Abstract
The aim of the present meta-analysis was to determine the efficacy and safety of metformin for the treatment of women with gestational diabetes mellitus (GDM). We searched databases, including PubMed, Embase and the Cochrane Central Register of Controlled Trials, for randomized controlled trials (RCTs) comparing metformin and insulin treatments in women with GDM. We carried out statistical analyses using RevMan 2011 and used the Grading of Recommendations, Assessment, Development, and Evaluations profiler to rate the quality of evidence of the primary outcomes. We analysed eight studies involving 1592 subjects. Meta-analysis of the RCTs showed that metformin had statistically significant effects on pregnancy-induced hypertension [PIH; risk ratio (RR) 0.54; 95% confidence interval (CI) 0.31, 0.91]. However, its effects on neonatal hypoglycaemia (RR 0.80; 95% CI 0.62, 1.02), rate of large-for-gestational age infants (RR 0.77; 95% CI 0.55, 1.08), respiratory distress syndrome (RR 1.26; 95% CI 0.67, 2.37), phototherapy (RR 0.94; 95% CI 0.67, 1.31) and perinatal death (RR 1.01; 95% CI 0.11, 9.53) were not significant. Our analyses suggest that there is no clinically relevant difference in efficacy or safety between metformin and insulin; however, metformin may be a good choice for GDM because of the lower risk of PIH. The advantages of metformin in terms of glycaemic control, PIH incidence and gestational age at birth are unclear, and should be verified in further trials.
Keywords: gestational diabetes, insulin, metformin, randomized controlled trial
Introduction
Gestational diabetes mellitus (GDM) is defined as ‘any degree of glucose intolerance with onset or first recognition during pregnancy’ 1. It is observed in 7–18% of pregnancies 2 and is associated with an increased risk of a variety of maternal and perinatal complications, including preeclampsia, Caesarean section, macrosomia, shoulder dystocia, birth injuries, hypoglycaemia and respiratory distress syndrome (RDS) 3,4.
If maternal normoglycaemia cannot be achieved by diet and lifestyle changes, medication will be needed. The standard treatment for achieving adequate glucose levels is insulin therapy. However, the disadvantages of insulin for the mother include the need for injections, risk of hypoglycaemia, increased appetite and weight gain 5. Furthermore, this treatment requires modification based on the patient's body mass index, glucose levels and lifestyle 6, and involves substantial costs of health education for the safe use of insulin and the cost of insulin-administration devices. Oral hypoglycaemic agents have traditionally been avoided in pregnant women with diabetes because of the potential risks of neonatal hypoglycaemia and teratogenicity associated with placental transfer to the fetus. Metformin is an anti-hyperglycaemic agent which is not known to cause hypoglycaemia in adult users. Metformin reduces hyperglycaemia by suppressing hepatic glucose output (hepatic gluconeogenesis), increasing insulin sensitivity and enhancing peripheral glucose uptake 6,7. Metformin readily crosses the placenta in women with GDM, exposing the fetus to concentrations approaching those in the maternal circulation 8, as well as in women with polycystic ovary syndrome 9. Fortunately, metformin is not associated with fetal anomalies when used during the first trimester of pregnancy 10. In addition, metformin appears to be safe in the second and third trimesters of pregnancy 11.
An early systematic review that included only two randomized controlled trials (RCTs) did not find any differences in maternal or neonatal outcomes between GDM patients using metformin and those using insulin 12. However, the latest systematic review on metformin and insulin in GDM, which included five RCTs, found differences between metformin and insulin, and favoured the use of metformin 13.
The existing systematic reviews on metformin and insulin, particularly for GDM, included a few RCTs. Interestingly, new RCTs with differing results have been published recently. Metformin was found to be efficient at controlling hyperglycaemia in pregnancy, the levels of HbA1c at the end of pregnancy, maternal weight gain during pregnancy, preterm labour, neonatal jaundice and respiratory distress (RDS). Additionally, metformin provides adequate glycaemic control, with lower mean glucose levels throughout the day, and results in a lower frequency of neonatal hypoglycaemia 14. By contrast, the hospitalization of infants was observed to be higher in the group treated with insulin 15. The quality of the evidence given in these RCTs has not yet been analysed and evaluated. We therefore performed the present meta-analysis, with the aim of determining the efficacy and safety of metformin compared with insulin for GDM.
Methods
Search strategy
We searched databases, including PubMed, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL; the last search was updated on September 2013). We used the key words gestational diabetes, pregnancy and metformin, in combination with RCT. We contacted the authors of the original studies for additional data, if necessary. Two reviewers performed the literature search, evaluated potentially eligible studies for inclusion and extracted the data independently. Any discordance between the findings of the two reviewers was resolved through meetings.
Selection criteria
Any of the identified trials were included in the meta-analysis if they had a randomized controlled design; involved women with GDM that had been diagnosed by the authors, using any method, at any stage of the pregnancy; and compared metformin and insulin for the treatment of GDM, and reported one or more maternal and neonatal outcomes. Quasi-RCTs, retrospective studies, observational studies, case series and studies with a crossover design were excluded.
Data extraction and management
We designed a form by which to extract data. Two review authors independently extracted data from the eligible studies by using this form, and resolved discrepancies through discussion. We entered the data into the Review Manager software (RevMan 2011) (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration). When any of the afore-mentioned information was unclear, we attempted to contact the authors of the original reports for further details. There was no blinding of authorship.
Assessment of risk of bias
Two review authors independently assessed the risk of bias for each included study by using the Cochrane Collaboration's risk-of-bias tool in the Cochrane Handbook for Systematic Reviews of Interventions 16. This tool was used to assess the risk of bias in randomization methods (allocation sequence generation and allocation concealment), blinding (of participants, personnel and investigators), completeness of outcome data, reporting of data, and other issues. We summarized the risk of bias in all six domains to produce an overall risk of bias. The following classification was used: low risk, high risk or unclear risk (either lack of information or uncertainty over the potential for bias). We resolved any disagreement by discussion or by involving a third assessor. The results of the assessments were directly applied to the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) profiler.
We used Egger's publication plots to test for publication bias with STATA 12.0 (StataCorp., College Station, Texas, USA), and considered that there was no obvious publication bias at P > 0.1. The results of the assessments were directly applied to the GRADE profiler.
Statistical analysis
We pooled the data from the RCTs by using the Review Manager software (RevMan 2011). For dichotomous data, we presented the results as the summary risk ratio (RR) and 95% confidence intervals (CIs). We used the standardized mean difference and 95% CI for the analysis of continuous data. All pooled outcomes were calculated using the inverse–variance random-effects model.
We assessed statistical heterogeneity in each meta-analysis by using the \2 and I2 statistics. We planned to explore heterogeneity by prespecified subgroup analyses and sensitivity analyses when the I2 statistic was greater than 50%.
We also assessed the quality of evidence in these studies by using the GRADE profiler (GRADEpro) software, version 3.2 (McMaster University and Evidence Prime Inc, Hamilton, Ontario, Canada). The illustrative comparative risks, relative effect, no. of participants and quality of evidence of every outcome will be summarized. When grading the evidence, the reviewers evaluated the domains of study limitations (risk of bias), inconsistency, indirectness, imprecision and publication bias and downgraded wherever important limitations existed. Studies were to be upgraded based on a strong magnitude of effect that was not due to known biases, a dose–response gradient and residual confounding that would have reduced the observed effect. The overall quality-of-evidence grade was designated as high (confident that the true effect is close to the estimate), moderate (moderately confident in the effect estimate but may be substantially different), low (confidence in the effect estimate is limited) or very low (very little confidence in the effect estimate) 16.
Results
Literature search and study characteristics
A total of 154 articles were identified through the literature search, of which 38 were duplicates (n = 116). After review of the titles and abstracts, another 101 were excluded. Thus, 15 articles were reviewed in detail, and 12 were finally assessed for inclusion in the meta-analysis. The process of RCT selection is illustrated in Figure1. The main study characteristics of the eight included RCTs are presented in Table1. The criteria for diagnosis and commencing medical treatment for GDM are shown in Table2.
Figure 1.
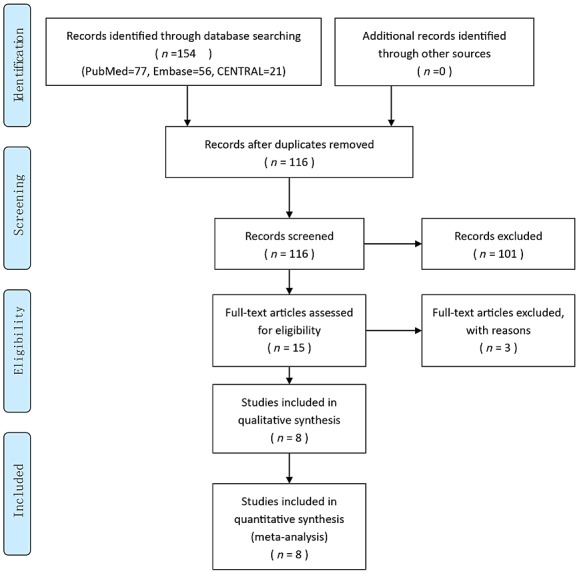
Study flow diagram
Table 1.
Characteristics of randomized controlled trials
| Author (year) | Country | Study period | Participants | No. of participants | Dose | The types of insulin used | Initial dosage (units kg–1) | No. in metformin group requiring insulin | No. of outcomes | Informed consent | Ethical approval | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metformin | Insulin | Metformin (mg) | Insulin (units) | ||||||||||
| Hague et al. 2003 | New Zealand, Australia | NR | NR | 16 | 14 | NR | NR | NR | NR | NR | 15 | Y | Y |
| Ijäs et al. 2011 | Finland | June 2005 to June 2009 | Singleton; gestational age 12–34 weeks | 47 | 50 | 750–2250 | 30 | Protaphan and Humalog | NR | 15 (31.9%) | 21 | Y | Y |
| Mesdaghinia et al. 2013 | Iran | NR | Women aged 18–45 years; singleton; gestational age 24–34 weeks | 100 | 100 | NR | NR | NPH and regular insulin | 0.5 (NPH) | 22 (22%) | 13 | Y | Y |
| Moore et al. 2007 | America | 2001 to 2004 | Gestational age, 24–30 weeks | 32 | 31 | 1000–2000 | NR | Regular insulin and NPH | 7 | 0 (0%) | 14 | Y | Y |
| Niromanesh et al. 2012 | Iran | December 2010 to January 2012 | Women aged 18–40 years; singleton; gestational age 20–34 weeks | 80 | 80 | 1000–2500 | NR | NPH | 0.2 | 11 (14%) | 26 | Y | Y |
| Rowan et al. 2008 | New Zealand, Australia | October 2002 to October 2006 | Women aged 18–45 years; singleton; gestational age 20–33 weeks | 363 | 370 | 1750–2500 | 30–90 | NR | NR | 168 (46.3%) | 21 | Y | Y |
| Spaulonci et al. 2013 | Brazil | November 2007 to January 2010 | Singleton | 46 | 46 | 1700–2550 | NR | NPH | 0.4 | 12 (26.1%) | 15 | Y | Y |
| Tertti et al. 2013 | Finland | June 2006 to December 2010 | Singleton | 110 | 107 | 500–2000 | 2–42 | NPH and/or Humalog or Novorapid | NR | 23 (20.9%) | 24 | Y | Y |
Abbreviations are as follows: NR, not reported; NPH, Neutral Protamine Hagedorn; Y, yes
Table 2.
Criteria for diagnosis and starting medical treatment for gestational diabetes mellitus
| Author (year) | Criteria for diagnosis | Criteria for starting medical treatment | |||||
|---|---|---|---|---|---|---|---|
| Loading | Fasting | 1 h, | 2 h, | 3 h, | Fasting, mg dl–1 | Postprandial 2 h, mg dl–1 | |
| mg dl–1 | mg dl–1 | mg dl–1 | |||||
| Hague et al. 2003 | 75 g | 99 | 126 | – | 97.2 | 120.6 | |
| Ijäs et al. 2011 | 75 g | 95.4 | 198 | 172.8 | – | 95.4 | 120.6 |
| Mesdaghinia et al. 2013 | 100 g | 95 | 180 | 155 | 140 | 95 | 120 |
| Moore et al. 2007 | 100 g | 105 | 190 | 165 | 145 | 105 | 120 |
| Niromanesh et al. 2012 | 100 g | 95 | – | 120 | – | 95 | 120 |
| Rowan et al. 2008 | 75 g | 99 | – | 126 | – | 97.2 | 120.6 |
| Spaulonci et al. 2013 | 100 g or 75 g | 95 | 180 | 155 | 140 | 123.5 | 156 |
| Tertti et al. 2013 | 75 g | 95.4 | 180 | 154.8 | – | 99 | 140.4 (1 h) |
= None reported.
The search of the PubMed, Embase and CENTRAL databases identified 15 reports that could be included in the review. We found that eight of these studies satisfied the eligibility criteria; the remaining seven studies, including four follow-up articles about the Metformin in Gestational Diabetes (MiG) trial, did not. The included trials were by Hague et al., 2003 17, Ijäs et al., 2011 18, Mesdaghinia et al., 2013 15, Moore et al., 2007 19, Niromanesh et al., 2012 20, Rowan et al., 2008 21, Spaulonci et al., 2013 14 and Tertti et al., 2013 22. The excluded trials (apart from the four follow-up trials) were Hickman et al., 2013 23, Hassan et al., 2012 24 and Rowan et al., 2007 25. The follow-up articles about the MiG trial were Barrett et al., 2013 26,27, Rowan et al., 2010 28 and Rowan et al., 2011 6; only Rowan et al., 2010 28 provided useful follow-up data from the MiG trial. Thus, eight trials, including a total of 1592 women, were eligible for the review of metformin vs. insulin. The earliest study began in 2001 19, and the latest study was completed in 2012 20.
Risk of bias for all studies
The summary of the risk of bias for each included study is shown in Figure2. The studies by Mesdaghinia et al. 15 and Spanlonci et al. 14 are the new RCTs included in the present meta-analysis. The designs of all the RCTs were without selection bias, attrition bias and selective bias, which indicate that they were of a relatively high quality. As insulin was given by injection and metformin was given orally, all the included studies involved open allocation, which did not affect the outcomes, as these were all objective.
Figure 2.
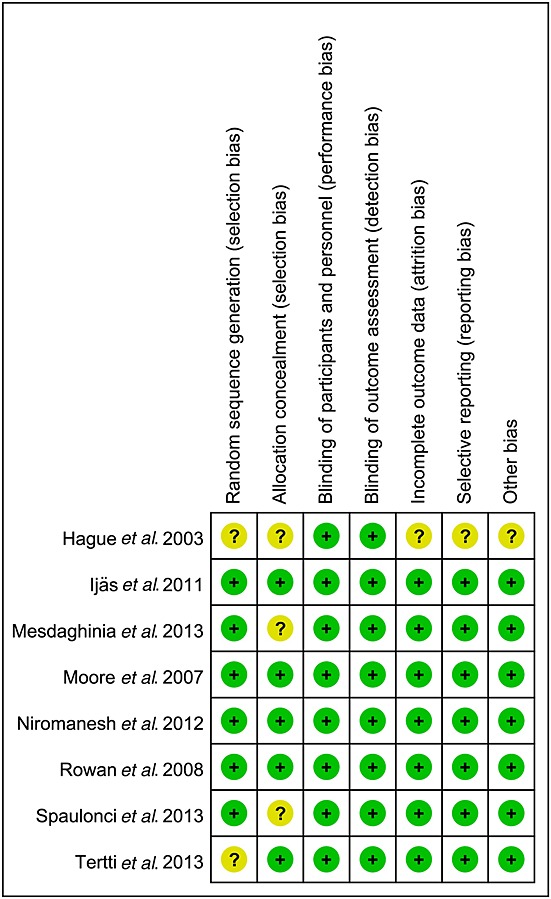
Summary of risk of bias for each included study. +, low risk of bias; ?, unknown risk of bias.
The quality of the evidence (GRADE) for the primary outcomes, including neonatal hypoglycaemia, rate of large-for-gestational age (LGA) infants, RDS, phototherapy, preterm birth, pregnancy-induced hypertension (PIH) and perinatal death, was very low to moderate. The GRADE system evidence for the above outcomes and reasons for upgrade and downgrade are shown in Table3, and the primary outcomes of the meta-analysis are presented in Figure3.
Table 3.
Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) summary of primary outcomes of meta-analysis
| Metformin vs. insulin for gestational diabetes mellitus | |||||
|---|---|---|---|---|---|
| Patient population:patients with gestational diabetes mellitus | |||||
| Intervention:metformin | |||||
| Comparison:insulin | |||||
| Outcome | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Insulin | Metformin | ||||
| Neonatal hypoglycaemia | Study population | RR 0.8 (0.62 to 1.02) | 1591 (8 studies) | ⊕ ⊕ ⊕⊝ moderate† | |
| 155 per 1000 | 124 per 1000 (96 to 158) | ||||
| Moderate | |||||
| 145 per 1000 | 116 per 1000 (90 to 148) | ||||
| Large for gestational age | Study population | RR 0.77 (0.55 to 1.08) | 1499 (6 studies) | ⊕ ⊕ ⊕⊝ moderate† | |
| 171 per 1000 | 132 per 1000 (94 to 185) | ||||
| Moderate | |||||
| 143 per 1000 | 110 per 1000 (79 to 154) | ||||
| Respiratory distress syndrome | Study population | RR 1.26 (0.67 to 2.37) | 1265 (6 studies) | ⊕⊝⊝⊝ very low†‡§ | |
| 25 per 1000 | 32 per 1000 (17 to 60) | ||||
| Moderate | |||||
| 45 per 1000 | 57 per 1000 (30 to 107) | ||||
| Phototherapy | Study population | RR 0.94 (0.67 to 1.31) | 1236 (5 studies) | ⊕⊝⊝⊝ very low†‡¶ | |
| 100 per 1000 | 94 per 1000 (67 to 131) | ||||
| Moderate | |||||
| 84 per 1000 | 79 per 1000 (56 to 110) | ||||
| Preterm birth | Study population | RR 1.24 (0.69 to 2.23) | 1499 (6 studies) | ⊕ ⊕ ⊝⊝ low†‡ | |
| 69 per 1000 | 86 per 1000 (48 to 154) | ||||
| Moderate | |||||
| 68 per 1000 | 84 per 1000 (47 to 152) | ||||
| Pregnancy- induced hypertension | Study population | RR 0.54 (0.31 to 0.91) | 1110 (3 studies) | ⊕ ⊕ ⊕⊝ moderate† | |
| 68 per 1000 | 37 per 1000 (21 to 62) | ||||
| Moderate | |||||
| 62 per 1000 | 33 per 1000 (19 to 56) | ||||
| Perinatal death | Study population | RR 1.01 (0.11 to 9.53) | 1253 (2 studies) | ⊕ ⊕ ⊝⊝ low†‡ | |
| 2 per 1000 | 2 per 1000 (0 to 15) | ||||
| Moderate | |||||
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE Working Group grades of evidence:
High quality: Further research is very unlikely to change our confidence in the estimate of the effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of the effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of the effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Total (cumulative) sample size is lower than the calculated optimal information size.
The relative risk reduction or relative risk increase is greater than 25%.
Egger's test, P > |t| = 0.040.
Egger's test, P > t| = 0.043. Abbreviations are as follows: CI, confidence interval; RR, risk ratio.
Figure 3.
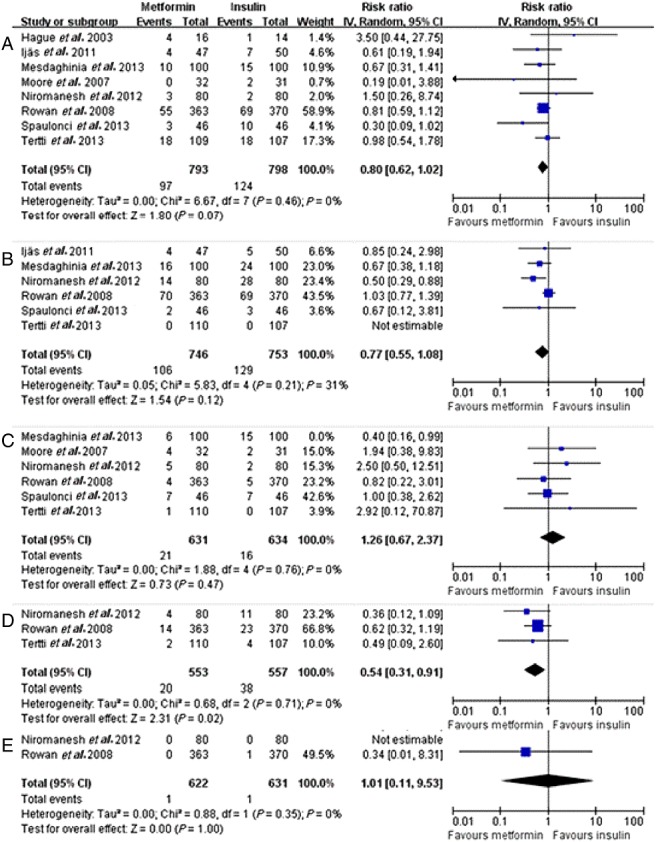
Forest plots of primary outcomes of meta-analysis. (A) Neonatal hypoglycaemia; (B) Large for gestational age (LGA); (C) Respiratory distress syndrome (RDS); (D) Pregnancy-induced hypertension (PIH); (E) Perinatal death. CI, confidence interval; IV, intravenous.
Meta-analysis of effects of metformin in GDM
Neonatal hypoglycaemia
Neonatal hypoglycaemia was reported in eight studies. There was no significant heterogeneity between these studies (P = 0.42, I2 = 0%). The pooled results showed no significant difference between the metformin and insulin groups in terms of neonatal hypoglycaemia (P = 0.07, RR = 0.80, 95% CI 0.62, 1.02) (Figure3a).
LGA
The frequency of LGA infants was reported in five studies. There was no significant heterogeneity between these studies (P = 0.46, I2 = 31%). The pooled result showed no significant difference in the frequency of LGA infants between the metformin and insulin groups (P = 0.12, RR = 0.77, 95% CI 0.55, 1.08) (Figure3b).
RDS
RDS was reported in five studies. There was no significant heterogeneity between these studies (P = 0.76, I2 = 0%). The pooled result showed no significant difference between the metformin and insulin groups in terms of RDS (P = 0.47, RR = 1.26, 95% CI 0.67, 2.37) (Figure3c).
PIH
PIH was reported in three studies. There was no significant heterogeneity between these studies (P = 0.71, I2 = 0%). The standardized difference in the mean PIH incidence was 0.54 (P = 0.02, 95% CI 0.31, 0.91) (Figure3d) times greater in the insulin group than in the metformin group.
Perinatal death
Perinatal death was reported in two studies. There was no significant heterogeneity between these studies (P = 0.35, I2 = 0%). The pooled result showed no significant difference between the metformin and insulin groups in terms of perinatal death (P = 1.00, RR = 1.01, 95% CI 0.11, 9.53) (Figure3e).
Other outcomes
Table4 shows a summary of the meta-analysis outcomes, which include the following.
Table 4.
Summary of meta-analysis outcomes
| Outcome | No. of studies | No. of participants | Type of meta-analysis | Effect estimate (95% CI) | P value | I2 (%) | Egger's test (P value) |
|---|---|---|---|---|---|---|---|
| Perinatal death | 5 | 1253 | RR | 1.01 | 1.00 | 0 | – |
| (Random) | [0.11, 9.53] | ||||||
| Respiratory distress syndrome | 6 | 1265 | RR | 1.26 | 0.47 | 0 | 0.040 |
| (Random) | [0.67, 2.37] | ||||||
| Admission to neonatal intensive care unit | 6 | 1469 | RR | 0.75 | 0.07 | 37 | 0.770 |
| (Random) | [0.55, 1.03] | ||||||
| Birth trauma | 3 | 1047 | RR | 0.86 | 0.64 | 0 | 0.076 |
| (Random) | [0.45, 1.63] | ||||||
| Birth defect | 4 | 1310 | RR | 0.74 | 0.33 | 0 | 0.770 |
| (Random) | [0.41, 1.34] | ||||||
| Preterm birth | 6 | 1499 | RR | 1.24 | 0.47 | 34 | 0.252 |
| (Random) | [0.69, 2.23] | ||||||
| 5-min Apgar score < 7 | 2 | 933 | RD (Random) | 0.00 | 0.37 | 0 | - |
| [−0.01,0.01] | |||||||
| 5-min Apgar score | 3 | 376 | SMD | −0.11 | 0.31 | 0 | 0.862 |
| (Random) | [−0.31,0.10] | ||||||
| Phototherapy | 5 | 1236 | RR | 0.94 | 0.71 | 0 | 0.043 |
| (Random) | [0.67, 1.31] | ||||||
| Neonatal hypoglycaemia | 8 | 1591 | RR | 0.80 | 0.07 | 0 | 0.568 |
| (Random) | [0.62, 1.02] | ||||||
| pH of umbilical-cord blood | 5 | 757 | SMD | 0.04 | 0.62 | 0 | 0.549 |
| (Random) | [−0.11, 0.18] | ||||||
| Shoulder dystocia | 6 | 1373 | RR | 0.67 | 0.30 | 0 | 0.232 |
| (Random) | [0.32, 1.43] | ||||||
| Birth weight (g) | 8 | 1592 | SMD | −0.05 | 0.35 | 0 | 0.372 |
| (Random) | [−0.15,0.05] | ||||||
| Birth weight > 4000 g | 7 | 859 | RR | 0.81 | 0.27 | 6 | 0.164 |
| (Random) | [0.56, 1.18] | ||||||
| Large-for-gestational age infants | 6 | 1499 | RR | 0.77 | 0.12 | 31 | 0.313 |
| (Random) | [0.55, 1.08] | ||||||
| Small-for-gestational age infants | 5 | 1402 | RR | 0.86 | 0.50 | 0 | 0.021 |
| (Random) | [0.56, 1.33] | ||||||
| Head circumference (cm) | 2 | 893 | SMD | −0.20 | 0.22 | 71 | - |
| (Random) | [−0.51,0.12] | ||||||
| Arm circumference (cm) | 2 | 748 | SMD | −0.13 | 0.56 | 84 | - |
| (Random) | [−0.57,0.31] | ||||||
| Chest circumference (cm) | 2 | 760 | SMD | −0.20 | 0.17 | 64 | - |
| (Random) | [−0.49,0.09] | ||||||
| Pregnancy-induced hypertension | 3 | 1110 | RR | 0.54 | 0.02 | 0 | 0.470 |
| (Random) | [0.31, 0.91] | ||||||
| Preeclampsia | 6 | 1329 | RR | 0.86 | 0.42 | 0 | 0.610 |
| (Random) | [0.59, 1.25] | ||||||
| Gestational age at birth (weeks) | 7 | 1392 | SMD | −0.13 | 0.02 | 0 | 0.927 |
| (Random) | [−0.23,-0.02] | ||||||
| Induction of labour | 4 | 1077 | RR | 0.85 | 0.14 | 49 | 0.312 |
| (Random) | [0.68, 1.06] | ||||||
| Assisted vaginal delivery | 2 | 314 | RR | 1.34 | 0.42 | 0 | - |
| (Random) | [0.65, 2.75] | ||||||
| Caesarean section | 7 | 1392 | RR | 1.04 | 0.73 | 39 | 0.232 |
| (Random) | [0.84, 1.28] | ||||||
| Weight gain (kg) | 4 | 565 | SMD | −0.36 | 0.07 | 80 | 0.502 |
| (Random) | [−0.74, 0.02] | ||||||
| Weight gain after randomization (kg) | 4 | 1067 | SMD | −0.52 | 0.00 | 71 | 0.735 |
| (Random) | [−0.78, −0.25] | ||||||
| Glycated haemoglobin in weeks 36–37 (%) | 4 | 1144 | SMD | −0.15 | 0.02 | 12 | 0.693 |
| (Random) | [−0.28,-0.02] | ||||||
| Fasting glucose from randomization until delivery (mg dl–1) | 4 | 1048 | SMD | 0.03 | 0.73 | 39 | 0.220 |
| (Random) | [−0.16, 0.23] | ||||||
| 2-h postprandial glucose from randomization until delivery (mg dl–1) | 4 | 1048 | SMD | −0.10 | 0.32 | 40 | 0.063 |
| (Random) | [−0.30, 0.10] | ||||||
| Fasting glucose in the first week after randomization (mg dl–1) | 2 | 893 | SMD | 0.05 | 0.59 | 28 | - |
| (Random) | [−0.13, 0.23] | ||||||
| 2-h postprandial glucose in the first week after randomization (mg dl–1) | 2 | 893 | SMD | −0.21 | 0.00 | 0 | - |
| (Random) | [−0.34,-0.07] |
Abbreviations are as follows: CI, confidence interval; RR, risk ratio; SMD, standardized mean difference.
Neonatal outcomes: perinatal death, RDS, admission to the neonatal intensive care unit (NICU), birth trauma, birth defect, preterm birth, 5-min Apgar score < 7, 5-min Apgar score, phototherapy, neonatal hypoglycaemia, pH of umbilical-cord blood, shoulder dystocia, birth weight (g), birth weight > 4000 g, rates of LGA and small-for-gestational age infants, head circumference (cm), arm circumference (cm) and chest circumference (cm).
Maternal outcomes: PIH, preeclampsia, gestational age at birth (weeks), induction of labour, assisted vaginal delivery, Caesarean section, weight gain (kg) and weight gain after randomization (kg).
Glycaemic control: glycated haemoglobin (HbA1c) level in weeks 36–37 (%), fasting glucose from randomization until delivery (mg dl–1), 2-h postprandial glucose from randomization until delivery (mg dl–1), fasting glucose in the first week after randomization (mg dl–1), and 2-h postprandial glucose in the first week after randomization (mg dl–1).
Discussion
The Rowan et al., 2010 28 paper on the MiG trial found that glycaemic control in women with GDM treated with metformin and/or insulin was strongly related to the following: HbA1c% predicted LGA infants (P = 0.003); fasting capillary glucose predicted neonatal complications (P < 0.001); and postprandial glucose predicted preeclampsia (P = 0.016) and LGA infants (P = 0.001). However, there were no statistical differences in the frequency of LGA infants between the metformin group and the insulin group. Therefore, the small statistical differences in the HbA1c% levels at the gestational age of 36–37 weeks, a finding which differs from that of a previous review 13, might be of no clinical importance.
In addition, we found that the average 2-h postprandial glucose levels in the first week after randomization were significantly lower in the metformin group, which is consistent with the result of the previous review 13. This finding indicates that in the metformin group, glucose targets might be reached sooner, possibly because metformin reduces hyperglycaemia by suppressing hepatic glucose output, increasing insulin sensitivity and enhancing peripheral glucose uptake 7. These effects are potentially useful during pregnancy, when glucose control deteriorates with changes to insulin resistance 29. Furthermore, for insulin, it takes time for participants to master the usage and dose computation. The glycaemic control outcomes were not worse in metformin-treated women; however, a large proportion of women randomized to the metformin group required the addition of insulin to achieve adequate glycaemic control.
Hypertensive disorders of pregnancy represent the second most common cause of direct maternal death and are known to complicate an estimated 5–10% of pregnancies 29. Notably, women with diabetes have an increased risk for de novo hypertension during pregnancy compared with nondiabetic subjects 30. Therefore, the better choice of antidiabetic agents will not only efficiently control the blood glucose level during pregnancy, but will also be useful in preventing PIH. Our meta-analysis revealed that the PIH-related morbidity was lower in the metformin group, which is consistent with the findings of a previous review 13. In addition, the GRADE results were found to be moderate. The data showed that metformin will prove beneficial for the prevention of GDM in patients with complications of PIH. This could be attributed to the complex effects of metformin on endothelial functions and the production of reactive oxygen species 31; additionally, it reduces endothelial activation and the maternal inflammatory response to insulin resistance. Furthermore, some studies indicate that the lower gestational age at delivery in the metformin group may account for the lower incidence of PIH. This could be due either to chance or an unrecognized effect of metformin on the labour process. However, a lower gestational age at delivery is not associated with higher rates of other complications 28. The sample sizes of metformin and insulin groups in the present study were 553 and 557, respectively. However, only three studies in our meta-analysis used gestational hypertension as an outcome. Therefore, further studies should be performed, with a larger sample size, in order to evaluate the effect of metformin on PIH-related morbidity during pregnancy. The incidence of preterm birth, perinatal death, RDS and phototherapy did not differ between the two groups, and the frequency of preterm births found was consistent with that found in the previous review 13. However, the GRADE results of RDS and phototherapy were of ‘low quality’, and the perinatal death rate was low.
Our results for the neonatal outcomes of neonatal hypoglycaemia and transfer to the NICU were consistent with the results of previous reviews 13,32. The GRADE result for these parameters was ‘moderate quality’.
Our other findings about the neonatal outcomes were in accordance with the results of previous reviews 12,13, which suggests that, compared with insulin, metformin does not harm the fetus. Although it is reassuring that the outcomes were not worse in the metformin-treated women, a large proportion of the women randomized to the metformin group required the addition of insulin to achieve adequate glycaemic control.
Of the eight RCTs, only the MiG trial assessed follow-up data. Rowan et al. 28 measured the body composition of 154 and 164 children whose mothers had been randomized to the metformin and insulin groups, respectively, when the children were aged 2 years. Children exposed to metformin had larger measures of subcutaneous fat but the same overall body fat as those whose mothers had been treated with insulin alone. Further follow-up is required to examine whether these findings persist into later life and whether children exposed to metformin will develop less visceral fat and be more insulin sensitive. More long-term data on offspring outcomes are required to confirm the safety of metformin use in pregnancy.
The GRADE framework was applied to assess the quality of evidence for our outcomes. The results showed that the quality of evidence for RDS and phototherapy was very low, and for preterm birth and perinatal death was low. According to the GRADE approach, the rating ‘low’ is ascribed to double-downgraded randomized trials and ‘very low’ to triple-downgraded randomized trials. The low quality may be due to: (i) imprecision of the results: the RR and 95% CI of the meta-analysis for preterm birth and perinatal death were 1.24 (0.69, 2.23) and 0.34 (0.01, 8.23), and there were no significant differences between the two groups; and (ii) limitation in the design, suggesting a likelihood of bias: the sample size was smaller than the optimal size. Besides, the very low quality of the evidence obtained for RDS and phototherapy resulted from a high probability of publication bias, as indicated by their Egger's test P values of 0.040 and 0.043, respectively.
The present meta-analysis had several limitations. Only a few studies fulfilled the inclusion criteria. Moreover, original studies that were not written in English were not included, which could have resulted in bias.
Conclusion
The present meta-analysis found that, compared with insulin, metformin has similar efficacy and safety in terms of neonatal hypoglycaemia, the frequency of LGA infants, RDS, phototherapy and perinatal death, but is safer in terms of PIH incidence in women with GDM. The advantages of metformin with regard to glycaemic control, PIH and gestational age at birth are unclear and should be verified in further trials. Future research, with larger sample sizes, is needed to assess the maternal and neonatal complications of GDM and evaluate the long-term follow-up data of children born to women with GDM in order to determine the safety of metformin.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2003;26:S103–5. doi: 10.2337/diacare.26.2007.s103. [DOI] [PubMed] [Google Scholar]
- Coustan DR, Lowe LP, Metzger BE, Dyer AR; International Association of Diabetes and Pregnancy Study Groups. Am J Obstet Gynecol. 2010;202:e1–6. doi: 10.1016/j.ajog.2010.04.006. . The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus.: 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korucuoglu U, Biri A, Turkyilmaz E, Doga Yildirim F, Ilhan M, Hirfanoglu IM, Atalay Y. Glycemic levels with glucose loading test during pregnancy and its association with maternal and perinatal outcomes. Diabetes Res Clin Pract. 2008;80:69–74. doi: 10.1016/j.diabres.2007.10.028. [DOI] [PubMed] [Google Scholar]
- HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- United Kingdom Prospective Diabetes Study (UKPDS) Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310:83–8. [PMC free article] [PubMed] [Google Scholar]
- Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition at 2 years of age. Diabetes Care. 2011;34:2279–84. doi: 10.2337/dc11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- Charles B, Norris R, Xiao X, Hague W. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit. 2006;28:67–72. doi: 10.1097/01.ftd.0000184161.52573.0e. [DOI] [PubMed] [Google Scholar]
- Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 2005;83:1575–78. doi: 10.1016/j.fertnstert.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertil Steril. 2006;86:658–63. doi: 10.1016/j.fertnstert.2006.02.098. [DOI] [PubMed] [Google Scholar]
- Nanovskaya TN, Nekhayeva IA, Patrikeeva SL, Hankins GD, Ahmed MS. Transfer of metformin across the dually perfused human placental lobule. Am J Obstet Gynecol. 2006;195:1081–5. doi: 10.1016/j.ajog.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Nicholson W, Baptiste-Roberts K. Oral hypoglycaemic agents during pregnancy: the evidence for effectiveness and safety. Best Pract Res Clin Obstet Gynaecol. 2011;25:51–63. doi: 10.1016/j.bpobgyn.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Gui J, Liu Q, Feng L. Metformin vs insulin in the management of gestational diabetes: a meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064585. : e64585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulonci CP, Bernardes LS, Trindade TC, Zugaib M, Francisco RP. Randomized trial of metformin vs insulin in the management of gestational diabetes. Am J Obstet Gynecol. 2013;209:e1–e7. doi: 10.1016/j.ajog.2013.03.022. : 34. [DOI] [PubMed] [Google Scholar]
- Mesdaghinia E, Samimi M, Homaei Z, Saberi F, Moosavi SG, Yaribakht M. Comparison of newborn outcomes in women with gestational diabetes mellitus treated with metformin or insulin: a randomised blinded trial. Int J Prev Med. 2013;4:327–33. [PMC free article] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Hague WM, Davoren PM, Oliver J, Rowan J. Contraindications to use of metformin. Metformin may be useful in gestational diabetes. BMJ. 2003;326:762. [PubMed] [Google Scholar]
- Ijäs H, Vääräsmäki M, Morin-Papunen L, Keravuo R, Ebeling T, Saarela T, Raudaskoski T. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG. 2011;118:880–5. doi: 10.1111/j.1471-0528.2010.02763.x. [DOI] [PubMed] [Google Scholar]
- Moore LE, Briery CM, Clokey D, Martin RW, Williford NJ, Bofill JA, Morrison JC. Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. J Reprod Med. 2007;52:1011–5. [PubMed] [Google Scholar]
- Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract. 2012;98:422–9. doi: 10.1016/j.diabres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Rowan JA, Hague WM, Gao W, Battin MR. Moore MP; MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- Tertti K, Ekblad U, Koskinen P, Vahlberg T, Rönnemaa T. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes Metab. 2013;15:246–51. doi: 10.1111/dom.12017. [DOI] [PubMed] [Google Scholar]
- Hickman MA, McBride R, Boggess KA, Strauss R. Metformin compared with insulin in the treatment of pregnant women with overt diabetes: a randomized controlled trial. Am J Perinatol. 2013;30:483–90. doi: 10.1055/s-0032-1326994. [DOI] [PubMed] [Google Scholar]
- Hassan JA, Karim N, Sheikh Z. Metformin prevents macrosomia and neonatal morbidity in gestational diabetes. Pak J Med Sci. 2012;28:384. [Google Scholar]
- Rowan JA MiG Investigators. A trial in progress: gestational diabetes: treatment with metformin compared with insulin (the metformin in gestational diabetes [MIG] trial) Diabetes Care. 2007;30(Suppl. 2):S214–9. doi: 10.2337/dc07-s219. [DOI] [PubMed] [Google Scholar]
- Barrett HL, Gatford KL, Houda CM, De Blasio MJ, McIntyre HD, Callaway LK, Dekker Nitert M, Coat S, Owens JA, Hague WM, Rowan JA. Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the metformin in gestational diabetes (MIG) trial: responses to maternal metformin versus insulin treatment. Diabetes Care. 2013;36:529–36. doi: 10.2337/dc12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett HL, Dekker Nitert M, Jones L, O'Rourke P, Lust K, Gatford KL, De Blasio MJ, Coat S, Owens JA, Hague WM, McIntyre HD, Callaway L, Rowan J. Determinants of maternal triglycerides in women with gestational diabetes mellitus in the metformin in gestational diabetes (MIG) study. Diabetes Care. 2013;36:1941–6. doi: 10.2337/dc12-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan JA, Gao W, Hague WM, McIntyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care. 2010;33:9–16. doi: 10.2337/dc09-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest AR, Cho LS. Hypertension in pregnancy. Curr Atheroscler Rep. 2014;16:395. doi: 10.1007/s11883-013-0395-8. [DOI] [PubMed] [Google Scholar]
- Gordin D, Groop PH, Teramo K, Kaaja R. Hypertensive pregnancy in diabetes – risk factors and influence on future life. Duodecim. 2013;129:932–8. [PubMed] [Google Scholar]
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253–70. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh N, Royle P, Clar C, Henderson R, Cummins E, Hadden D. Screening for hyperglycaemia in pregnancy: a rapid update for the National Screening Committee. Health Technol Assess. 2010;14:1–183. doi: 10.3310/hta14450. [DOI] [PubMed] [Google Scholar]


