Examples of modifications performed in informed consent documents
| Original informed consent document | Modified informed consent document |
|---|---|
| Le Dr..........................................................vous propose de participer à une recherche organisée par le C.H.U. de GRENOBLE portant sur un essai clinique | Le Dr..................................................... vous propose de participer à un essai clinique. Il est organisé par le C.H.U. de GRENOBLE. |
| Les visites de suivi se feront ensuite après 6, 12, 18 et 24 mois de traitement. Elles dureront environ 45 mn. A 6 mois des prélèvements sanguins à jeun le matin seront réalisés (marqueurs du remodelage osseux, prélèvement de 5 CC de sang) ainsi qu'un questionnaire sur une éventuelle survenue de fractures. A 12 mois et 24 mois, les mêmes prélèvements sanguins seront faits pour le dosage des marqueurs du remodelage et de l'estradiol (deux tubes de 5 CC de sang), ainsi qu'une mesure de la densité minérale osseuse et le questionnaire sur les fractures. | Les visites de suivi se feront ensuite: |
| - après 6 mois de traitement, | |
| - après 12 mois de traitement, | |
| - après 18 mois de traitement | |
| - après 24 mois de traitement. | |
| Elles dureront environ 45 mn. | |
| A 6 mois, 5 cc de sang seront prélevés. Ils permettront de doser les marqueurs du remodelage osseux. Le prélèvement devra être fait: | |
| - à jeun | |
| - le matin. | |
| On vous posera un questionnaire pour savoir si vous avez eu une éventuelle survenue de fractures. | |
| A 12 mois et 24 mois, deux tubes5 cc de sang seront prélevés. Cela fait donc 10 cc au total. Leur analyse permettra de doser: | |
| - les marqueurs du remodelage | |
| - l'estradiol. | |
| Une mesure de la densité minérale osseuse sera aussi faite. On vous reposera le questionnaire sur les fractures. | |
| Les dyskinésies (mouvements involontaires) induites par le lévodopa constituent un effet indésirable majeur chez les patients présentant une maladie de Parkinson.Ces mouvements involontaires peuvent altérer la qualité de vie et limiter les possibilités thérapeutiques. XXXX est le seul médicament dont l'efficacité est reconnue en tant que traitement symptomatique des dyskinésies induites par la levodopa chez le parkinsonien. Néanmoins, les effets antidyskinétiques de XXXX au long cours demeurent mal connus. | Les dyskinésies induites par la lévodopa sont un effet indésirable majeur chez les parkinsoniens. Elles peuvent: |
| - altérer la qualité de vie | |
| - limiter les possibilités thérapeutiques. | |
| XXXX est le seul médicament dont l'efficacité est reconnue comme traitement symptomatique de ces dyskinésies chez le parkinsonien. Mais, ses effets antidyskinétiques au long cours sont mal connus. | |
| Conformément à la loi n°2004-806 du 9 août 2004 relative à la Politique de Santé Publique: | Conformément à la loi n°2004-806 du 9 août 2004 relative à la Politique de Santé Publique, cette étude a obtenu: |
| - cette étude a obtenu un avis favorable du Comité de Protection des Personnes le ……/……/…… ainsi que l'autorisation préalable de l'autorité compétente de santé. | - un avis favorable du Comité de Protection des Personnes Sud Est VI le 06/05/2011. |
| - l'autorisation préalable de l'autorité compétente de santé. | |
| -Il est possible que cette recherche soit interrompue, si les circonstances le nécessitent, par le promoteur ou à la demande de l'autorité de santé. | |
| Il se peut que cette étude soit interrompue, si les circonstances le nécessitent: | |
| - lorsque cette étude sera terminée, vous serez tenu informé personnellement des résultats globaux par votre médecin dès que ceux-ci seront disponibles, si vous le souhaitez (art L1122-1 du code de la Santé Publique). | - par le promoteur |
| - ou à la demande de l'autorité de santé. | |
| Quand cette étude sera finie, vous serez informé(e) des résultats globaux: | |
| - personnellement | |
| - par votre médecin | |
| - dès qu'ils seront disponibles | |
| - si vous le voulez. | |
| C'est conforme à l'article L1122-1 du code de la Santé Publique. |
For each study protocol, the two versions of the ICD were reviewed and approved by the referent French IRB (Comité de Protection des Personnes), according to French law.
Questionnaire
Patients’ comprehension was evaluated using the questionnaire ‘Questionnaire d'Evaluation de la Compréhension de l'Information Ecrite chez des Malades’ (QECEM) based on the American Quality of Informed Consent questionnaire 15 and validated in French in a previous study 12. Briefly, the questionnaire is composed of two parts. The first part is composed of 28 questions and measures the objective comprehension, i.e. what the subject really understands. The second part is composed of 12 questions and evaluates the subjective comprehension, i.e. what the subject thinks he understood. Each question is scored from 0 to 100 (0 being poor understanding and 100 very high). The objective and subjective scores are the mean of 28 and 12 questions, respectively. The global score is the mean of objective and subjective score. The details for calculating the different scores are specified in our previous work 12.
LISYCOM study design
The LISYCOM study was a multicentre randomized controlled study performed in real conditions. The original and modified ICDs were inserted in separate sealed opaque envelopes. Each envelope was given a unique ordered identification number. Then, envelopes were randomized electronically (Clininfo, Lyon, France) using variable block sizes and stratified by study and centre. During the information/screening visit for a participating clinical study the investigator opened the consecutive randomized envelope and gave the patient the ICD and the questionnaire inside. In order to avoid randomization bias, the consecutive allocation of sealed envelopes was checked in each centre by a clinical research assistant. At the end of the visit the patient was given the comprehension questionnaire to fill in within 24 h and return it to the centre by hand or by post. Investigators completed a specific case report form with information about demography, illness and, subsequently, signature of the consent form.
One year after the information visit, the patients were asked to answer the same comprehension questionnaire, to evaluate long term comprehension.
Objective and end points
The study's main objective was the impact of improving ICDs used in biomedical research on the patients’ comprehension of the information. The primary end point was the score of objective comprehension, using the QECEM comprehension questionnaire, within 24 h of the information/screening visit.
Secondly, we studied the impact of improved consent documents on patient recruitment. We also explored the effect of the patient's characteristics, such as gender, educational level and sphere of professional activity, on comprehension. Secondary end-points were the enrolment level in each group, the score of objective comprehension in the different sub-populations, the overall score, the score of subjective comprehension and the scores in the different domains of information.
Updated systematic review of improved informed consent forms
An updated systematic review of improved informed consent forms tested in randomized ‘clinical trials’ was performed according to the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 16, using the methodology published by Nishimura et al. in 2013 14. Briefly, we included randomized controlled trials that evaluated interventions designed to improve the informed consent process for patients in biomedical research through improvement of ICDs. The initial search was run on MEDLINE using a combination of medical subject headings (MeSH) and text-words, and then run on EMBASE. The ISI Web of Science and Scopus databases were searched as well, using text-words. The primary subject headings used were ‘informed consent’ or ‘consent’. We cross referenced our results with those of the two systematic reviews on the topic published in 2004 13 and 2013 14. Our primary outcome was participant understanding/knowledge. Data were analyzed using Review Manager (RevMan) v5.2.7. Briefly, we included 14 published randomized controlled trials evaluating modified ICDs for clinical research 11,12,17–28, whether tested in simulated or in real conditions, and we added the results of the present study.
Statistical analysis
Based on our previous experience, the expected mean comprehension score was 70.8, with a standard deviation of 7.42 12. A sample size of 167 patients in each group would have 90% power to detect a difference in means of 6% (4.248) assuming that the common standard deviation would be 10, using a two group t-test with a two-sided significance level of 0.01 (NQuery Advisor® 6.01). In the present multicentre multistudy trial, the standard deviation of 7.42 was expected to increase. Therefore, we decided to enrol 200 patients in each group to maintain an adequate study power should the standard deviation be higher. Due to a higher rate of recruitment than expected, we decided to continue the recruitment until the end of the financial support for the study.
The groups, stratified by study and centre, were labelled such as to conceal the identity of the intervention and permit blind statistical analysis. All analyses are presented by intent-to-treat. Quantitative data are described by mean and 95% confidence interval or standard deviation. Qualitative data were described by size and percentage. An independent t-test was used for the primary end point. Comparison between groups was also performed by ancova, with change in age, gender and school level used as the covariates. In order to assess the impact of missing data, both, intention-to-treat using imputed dataset and intention-to-treat using complete dataset (individuals with complete data for the score of objective comprehension) analyses were conducted for the primary end point. We used an iterative Markov Chain Monte Carlo (MCMC) method, to impute missing values under a multivariable normal model for multiple continuous variables. For the secondary end points, we used the t-test for variables that showed normal distribution and the non-parametric Mann–Whitney test for variables that were not normally distributed. Categorical analysis of the frequency distributions was tested for statistical significance by use of the chi-square statistic. We used multiple regression to identify the parameters associated with better objective comprehension, and checked for assumptions in regression.
Statistical tests were two-tailed and significance was set at 5%. All data analysis was performed with Stata V.13.0 for Windows 29 software.
Results
Description of the population
The six centres included 481 patients between April 2009 and March 2013. The study flow chart is shown in Figure1. One patient who did not meet the LYSICOM inclusion criteria was randomized in error and then dropped from the study. The Grenoble centre included 338 patients, Saint-Etienne 57, Lyon 44, Toulouse 21, Créteil 13 and Clermont-Ferrand 7 (Table1). The characteristics of the population in each ICD group are shown in Table3.
Figure 1.
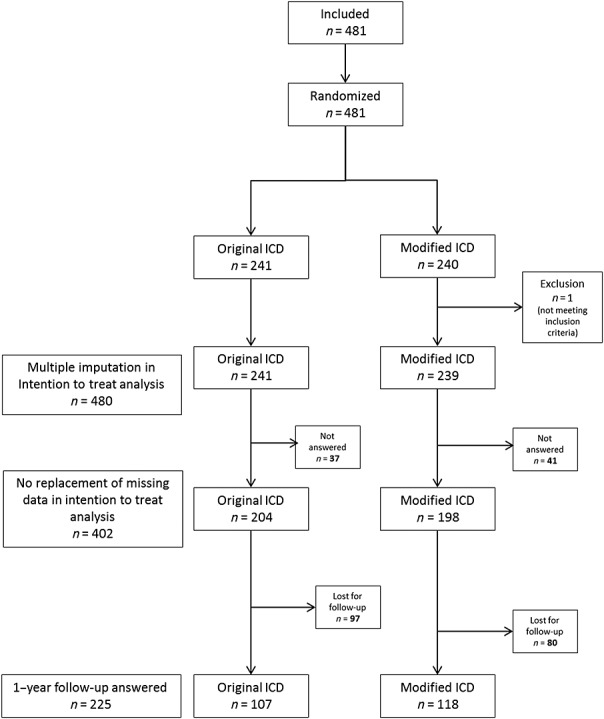
Study flow chart
Table 3.
Patients’ characteristics. Values are numbers (percentages) unless stated otherwise
| Original informed consent form | Modified informed consent form | P | |
|---|---|---|---|
| (n =241) | (n =239) | ||
| Male gender | 142 (59.4) | 97 (40.6) | 0.65 |
| Mean age (SD) | 46.8 (19.4) | 46.7 (18.2) | 0.95 |
| Highest educational level | 0.20 | ||
| School leaving certificate | 45/191 (23.6) | 32/184 (17.4) | |
| High school graduation | 36/191 (18.8) | 151/184 (26.0) | |
| Undergraduate degree | 74/191 (38.8) | 46/184 (40.8) | |
| Postgraduate degree | 36/191 (18.8) | 29/184 (15.8) | |
| Previous participation in a clinical study | 35/205 (17.1) | 36/198 (18.2) | 0.77 |
| Medical/Paramedical profession | 56/199 (28.1) | 49/186 (26.3) | 0.69 |
| Socio-economic class | 0.74 | ||
| Farmers | 2/199 (1.0) | 1/184 (0.5) | |
| Tradesmen, shopkeepers and business owners | 4/199 (2.0) | 2/184 (1.1) | |
| Managerial and professional occupations | 18/199 (9.0) | 19/184 (10.3) | |
| Intermediary professions n (%) | 32/199 (16.1) | 30/184 (16.3) | |
| Employees n (%) | 51/199 (25.6) | 54/184 (29.4) | |
| Workers n (%) | 19/199 (9.6) | 9/184 (4.9) | |
| Pensioners n (%) | 40/199 (20.1) | 36/184 (19.6) | |
| Others with no employment n (%) | 33/199 (16.6) | 33/184 (17.9) | |
| Mean time since diagnosis (months, SD) | 84.4 (106.5) | 93.2 (126.2) | 0.72 |
Main end point
In intention to treat analysis and following multiple imputations for missing data, there was no difference between the two groups for the score of objective comprehension (72.7, 95% CI 71.3, 74.1) for the original vs. 72.5 (95% CI 71.0, 74.0) for the modified document, P = 0.81). When age, gender and educational level were introduced as covariables, no difference between groups was detected (ancova, P = 0.49).
Secondary end points
In intention to treat analysis of objective comprehension using the complete dataset, there was no difference between the two groups (72.5 (95% CI 71.2, 73.8) for the original vs. 72.3 (95% CI 70.9, 74.7) for the modified document, P = 0.83). As a sensitivity analysis, data from the largest clinical trial, accounting for 46% of the patients, were removed, but this did not change the results (71.5 (95% CI 69.8, 73.2) for the original vs. 69.6 (95% CI 67.8, 71.4) for the modified document, P = 0.15). No difference was found for the different domains of the score, objective and subjective comprehension (Table4).
Table 4.
Scores of objective and subjective comprehension on the different comprehension domains (ranging from 0 = no comprehension to 100 = full comprehension), in the intention to treat analysis without replacement of missing data
| Original informed consent form | Modified informed consent form | P | |
|---|---|---|---|
| (n = 204) | (n = 198) | ||
| Objective comprehension total score | 72.5 (9.6) | 72.3 (10.0) | 0.83 |
| Notion of experimentation | 75.7 (21.8) | 78.7 (20.8) | 0.19 |
| Study objective | 65.7 (18.9) | 65.6 (19.6) | 0.89 |
| Methodology | 72.1 (13.7) | 70.8 (14.3) | 0.44 |
| Benefits/risks and constraints | 61.0 (18.6) | 60.9 (17.1) | 0.81 |
| Legal obligations | 65.5 (23.9) | 68.1 (22.7) | 0.27 |
| Subject protection | 83.5 (13.5) | 82.1 (15.7) | 0.58 |
| Subjective comprehension total score | 87.7 (13.4) | 87.6 (15.3) | 0.40 |
| Notion of experimentation | 92.4 (17.4) | 90.8 (20.3) | 0.75 |
| Study objective | 90.8 (19.6) | 91.4 (17.4) | 0.98 |
| Methodology | 87.6 (17.2) | 87.9 (17.6) | 0.92 |
| Benefits/risks and constraints | 89.5 (16.9) | 88.5 (19.5) | 0.98 |
| Legal obligations | 77.9 (25.2) | 76.7 (26.3) | 0.78 |
| Subject protection | 93.5 (14.1) | 93.8 (15.7) | 0.33 |
Data are given as mean (SD).
The rate of enrolment in the studies was lower in the group that received the modified ICD (64.4% (95% CI 58.3, 70.5)) than for the original ICD (73.0% (95% CI 67.4, 78.7)) (P = 0.042). The specific reasons for non-enrolment were refusal to sign the consent document (16.7% vs. 11.6%, respectively, P = 0.108), presence of non-inclusion criteria (13.4% vs. 10%, P = 0.242) or other reasons (5.4% vs. 5.4%, P = 0.983).
In univariable analysis, women (73.5 (9.5)) had a better objective comprehension than men (70.7 (10.1), P = 0.012). Age was inversely correlated with the score of objective comprehension (r = −0.21, P < 0.001). In addition, the score of objective comprehension increased according to the participant's highest academic qualification obtained: school leaving certificate (68.6 (10.4), n = 77), high school graduation (71.1 (9.6), n = 84), undergraduate degree (74.0 (9.5), n = 149), and postgraduate degree (77.3 (7.0), n = 65) (P = 0.001 for trends). Finally, patients currently or previously working in medical/paramedical fields had higher scores (76.1 (8.4)) than others (71.2 (9.8), P < 0.001).
In a multivariable analysis, female gender (P = 0.01) and educational level (P < 0.001) were the only parameters associated with better objective comprehension.
For the analysis at 1 year, 225 patients answered the comprehension questionnaire. No difference was shown between the two groups for the objective score 77.0 (95% CI 75.0, 79.0) for the original vs. 77.6 (95% CI 74.5, 78.6) for the modified ICD (P = 0.82) and for each dimension.
Updated systematic review of improved informed consent forms
The systematic review showed no improved understanding in studies performed in real conditions, while an improvement was found for those performed in simulated situations (Figure2). However, there was substantial heterogeneity among studies performed in simulated situations, whereas heterogeneity remained low among studies performed in real conditions. When funnel plots were constructed, we observed a trend towards asymmetry (positive publication bias, Figure3) for the simulated trials (Egger's regression P = 0.11), while no asymmetry was apparent for the real trials. When considering the rate of enrolment, heterogeneity was low and results showed a decreased rate of enrolment when using modified ICDs (Figure4).
Figure 2.
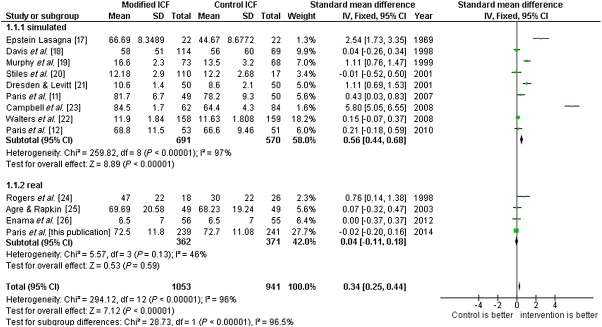
Meta-analysis of the effect of improved informed consent documents on subjects comprehension, stratified by the simulated or real condition
Figure 3.
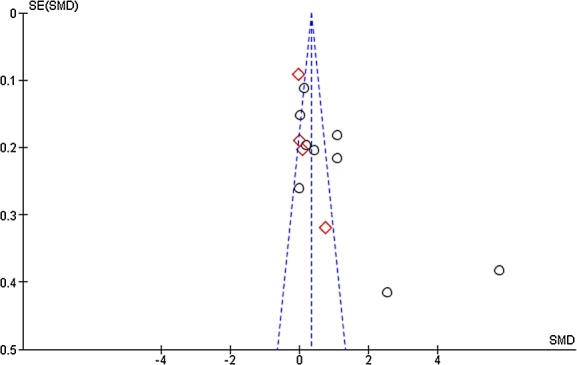
Funnel plot of the effect of improved informed consent documents on subjects comprehension, stratified by the simulated or real condition.  simulated,
simulated,  real
real
Figure 4.
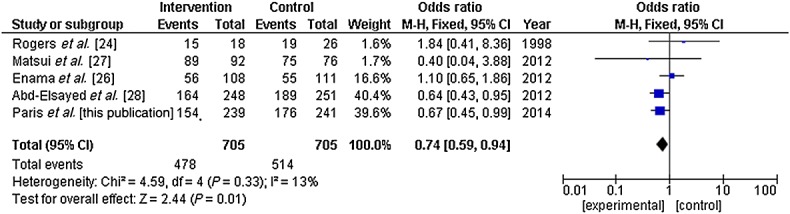
Meta-analysis of the effect of improved informed consent documents on subjects enrolment rate
Discussion
Improving ICDs in terms of readability and applying French recommendations of good writing practice for ICDs were not associated with better objective comprehension. In addition, the rate of study enrolment decreased in the group of volunteers who were informed with modified ICDs. This was unexpected given that our initial hypothesis was that improving information may improve the enrolment rate.
The present study had the strength that it was performed in real conditions implicating six different research centres and 18 different clinical trials, making it the largest trial testing ‘improved’ ICDs to date. The conclusion remained unchanged whether data were analyzed using multiple imputation for missing data or without replacement of missing data, and after removal of the largest clinical trial. Furthermore, while comprehension in the short term is most relevant to patient enrolment, we also showed that there was no difference in comprehension 1 year after the consent process.
It could be asked whether our tool to assess patients’ comprehension was sensitive enough. Indeed, in the literature a variety of tools have been used, from multiple choice questions to a few formally validated questionnaires. Our score of objective comprehension was sensitive enough to differ in our population according to educational level and female gender, in multivariable analysis. Therefore, it is reasonable to believe that our questionnaire would have demonstrated a difference in comprehension, had there been any difference.
There is a consistent trend in the literature showing that whatever the way of improving the ICDs in real clinical trials, there is no gain in objective understanding. This observation seems to be true whatever the language used 27,30. No difference was found between a very short (five pages) and a much longer (11 pages) consent document in terms of comprehension and final enrolment. Interestingly, while no objective difference can be seen, some authors have shown that the participants felt better informed when given concise ICDs 26. The enhanced comprehension observed in Nishimura et al.'s systematic review was due to the combination of studies of enhanced ICDs used in real clinical trials and those performed in a simulated setting. Indeed, the authors highlighted the high heterogeneity (I2 was 92%) of the meta-analysis. When we updated the meta-analysis and separated studies performed in real and simulated conditions, the heterogeneity of studies performed in real conditions was moderate (I2 was 46%) while that of simulated studies was considerable. In addition, the funnel plot of simulated studies showed a non-significant trend towards asymmetry, with two studies with low precision showing a high benefit of improved consent documents in terms of understanding, suggesting a publication bias. Our data show that when performing research on the informed consent process, no conclusion can be drawn from simulated studies. It should be noted that in our meta-analysis data from the recent large (more than 500 patients) study by Abd-Elsayed et al. undertaken in real conditions, only 'rate of enrolment” data could be included as no total comprehension score was available 28. However, the rate of qualitative responses was similar for the standard and enhanced forms and thus would not change our conclusion.
One key question is to determine how time and money should be best spent to improve participant comprehension and participation in biomedical research. In addition to the lack of improvement in understanding, we showed an unexpected lower rate of enrolment in the modified ICD group, like recently reported in a large study enrolling 251 patients asked to participate in cardiac surgery clinical trials 28. Data from the systemic review of Nishimura et al. 14 clearly show that interaction with physicians and clinical research assistants through extended discussions may be the best way to improve comprehension, and detailed analysis suggests that when only those studies performed in real conditions are considered, this seems to be the best way to improve comprehension 14. While much time and money may be spent trying to improve ICDs through systematic analysis or through collaboration with patient associations, both our data and our updated systematic review do not support this. One potential limitation to our study was that we used overall non-enrolment as an end point, whether the reason was refusal to sign or the presence of non-inclusion criteria, and we observed only a trend in terms of refusal to sign the consent document. However, the meta-analysis showed low heterogeneity between studies for this end point, and confirmed our findings. Another limitation was that, similarly to Abd-Elsayed et al. 28, we cannot explain the unexpected higher rate of non-enrolment in patients receiving the modified ICD. The study design did not permit blinding of consenters and physicians, and we cannot rule out a bias, but this seems unlikely given that both physicians and IRB members preferred the modified consent documents and expected no effect on consent rates.
New technologies may be of help and a recent study showed, albeit in a simulated setting, that an interactive consent process using an iPad based interactive system was much better than standard paper consent documents in terms of understanding, although at the expense of more time being needed for the consent process 31. This result needs to be confirmed in a real setting.
In conclusion, improving ICDs had no effect on patients and decreased the enrolment rate. Our result was further supported by an updated systematic review. As a consequence, in order to improve understanding by participants in clinical research, efforts and future trials should be focused on other ways to improve comprehension.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare JLC, CC, CT, BD, CD and PM had support from French Ministry of Health (PHRC National) and AP, MK and EHhad no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We thank the staff of the participating clinical research centres and clinical units that included patients, the ethics committees that approved the ICDs and the study sponsor, Grenoble University Hospital. We are grateful to Alison Foote, PhD (Grenoble Clinical Research Centre) for critical reading and language editing.
Contributors
AP, BD, CC, CT, PM, CD and JLC participated in study concept and design and critical revision of the manuscript. AP and JLC participated in drafting of the manuscript. AP and JLC obtained funding. AP and MK participated in recruitment of centres, participants and studies, in data-management and in coordination of the study. EH and JLC conducted and are responsible for the data analysis. AP participated in administrative and study supervision.
AP and JLC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This work was supported by the French Ministry of Health – Programme for Hospital Clinical Research (PHRC) 2008.
References
- Falagas ME, Korbila IP, Giannopoulou KP, Kondilis BK, Peppas G. Informed consent: how much and what do patients understand? Am J Surg. 2009;198:420–35. doi: 10.1016/j.amjsurg.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Paris A, Cracowski J-L, Ravanel N, Cornu C, Gueyffier F, Deygas B, Guillot K, Bosson J-L, Hommel M. Readability of informed consent forms for subjects participating in biomedical research: updating is required. Presse Médicale Paris Fr 1983. 2005;34:13–8. doi: 10.1016/s0755-4982(05)83877-1. [DOI] [PubMed] [Google Scholar]
- Paris A, Cracowski J-L, Maison P, Radauceanu A, Cornu C, Hommel M. Impact of French ‘Comités de Protection des Personnes’ on the readability of informed consent documents (ICD) in biomedical research: more information, but not better information. Fundam Clin Pharmacol. 2005;19:395–9. doi: 10.1111/j.1472-8206.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- Paasche-Orlow MK, Taylor HA, Brancati FL. Readability standards for informed-consent forms as compared with actual readability. N Engl J Med. 2003;348:721–6. doi: 10.1056/NEJMsa021212. [DOI] [PubMed] [Google Scholar]
- Jiménez Alvarez C, Morales Torres JL, Pereira Rodríguez MJ. Evaluation of thoroughness and legibility of informed consent documents in pediatric surgery. Cir Pediátrica Organo Of Soc Esp Cir Pediátrica. 2001;14:53–6. [PubMed] [Google Scholar]
- Reinert C, Kremmler L, Burock S, Bogdahn U, Wick W, Gleiter CH, Koller M, Hau P. Quantitative and qualitative analysis of study-related patient information sheets in randomised neuro-oncology phase III-trials. Eur J Cancer. 2014;50:150–8. doi: 10.1016/j.ejca.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Pilegaard M, Ravn HB. Readability of patient information can be improved. Dan Med J. 2012;59:A4408. [PubMed] [Google Scholar]
- Benatar JR, Mortimer J, Stretton M, Stewart RAH. A Booklet on Participants’ Rights to Improve Consent for Clinical Research: A Randomised Trial. PLoS ONE [Internet] 2012;7 doi: 10.1371/journal.pone.0047023. : Oct 19 [cited 2014 Jul 22]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3477160/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassany O, Bernard-Harlaut M, Guy G, Billon N. the participants in Round Table N°3, Giens XXIV. Information sheets and informed consent forms for clinical study participants: towards standardised recommendations? Therapie. 2009;64:179–86. doi: 10.2515/therapie/2009035. [DOI] [PubMed] [Google Scholar]
- Hénin Y, de Boischevalier B, Reboul-Salze F, Cracowski J-L, Dualé C. Aide à la rédaction du document écrit destiné à l'information du participant à la Recherche BioMédicale et à l'attestation de son consentement éclairé. Therapie. 2010;65:71–4. doi: 10.2515/therapie/2010006. [DOI] [PubMed] [Google Scholar]
- Paris A, Nogueira da Gama Chaves D, Cornu C, Maison P, Salvat-Mélis M, Ribuot C, Brandt C, Bosson J-L, Hommel M, Cracowski J-L. Improvement of the comprehension of written information given to healthy volunteers in biomedical research: a single-blind randomised controlled study. Fundam Clin Pharmacol. 2007;21:207–14. doi: 10.1111/j.1472-8206.2007.00472.x. [DOI] [PubMed] [Google Scholar]
- Paris A, Brandt C, Cornu C, Maison P, Thalamas C, Cracowski J-L. Informed consent document improvement does not increase patients’ comprehension in biomedical research. Br J Clin Pharmacol. 2010;69:231–7. doi: 10.1111/j.1365-2125.2009.03565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: A systematic review. JAMA. 2004;292:1593–601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomised control trials. BMC Med Ethics. 2013;14:28. doi: 10.1186/1472-6939-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst. 2001;93:139–47. doi: 10.1093/jnci/93.2.139. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Greene S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] [Internet] The Cochrane Collaboration. 2011;2011 : [cited 2014 Jul 30]. Available from: http://handbook.cochrane.org. [Google Scholar]
- Epstein LC, Lasagna L. Obtaining informed consent. Form or substance. Arch Intern Med. 1969;123:682–8. [PubMed] [Google Scholar]
- Davis TC, Holcombe RF, Berkel HJ, Pramanik S, Divers SG. Informed consent for clinical trials: a comparative study of standard versus simplified forms. J Natl Cancer Inst. 1998;90:668–74. doi: 10.1093/jnci/90.9.668. [DOI] [PubMed] [Google Scholar]
- Murphy DA, O'Keefe ZH, Kaufman AH. Improving comprehension and recall of information for an HIV vaccine trial among women at risk for HIV: reading level simplification and inclusion of pictures to illustrate key concepts. AIDS Educ Prev Off Publ Int Soc AIDS Educ. 1999;11:389–99. [PubMed] [Google Scholar]
- Stiles PG, Poythress NG, Hall A, Falkenbach D, Williams R. Improving understanding of research consent disclosures among persons with mental illness. Psychiatr Serv Wash DC. 2001;52:780–5. doi: 10.1176/appi.ps.52.6.780. [DOI] [PubMed] [Google Scholar]
- Dresden GM, Levitt MA. Modifying a standard industry clinical trial consent form improves patient information retention as part of the informed consent process. Acad Emerg Med. 2001;8:246–52. doi: 10.1111/j.1553-2712.2001.tb01300.x. [DOI] [PubMed] [Google Scholar]
- Walters KA, Hamrell MR. Consent Forms, Lower Reading Levels, and Using Flesch-Kincaid Readability Software. Drug Inf J. 2008;42:385–94. [Google Scholar]
- Campbell HM, Raisch DW, Sather MR, Segal AR, Warren SR, Naik R. Impact of a clinical trials information handbook on patient knowledge, perceptions, and likelihood of participation. IRB. 2008;30:6–14. [PubMed] [Google Scholar]
- Rogers CG, Tyson JE, Kennedy KA, Broyles RS, Hickman JF. Conventional consent with opting in versus simplified consent with opting out: An exploratory trial for studies that do not increase patient risk. J Pediatr. 1998;132:606–11. doi: 10.1016/s0022-3476(98)70347-6. [DOI] [PubMed] [Google Scholar]
- Agre P, Rapkin B. Improving informed consent: a comparison of four consent tools. IRB. 2003;25:1–7. [PubMed] [Google Scholar]
- Enama ME, Hu Z, Gordon I, Costner P, Ledgerwood JE, Grady C. Randomization to standard and concise informed consent forms: Development of evidence-based consent practices. Contemp Clin Trials. 2012;33:895–902. doi: 10.1016/j.cct.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Lie RK, Turin TC, Kita Y. A randomised controlled trial of short and standard-length consent forms for a genetic cohort study: is longer better? J Epidemiol. 2012;22:308–16. doi: 10.2188/jea.JE20110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-Elsayed AA, Sessler DI, Mendoza-Cuartas M, Dalton JE, Said T, Meinert J, Upton G, Franklin C, Kurz A. A randomised controlled study to assess patients’ understanding of and consenting for clinical trials using two different consent form presentations. Minerva Anestesiol. 2012;78:564–73. [PubMed] [Google Scholar]
- 2009. StataCorp: Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.
- Foradori MA, Nolan MT. Effect of a study map intended to support informed consent in transplant research. Prog Transplant Aliso Viejo Calif. 2012;22:56–61. doi: 10.7182/pit2012553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Astin J, Greene K, Cummings SR. Interactive Informed Consent: Randomised Comparison with Paper Consents. PLoS One. 2013;8:e58603. doi: 10.1371/journal.pone.0058603. [DOI] [PMC free article] [PubMed] [Google Scholar]


