Abstract
Aim
Pharmacogenetic studies have identified the presence of the HLA-A*31:01 allele as a predictor of cutaneous adverse drugs reactions (ADRs) to carbamazepine. This study aimed to ascertain the preferences of patients and clinicians to inform carbamazepine pharmacogenetic testing services.
Methods
Attributes of importance to people with epilepsy and neurologists were identified through interviews and from published sources. Discrete choice experiments (DCEs) were conducted in 82 people with epilepsy and 83 neurologists. Random-effects logit regression models were used to determine the importance of the attributes and direction of effect.
Results
In the patient DCE, all attributes (seizure remission, reduction in seizure frequency, memory problems, skin rash and rare, severe ADRs) were significant. The estimated utility of testing was greater, at 0.52 (95% CI 0.19, 1.00) than not testing at 0.33 (95% CI –0.07, 0.81). In the physician DCE, cost, inclusion in the British National Formulary, coverage, negative predictive value (NPV) and positive predictive value (PPV) were significant. Marginal rates of substitution indicated that neurologists were willing to pay £5.87 for a 1 percentage point increase in NPV and £3.99 for a 1 percentage point increase in PPV.
Conclusion
The inclusion of both patients' and clinicians' perspectives represents an important contribution to the understanding of preferences towards pharmacogenetic testing prior to initiating carbamazepine. Both groups identified different attributes but had generally consistent preferences. Patients' acceptance of a decrease in treatment benefit for a reduced chance of severe ADRs adds support for the implementation of HLA-A*31:01 testing in routine practice.
Keywords: carbamazepine, cutaneous adverse drug reaction, discrete choice experiment, HLA-A*31:01, pharmacogenetics
What is Already Known about this Subject
Carbamazepine is associated with severe, immune-mediated adverse drug reactions that may be predicted, and potentially avoided, by testing for human leukocyte antigen alleles.
There is presently no evidence on the preferences of patients with epilepsy or neurologists towards pharmacogenetic testing prior to carbamazepine treatment.
What this Study Adds
Based on discrete choice experiments, patients were willing to accept a reduced chance of 1 year remission from seizures for a reduction in adverse drug reactions.
Neurologists' preference for testing was sensitive to the cost of the test, but they were willing to pay for a modest increase in negative predictive value.
Introduction
Carbamazepine is used widely as a first line treatment for focal onset seizures, and has proven benefits in terms of time to achieving 12 month remission 1,2. However, it is associated with common adverse drug reactions (ADRs) 3 and more serious, immune-mediated ADRs, including cutaneous hypersensitivity reactions such as drug induced hypersensitivity syndrome (DIHS), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). The estimated incidence of SJS-TEN is 1 to 6 per 10 000 persons exposed to carbamazepine with TEN being associated with mortality of up to 30% 4.
Pharmacogenetic association studies have identified significant genetic predictors of cutaneous ADRs associated with carbamazepine. While rare in European populations, the HLA-B*15:02 allele is a significant predictor of SJS-TEN in people of Han-Chinese descent 5, and testing significantly reduces the rate of SJS-TEN 6. Recommendations from regulators have consequently led to increased use of HLA-B*15:02 testing of people of Han-Chinese, Thai and other Asian origin in East Asia.
In European populations, the HLA-A*31:01 allele is a significant predictor of the full spectrum of carbamazepine-induced hypersensitivity ADRs 7, the risk being 26% in carriers of the allele and 3.8% in non-carriers. Based on the 10% prevalence of mild carbamazepine-induced cutaneous ADRs (maculopapular exanthema) in people of European descent 1, 39 people would need to be screened to prevent one carbamazepine-induced ADR 7. However testing for HLA-A*31:01, which has a prevalence of 2 to 5% in European populations, has yet to gain mainstream acceptance in Western countries. As for any new innovation, uptake will be dependent on many factors, not least patients' acceptance, and preferences for harm reduction vs. benefit maximzsation and prescribers' considerations of diagnostic value, clinical utility and cost, among other factors 8.
Discrete choice experiments (DCEs) are a method for measuring respondents' stated preference for healthcare interventions or services 9. In DCEs, respondents are asked to choose their preferred alternative from a set of hypothetical (but realistic) alternatives. The method allows for the estimation of the relative importance of different aspects of care, assessment of any tradeoffs between these aspects, and of respondents' total satisfaction (utility) associated with the intervention or service under consideration 9,10. DCEs have been used previously to elicit preferences for antiepileptic drugs (AEDs) 11,12 and for the delivery of pharmacogenetic testing services 13. The latter revealed differences in patient and prescriber preferences, with patients demanding accurate and timely information regarding why testing was necessary and what the test results meant, while health-care professionals focussed more on the predictive accuracy and waiting time for a test result 13.
In the present study, we aimed to ascertain the preferences of patients with epilepsy and neurologists when considering testing for HLA-A*31:01 prior to prescribing carbamazepine. Specifically, we estimated patients' threshold at which the incidence of serious ADRs would make testing worthwhile and neurologists' willingness to pay for testing. The results of this study may inform the delivery of pharmacogenetic testing services.
Methods
Overview
We identified attributes that patients with epilepsy and neurologists considered important in their respective consideration of pharmacogenetic testing prior to starting treatment with carbamazepine. Levels for each attribute were derived from appropriate sources of clinical evidence. Separate DCEs were designed and administered to samples of patients with epilepsy and neurologists from across the UK.
Participants and administration
Adults aged 18 years or over and who self-reported as being diagnosed with epilepsy by a doctor were eligible. Participants were not rewarded for their time but were informed of the potential benefits and risks to them, and had to consent before taking part. Recruitment was facilitated by the UK charity Epilepsy Action and included advertisements, articles and links using social media, members' magazine, e-forums and newsletters and website home page. An advertisement was placed in the local press and posters displayed in hospital clinics. The questionnaire was made available via a link to an anonymous online service (Snap Surveys, London, UK) between June and October 2013. Target sample size was 63 completed DCE responses, based on each main effect level of interest being represented across the design at least 500 times 14. Ethical approval was gained from the NHS National Research Ethics Service (reference 11/NW/0191).
Adult and paediatric neurologists registered in the UK were recruited via the International League Against Epilepsy and the Association of British Neurologists. The questionnaire was made available nationally via an anonymous online service (SurveyMonkey, Palo Alto, CA, USA) between July and October 2012. The target sample size for the main effects analysis was 47 completed DCE responses 14.
Attribute and level selection
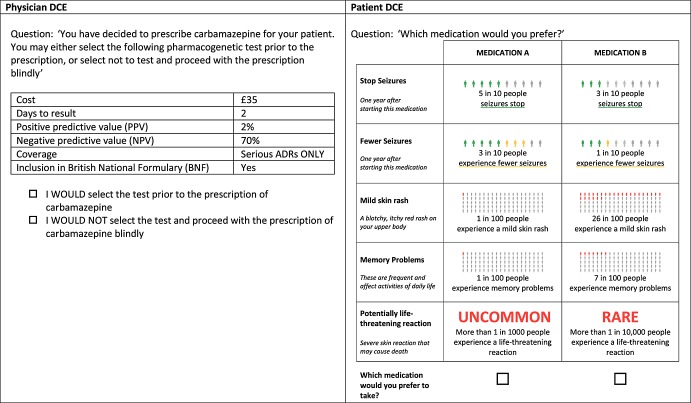
Attributes for the patient DCE were identified using semi-structured interviews with patients, a focus group with prescribers, and from published data. Patients (n = 56) were recruited from three clinical sites, and included 33 with established epilepsy (17 females, mean age 38 years) and 23 with a recent (≤1 year) diagnosis of epilepsy (10 females, mean age 43 years). Forty-one patients were first asked to list and rank attributes relating to the benefits, side effects and life impacts of treatment for epilepsy. The second stage of the interviews was designed to explore the framing of risk and the validity of risk communication. Fifteen patients participated in cognitive interviews to assess the face validity of the DCE (presentation of attributes and levels) and were provided with show cards depicting risk in pictograms alongside a written explanation of the risk being illustrated. Interviewers were given notes on how to explain risk. This exercise was repeated in the focus group with prescribers (n = 8), who were also asked to discuss the frequency and severity at which a side effect became a ‘clinically important adverse event’ that would require a change in treatment. Prescribers were also asked for feedback on the format of the patient DCE and the presentation of attributes and levels. The final DCE of patients contained five attributes to represent remission of seizures (the highest ranked benefit in the qualitative study), reduction in seizure frequency, memory problems (the highest ranked side effect in the qualitative study), skin rash, and rare or uncommon severe ADRs (associated with carbamazepine) (Table1). Appropriate levels for each category were identified from published clinical data 1,7,15.
Table 1.
Attributes and levels of the discrete choice experiments
| Attribute | Description | Levels (code) | Rationale |
|---|---|---|---|
| Physicians' DCE | |||
| Cost of test | The total cost of the pharmacogenetic test in pounds sterling. | 35 (0) | Cost attribute ranked highly by neurologists. Realistic levels based on expert opinion (M. Pirmohamed). |
| 100 (1) | |||
| 200 (2) | |||
| Time to result | The total time from initially requesting the pharmacogenetic test to receipt of result. | 2 (0) | Time attribute ranked highly by neurologists. Realistic levels based on expert opinion (M. Pirmohamed). |
| 4 (1) | |||
| 7 (2) | |||
| Positive predictive value (PPV) | The probability of experiencing the ADR if a positive result is identified on the pharmacogenetic test: the ‘true positives’. | 2 (0) | PPV attribute ranked highly by neurologists. Range of PPV values informed by literature review 5–7 |
| 35 (1) | |||
| 70 (2) | |||
| Negative predictive value (NPV) | The probability of not experiencing the ADR if a negative result is identified on the pharmacogenetic test: the ‘true negatives’. | 70 (0) | NPV attribute ranked highly by neurologists. Range of NPV values informed by literature review 5–7 |
| 85 (1) | |||
| 99 (2) | |||
| Coverage of test | The ability of the pharmacogenetic test to predict severe ADRs only, or mild in addition to severe ADRs. | Severe hypersensitivity adverse drug reactions (0), severe AND mild hypersensitivity adverse drug reactions (1) | Parameter informed by the attributes of current alleles: HLA-A*31:01 is associated with severe and mild ADRs 7, HLA-B*15:02 is associated with severe ADRs only 5 |
| British National Formulary (BNF) | The inclusion or exclusion of the pharmacogenetic test in the drug information detailed under carbamazepine. | Test NOT INCLUDED in the BNF (0) | Regulatory approval and inclusion in clinical guidelines ranked highly by neurologists. Inclusion in the British National Formulary 16 was included in the DCE as a pragmatic marker of regulatory approval and clinical availability. |
| Test INCLUDED in the BNF (1) | |||
| Patients' DCE | |||
| Seizures stop | The probability of patients achieving 1 year remission from seizures with AED | 5 in 10 people (0.5) | Primary outcome of AED studies is 12 month remission. Levels based on published clinical trial data 1. |
| 3 in 10 people (0.3) | |||
| Fewer seizures | The probability of patients experiencing fewer seizures after 1 year with AED | 3 in 10 people (0.3) | Seizure reduction was the highest ranked outcome by patients. Levels based on clinical trial data 1. |
| 1 in 10 people (0.1) | |||
| Mild skin rash | The probability of patients experiencing a mild adverse drug reaction but which is sufficient to warrant change in AED | 1 in 100 people (0.01) | HLA-A*31:01 allele is associated with mild hypersensitivity reaction with patients exposed to carbamazepine. Levels based on published data 1,7. |
| 26 in 100 people (0.26) | |||
| Memory problems | The probability of patients experiencing memory problems which are sufficient to warrant change in AED | 1 in 100 people (0.01) | Adults with established epilepsy and prescribing clinicians were most concerned about memory problems in ranking exercises. Levels based on published clinical trial data 1. |
| 7 in 100 people (0.07) | |||
| Potentially life-threatening reaction | The probability of patients experiencing a rare but severe skin reaction, described as hot, painful patches on the skin that can blister and risks death. | RARE: More than 1 in 10 000 people (0.0001) | HLA-A*31:01 allele is associated with drug induced hypersensitivity syndrome (DIHS), Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) with patients exposed to carbamazepine. Levels based on published data on allele associations 7 and SmPC for carbamazepine 17. |
| UNCOMMON: More than 1 in 1000 people (0.001) |
Attributes for the physician DCE were taken from Payne et al. 13, who identified cost, predictive accuracy and result turnaround time as being important when considering pharmacogenetic tests and from structured individual interviews with neurologists (n = 12) recruited from the North West of England. Initial interviews involved a discussion of attributes that would be of potential importance to neurologists when considering a pharmacogenetic test and included cost, predictive accuracy, turnaround time to result, coverage of test (severe ADRs only or severe and mild ADRs), inclusion in British National Formulary (BNF) 16, method of testing (blood, salivary swab), method of follow-up and subsequent prescribing, location of testing and method of presentation of results (‘raw data’, summarized interpretation).
A rating exercise was performed to identify the attributes of greatest importance. Subsequent interviews with neurologists discussed the presentation of the attributes and identified relevant levels. As this study targeted UK neurologists, cost was understood to be total cost to the National Health Service (NHS), rather than cost to the patient or cost for a privately requested test. Although there is no direct cost to the neurologist or patient, neurologists and physicians in general in the UK are cognisant of the costs of medical interventions and this characteristic was confirmed by the identification of the attribute as important in the interviews. Framing of the predictive attributes of the pharmacogenetic test was discussed. The negative predictive value (NPV) and positive predictive value (PPV) were understood and favoured by the neurologists compared with alternative methods of presentation including sensitivity, specificity or ‘risk of ADR following test’. The final attributes presented in the DCE were cost, time to result, inclusion in the BNF, coverage, NPV, and PPV (Table1). Data from published sources 5,7, together with discussion in individual interviews with neurologists and expert opinion led to identification of a range of plausible attribute levels.
Experimental design
Our qualitative findings did not reveal a common list of attributes that could be used to value both physician and patient preferences for pharmacogenetic testing services. We therefore conducted two separate DCEs that contained the most relevant and plausible attributes from both perspectives.
In clinical practice, patients who test positive for the HLA-A*31:01 allele would be prescribed an alternative AED, which is likely to have a different benefit and harm profile. To reflect this, the DCE asked patients to choose between two hypothetical medicines, from which we inferred their preference for pharmacogenetic testing. The DCE used a fractional factorial design 18 and folded into eight binary choices, one of which is presented as an example in Figure1. The DCE was administered as part of a larger survey containing 126 items in total and requiring an estimated 30 min for completion.
Figure 1.
Example of binary choice DCE questions
A binary design was selected for the DCE of neurologists in order to include a choice of no testing, which is aligned with current clinical practice. A fractional factorial design was selected from a design catalogue to ensure orthogonality 18. Sixteen choice scenarios were presented to respondents, following the example shown in Figure1.
Analysis
Random effects logit regression models were used to determine the importance of the attributes and direction of effect. Marginal rates of substitution (MRS, the rate at which respondents were willing to give up a unit change in one attribute in exchange for a unit change in another while maintaining the same level of utility) were calculated using each attribute as the value attribute with bootstrapped confidence intervals calculated using 1000 replications. All analyses were conducted in STATA version 10 (StataCorp, College Station, TX, USA). To test the validity of the patient DCE we identified a potentially dominant choice in which medicine A was superior in all but one attribute (higher chance of remission, lower risk of memory problems, mild rash and life-threatening ADR, but a higher frequency of seizures). We assumed that people who selected the alternative (medicine B) for this choice did not understand the task, and analyzed the DCE with and without these respondents by comparing the confidence intervals of all the coefficients in the regression to ascertain if there were statistically significant differences.
Patients' utility was calculated by weighting the results of the regression against potential outcomes of treatment with carbamazepine with or without pharmacogenetic testing. Clinical data 1,7,15 were used to model the scenarios of testing (in which carriers of the HLA-A*31:01 allele are prescribed lamotrigine) and standard care (Table2). The probability of test uptake was calculated as the exponential of the utility for testing divided by the sum of the exponential of the utilities for testing and not testing. We further calculated the threshold at which patients would prefer to be tested, defined when the utility of testing is at least as much as the utility of standard treatment:
where, MRS is the ratio of β coefficient for a given attribute divided by the β coefficient for severe ADRs (sADR), and Δattribute represents the actual difference in probabilities of occurrence of attribute-defined events between a testing strategy and standard treatment. The trade-off between the benefits and harms of interest provides the point of indifference from the patient's perspective and therefore represents the threshold at which patients would choose to be tested.
Table 2.
Values of regression variables used to estimate utility, probability of test uptake and maximally tolerated rate of severe ADR for patients to prefer testing. Data are taken from source, or derived according to standard epidemiological calculations
| Attributes | Expected probabilities conditional on AED and HLA-A*31:01 test result | Testing strategy | Reference | |||
|---|---|---|---|---|---|---|
| CBZ/-ve | CBZ/+ve | LTG/+ve | Test | No test | ||
| Remission | 36.000 | 36.000 | 29.000 | 35.8189 | 36.0000 | 1 |
| Fewer seizures | 17.370 | 17.370 | 21.430 | 17.4751 | 17.3700 | 1 |
| Memory problems | 3.1746 | 3.1746 | 2.6455 | 3.1609 | 3.1746 | 1 |
| Skin rash | 7.000 | 34.000 | 4.000 | 6.9224 | 7.6986 | 1,7 |
| Severe ADR | 0.0738 | 1.0895 | 0.0354 | 0.0728 | 0.1001 | 7,15,17 |
All data are reported as number of events per 100 patients.
AED, anti-epileptic drug; CBZ, carbamazepine; LTG, lamotrigine; ADR, adverse drug reaction.
Scenario analyses
While the base case focused on HLA-A*31:01, a scenario analysis was performed using the characteristics of testing for HLA-B*15:02. This was based on a meta-analysis of the association with SJS/TEN 19 and assumed a 10% allele prevalence, consistent with Asian populations 20.
A further exploratory analysis was conducted by identifying statistically significant subgroups based on log likelihood ratio tests of base case ‘restricted model’ (all cases) and unrestricted models for groups of n ≥ 30 and assuming P < 0.05 with Bonferroni correction.
For the DCE of neurologists, welfare estimates including total utility and probability of uptake were calculated for various testing scenarios which represented a less expensive test, higher PPV and NPV, and a reduced time to test result. A test which costs £100, takes 4 days for the result, with PPV 26%, NPV 96%, predictive of both severe and mild ADRs but not included in the BNF was selected as being representative of current clinical practice associated with HLA-A*31:01 testing. An assessment of validity using a dominant choice set was not possible in the DCE of neurologists. Pharmacogenetic testing for HLA-A*31:01 is not currently mandatory and therefore selecting a single scenario where a test should always or never be selected would not be appropriate in the context of a labelled DCE. We defined non-traders as respondents always selecting one response (test or no test) and examined the results of the regression with and without the inclusion of non-traders.
Results
Patients' DCE
Ninety-two people with epilepsy started the DCE, of whom 82 (89%) completed the survey. Respondents had a median age of 38 years and 61 (66%) were female (Table3). Almost all patients were taking AEDs (n = 85, 99%) and 31 (36%) had experienced changes to their AED treatment in the previous 3 months. Over a third of respondents (n = 31, 36%) had previously taken carbamazepine to treat epilepsy, of which one respondent reported a severe skin reaction requiring hospitalization and 10 (19%) had experienced rash of the upper body.
Table 3.
Patient characteristics
| Patients' characteristics | n | % |
|---|---|---|
| Age (years) median (range) | 38 | (18–72) |
| Female | 61 | 66.3 |
| Time since diagnosis | ||
| Less than 4 months | 1 | 1.1 |
| 4–12 months | 3 | 3.3 |
| 1–5 years | 14 | 15.4 |
| 6–10 years | 12 | 13.2 |
| More than 10 years | 61 | 67.0 |
| Seizure type | ||
| Focal | 27 | 31.4 |
| Complex focal | 40 | 46.5 |
| Absences, tonic, atonic | 45 | 52.3 |
| Tonic clonic | 56 | 65.1 |
| Time since last seizure | ||
| Less than a week | 38 | 44.2 |
| Less than a month | 16 | 18.6 |
| Less than 6 months | 14 | 16.3 |
| Less than 1 year | 2 | 2.3 |
| 1 year or over | 16 | 18.6 |
| Seizure frequency compared with 1 year ago | ||
| More often | 19 | 22.1 |
| Less often | 26 | 30.2 |
| About the same | 41 | 47.7 |
| Prescribed AED in past 3 months | 85 | 98.8 |
| Changes to AED in past 3 months | ||
| No change | 54 | 63.5 |
| Increased/decreased | 25 | 29.4 |
| Change of drug | 9 | 10.6 |
| Additional drug | 12 | 14.1 |
| Fewer drugs | 2 | 2.4 |
| Stopped altogether | 1 | 1.2 |
| Reason for changes | ||
| Lack of seizure control | 30 | 90.9 |
| Unpleasant side effects | 14 | 42.4 |
| Remission | 1 | 3.0 |
| Morisky non-adherence 21 | 16 | 50.0 |
| Experience of taking CBZ | 31 | 36.5 |
| Experience of adverse events | ||
| Change or stop due to memory problems | 8 | 24.2 |
| CBZ skin rash | 10 | 18.5 |
| CBZ severe ADR (requiring hospital treatment) | 1 | 1.9 |
| Living alone | 13 | 15.9 |
| In employment, education, or looking after home | 49 | 60.5 |
| Ethnicity | ||
| White | 74 | 90.2 |
| Black/African/Caribbean/Black British | 3 | 3.7 |
| Asian/Asian British | 1 | 1.2 |
| Mixed/Multiple ethnic groups | 2 | 2.4 |
All five attributes were significant and in the expected direction and the overall goodness of fit of the model was good (Table4). Five patients failed to select the dominant choice. However as there were no statistically significant differences between models by their inclusion or exclusion they were retained in the base case analysis. Patients were willing to accept a reduction in the chance of 12 month remission from seizures in exchange for a reduction in adverse events. Patients were willing to reduce the chance of remission by 0.58 percentage points (95% CI 0.39, 0.82) for a 1 percentage point reduction in skin rash, 3.2 percentage points (95% CI 2.32, 4.44) for a 1 percentage point reduction in memory problems and 1.76 percentage points (95% CI 1.21, 2.54) for a 0.001 percentage point reduction in the risk of a severe ADR.
Table 4.
Random effects logit regression model and marginal rates of substitution
| DCE of patients | |||
|---|---|---|---|
| Attribute | Coefficient (95% CI) | Odds ratio | Marginal rate of substitution with respect to remission (95% CI) |
| Remission | 0.037 (0.032, 0.054) | 1.04 | 1.00 |
| Fewer seizures | 0.011 (0.003, 0.024) | 1.01 | 0.29 (0.07, 0.58) |
| Memory | –0.119 (–0.182, –0.104) | 0.89 | –3.22 (–4.54, –2.35) |
| Skin rash | –0.021 (–0.034, –0.016) | 0.98 | –0.58 (–0.84, –0.38) |
| Severe ADR | –6.490 (–10.295, –5.467) | 0.00 | –175.83 (–253.30, –121.42) |
| Constant | 0.147 (–0.022, 0.392) | 1.16 | |
| Pseudo r2 = 0.2118; Wald χ2 140.34; Log likelihood = −382.74; P = 0.00. | |||
| DCE of neurologists | |||
|---|---|---|---|
| Attribute | Coefficient (95% CI) | Odds ratio | Willingness to pay (95% CI) |
| Cost | –0.012 (–0.016, –0.010) | 0.99 | – |
| Time to result | 0.027 (–0.077, 0.131) | 1.03 | – |
| PPV | 0.047 (0.042, 0.061) | 1.05 | 3.99 (3.00, 5.37) |
| NPV | 0.068 (0.056, 0.096) | 1.07 | 5.87 (4.04, 8.46) |
| Coverage of test | –0.365 (–0.774, –0.095) | 0.69 | –31.29 (–60.06, –7.20) |
| Included in BNF | 0.459 (0.140, 0.865) | 1.58 | 39.35 (10.97, 71.05) |
| Constant | –7.120 (–9.879, –5.824) | ||
Pseudo r2 value 0.2294; Wald χ2 199.74; Log likelihood = −529.66; P < 0.001.
Utility model
The estimated utility associated with testing for HLA-A*31:01 was greater, at 0.52 (95% CI 0.19, 1.00) than not testing at 0.33 (95% CI –0.07, 0.81). Consequently the choice model estimated the probability of test uptake at 55% (95% CI 54, 57) which would suggest that more patients would choose to be tested than not.
Patient-defined threshold for testing
The patient-defined threshold for testing for HLA-A*31:01, based on the rate of severe ADRs was 10.20 per 10 000 patients (95% CI 10.11, 10.33) which exceeds the actual number of severe ADRs identified through testing (7.28 per 10 000), suggesting that patients would accept a test.
Scenario analysis
Based on the characteristics of a test for HLA-B*15:02 which, if implemented, is estimated to reduce the risk of serious ADRs by 6.94 cases per 10 000 patients treated, the probability of patient uptake is calculated as 61%. Total utility of testing was 0.32 compared with –0.13 for the untested cohort. The patient-defined threshold for testing is 16.55 severe ADRs per 10 000, implying that testing for HLA-B*15:02 is also preferred, given that this value exceeds the true rate of serious ADRs of 9.70 per 10 000, if testing were implemented.
Two subgroups qualified for analysis, namely gender and age. Marginal rates of substitution indicated that females were more willing than males to trade a reduction in the chance of remission for reduction in the risk of the severe ADR. Females were willing to accept a 30.2 percentage point (95% CI 19.5, 52.9) reduction in remission for a 0.1% reduction in the risk of severe ADR, compared with males who were only willing to exchange a 4.6 percentage point (95% CI 0.7, 11.2) reduction in remission for the same 0.1% reduction in the risk of severe ADR. Differences in the rate of exchange for remission and side effects (MRS) were not statistically significant for age.
Physicians' DCE
Eighty-three neurologists completed the questionnaire, the majority (n = 69, 83%) were adult neurologists. Sixty-four (80%) respondents self-rated their knowledge of pharmacogenetic testing as ‘No/Superficial awareness’, with just 16 (20%) reporting ‘Detailed awareness’. Fifty-six (67%) respondents had not requested any pharmacogenetic test in the previous year, while 21 had requested tests on up to five occasions. Forty-three (52%) respondents had reviewed at least one patient with a cutaneous ADR associated with carbamazepine in the previous year and 69 (83%) respondents had initiated carbamazepine in at least one patient in the previous month.
Thirteen neurologists were non-traders, defined as respondents who always select A or B (‘test’ or ‘no test’) throughout the experiment, regardless of changes in the profiles. Ten neurologists selected ‘no test’ to all responses and three neurologists selected ‘test’ to all responses. As discussed in the methods, pharmacogenetic testing is not currently mandatory and the decision whether to request a test will depend on a number of professional factors and personal opinions. During the individual interviews, a minority of neurologists were opposed to the introduction of pharmacogenetic testing into routine clinical practice, even when presented with attributes demonstrating a clear clinical benefit. In order to optimise our assessment of the attributes of a pharmacogenetic test valued by neurologists, we excluded non-traders from the analysis presented. However, the statistically significant attributes remained significant when non-traders were included in the model. The coefficients of all attributes with the exception of time to test result were significant and in the expected direction. Overall goodness of fit of the model was good. The odds that respondents selected the test decreased by 1% for every £1 increase in the cost of testing. An increase of 1 percentage point in PPV increased the odds of preferring pharmacogenetic testing by 7%, reference to HLA-A*31:01 testing in the BNF increased the odds that respondents would test by 58% and a test that predicts both severe and mild ADRs decreased the odds of testing by 31% (Table4).
Marginal rates of substitution for the significant attributes indicated that neurologists were willing to pay £5.87 for a 1 percentage point increase in NPV and £3.99 for an equivalent increase in PPV. Respondents were willing to pay £31.29 for the coverage of mild in addition to severe cutaneous ADRs, and £39.35 for the inclusion of testing advice in the BNF (Table4).
Utility model
The total utility of testing for HLA-A*31:01 is positive at 6.36 (95% CI 3.74, 10.22), indicating a general tendency to request the test (Table5). Reducing the cost of testing from £100 to £35 increased the probability of requesting the test to 68.1%. A scenario in which the time to test result is reduced from 4 to 2 days had little influence on the probability of requesting the test, but an improvement in PPV from 26% to 70%, increased the probability of requesting the test almost 8-fold, to 88.6%. An improved NPV of 99% compared with the existing 96% increased the probability of requesting the test to 55.1%.
Table 5.
Results of scenario analysis of varying attribute levels within plausible ranges on the total utility and probability of test uptake
| Parameter | Attribute and levels | Utility (95% CI) | Probability of uptake |
|---|---|---|---|
| Base case | Cost £100 | 6.3584 (3.739, 10.2210) | 49.9% |
| Time to result 4 days | |||
| PPV 26% | |||
| NPV 96% | |||
| Coverage of test: Severe and mild | |||
| Included in BNF: No | |||
| Reduced cost | Cost £35 | 7.117 (4.801, 10.8525) | 68.1% |
| Reduced time to result | Time to result 2 days | 6.3046 (3.8939, 9.9629) | 48.6% |
| Improved PPV | PPV 70% | 8.4055 (5.565, 12.8900) | 88.6% |
| Improved NPV | NPV 99% | 6.5639 (3.907, 10.5111) | 55.1% |
Discussion
Using a structured ranking exercise, we found that patients prioritized health outcomes relating to the benefits of treatment, in terms of seizure freedom and associated adverse events. The results of the DCE suggested that patients were willing to accept a less effective AED if that treatment had less risk of harm. They were willing to forego a 1760 per 100 000 chance of improvement in remission for each 1 in 100 000 reduction in the risk of a severe ADR. When patient preferences were analyzed alongside data of actual event rates and characteristics of a test for HLA-A*31:01, the results indicated that patients would prefer testing and being prescribed lamotrigine (conditional on test result) to the current standard of care. The current rate of ADR for patients who have the test is 7.28 per 10 000. If this were to increase by an additional 19 (or more) per 10 000, patients would prefer standard care.
In contrast to patients, neurologists highlighted process-related outcomes. Their preference for higher NPV might indicate a degree of caution in terms of wanting tests with a reduced likelihood of false negative results that would require the prescribing of a second choice AED. They were willing to pay an additional £58.67 per 10 percentage point increase in NPV. Neurologists were willing to pay an additional £39.35 for a test which was included in the BNF. This attribute captures tests that are recommended by regulatory agencies or included in clinical guidelines and are more likely to have high PPV and NPV 22. A pharmacogenetic test that was less expensive was predictably preferred, but reduced turnaround time did not significantly influence the probability of requesting the test.
The study benefitted from having taken a systematic and rigorous approach to identifying attributes and levels that were both plausible and relevant to each perspective. For the DCE of patients, these were derived from interviews, with the final selection of attributes and levels piloted in cognitive interviews and presented in numerical and pictogram format to aid interpretation. A recent systematic review found that DCE studies have been notoriously poor at reporting the methodology supporting the explanation of risk and the validity of risk communication 23. This study represents a thorough application of cognitive interviews to support the face validity of the design of the DCE and the presentation of risk attributes, and associated trading tasks. A comparable approach was taken with neurologists, which included a literature review and structured interviews, consistent with guidelines for DCE attribute selection 24.
Our inclusion of both patients' and clinicians' perspectives represents an important addition to the emerging literature on preference-elicitation in pharmacogenetics. The finding that both groups identified very different attributes but generally consistent preferences is reassuring in the context of implementing a new health technology. Patients' acceptance of a decrease in treatment benefit for a reduced chance of serious adverse drug reactions, even if that chance is very small, implies that patients will be satisfied with a prescription for a second choice AED which might not necessarily be as effective as the first.
Payne et al. 13 evaluated patient and health care professionals' preferences, using DCE methods, for pharmacogenetic testing of thiopurine s-methyltransferase prior to treatment with azathioprine. Their study focused on service delivery and found that patients valued accurate and timely information about the necessity of the test and interpretation of the results. Our patient study differed as it focused on their preference for different AEDs, accepting that the key consequence of a pharmacogenetic test is the possibility of being prescribed an alternative medicine with a different safety profile, and potentially reduced effectiveness. We subsequently modelled the scenario of pharmacogenetic testing using additional information on the actual benefits of AEDs and test characteristics. This approach has the advantage of acknowledging the broader clinical context of testing as opposed to the specific action of whether or not to test. Importantly, we have derived the threshold at which patients' utility will be maximized through testing prior to taking carbamazepine.
We are aware of two other DCEs of patients with epilepsy. Lloyd et al. 11 used a DCE to elicit the importance of adverse events compared with seizure control for people with epilepsy and found that patients preferred AEDs with less severe adverse events, greater control and least cost. This direction of preferences was the same in our study. However, the amount of remission patients were willing to forego for a 1% reduction in rash differed: 4.45% seizure control for 1% reduction in risk of rash compared with a 0.58 percentage point reduction in remission for a 1 percentage point reduction in rash in our study. This may be explained by differences in how attributes were presented in the DCE. In our study we considered a ‘potentially life threatening adverse drug reaction’ that may influence the strength of preference for other attributes. Lloyd et al. 11 also included cost, whereas our study only focused on treatment benefits and harms. More recently, Manjunath et al. 12 included attributes for seizure frequency and, among others, ‘short term’ side effects (sleepiness, dizziness, headache, nausea, tremor, double or blurred vision and skin rash) and ‘long term’ side effects (fatigue, moodiness, confusion or memory problems). Patients with epilepsy considered seizure reduction to be the top priority when ranked against the reduction or elimination of side effects. However as with Lloyd et al. 11, there was no consideration of more serious ADRs which respondents to our DCE considered important.
Our study had some limitations. The survey was conducted online which resulted in a self-selected sample of patients. This may affect the generalizability of the findings, particularly given that access to and use of the internet will be variable among patients with epilepsy. Moreover, the sample primarily represented prevalent cases with long-standing experience of epilepsy, compared with incident cases who will be most commonly offered testing. In addition, the severity of epilepsy, defined as the frequency of seizures, was not recorded in the survey. It is foreseeable that patients with more severe epilepsy may be willing to trade a greater risk of ADR for an improvement in seizure control. Nevertheless, the agreement of our findings with other such studies lends support to the validity of the results. Common to all DCEs is the balance of comprehensiveness in the selection of attributes included and ability of respondents to make rational choices. Our DCE of patients was restricted to the five highest ranked attributes each with two levels, and only five patients did not select the choice which was marginally dominant and this had no impact on the result. By contrast, the DCE of physicians was somewhat more extensive with six attributes and 16 levels in total, and 13 respondents were non-traders. Overall, however, we considered the impact of the DCE designs not to have adversely affected the study conclusions. Finally, the study included a sample of UK patients and neurologists and the characteristics of these groups as well as the nationally funded healthcare system where patient care takes place, may limit the generalizability of results. In particular, the extent to which the results of the assessment of neurologists' preferences for pharmacogenetic testing can be extrapolated to other populations may be limited both by different healthcare systems (for example privatised systems) and different ethnic populations where the risk of ADRs associated with carbamazepine may be different. However, importance of the significant attributes of predictive accuracy (PPV, NPV) will likely translate across all populations.
In conclusion, our analysis of patient preferences indicates that patients value the reduction in risk of severe ADRs which could be achieved by pharmacogenetic testing prior to prescribing carbamazepine. The DCE of neurologists would suggest that the most effective method of ensuring that current pharmacogenetic tests are used more widely would be for the cost of testing to reduce. Reassuringly, testing for HLA-A*31:01 is cost-effective 25 meaning that turnaround time to result will likely become important given there is often a clinical urgency and patient expectation for treatment of uncontrolled seizures.
Competing Interests
The authors declare no conflict of interest and confirm to have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
The authors wish to thank Ana Alfirevic and Vincent Yip (University of Liverpool), and Margaret Rawnsley and Angela Pullen (Epilepsy Action). The research was supported by the NIHR Research for Patient Benefit (RfPB) Programme: PB-PG-0909-20 161 – Defining patient preferences and priorities for treatment options and outcomes in epilepsy (EAFH, RA, GAB, AJ, ADG, DAH), the MRC North West Hub in Trial Methodological Research (COP, DAH), G0800792 and the NIHR Academic Clinical Fellowship Programme (GP).
Contributors
GP, EAFH, MP, AGM, DAH contributed substantially to the conception and design of the work. All authors made contributions to the acquisition, analysis or interpretation of data. GP, EAFH, DAH drafted the paper and all authors revised it critically for important intellectual content and gave their final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, Cramp C, Cockerell OC, Cooper PN, Doughty J, Eaton B, Gamble C, Goulding PJ, Howell SJ, Hughes A, Jackson M, Jacoby A, Kellett M, Lawson GR, Leach JP, Nicolaides P, Roberts R, Shackley P, Shen J, Smith DF, Smith PE, Smith CT, Vanoli A, Williamson PR SANAD Study group. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–15. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudur Smith C, Marson AG, Chadwick DW, Williamson PR. Multiple treatment comparisons in epilepsy monotherapy trials. Trials. 2007;8:34. doi: 10.1186/1745-6215-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GM, Lhatoo SD. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22:739–60. doi: 10.2165/00023210-200822090-00003. [DOI] [PubMed] [Google Scholar]
- Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, Kardaun S, Sidoroff A, Liss Y, Schumacher M, Roujeau JC RegiSCAR study group. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Investig Dermatol. 2013;133:1197–204. doi: 10.1038/jid.2012.510. [DOI] [PubMed] [Google Scholar]
- Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, Wu JY, Chen YT. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, Tai CT, Wu SL, Lu CH, Hsu YC, Yu HY, Ro LS, Lu CT, Chu CC, Tsai JJ, Su YH, Lan SH, Sung SF, Lin SY, Chuang HP, Huang LC, Chen YJ, Tsai PJ, Liao HT, Lin YH, Chen CH, Chung WH, Hung SI, Wu JY, Chang CF, Chen L, Chen YT, Shen CY, Taiwan SJS. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. New Eng J Med. 2011;364:1126–33. doi: 10.1056/NEJMoa1009717. Consortium. [DOI] [PubMed] [Google Scholar]
- McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperavičiūtė D, Carrington M, Sills GJ, Marson T, Jia X, de Bakker PI, Chinthapalli K, Molokhia M, Johnson MR, O'Connor GD, Chaila E, Alhusaini S, Shianna KV, Radtke RA, Heinzen EL, Walley N, Pandolfo M, Pichler W, Park BK, Depondt C, Sisodiya SM, Goldstein DB, Deloukas P, Delanty N, Cavalleri GL, Pirmohamed M. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. New Eng J Med. 2011;364:1134–43. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. Criteria influencing the clinical uptake of pharmacogenomic strategies. BMJ. 2004;328:1482–6. doi: 10.1136/bmj.328.7454.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–3. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics; a review of the literature. Health Econ. 2012;21:145–72. doi: 10.1002/hec.1697. [DOI] [PubMed] [Google Scholar]
- Lloyd A, McIntosh E, Price M. The impacts of drug adverse effects compared with seizure control for people with epilepsy. Pharmacoeconomics. 2005;23:1167–81. doi: 10.2165/00019053-200523110-00008. [DOI] [PubMed] [Google Scholar]
- Manjunath R, Yang JC, Ettinger AB. Patients' preferences for treatment outcomes of add-on antiepileptic drugs: a conjoint analysis. Epilepsy Behav. 2012;24:474–9. doi: 10.1016/j.yebeh.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Payne K, Fargher EA, Roberts SA, Tricker K, Elliott RA, Ratcliffe J, Newman WG. Valuing pharmacogenetic testing services: a comparison of patients' and healthcare professionals' preferences. Value Health. 2011;14:121–34. doi: 10.1016/j.jval.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Orme BK. Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research. 2nd edn. Madison, Wisconsin: Research Publishers LLC; 2010. [Google Scholar]
- Mockenhaupt M, Messenheimer J, Tennis P, Schlingmann J. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology. 2005;64:1134–8. doi: 10.1212/01.WNL.0000156354.20227.F0. [DOI] [PubMed] [Google Scholar]
- Joint Formulary Committee. 2014. British National Formulary. 68th ed. London: British Medical Association and Royal Pharmaceutical Society,
- 2014. Summary of Product Characteristics for carbamazepine. Electronic Medicines Compendium. London: Datapharm Communications Limited,
- Hahn GJ, Shapiro SS. 1966. A catalog and computer program for the design and analysis of orthogonal symmetric and asymmetric fractional factorial experiments. Schenectady, New York: General Electric, Research and Development Center,
- Yip VL, Marson AG, Jorgensen AL, Pirmohamed M, Alfirevic A. HLA genotype and carbamazepine induced cutaneous adverse drug reactions: a systematic review. Clin Pharmacol Ther. 2012;92:757–65. doi: 10.1038/clpt.2012.189. [DOI] [PubMed] [Google Scholar]
- Lim KS, Kwan P, Tan CT. Association of HLA-B*1502 allele and carbamazepine-induced severe adverse cutaneous drug reaction among Asians, a review. Neurol Asia. 2008;13:15–21. [Google Scholar]
- Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure for hypertension control. J Clin Hypertens. 2008;10:348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kendall M, Enright D. Provision of medicines information: the example of the British National Formulary. Br J Clin Pharmacol. 2012;73:934–8. doi: 10.1111/j.1365-2125.2012.04241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M, Rigby D, Vass C, Flynn T, Louviere J, Payne K. Risk as an attribute in discrete choice experiments: a systematic review of the literature. Patient. 2014;7:151–70. doi: 10.1007/s40271-014-0048-1. [DOI] [PubMed] [Google Scholar]
- Coast J, Al-Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, Flynn TN. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ. 2012;21:730–41. doi: 10.1002/hec.1739. [DOI] [PubMed] [Google Scholar]
- Plumpton CO, Yip VL, Alfirevic A, Marson AG, Pirmohamed M, Hughes DA. Cost-effectiveness of screening for HLA-A*31:01 prior to initiation of carbamazepine in epilepsy. Epilepsia. 2015;56:556–63. doi: 10.1111/epi.12937. [DOI] [PubMed] [Google Scholar]