Abstract
Aims
Physiological changes in pregnancy are expected to alter the pharmacokinetics of various drugs. The objective of this study was to evaluate systematically the pharmacokinetics of oseltamivir (OS), a drug used in the treatment of influenza during pregnancy.
Methods
A multicentre steady-state pharmacokinetic study of OS was performed in 35 non-pregnant and 29 pregnant women. Plasma concentration–time profiles were analyzed using both non-compartmental and population pharmacokinetic modelling (pop PK) and simulation approaches. A one compartment population pharmacokinetic model with first order absorption and elimination adequately described the pharmacokinetics of OS.
Results
The systemic exposure of oseltamivir carboxylate (OC, active metabolite of OS) was reduced approximately 30 (19–36)% (P < 0.001) in pregnant women. Pregnancy significantly (P < 0.001) influenced the clearance (CL/F) and volume of distribution (V/F) of OC. Both non-compartmental and population pharmacokinetic approaches documented approximately 45 (23–62)% increase in clearance (CL/F) of OC during pregnancy.
Conclusion
Based on the decrease in exposure of the active metabolite, the currently recommended doses of OS may need to be increased modestly in pregnant women in order to achieve comparable exposure with that of non-pregnant women.
Keywords: oseltamivir, population pharmacokinetics, pregnancy
What is Already Known about this Subject
Oseltamivir (OS) is approved for the prevention and treatment of influenza viral infections.
Pregnant women are at great risk for complications associated with influenza infections.
The current dosing recommendation of OS in pregnant women is based on studies in men and non-pregnant women with influenza infections. However, physiological changes during pregnancy may alter the pharmacokinetics of drugs in pregnant women.
What this Study Adds to our Knowledge
The clearance of oseltamivir carboxylate was higher during pregnancy, resulting in lower systemic exposure of oseltamivir carboxylate in pregnant women.
We suggest an increase in dose of OS during pregnancy in order to achieve comparable drug exposure to that of non-pregnant women.
Introduction
Pregnant women with influenza viral infections are at higher risk for severe illness, hospitalizations and mortality than non-pregnant women with influenza 1,2. Influenza infections may also adversely affect the pregnancy, leading to complications including premature delivery and fetal growth alterations 1,3,4. The World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have recommended oseltamivir (OS, Tamiflu®) as one of the primary pharmacological options for disease mitigation 5.
OS is an orally administrated pro-drug. It is extensively hydrolyzed by hepatic carboxylesterases to the active drug oseltamivir carboxylate (OC) 6. The oral bioavailability of OS is approximately 80%. The active metabolite, OC, is a major circulating component (95%) in plasma, and the pro-drug OS accounts for only approximately 5% of the circulating component in plasma 7. OS is moderately bound to plasma proteins (42%) with a steady-state volume of distribution (Vss/F) of 1768 ± 1445 l, and a half-life of 1–3 h 6,8. Oral intake does not appear to have significant effects on the pharmacokinetics of OS 9. OC is poorly bound to plasma protein (<3%), and the volume of distribution is 23 to 26 l. It is eliminated entirely (>99%) through renal excretion by glomerular filtration and active tubular secretion with a half-life of 6–10 h 6.
Physiological alterations that occur during pregnancy have been reported to alter the exposure of various therapeutic agents in pregnant women 10. A previous report from our group has shown that exposure to the active metabolite, OC, was reduced by approximately 30% in pregnant women compared with non-pregnant women. It was proposed that an increase in the dose of OS may be considered for the treatment of influenza infections in pregnant women 8. However, the conclusions of this earlier study were limited by a relatively small sample size, demographic differences (race and age) between the pregnant and non-pregnant groups, and the use of an empirical method of data analysis. These limitations suggested that additional clinical pharmacokinetic analysis of OS in pregnant women was warranted.
Population pharmacokinetic (pop PK) modelling (with simulations) is a powerful tool often used to estimate the pharmacokinetics of a therapeutic agent using the concentration–time profiles from a small number of subjects and limited sampling from a large number of subjects. Until recently, very limited pop PK studies of OS have been reported in healthy and influenza infected subjects 11–17. However, no pop PK studies have been reported comparing the exposure of OC between pregnant and non-pregnant women. The objective of this study was to develop and validate a pop PK model for OS and OC in a large group of demographically-balanced pregnant and non-pregnant women. We also evaluated the covariates that influence the exposure of OC in pregnant women.
Methods
Data
Data were obtained from non-pregnant reproductive-aged women and pregnant women who were recruited between 2007 and 2012 from four clinical research centres, including Magee Women's Hospital of the University of Pittsburgh Medical Center, Pittsburgh, PA, University of Texas Medical Branch, Galveston, TX, University of Washington Medical Center, Seattle, WA and Georgetown University, Washington, DC, USA Part of the data was previously analyzed using non-compartmental pharmacokinetic analysis and was published elsewhere 8. Additional subjects were only enrolled at the Pittsburgh site. All protocols were approved by each site's institutional review board and all participants underwent the informed consent process before entering the study. All of the non-pregnant control subjects received standard oral doses of OS at 75 mg once daily or 75 mg twice daily for at least 48 h prior to reporting for study procedures, and pregnant subjects were continued on their clinical doses either for prophylaxis or for treatment of influenza viral infection/influenza like illness, with no changes made in the dose of OS for study purposes. A previous publication 18 has reported no significant differences in the pharmacokinetics of OC between healthy subjects and influenza viral infected patients.
Sample collection and bioanalysis
On the study day, all participants underwent a pre-dose blood draw (trough) and received a 75 mg oral dose of OS. Subsequently, serial blood samples were collected over one dosing interval at 0.5, 1, 2, 3, 4, 6, 8, 10 and 12 h after the dose. For once daily dosing, an additional blood sample was collected at 24 h after the dose. Blood samples were collected into K3-EDTA/sodium fluoride tubes and centrifuged at 3000 rev min–1 for 10 min, and plasma was separated and stored at –80 °C until analysis.
OS and OC were analyzed by a previously validated method 19 using liquid chromatography with tandem mass spectrometry and positive electrospray ion mode. The assay sensitivity in terms of lower limit of quantification for OS was 1 ng ml–1 and OC was 10 ng ml–1. The coefficient of variation (CV%) for the accuracy and precision of the assay was <3% and <6%, respectively.
Non-compartmental pharmacokinetic analysis
Plasma concentration–time profiles of OS and OC in non-pregnant and pregnant women are shown in Figure1. The maximum plasma concentration (Cmax), area under the concentration–time curve (AUC(0,tlast)), clearance (CL/F), volume of distribution (Vd,ss) and half-life (t1/2) were calculated with a non-compartmental approach using Phoenix WinNonlin® version 6.1 (Pharsight Corp, Cary, North Carolina, USA).
Figure 1.
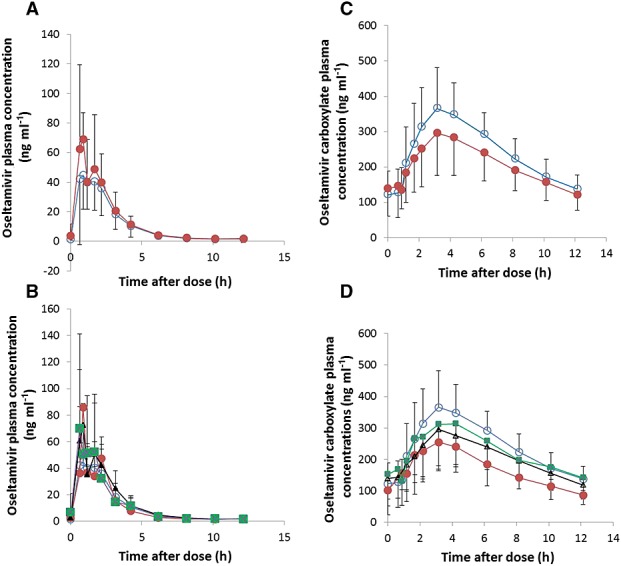
Mean plasma concentration–time profiles of oseltamivir (A and B) and oseltamivir carboxylate (C and D) in non-pregnant and pregnant women. A and C  non-pregnant,
non-pregnant,  pregnant. B and D
pregnant. B and D  non-pregnant,
non-pregnant,  trimester 1,
trimester 1,  trimester 2,
trimester 2,  trimester 3
trimester 3
Population pharmacokinetic modelling
Pop PK modelling was developed using non-linear mixed effects modelling software (nonmem version 7.1; ICON Development Solutions, Hanover, MD, USA), GNU Fortran 95 compiler and PLT tools (version 5.1.0). Earlier reports suggested that the pharmacokinetics of OS were linear up to 500 mg oral dose 20. OS is extensively metabolized (∼95%) to its active metabolite, OC in humans 7. Approximately 94.5% of OS was recovered as OC in urine samples from both non-pregnant and pregnant women. The elimination of OC is predominantly through the renal route 21. Therefore, the following assumptions were made prior to model building, 1) the pharmacokinetics of OS are linear, 2) the fraction of OS metabolized to OC (fm) is fixed to 0.945 and 3) OC and the remaining OS are completely eliminated through renal route.
The general linear model under steady-state was constructed using ADVAN5 SS5 subroutines. Both zero and first order absorption models were tested. Similarly, one compartment and two compartment models were fitted to the current data. The first order conditional estimation method with interaction was used for the parameter estimation. The inter-individual variability (IIV) in pharmacokinetic model parameters was estimated using an exponential model,
where Pij is the ith individual's estimate of the jth pharmacokinetic parameter, TV(Pj) is the typical value of the jth pharmacokinetic parameter, and ηij is a random variable for the ith individual and the jth pharmacokinetic parameter distributed with mean zero and variance of ωj2.
The random residual variability was modelled using combined error model,
where Cobs and Cpred are the observed and predicted concentrations, respectively, and ε and ε' are normal random variables with means of zero and variances of σ2 and σ'2, respectively.
In order to identify the covariates that influence the pharmacokinetics of OC, the following covariates including age (years), body weight (kg), height (cm), race, pregnancy, gestational age, trimester of pregnancy, albumin, serum creatinine (mg dl–1), creatinine clearance (CLCR, ml min–1), blood urea nitrogen (BUN, mg dl–1), aminotransferases (ALT and AST) and total bilirubin were evaluated. The urinary creatinine was quantitated and used for the calculation of creatinine clearance (Cumulative amount of creatinine excreted in urine (mg)/(serum creatinine (mg dl–1) × time (h)). For covariate selection, univariate analysis with stepwise forward addition (P < 0.01) and backward elimination (P < 0.001) procedures were followed. Covariate-parameter relationships were evaluated as random variability of model parameter vs. covariate plots and the graphical trend was assessed by univariate linear regression analysis (data not shown). The covariates that significantly influence the pharmacokinetics of OS and its metabolite were incorporated in the final model.
The adequacy of the model was evaluated using the following criteria: examination of goodness of fit diagnostic plots for observed vs. population predicted or individual predicted concentration–time profiles, weighted residuals vs. predicted concentrations, change in minimum objective function values (OFV) (>3.84, considered statistically significant based on chi-square distribution with 1 degree of freedom) and extent of interindividual and residual random variability. Precision of parameter estimation and stability of the model parameter estimates were evaluated with bootstrapping (resampling repeated 500 times) using Wings for nonmem® 22. Non-parametric statistics (median, 95% confidence interval) of parameter estimates obtained from bootstrapping were compared with the point parameter estimates obtained from the model.
The predictive performance of the model was internally evaluated using a prediction corrected visual predictive check, in which 1500 data sets were simulated using the parameter estimates in the final model. The 50th percentile concentration (median) and the 5th and 95th percentile concentrations (90% prediction interval) were plotted and compared with the observed concentrations.
Additional statistical analysis
The statistical analysis was performed using Graph Pad Prism version 5.04 (La Jolla, CA, USA). Statistical comparison between two groups was analyzed using two-sided Student's t-test, whereas three or more groups were statistically compared using one way analysis of variance (anova) followed by Tukey's post hoc test. A P value of < 0.05 was considered statistically significant.
Results
Demographic data
A total of 763 samples were collected from 35 non-pregnant and 29 pregnant women enrolled in this study. The number of pregnant subjects in trimester 1 (TRI1), trimester 2 (TRI2) and trimester 3 (TRI3) of pregnancy were 4, 16 and 9, respectively. For pregnant subjects, 18 out of 29 subjects tested positive for polymerase chain reaction proven influenza virus A/B and the remaining subjects either tested as negative or were not tested. Among the 64 subjects, one subject from the non-pregnant and two subjects from the pregnant categories possessed atypical concentration–time profiles with fewer than three points in the terminal disposition phase, and were subsequently not included for non-compartmental analysis. However, all the subjects were included in the population pharmacokinetic analysis. The demographics and clinical characteristics of the non-pregnant and pregnant women are presented in Table1. The median age of the pregnant women was slightly, but significantly, lower than non-pregnant women. Serum albumin, serum creatinine and blood urea nitrogen were significantly reduced whereas body weight and creatinine clearance were significantly increased in pregnant women as compared with non-pregnant women.
Table 1.
Demographic and clinical characteristics of non-pregnant and pregnant women
| Parameter | Non-pregnant | Pregnant |
|---|---|---|
| Number of subjects | 35 | 29 |
| Trimester 1 | 4 | |
| Trimester 2 | 16 | |
| Trimester 3 | 9 | |
| Age (years) | 28 (18–50) | 24 (18–40)* |
| Gestational age (weeks) | 0 | 25 (6–37) |
| Body weight (kg) | 71 (52–113) | 82 (48–147)* |
| BMI (kg m–2) | 28 (19–44) | 30 (18–50) |
| Race | ||
| Caucasian | 24 | 11 |
| African American | 11 | 18 |
| Site | ||
| UPMC, Pittsburgh | 3 | 27 |
| UT, Galveston | 20 | 2 |
| UW, Seattle | 10 | |
| GU, Washington, DC | 2 | |
| Albumin (g dl–1) | 4.3 (2.8–5.6) | 2.8 (2.2–3.8)* |
| Serum creatinine (mg dl–1) | 0.7 (0.55–1) | 0.6 (0.4–0.9)* |
| Blood urea nitrogen (mg dl–1) | 12 (7–19) | 7 (2–13)* |
| Creatinine clearance (ml min–1) | 128 (69–254) | 163 (52–327)* |
| AST (IU l–1) | 23 (10–62) | 18 (11–79) |
| ALT (IU l–1) | 29 (10–116) | 29 (10–72) |
| ALK (IU l–1) | 82 (40–127) | 78 (42–176) |
| BIL (mg dl–1) | 0.5 (0.1–1.6) | 0.2 (0.1–0.7) |
Values are expressed as median (range).
*P < 0.05 vs. non-pregnant, unpaired, two sided t-test
Non-compartmental pharmacokinetic analysis
The pharmacokinetic parameters estimated from the plasma concentration–time profiles of OS and OC are shown in Table2. The exposure to the parent compound OS was only slightly but significantly (*P < 0.05) increased in pregnant women (178 ± 68 ng ml–1 h) compared with non-pregnant women (145 ± 46 ng ml–1 h) with a corresponding minimal decrease in CL/F (478 ± 168 l h–1 vs. 569 ± 173 l h–1) during pregnancy. The Vd,ss/F of OS was significantly (P < 0.05) reduced (1909 ± 860 vs. 1342 ± 635 l) during pregnancy. The Cmax and t1/2 of OS were not different between pregnant (71 ± 36 ng ml–1 and 3.8 ± 1.7 h, respectively) and non-pregnant (85 ± 43 ng ml–1 and 3.0 ± 1.9 h, respectively) women.
Table 2.
Parameter estimates from non-compartmental pharmacokinetic analysis of oseltamivir and oseltamivir carboxylate
| Non-pregnant | Pregnant | |||
|---|---|---|---|---|
| OS | OC | OS | OC | |
| Cmax (ng ml–1) | 71 ± 36 | 397 ± 113 | 85 ± 43 | 318 ± 107** |
| AUC(0,tlast) (ng ml–1 h) | 145 ± 46 | 3507 ± 992 | 178 ± 68* | 2653 ± 928** |
| CL/F (l h–1) | 569 ± 173 | 23 ± 6 | 478 ± 168* | 33 ± 16** |
| Vd,ss/F (l) | 1909 ± 860 | 149 ± 35 | 1342 ± 635** | 184 ± 73* |
| t1/2 (h) | 3.0 ± 1.9 | 5.9 ± 1.5 | 3.8 ± 1.7 | 6.3 ± 2.2 |
Values are represented as mean ± SD;
*P < 0.05 and
**P < 0.01 vs. non-pregnant, unpaired two sided t-test
The Cmax and exposure (AUC(0,tlast)) of the active metabolite, OC, were significantly lower (P < 0.01) in pregnant women (318 ± 107 ng ml–1 and 2653 ± 928 ng ml–1 h, respectively) compared with non-pregnant women (397 ± 113 ng ml–1 and 3507 ± 992 ng ml–1 h, respectively). This was associated with an increase (P < 0.05) in clearance (CL/F) of OC (33 ± 16 l h–1 vs. 23 ± 6 l h–1) during pregnancy. The Vd,ss/F of OC was also significantly (P < 0.05) increased (184 ± 73 vs. 149 ± 35) during pregnancy. However, the half-life (t1/2) of OC was not different between pregnant (6.3 ± 2.2 h) and non-pregnant (5.9 ± 1.5 h) women.
Population pharmacokinetic modelling
The structure of population pharmacokinetic model is shown in Figure2. A model incorporating first order absorption was selected due to its superiority over zero order absorption of OS. Similarly, a one compartment model produced a better fit than a two compartment model using the current data. The final model selection was supported by the symmetrical distribution of observed vs. model predicted concentrations around the lines of identity (goodness of fit diagnostic plots), and homoscedastic distribution of weighted residuals over time (weighted residuals vs. time after dose plots) (Figure3). Parameters that were estimated using this model included absorption rate constant (ka) of OS, and clearance/bioavailability (CL/F) and volume of distribution/bioavailability (Vd/F) of OS and OC. Since the oral bioavailability could not be calculated in this study, the clearance and volume of distribution were reported as CL/F and Vd/F. The first value below the quantitation limit value (BLQ) was replaced with lower limit of quantitation value (LOQ)/2 and the remaining BLQ values were ignored for the analysis. The BLQ values observed in this study were less than 7%. Only two non-pregnant subjects whose missing covariate values (weight, height and serum biochemistry values such as serum creatinine, blood urea nitrogen, creatinine clearance, AST, ALT, ALK and bilirubin) were substituted with median covariate values from the population.
Figure 2.
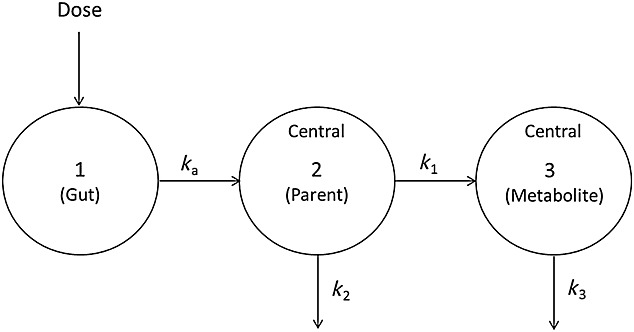
Structure of oseltamivir carboxylate population pharmacokinetic model. ka, absorption rate constant; k1, conversion rate constant of oseltamivir to oseltamivir carboxylate; k2 and k3, elimination rate constant of oseltamivir and oseltamivir carboxylate, respectively
Figure 3.
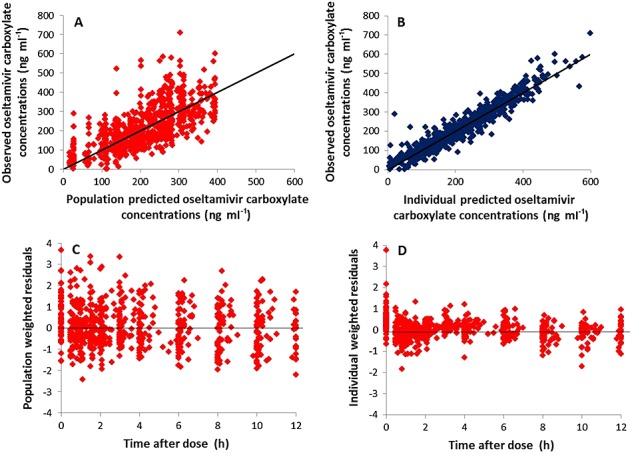
Goodness of fit diagnostic plots for oseltamivir carboxylate concentrations fitted using one compartment model with first order absorption and elimination. Observed plasma concentrations vs. population predicted concentrations (A), observed concentrations vs. individual predicted concentrations (B), population weighted residuals vs. time after dose (C) and individual weighted residuals vs. time after dose (D). The solid line indicates the line of identity
Using univariate analysis, the influential covariates were selected based on the initial screening of individual covariate followed by forward addition (P < 0.01) and backward elimination (P < 0.001) processes. Covariate analysis suggested that weight and pregnancy significantly influence the CL/F of OS whereas renal function markers such as creatinine clearance and serum creatinine significantly influence the CL/F of OC. In addition, the covariate pregnancy also significantly influenced the Vd/F of OC. We observed a significant interaction between the covariates of pregnancy and weight, as well as pregnancy and renal function markers. Therefore, we chose pregnancy as a covariate of OC CL/F and Vd/F in the final model. The final model showed significant decreases in the OFV (25.4, P < 0.001, Chi-square distribution, df = 2). The inclusion of pregnancy as a covariate modestly reduced the IIV of CL/F and Vd/F of OC from 35% to 31% and 40% to 36%, respectively. The pop PK parameter estimates, IIV and random residual variability are represented in Table3.
Table 3.
Parameter estimates from population pharmacokinetic analysis and bootstrap analysis of oseltamivir and oseltamivir carboxylate
| Parameter | Final model | Bootstrap(n = 500) | ||
|---|---|---|---|---|
| Estimate | %RSE | Median | 2.5–97.5 percentile | |
| ka | 0.6 | 8.3 | 0.65 | 0.56–1.09 |
| CL/F OS | 532 | 4.9 | 546 | 492–600 |
| CL/F OC | 20 | 4.9 | 20 | 18–23 |
| Vd/F OS | 289 | 17.6 | 346 | 224–697 |
| Vd/F OC | 152 | 5.6 | 155 | 137–172 |
| PG effect on CL/F OC | 8.1 | 25.9 | 8.3 | 4.2–12.9 |
| PG effect on Vd /F OC | 56 | 28.8 | 68 | 27–114 |
| Inter-individual variability (%CV) | ||||
| ka | 38.1 | 15.4 | 30.3 | 0.3–60 |
| CL/F OS | 39.2 | 10.4 | 34.1 | 25–43 |
| CL/F OC | 30.8 | 4.8 | 30.8 | 26–36 |
| V/F OS | 92.7 | 27.1 | 78.0 | 30–110 |
| V/F OC | 35.9 | 14.0 | 31.1 | 23–45 |
| Residual variability | ||||
| Proportional error for OS | 50.3 %CV | 6.6 | 46.3 %CV | 42–51 %CV |
| Additive error for OS | 1.5 ng ml–1 (LOQ 2 ng ml–1) | 58.4 | 1.6 ng ml–1 | 1.1–5.2 ng ml–1 |
| Proportional error for OC | 15 %CV | Fixed | 5 %CV | Fixed |
| Additive error for OC | 35.7 ng ml–1 (LOQ 10 ng ml–1) | 0.1 | 34.9 ng ml–1 | 28–41 ng ml–1 |
The CL/F of OS was minimally but significantly reduced in pregnant women (504 ± 178 l h–1; *P < 0.05) compared with non-pregnant women (606 ± 178 l h–1). Although Vd,ss/F of OS was increased slightly in pregnant women (404 ± 315 l), the data were not significantly different from non-pregnant women (369 ± 235 l). The CL/F of OC was relatively higher in pregnant women (∼43%; 30.0 ± 10.7 l h–1; P < 0.001) than non-pregnant women (21.0 ± 5.4 l h–1). When compared with the non-pregnant women, the first trimester of pregnancy appears to have higher CL/F (66%) of OC than second (45%) and third trimester (28%) of pregnancy (Figure4). The Vd,ss/F of OC was also significantly (P < 0.001) increased (213 ± 63 vs. 155 ± 39 l (non-pregnant)) during pregnancy. The AUC distribution of OC was shifted towards the lower side in agreement with an increase in clearance during pregnancy (Figure5).
Figure 4.
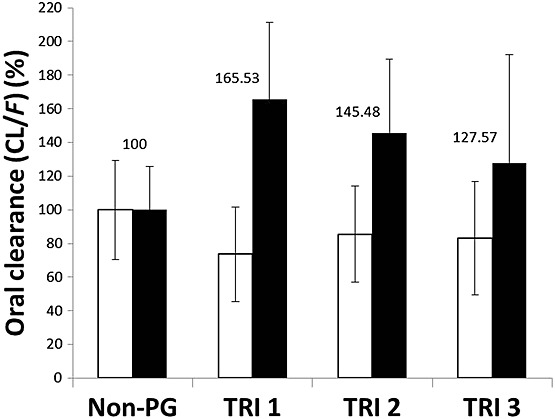
% oral clearance (CL/F) of oseltamivir and oseltamivir carboxylate in non-pregnant and first (TRI1), second (TRI2) and third (TRI3) trimester of pregnancy. Open bar indicates CL/F of oseltamivir whereas the filled bar represents CL/F of oseltamivir carboxylate. The results are expressed as mean ± SD. *P < 0.05 and **P < 0.01 vs. non-pregnant oseltamivir carboxylate, one way anova followed by Tukey's multiple comparison post hoc test
Figure 5.
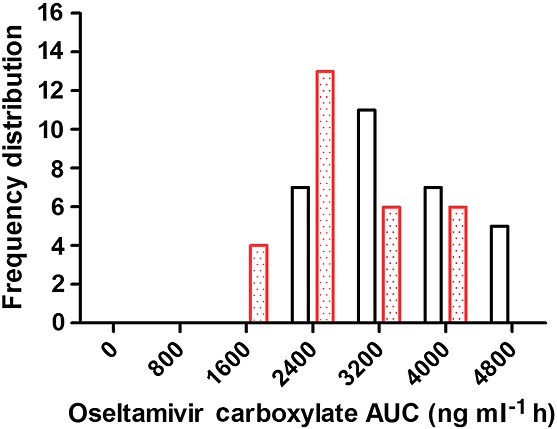
Frequency distribution of oseltamivir carboxylate area under the concentration–time (AUC) curve between non-pregnant and pregnant women. The frequency distribution of AUC of non-pregnant and pregnant women is shown as the open and dotted bar, respectively
Model validation
The stability of the model was evaluated by generating 500 datasets (resampling with replacement) using bootstrapping analysis. The median and 2.5–97.5th percentiles of parameter estimates from the bootstrapping analysis were compared with final parameter estimates (Table3). Additionally, the model performance was evaluated by simulating 1500 virtual datasets using the final model by visual predictive check (VPC). The visual inspection was performed at observed concentrations that were plotted against 5, 50 and 95 percentiles of the simulated concentrations at each time point (Figure6).
Figure 6.
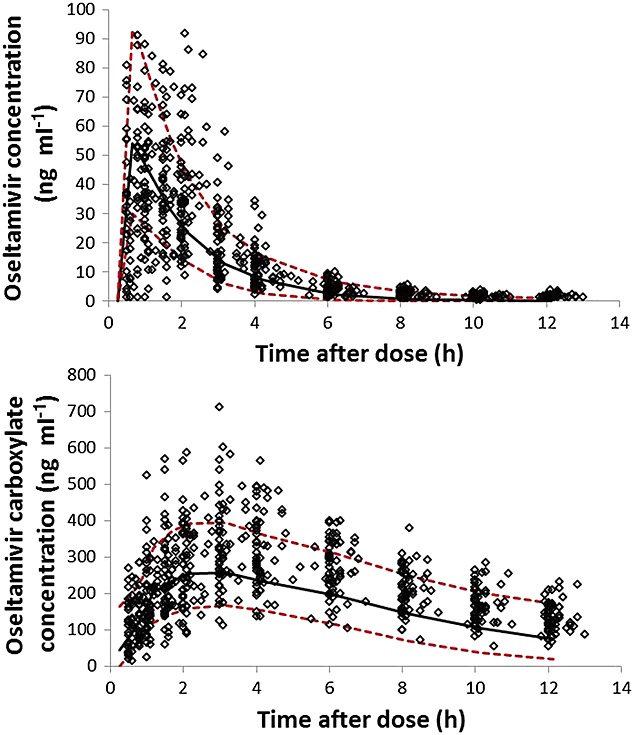
Predictive performance of the final model was analyzed using visual predictive check plots. The model predicted concentrations of oseltamivir (top) and oseltamivir carboxylate (bottom) were shown as 5, 50 and 95 percentiles lines and were plotted against observed concentrations at each time after dose.  5th and 95th percentile,
5th and 95th percentile,  50 percentile (median),
50 percentile (median),  Observants.
Observants.
Discussion
Pregnant women are at greater risk for poor clinical outcomes from influenza infections. The detrimental effects of influenza in pregnancy include higher rates of severe maternal illness, need for hospitalization and maternal death, as well as adverse effects on the pregnancy itself, including preterm birth and adverse fetal growth 23–25. Although influenza infections can be primarily controlled by influenza vaccination, the vaccine uptake rate still needs to be improved 26. Given these noted increased risks, precise and accurate pharmacological therapy with neuraminidase inhibitors such as OS are of prime importance 5.
Delineating the various pharmacokinetic parameters of drugs during pregnancy presents a challenge. This is partly due to the physiological changes during pregnancy which impact gastrointestinal absorption, plasma volume and protein binding, thereby influencing volume of distribution, gut and liver metabolism and renal elimination of drugs 10. On the basis of non-compartmental pharmacokinetic analysis, our earlier study suggested that the systemic exposure of OC was lowered approximately 30% in pregnant women compared with non-pregnant women 8. For this report we recruited additional subjects to balance better the demographic parameters between pregnant and non-pregnant women, and we performed both non-compartmental pharmacokinetic analysis and pop PK modelling to evaluate the pharmacokinetics of OS during pregnancy.
Non-compartmental pharmacokinetic analysis from this study suggested that the clearance (CL/F) of OC was increased by approximately 45% in pregnant women compared with non-pregnant women. These findings with an increased number of subjects and demographically comparable subjects were similar to our earlier observations. Considering the limitations associated with non-compartmental analysis, we performed a pharmacokinetic analysis using non-linear mixed effects modelling in non-pregnant and pregnant women.
Pop PK modelling provides a greater flexibility for estimation of the pharmacokinetic parameters of drugs under various scenarios including different dosing intervals, various sampling times, alternate routes of drug administration, and a multitude of demographic and physiologic covariates 27. This is the first study to evaluate the pharmacokinetics of OS in pregnant women based on pop PK approach. In this study, the plasma concentration–time profile of OC fitted well with a one compartment model with first order absorption and elimination. In order to avoid any erroneous covariate vs. parameter relationship, a parsimonious approach (P < 0.001) was used for the identification of significant covariates in the initial covariate screening. The significant covariate identified in this study was pregnancy. Population pharmacokinetic analysis indicates that pregnant women exhibit approximately 43% higher clearance (CL/F) with a corresponding decrease (∼30%) in exposure (AUC) of OC compared with non-pregnant women. The magnitude of increase in CL/F of OC for the first, second and third trimester of pregnancy was 66%, 45% and 28%, respectively (Figure3). Since OC is primarily eliminated through renal pathways, the higher clearance of OC during the first trimester could be due to the ∼50% increase in renal blood flow and glomerular filtration rate during the first trimester of pregnancy 28. It should be noted that the number of subjects in the first and third trimester of pregnancy was relatively low. Moreover, the trimester of pregnancy did not influence the pharmacokinetics of OS in this model. Interestingly, our results are in agreement with Greer et al. 29 that the trimester of pregnancy does not significantly affect the pharmacokinetics of OS during pregnancy.
In conclusion, both non-compartmental and pop PK approaches suggest that approximately 30% decrease in exposure to OC during pregnancy. Additionally, the trimester of pregnancy does not affect the pharmacokinetics of OS. In order to attain comparable systemic exposure with non-pregnant women, the currently recommended doses of OS may need to be increased (105 mg once daily for prophylaxis and 105 mg twice daily for treatment of influenza infections) in pregnant women. There is a lack of clinical evidence that supports OC exposure (AUC) and influenza viral suppression in humans. Considering a large variability in effective concentration that inhibits 90% of viral activity (0.004 to >100 µm; 1.14 ng ml–1 to >24800 ng ml–1; www.fda.gov), emerging strains of influenza viruses, OS resistant cases, and the complications associated with influenza infections during pregnancy, we suggest a pharmacokinetic guided approach to increase the dose of OS in the pregnant population in order to achieve comparable exposure with that of non-pregnant women. However, additional pharmacodynamic studies are necessary to support the dosing guidelines of OS during pregnancy.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.or/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work and no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years. K.H. was working with Roche Pharmaceuticals in the previous 3 years. There are no other relationships or activities that could appear to have influenced the submitted work.
This work is funded by grants (5U10 HD 047905, U10 HD047892 and 3U10 HD047891-05S2) from Obstetric Pharmacology Research Unit Network, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). We acknowledge the clinical staff (Donna Deangetlis and Dawn Fisher from University of Pittsburgh Medical Center, Holly West from University of Texas Medical Branch, Claudine Hernandez and Karen Hays from University of Washington) who helped for study conduct/data collection for this research. The study team also acknowledges Roche Pharmaceuticals for the logistic support for bio-analysis of oseltamivir and oseltamivir carboxylate.
References
- Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–5. doi: 10.1016/0002-9378(59)90570-8. [DOI] [PubMed] [Google Scholar]
- Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205:10–8. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Available at http://www.cdc.gov/flu/protect/vaccine/pregnant.htm (last accessed 2014). In, Atlanta, GA, 2014.
- Cervantes-Gonzalez M, Launay O. Pandemic influenza A (H1N1) in pregnant women: impact of early diagnosis and antiviral treatment. Expert Rev Anti Infect Ther. 2010;8:981–4. doi: 10.1586/eri.10.83. [DOI] [PubMed] [Google Scholar]
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recommendations and reports: morbidity and mortality weekly report and reports / Centers for Disease Control. 2011;60:1–24. [PubMed] [Google Scholar]
- He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet. 1999;37:471–84. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
- Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 2000;355:827–35. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- Beigi RH, Han K, Venkataramanan R, Hankins GD, Clark S, Hebert MF, Easterling T, Zajicek A, Ren Z, Mattison DR, Caritis SN. Pharmacokinetics of oseltamivir among pregnant and nonpregnant women. Am J Obstet Gynecol. 2011;204:S84–8. doi: 10.1016/j.ajog.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiflu Product Monograph. Available at https://www.healthunit.com/uploads/tamiflu-product-monograph.pdf. (last accessed 2013) Hoffmann-La Roche Limited, 2013.
- Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33:328–43. doi: 10.2165/00003088-199733050-00002. [DOI] [PubMed] [Google Scholar]
- Canini L, Conway JM, Perelson AS, Carrat F. Impact of different oseltamivir regimens on treating influenza A virus infection and resistance emergence: insights from a modelling study. PLoS Comput Biol. 2014;10:e1003568. doi: 10.1371/journal.pcbi.1003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal MA, Acosta EP, Kimberlin DW, Gibiansky L, Jester P, Niranjan V, Rath B, Clinch B, Sanchez PJ, Ampofo K, Whitley R, Rayner CR. The Posology of Oseltamivir in Infants With Influenza Infection Using a Population Pharmacokinetic Approach. Clin Pharmacol Ther. 2014;96:380–9. doi: 10.1038/clpt.2014.120. [DOI] [PubMed] [Google Scholar]
- Kamal MA, Van Wart SA, Rayner CR, Subramoney V, Reynolds DK, Bulik CC, Smith PF, Bhavnani SM, Ambrose PG, Forrest A. Population pharmacokinetics of oseltamivir: pediatrics through geriatrics. Antimicrob Agents Chemother. 2013;57:3470–7. doi: 10.1128/AAC.02438-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai MP, Lodise TP., Jr Oseltamivir and oseltamivir carboxylate pharmacokinetics in obese adults: dose modification for weight is not necessary. Antimicrob Agents Chemother. 2011;55:5640–5. doi: 10.1128/AAC.00422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner CR, Bulik CC, Kamal MA, Reynolds DK, Toovey S, Hammel JP, Smith PF, Bhavnani SM, Van Wart SA, Ambrose PG, Forrest A. Pharmacokinetic-pharmacodynamic determinants of oseltamivir efficacy using data from phase 2 inoculation studies. Antimicrob Agents Chemother. 2013;57:3478–87. doi: 10.1128/AAC.02440-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner CR, Chanu P, Gieschke R, Boak LM, Jonsson EN. Population pharmacokinetics of oseltamivir when coadministered with probenecid. J Clin Pharmacol. 2008;48:935–47. doi: 10.1177/0091270008320317. [DOI] [PubMed] [Google Scholar]
- Standing JF, Nika A, Tsagris V, Kapetanakis I, Maltezou HC, Kafetzis DA, Tsolia MN. Oseltamivir pharmacokinetics and clinical experience in neonates and infants during an outbreak of H1N1 influenza A virus infection in a neonatal intensive care unit. Antimicrob Agents Chemother. 2012;56:3833–40. doi: 10.1128/AAC.00290-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BE. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J Antimicrob Chemother. 2010;65(Suppl 2):ii5–ii10. doi: 10.1093/jac/dkq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshire H, Wiltshire B, Citron A, Clarke T, Serpe C, Gray D, Herron W. Development of a high-performance liquid chromatographic-mass spectrometric assay for the specific and sensitive quantification of Ro 64-0802, an anti-influenza drug, and its pro-drug, oseltamivir, in human and animal plasma and urine. J Chromatogr B Biomed Sci Appl. 2000;745:373–88. doi: 10.1016/s0378-4347(00)00300-5. [DOI] [PubMed] [Google Scholar]
- Massarella JW, He GZ, Dorr A, Nieforth K, Ward P, Brown A. The pharmacokinetics and tolerability of the oral neuraminidase inhibitor oseltamivir (Ro 64-0796/GS4104) in healthy adult and elderly volunteers. J Clin Pharmacol. 2000;40:836–43. doi: 10.1177/00912700022009567. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. 2008. . Available at http://www.fda.gov/downloads/drugs/drugsafety/informationbydrugclass/ucm147992.pdf.
- Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM user's guides (1989–2009) Icon Development Solutions: Ellicott City, MD; 2009. [Google Scholar]
- Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–57. doi: 10.4049/jimmunol.1000289. (Baltimore, Md: 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez J, Velez G, Zuleta J, Franco F, Gomez J. H1N1 influenza pandemic and maternal mortality in Antioquia, Colombia. Int J Gynaecol Obstet. 2011;115:144–7. doi: 10.1016/j.ijgo.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Dede FS, Celen S, Bilgin S, Ure G, Ozcan AO, Buzgan T, Kose R. Maternal deaths associated with H1N1 influenza virus infection in Turkey: a whole-of-population report. BJOG. 2011;118:1216–22. doi: 10.1111/j.1471-0528.2011.03002.x. [DOI] [PubMed] [Google Scholar]
- Influenza vaccination coverage among pregnant women--United States. 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2013;62:787–92. [PMC free article] [PubMed] [Google Scholar]
- Aarons L, Karlsson MO, Mentre F, Rombout F, Steimer JL, van Peer A. Role of modelling and simulation in Phase I drug development. Eur J Pharm Sci. 2001;13:115–22. doi: 10.1016/s0928-0987(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18:152–61. doi: 10.1038/ki.1980.124. [DOI] [PubMed] [Google Scholar]
- Greer LG, Leff RD, Rogers VL, Roberts SW, McCracken GH, Jr, Wendel GD, Jr, Sheffield JS. Pharmacokinetics of oseltamivir according to trimester of pregnancy. Am J Obstet Gynecol. 2011;204:S89–93. doi: 10.1016/j.ajog.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


