Abstract
BACKGROUND
Lichen planopilaris is a frequent presentation of primary cicatricial alopecia. Scalp distribution characterizes the main clinical presentations: classic lichen planopilaris, frontal fibrosing alopecia and Graham-Little Piccardi-Lassueur Syndrome (GLPLS).
OBJECTIVE
Description of the clinical, dermoscopic and histopathological findings of Lichen planopilaris in public and private practices.
METHOD
A retrospective observational study was performed by reviewing medical records of patients with lichen planopilaris.
RESULTS
Eighty patients were included, 73 (91,25%) were female. Prototype II was seen in 53 (66,25%) patients. Classic lichen planopilaris was seen in 62,5% of the cases. Frontal fibrosing alopecia was seen in 31% of the patients and only one patient presented Graham-Little Piccardi-Lassueur Syndrome (GLPLS). Scalp lesions were scattered throughout the scalp in 47 (58,75%) of the patients, while 24 (30%) presented mainly central scalp lesions, 29 (36,25%) presented marginal lesions and only 4 (5%) patents had vertex lesions.
CONCLUSIONS
Clinical presentation of Lichen planopilaris varies. To recognize the heterogeneity of the clinical appearance in lichen planopilaris is important for differential diagnosis.
Keywords: Alopecia, Cicatrix, Hair, Lichen planus, Postmenopause, Scalp, Scalp dermatoses, Women
INTRODUCTION
Lichen planopilaris is a common form of primary cicatricial alopecia. Lymphocytic inflammatory process occurs at isthmus level destroying the follicle germ cells. Distribution of the lesions on the scalp characterize the main clinical forms.1 Its variable clinical features postpone the diagnosis hindering the dermatologist daily practice.
Three clinical variants of the lichen planopilaris can be classically observed: the classic form (LPP), the frontal fibrosing alopecia (FFA) and the Graham-Little-Piccardi-Lassueur syndrome (GLPLS).2 The classic form manifests with alopecia plaques preferably at the vertex, but the scalp may be affected anywhere (Figure 1).1 FFA, described by Kossard in 1994, is characterized by progressive cicatricial alopecia affecting the hair implant in the frontotemporal region (Figure 2). It was initially described only in postmenopausal women, but several cases have been reported in premenopausal women and even in men.3 The third form, GLPLS, is characterized by the triad: cicatricial alopecia of the scalp, non-cicatricial alopecia in the armpit and pubis, and lichenoid papules on the trunk and extremities.4 In some cases, FFA may have some characteristics of GLPLS, showing that these forms may overlap.5
Figure 1.
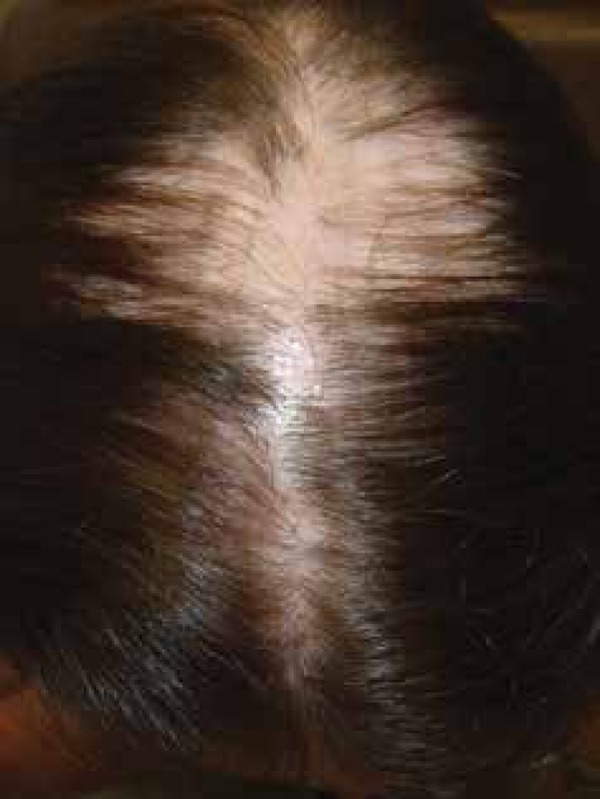
Patient presenting classic form of lichen planopilaris with alopecia plaques at the vertex
Figure 2.
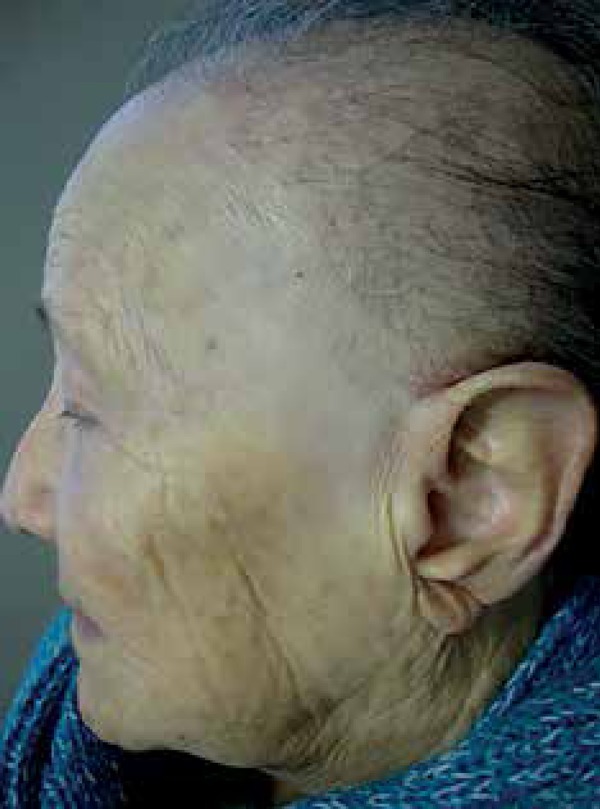
Patient with advanced frontal fibrosing alopecia characterized by progressive cicatricial alopecia affecting the implant hairline in the frontotemporal region
Objectives
Description of clinical, dermoscopic and histopathologic findings of lichen planopilaris and its variants in the city of Curitiba, in the State of Paraná (South region of Brazil) in two healthcare centers, one public and one private.
METHODS
An observational and retrospective study was performed through review of medical records of patients diagnosed with lichen planopilaris. The study was approved by the Ethics Committee of the Universidade Federal do Paraná (UFPR) and registered in Brazil research platform.
The study included patients assisted at the scalp disorders outpatient clinic of the Hospital das Clínicas de Curitiba - UFPR between 2010 and 2013 and at the private clinic CEPELLE - Centro Especializado da Pele between 2008 and 2013. In addition to clinical diagnosis, patients should present histological and/or dermoscopic criteria compatible with LPP. Histological findings of lymphocytic cicatricial alopecia with perifollicular infiltrate at the isthmus were essential for inclusion in the study. This finding should be linked to at least one of the following: presence of Civatte bodies, perifollicular lichenoid infiltrate or a suggestive dermoscopic finding of cicatricial alopecia by LPP.6 Patients who had associated periannexal infiltrate, basement membrane thickening or dermal mucinosis, in addition to cases with other findings suggesting systemic lupus erythematosus or discoid lupus, were excluded. Dermoscopic criteria for exclusion were: presence of atrophic white spots, periplaque perifollicular hyperkeratosis, isolated terminals stalk or, more rarely, hyperpigmentation of perifollicular halo.
The study excluded patients without histological confirmation of lymphocytic cicatricial alopecia. A total of 80 patients was included in the study. Among these, 16 were from the scalp disorders outpatient clinic at Hospital de Clínicas - UFPR. The remaining 64 patients were treated at the private clinic CEPELLE - Centro Especializado da Pele.
A standard questionnaire was formulated. Among the questions assessed were demographics (age, gender and skin type) and also data related to the disease, such as disease duration, clinical form, distribution of lesions on the scalp, involvement of other hairy areas, simultaneous diagnoses on the scalp, other sites of lichen planus, local symptoms, findings on clinical and dermoscopic examination, histopathologic findings, previous and current treatments and serology for hepatitis. Questions were answered by the researchers using data obtained from medical records. In some cases it was not possible to fill the questionnaire due to lack of data in retrospective notes.
The descriptive analysis of the data was made by building contingency tables for categorical variables. For continuous variables, some descriptive measures were calculated: mean, standard deviation, minimum and maximum.
To compare patient groups, the chi-square test was applied when the conditions for using the test were met.
RESULTS
Of the 80 patients, 73 (91.25%) were women and 7 (8.75%) were men. Regarding the phototype, 53 (66.25%) were phototype II, 24 (30%) were phototype III and 3 (3.75%) were phototype IV. The two groups of patients (public and private practice) showed similar profiles. There was no significant difference between the groups regarding age.
Classic form of LPP was found in 50 (62.5%) patients whereas fibrosing alopecia in a pattern distribution (FAPD) was evident in 9 (11.25%) patients and FFA in 29 (31%) patients. Only one patient presented GLPLS.
Concerning the distribution of alopecia plaques on the scalp, 47 (58.75%) patients had random plaques, 24 (30%) had central distribution, 29 (36.25%) presented frontal marginal involvement, and 4 (5%) patients showed vertex involvement.
Involvement of axillary hair was observed in 16 (20%) patients. Decrease in genital hair occurred in 8 (10%) patients as well as involvement of hair of upper limbs. Loss of hair in the lower limbs was seen in 9 (11.25%) patients. Involvement of eyelashes was noted in 3 (3.75%) patients.
Seven patients presented involvement of other areas by LP. Oral lesions occurred in 2 patients, one with FFA and the other with FAPD. Two patients with LPP had pterygium nail. A patient with FFA had involvement of the 20 nails. LP skin lesions have been reported in one patient with LPP form of the disease and in one patient with FAPD, synchronously with scalp expression.
All included patients underwent at least a scalp biopsy; 29 patients underwent more than one biopsy at different times. Sixty-three (78.75%) patients had lymphocytic cicatricial alopecia with involvement, in line or sparse, of the radicular sheath, on follicular isthmus level. The remaining 17 cases showed scattered lymphocytes and perifollicular fibrosis, "onion skin-like", with follicular consumption. Of these 17 cases, 11 presented the classical clinical form of LPP and 6 presented FFA, but also showing clinical and dermoscopic findings suggestive of LPP. Among the dermoscopic findings, perifollicular hyperkeratosis was observed in 15 patients, white spots in 12 patients, isolated strands in 6 patients and perifollicular hyper-pigmentation in 2 patients. Patients were subdivided into 3 groups according to clinical aspect. Classic LPP was considered when confluent random plaques were observed. FFA was considered in patients with alopecia in the line of hair implant of frontotemporal region. And GLPLS was considered in cases of cicatricial alopecia of scalp associated with non-cicatricial alopecia in armpit and/or pubis and lichenoid papules on the trunk and/or limbs.
In the classic LPP group it was possible to characterize 2 distinct clinical features. One group with confluent random plaques distributed throughout the scalp and other with confluent plaques in the central region of the scalp, clinically similar to male pattern or female pattern androgenetic alopecia, what is called fibrosing alopecia in a pattern distribution (FAPD). This variant has increasingly been described as a variation of LPP; although clinically similar to male pattern or female pattern androgenetic alopecia, the perifollicular linchenoid inflammatory process histologically characterizes this entity (Figure 3).7 Both in FFA as in FAPD the trigger for lichenoid process seems to be associated with the miniaturization of the follicles.1
Figure 3.
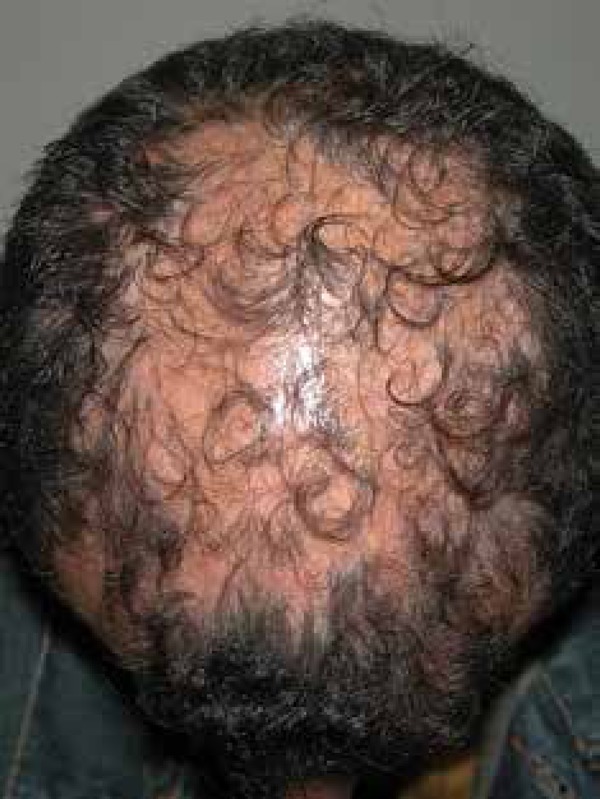
Patient with fibrosing alopecia in a pattern distribution showing confluent alopecia plaques in the central region of the scalp
Regarding the dermoscopic findings, 54 of the 80 cases presented description of dermoscopic examination in the medical records. The dermoscopic findings are shown in table 1.
Table 1.
Dermatoscopic findings
| DERMATOSCOPIC FINDING | Number of patients with positive finding | % of the patients with positive finding |
|---|---|---|
| REDUCTION OF NUMBER OF FOLLICLES | 48 | 88.88% |
| PERIFOLLICULAR HYPERKERATOSIS | 47 | 87.03% |
| POLYTRICHA | 20 | 37.03% |
| WHITE DOTS | 21 | 38.88% |
| VISIBLE VESSELS | 30 | 55.55% |
| BLACK DOTS | 03 | 5.55% |
| PIGMENTED HALO | 05 | 9.255 |
| ISOLATED TERMINAL STALK | 19 | 35.18% |
Among the treatments employed, 65 patients used antimalarials, 53 used topical minoxidil and 30 patients used intralesional corticosteroids. The use of topical corticosteroids was observed in 52 patients, of systemic corticosteroids in 19 patients, of antibiotics in 9 patients and of mycophenolate mofetil in 3 patients.
Phototype II was the most frequently affected: 64% of patients with LPP, 72% of patients with FFA.
DISCUSSION
Lichen planopilaris, also called follicular lichen or follicular lichen planus, was initially described by Pringle in 1895.1 It is a form of lichen planus with primary involvement of the hair follicle. As in lichen planus, its etiology is not well understood and most often affects white women.2 Around 17-28% of cases may be associated with lichen planus lesions in hairless skin, mucous membranes and nails.2 In the described sample, the first major Brazilian group of LPP, association was found with other forms of LP in 8.75% of cases. Among them, 2.5% affecting the skin and the same percentage affecting the mucosa. Nail involvement occurred in 8.75% of cases. One case of FFA presented associated LP pigmentosum.
Differential diagnosis of cicatricial alopecia is a clinical challenge to the dermatologist. LPP can mask alopecia areata (AA) in its classical form and the ophiasic form of AA can be masked by FFA. FAPD and FFA might be mistaken for androgenetic alopecia. Diagnosis of LPP is suspected in the presence of cicatricial alopecia plaques, single or multiple, presenting perifollicular hyperkeratosis, erythema, mainly on the periphery of scarring lesion. Itching, stinging and burning may also be present.1 In such cases, histological evaluation is essential.
Scalp trichoscopy/dermoscopy allows analysis of the epidermal portion of the hair follicle and also the epidermis of the perifollicular scalp.8
Among the most frequently observed dermoscopic findings, perifollicular hyperkeratosis and reduction of the number of follicles was observed in 95.92% and 96% of the cases examined, respectively. These data are similar to those previously described in the literature.1,8,9 In the described sample, black dots were observed on dermoscopic examination of 3 patients. Black dots are classically described in patients with AA and trichotillomania but had not yet been observed in patients with LP. These patients had no other associated diagnoses that could justify these findings. As with AA, these dots may be due to disease activity or external manipulation of the stalks in processes to camouflage the flaws, which may break strands and form black dots, similar to what occurs in trichotillomania. LPP is a diagnostic challenge. Clinical, dermoscopic and histopathologic findings may provide data for a definitive diagnosis. Dermoscopy of LPP is not characteristic, but associated with clinical and histological findings of cicatricial alopecia, to define the diagnosis. Clinical spectrum of LPP is varied. Classic form of LPP was responsible for more than half (62.5%, n=50) of cases. In this group, 18% (n=9) of patients presented FAPD, accounting for 11.25% of the total patients. In the current sample, an uncharacteristic group was evident, but already discussed in cicatricial alopecia consensus. It was possible to distinguish FAPD from the other 3 groups by the central and diffuse involvement of the scalp (Figure 1). LPP cases have been described after hair transplant surgery and thus considered as a Koebner phenomenon, but they might actually be misdiagnosed FAPD, treated as androgenetic alopecia.10
White areas described classically in LPP are irregular white spots that can come together forming white-cicatricial areas.8 These structures correspond histopathologically to fibrous bands and can also be seen in other types of folliculocentric cicatricial alopecias.8 Perifollicular scales or perifollicular hyperkeratosis are highly suggestive of LPP, presenting white-gray color with tubular aspect emerging around the strand axis, which is better visualized by dry dermatoscopy without immersion.8 They histologically correspond to perifollicular hyperkeratosis and vacuolar changes with presence of necrotic keratinocytes in the basal layer of the hair follicle and outer root sheath.8
Isolated strands are seen in cicatricial alopecia and correspond to the previous sites of inflammation with loss of follicles on the site.8 In FFA they can be evidenced in frontal and marginal implant hairline in the scalp.8
Perifollicular halo or target hyperpigmentation are seen as perifollicular blue-gray dots that correspond histologically to pigment leakage that occurs in LPP interface dermatites.9
Presence of elongated blood vessels arranged parallel to each other can be found in the periphery of LPP active plaques.8 There is no histopathological correlation known to date for these findings.8 However, as in most cases, only dermatoscopy with 10x magnification was performed and often this finding was not valued.
FFA, considered a LPP rare variant, was observed in 29 (31%) patients. Since the description of the first 6 cases in Brazil in 2007, several cases have been evaluated, suggesting that this entity is actually underdiagnosed and not rare.11 Only 1 patient presented criteria for GLPLS, which matches the rarity described in the literature.
Treatment of LPP is controversial and often disappointing. As a cicatricial alopecia, the main goal of therapy is to prevent the progression of the lesions. Early diagnosis and intervention can change the course of the disease. Among the treatment options available, the evaluated group used topical, intralesional and systemic corticosteroids; hydroxychloroquine; acitretin; and mycophenolate mofetil. Other options, such as cyclosporine, thalidomide and griseofulvin, have been used with variable response, but were not used by the patients included in this sample.1,2,3
CONCLUSION
Clinical presentation of LPP is diverse. Recognition of forms of LPP initial presentation is critical to the dermatologist, since this is a cicatricial alopecia that requires early intervention. Early forms may present mild hair loss so other differential diagnoses must be considered. Dermatoscopy and scalp biopsy complement the evaluation of LPP.
The study presents as limitation the fact that data were collected through medical records review, which may present some incomplete or distorted information.
Footnotes
Conflict of Interest: None.
Financial Support: None.
How to cite this article: Soares VC, Mulinari-Brenner F, De Souza TE. Lichen planopilaris epidemiology: a retrospective study of 80 cases. An Bras Dermatol. 2015;90(5):666-70.
Study performed at Hospital de Clínicas de Curitiba - Universidade Federal do Paraná (HC-UFPR) – Curitiba (PR), Brazil.
REFERENCES
- 1.Assouly P, Reygagne P. Lichen Planopilaris: Uptade on Diagnosis and Treatment. Semin Cutan Med Surg. 2009;28:3–10. doi: 10.1016/j.sder.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Kang H, Alzolibani AA, Otberg N, Shapiro J. Lichen planopilaris. Dermatol Ther. 2008;21:249–256. doi: 10.1111/j.1529-8019.2008.00206.x. [DOI] [PubMed] [Google Scholar]
- 3.Samrao A, Chew AL, Price V. Frontal Fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296–1300. doi: 10.1111/j.1365-2133.2010.09965.x. [DOI] [PubMed] [Google Scholar]
- 4.Zegarska B, Kallas D, Schwartz RA, Czajkowski R, Uchanska G, Placek W. Graham-Little Syndrome. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:39–42. [PubMed] [Google Scholar]
- 5.Abbas O, Chedraoui A, Ghosn S. Frontal fibrosing alopecia presenting with components of Piccardi-Lassueur-Graham-Little syndrome . J Am Acad Dermatol. 2007;57:S15–S18. doi: 10.1016/j.jaad.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Tayyebi Meibodi N, Asadi Kani F, Nahidi Y, Bordbar Azari J, Sadeghian H. Lichen Planopilaris: Histopathological Study of Vertical Sections of Scalp Biopsies in 44 Patients. Iran Red Crescent Med J. 2012;14:501–502. [PMC free article] [PubMed] [Google Scholar]
- 7.Zinkernagel MS, Trüeb RM. Fibrosing alopecia in a pattern distribution: Patterned lichen planopilaris or androgenetic alopecia with a lichenoid tissue reaction pattern? Arch Dermatol. 2000;136:205–211. doi: 10.1001/archderm.136.2.205. [DOI] [PubMed] [Google Scholar]
- 8.Rakowska A, Slowinska M, Kowalska-Oledzka E, Warszawik O, Czuwara J, Olszewska M, et al. Trichoscopy of cicatricial alopecia. J Drugs Dermatol. 2012;11:753–758. [PubMed] [Google Scholar]
- 9.Duque-Estrada B, Tamler C, Sodré CT, Barcaui CB, Pereira FB. Padrão dermatoscópio das alopecias cicatriciais causadas por lúpus eritematoso discoide e líquen plano pilar. An Bras Dermatol. 2010;85:179–183. doi: 10.1590/s0365-05962010000200008. [DOI] [PubMed] [Google Scholar]
- 10.Donovan J. Lichen planopilaris after hair transplantation: Report of 17 cases. Dermatol Surg. 2012;38:1998–2004. doi: 10.1111/dsu.12014. [DOI] [PubMed] [Google Scholar]
- 11.Mulinari-Brenner F, Rosas FM, Sato MS, Werner B. Frontal fibrosing alopecia: report of six cases. An Bras Dermatol. 2007;82:439–444. [Google Scholar]


