Abstract
Background
We aimed to investigate the impact of RRM1 and ERCC1 expression on response to cisplatin and/or gemcitabine chemotherapy in patients with lung, ovarian or pancreatic cancer.
Material and methods
Patients with lung, ovarian or pancreatic cancer, who used cisplatin and/or gemcitabine therapy were included; hospital files were examined and RRM1 and ERCC1 expression were evaluated with an immunohistochemical method on tissue cross sections from paraffin blocks of the tumour.
Results
Out of 89 patients, 51%, 30% and 19% had lung, ovarian and pancreatic cancer, respectively. The response rates to the therapy in patients with lung and ovarian cancer having low ERCC1 expression were 62% and 90%, respectively (p = 0.028 and p = 0.044, respectively). No significant association was found between ERCC1 expression and response to therapy in patients with pancreatic cancer (p = 0.354). Therapeutic response rates in patients with lung and pancreatic cancer with low RRM1 expression were 60% and 82%, respectively. Survival rates were higher in patients with lung cancer in which ERCC1 and RRM1 expressions were low. Median survival duration in patients with ovarian cancer showing low ERCC1 and RRM1 expressions was longer than that seen in patients with high expressions. Although no significant correlation was found between ERCC1 and the survival in ovarian cancer (p = 0.183), there was a significant correlation between RRM1 expression and survival in patients with pancreatic cancer (p = 0.005).
Conclusions
Our results suggest a predictive value of ERCC1 in lung and ovarian cancers, and also RRM1 in lung and pancreatic cancers.
Keywords: lung cancer, ovarian cancer, pancreatic cancer, ERRC1, RRM1, prognostic factors
Introduction
The main reason for the failure of chemotherapy in many types of cancer is resistance to drugs. Several resistance mechanisms have been identified. The genes playing a role in the DNA repair pathway, the excision repair cross-complementation 1 (ERCC1) gene and ribonucleotide reductase 1 (RRM1), have been correlated with the drug resistance and their correlation with survival has been suggested in numerous studies [1–21]. ERCC1 was studied as a prognostic factor in different cancer types, such as breast cancer [22].
DNA damage caused by the covalent binding of platin-DNA adducts blocks DNA replication by initiating cell death. However, some cancer cells enhance their own DNA repair capacity and hence become resistant to the effects of the chemotherapy. Cancer cells lead this effect especially via activation of the nucleotide excision repair (NER) pathway [3]. Damage caused by platinum is recognised and repaired by ERCC1. Consequently, the tumour cell protects itself from the effects of the chemotherapeutic agent and develops resistance to the drug. Ribonucleotide reductase (RRM) is the rate-limiting enzyme in DNA synthesis. It synthesises deoxyribonucleotide diphosphate, which has a role in DNA synthesis as well as in its repair. Ribonucleotide reductase consists of 2 subgroups: RRM1 and RRM2 [4, 5]. The M1 subunit is the bonding site for RR, whereas the M2 subunit consists of the group of organic free radicals responsible for enzymatic activity [6, 7]. Although the relationship between RRM1 level and response to gemcitabine therapy has been determined, it has been shown that RRM2 is not expressed and the ribonucleotide enzyme activity remains unchanged [8].
Due to the role of RRM1 in gemcitabine sensitivity and the synergistic effect with other chemotherapeutic agents (especially platinum), its pharmacology and pharmacogenetics have gained importance. There is a correlation between RRM1 levels and response to gemcitabine therapy. In a prospective study performed on non-small cell lung cancer (NSCLC) patients, an inverse relationship has been shown between RRM1 levels and response rate to the therapy. ERCC1 represses the platinum-containing DNA strand while RRM1 replaces the region evacuated by the repressed nucleotides. This fact might explain the synergy between cisplatin and gemcitabine [9].
In the present study, we investigated the value of RRM1 and ERCC1 expressions in paraffinised tissues from patients with NSCLC, ovarian cancer or pancreatic cancer by using an immunohistochemical method in predicting the survival as well as the response to platinum and gemcitabine. The potential utility of biomarkers in response prediction and selection of appropriate chemotherapy is receiving growing interest in oncology. More personalised treatment choices were acceptable for good response to therapy. These biomarkers are helpful both in response prediction and selection of true treatment choices. Consequently, we aimed to investigate the impact of ERCC1, RRM1 expression on cisplatin/gemcitabine chemotherapy response for lung, ovarian and pancreatic carcinoma in this study.
Material and methods
The total of 89 cancer patients were enrolled in this study. The archive system of the hospital was scanned. Patients with a diagnosis of inoperable stage III and IV, who were treated with cisplatin, gemcitabine or a combination of both, and with radiological response evaluated after chemotherapy were included in the study.
At the start of the study a total 271 patients were analysed. Different malignancy groups that had been treated with cisplatinum +/– gemcitabine were analysed. Lung, ovarian and pancreatic cancer patients were included to the study. Early-stage patients and patients diagnosed with fine needle aspiration were excluded. Treatment modalities were assessed and the patients who had been treated without cisplatinum +/– gemcitabine were excluded. Patients who were at inoperable stage III and Stage IV at the time of diagnosis were included in the study. Patients who were at an early stage at the time of diagnosis and then progressed to late stage were excluded.
Evaluation of response to the therapy was made in accordance with RECIST version 1.1 23. In order to determine the survival time, the patients’ hospital files were examined and phone calls to the patients’ relatives were made.
The study was approved by the Ethical Committee of Çukurova University Faculty of Medicine prior to clinical data collection and specimen handling. Informed consent was obtained from the patients (or from their relatives when the patient had died) before clinical data collection and specimen handling.
ERCC1 and RRM1 immunohistochemical staining
Haematoxylin and eosin (HE)-stained preparations were evaluated by light microscope and an appropriate block for the immunohistochemical staining was selected. Cross sections with a width of 4–5 µm taken from the tissues embedded in paraffin, were put onto a slide. First, the xylol was incubated at 60°C for 10–15 minutes and then the deparaffinisation process was ended by the process in which the xylol was repeatedly washed with alcohol and water at room temperature. Rabbit Anti-Human RRM1 polyclonal antibody (Spring/Bioscience Pleasanton, CA 94566; #E 18044) was diluted to 1/100 and Rabbit Anti-Human ERCC1 Monoclonal Antibody (Spring/Bioscience Pleasanton, CA 94566; #M 3684) was diluted to 1/80, followed by distillation and incubation in a closed and humid medium for 1 hour. Haematoxylin was separated by washing in distilled water, kept for 7–10 minutes, and stained to blue by being submersed in tap water for approximately 10 minutes. The tissue was dried around and closed with a water-based liquid closing substance.
Immunohistochemical evaluation
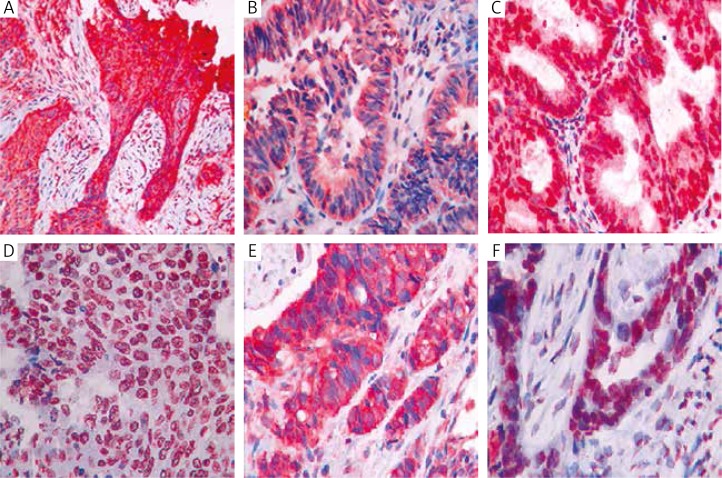
All pathological specimens were evaluated by the same expert pathologist at the Department of Pathology at Çukurova University. The pathologist was not informed about the clinical and demographic data of the patients. Preparations stained with ERCC1 and RRM1 antibodies were evaluated under a light microscope at × 400 magnification. Cytoplasmic and nuclear staining for ERCC1, and cytoplasmic staining for RRM1 were considered as positive staining. ERCC1 and RRM1 expression of tumour cells was ranked from 0 to 3. No staining in tumour cells was considered as 0, whereas 1–10% staining in tumour cells was +1, 10–50% staining in tumour cells was +2, and > 50% staining in tumour cells was +3 (Fig. 1).
Fig. 1.
Immunohistochemical staining in lung, ovarian and pancreatic cancer. A) Cytoplasmic RRM1 +3 staining in lung squamous cell carcinoma. B) Cytoplasmic RRM1 +3 staining in lung adenocarcinoma. C) Cytoplasmic and nuclear RRM1 +3 staining in lung adenocarcinoma. D) Cytoplasmic and nuclear ERCC1 +3 staining in lung squamous cell carcinoma. E) Cytoplasmic RRM1 +3 staining in pancreas cancer. F) Cytoplasmic and nuclear ERCC1 +3 staining in ovarian cancer
Statistical analysis
The SPSS 18.0 package program was used in the statistical analysis of data. Results were evaluated by an expert at the Department of Biostatistics. Categorical measurements were summarized as numbers and percentages whereas numeric measurements were given as average and standard deviation. The χ2 test was used in the comparison of categorical measurements between different groups. The log-rank test under the Kaplan-Meier analysis was performed in the evaluation of the difference in survival curves between two groups. Cox regression analysis was used in the modelling of the factors affecting the survival, and hence the related odds ratio (OR) was obtained. The level of statistical significance was considered as 0.05 in all tests.
Results
Of the patients enrolled in the study, 56% (n = 25) were male and 44% (n = 39) were female. Of these 51% (n = 47) had NSCLC, 30% (n = 27) had epithelial ovarian cancer and 19% (n = 17) had pancreatic cancer. Of the 47 lung cancer patients, 45% (n = 21) had adenocarcinoma while 55% (n = 26) had squamous cell carcinoma. All the ovarian cancer patients had serous adenocarcinoma. The average ages of the lung cancer patients, ovarian cancer patients and pancreatic cancer patients were 58.82 ±9.02, 55.8 ±11.6 and 55.18 ±8.32 years (average age ± SD), respectively. Patients with lung cancer received either received platinum-based therapy (44% of cases [n = 20]) or platinum + gemcitabine chemotherapy (56% of cases [n = 25]), and no patients received gemcitabine only. All ovarian cancer patients received platinum-based therapy. As for pancreas cancer patients, 72% (n = 12) received platinum + gemcitabine, whereas 28% (n = 5) received gemcitabine therapy.
Median overall survival time for lung cancer, ovarian cancer and pancreatic cancer were 13, 23 and 16 months, respectively. The demographic features and stage status of the patients are given in Table 1.
Table 1.
Demographic features
| NSCLC | Epithelial ovarian cancer | Pancreas cancer | |
|---|---|---|---|
| Sex | Male 84% (n = 38) Female 16% (n = 7) |
Female 100% (n = 27) | Male 71% (n = 12) Female 29% (n = 5) |
| Histologic type | Adenocarcinoma 45% (n = 21) Squamous cell carcinoma 55% (n = 24) |
Adenocarcinoma 100% (n = 27) | Adenocarcinoma 100% (n = 17) |
| Stage | Stage III 33% (n = 15) Stage IV 67% (n = 30) |
Stage III 59% (n = 16) Stage IV 41% (n = 11) |
Stage III 29% (n = 5) Stage IV 71% (n = 12) |
| Therapy | Platin 44% (n = 20) Gemcitabine 0% (n = 0) Platinum + gemcitabine 56% (n = 25) |
Platin 100% (n = 27) Gemcitabine 0% (n = 0) Platinum + gemcitabine 0% (n = 0) |
Platin 0% (n = 0) Gemcitabine 28% (n = 5) Platinum + gemcitabine 72% (n = 12) |
| Response to the therapy | Full response 31% (n = 14) Stable disease 13% (n = 6) Progression 56% (n = 25) |
Full response 67% (n = 18) Stable disease 11% (n = 3) Progression 22% (n = 6) |
Full response 41% (n = 7) Stable disease 18% (n = 3) Progression 41% (n = 7) |
| Median survival | 13 months | 23 months | 16 months |
There was no significant relationship between ERCC1 and RRM1 expressions and sex or histological type. The rate of ERCC1 and RRM1 expression positivity increased as diagnosis stage of patients increased (p = 0.018 and p = = 0.001, respectively). The threshold was significantly different in between ERCC1 and cancer type (p = 0.07). ERCC1 expression increased in patients with NSCLC and pancreatic cancer as 53% and 59%, respectively. ERCC1 expression rate was lower (30%) in epithelial ovarian cancer than NSCLC and pancreatic cancer (p = 0.074). No significant relationship was observed between RRM1 and cancer type (p = 0.315).
Zero, +1 and +2 were assumed as a low expression for ERCC1 and RRM1 while +3 was assumed as a high expression. Lung and ovarian cancer patients showed low levels of ERCC1 expressions: the response ratios to the therapy were 62% and 90%, respectively, and were statistically significant (p = 0.028 and p = 0.044, respectively). In pancreatic cancer, no statistical significance was detected between low expression of ERCC1 and the response to the therapy (p = 0.354). Five pancreatic cancer patients with low level of ERCC1 expression responded to the therapy. Four of these 5 received platinum + gemcitabine therapy and 1 received gemcitabine only. In lung and pancreatic cancer patients whose RRM1 expressions were low, the response to the therapy ratios were 60% and 80%, respectively when RRM1 was assessed in response to the therapy (p = 0.020 and 0.018, respectively). On the other hand, when the ovarian cancer patients were considered, there was no statistical significance between RRM1 expression and the response to the therapy (p = 0.695).
Focusing on the relationship between ERCC1 and RRM1, we detected that RRM1 expressions were high in most of the patients with high levels of ERCC1 expression.
ERCC1 expression was high when RRM1 expression was high in 27 patients (30%), and both expressions were low in 39 patients (44%), among all patients. When all of the patients were assessed both for ERCC1 and RRM1 expression, high-level patients responded to the therapy in the rate of 26% and failed to respond in the rate of 74% (p = 0.001).
When we compared other patients with the ones with high expressions of both ERCC1 and RRM1, the response ratio to the therapy of lung cancer was 19% and was statistically significant (p = 0.011). In epithelial ovarian cancer patients showing positive results for both markers, the response ratio to the therapy was 68%, but it was not statistically significant (p = 0.404). A lack of response to the therapy (p = 0.003) was observed in pancreatic cancer patients with positive results for ERCC1 and RRM1. When all patients were considered, it was observed that 26% of the patients who showed combined high expressions of ERCC1 and RRM1 responded to the therapy, while 74% of the patients did not respond to the therapy (p = 0.001). Among all patients enrolled in the study, in those with low level of ERCC1 or RRM1 expression, the response rate to the therapy was 75% and 70%, respectively (p = 0.001 and p = 0.002, respectively).
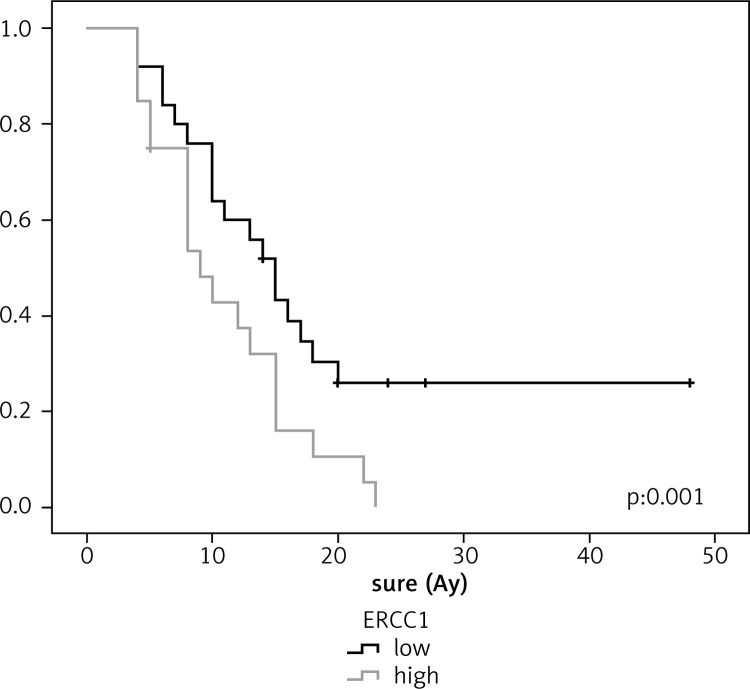
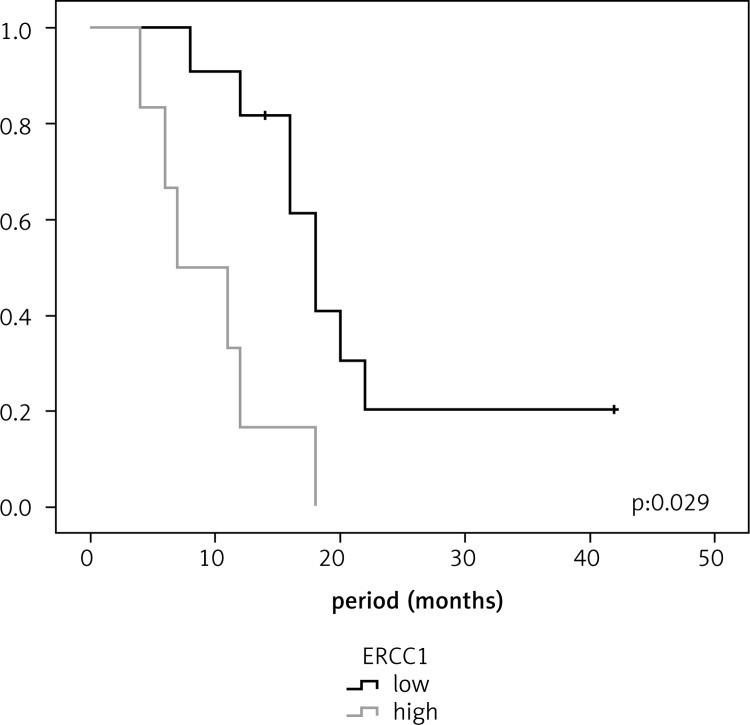
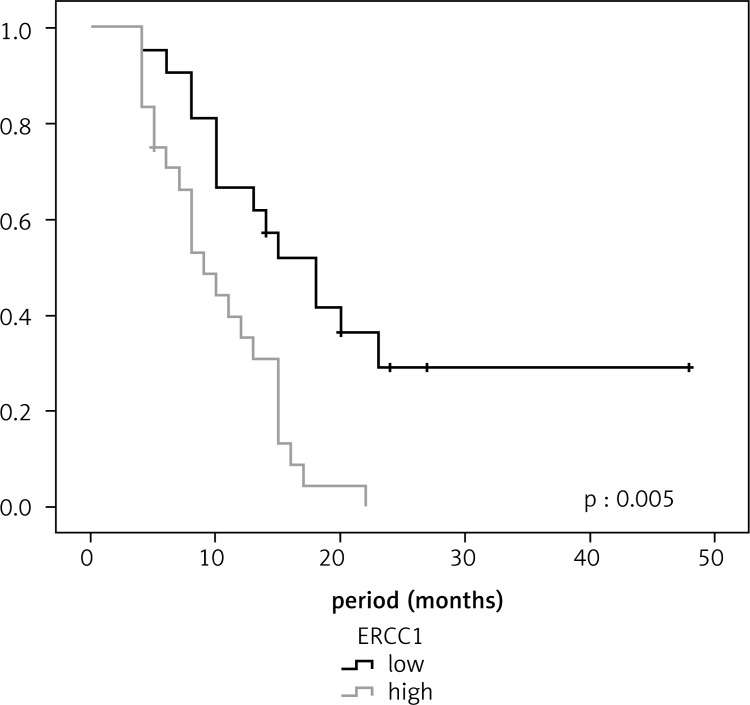
In lung cancer patients, the survival was longer in patients with low ERCC1 expression than in those with high ERCC1 expression (18 and 9 months, respectively, p = 0.001). Similarly, the median survival for lung cancer patients with low RRM1 expression was longer than for those with high RRMI expression (15 and 9 months, respectively, p = 0.029). In ovarian cancer patients, the median survival for those with low levels of ERCC1 and RRM1 expressions was longer than for those with high expressions, and no statistically significant difference was observed (p = 0.183 and p = 0.490, respectively). In pancreatic cancer patients, no statistically significant difference was found between low level of ERCC1 expression and survival (p = 0.684); however, the median survival rate in patients with low RRM1 expression was 18 months, which was statistically significant (p = 0.005) (Figures [2–4]; Table 2).
Table 2.
The relationship between survival and ERCC1 and RRM1 expression in lung, ovarian and pancreas cancer
| Median 95% confidence interval | Median 95% confidence interval | p | |||||
|---|---|---|---|---|---|---|---|
| NSCLC | ERCC1 | Low | 23.3 | 15.8–30.6 | 18 | 12.4–23.5 | 0.001 |
| High | 10.2 | 8.1–12.2 | 9 | 6.2–11.7 | |||
| RRM1 | Low | 20.9 | 14.3–27.5 | 15 | 11.8–18.1 | 0.029 | |
| High | 10.9 | 8.3–13.5 | 9 | 5.5–12.4 | |||
| Ovarian cancer | ERCC1 | Low | 30.0 | 23–37 | 27 | 17.8–36.1 | 0.183 |
| High | 15.8 | 9.3–22.4 | 10 | 0–23.8 | |||
| RRM1 | Low | 29.3 | 21.1–37.5 | 27 | 15.8–38.1 | 0.490 | |
| High | 20.1 | 14.3–26 | 23 | 10.7–35.2 | |||
| Pancreas cancer | ERCC1 | Low | 16.7 | 12.9–20.5 | 18 | 11.4–24.5 | 0.684 |
| High | 17.4 | 9.3–25.4 | 12 | 6.8–17.1 | |||
| RRM1 | Low | 21.6 | 14.9–28.4 | 18 | 15–20.9 | 0.005 | |
| High | 9.6 | 5.5–13.7 | 7 | 1–13 | |||
Fig. 2.
Relationship between survival and RRM1 in ovarian cancer
Fig. 3.
Relationship between survival and RRM1 in pancreatic cancer
Fig. 4.
Relationship between survival and ERCC1 in lung cancer
The median survival was 10 months in patients with combined high levels of ERCC1 and RRM1, and it was 18 months in the other patients (p = 0.001).
Discussion
RRM1 and ERCC1 genes are proposed as useful determinants of prognosis as well as drug resistance [1–23]. The rates of recurrence and mortality remain high in NSCLC although it is a first-line treatment [24]. Some studies have shown that patients with a tumours who had low levels of ERCC1 expression in NSCLC had longer survival than those with high level of ERCC1 levels [2, 15]. Some other studies have reported that there was no correlation with ERCC1 and survival when they studied its correlation with response to therapy and survival in advanced stage NSCLC patients treated with gemcitabine and cisplatinum [12].
The present study shows that survival time for NSCLC patients with low levels of ERCC1 expression were lon-ger than for those with higher expression (18 months vs. 9 months). Other studies have shown the median survival period for ERCC1-negative NSCLC patients as 13.7–20 months [2, 4, 15].
In the in vitro study carried out by Bepler et al., it was shown that cells with higher RRM1 expression displayed lower response to cisplatin + gemcitabine, and RRM1 was much more sensitive than ERCC1 with regard to drug resistance in the same study [2]. Rossel et al. was observed that patients receiving cisplatin + gemcitabine with low RRM1 expression had longer survival (13.7 months vs. 3.6 months). The same authors also indicated that longer survival was detected for patients with low expressions of RRM1 and ERCC1 combination compared to those with high expressions [19]. In parallel with scientific literature, the median survival for low RRM1 expression patients was 15 months and for high RRM1 expression patients it was 9 months, in our study.
In NSCLC patients with high RRM1 and ERCC1 expressions, the response rate was only 19%. The results of the in vitro study by Bepler et al. [2] support the findings of the present study.
In the advanced stage (stages III–IV) epithelial ovarian cancer patients treated with cisplatinum therapy, ERCC1-negative patients had longer survival [11, 21].
All the patients with epithelial ovarian cancer who enrolled in our study were advanced-stage (stages III–IV) patients who received platin based chemotherapy. Thirty-three percent of the patients had high levels of ERCC1 expression whereas 67% had low expression. The median survival was 27 and 10 months for patients with low and high level ERCC1, respectively. No significant relationship was observed between ERCC1 and survival. We observed a lower survival than seen in the literature. This may be explained by the small number of patients enrolled in our study.
There is limited number of studies that show ERCC1 and response to platinum in ovarian cancer. In the study performed by Codegoni et al., the relationship between response to platin and the genes playing a role in DNA repair mechanism ERCC1, was investigated and no statistically significant relationship was found between response to therapy and the gene expression. High ERCC1 expression in tissues from ovarian cancer patients who had received platinum-based therapy was associated with treatment resistance in this study showed a threshold of statistical significance and [5].
We found in our study that 90% of the ovarian cancer patients with low levels of ERCC1 expression responded to therapy whereas 10% remained unresponsive. ERCC1 has been found to be responsible for platinum resistance in the studies. We determined that response to therapy was expectedly high in patients with low levels of ERCC1 expression and this result was statistically significant. On the other hand, the small number of patients accounted for the equal treatment response in patients with high levels of ERCC1 expression.
RRM1 has been shown to play a role in resistance to gemcitabine in many studies. However, there are no studies relating with RRM1, response to gemcitabine therapy and survival in epithelial ovarian cancer. There was a significant relationship between RRM2 and survival in the study by Gabriella et al.; however there was no relationship between RRM1 and survival [9]. RRM1 expression was determined by PCR method in the aforementioned study, unlike in our study. Whereas all the patients enrolled in our study were with epithelial serous ovarian cancer, the aforementioned study involved a heterogeneous group with undifferentiated, transparent cell as well as serous adenocarcinoma patients. There was no statistically significant relationship between RRM1 and response to therapy. Besides, the fact that the patients in the study had not received gemcitabine therapy may be the explanation for the statistically insignificant relationship between response to the therapy and RRM1. There was no statistically significant relationship between RRM1 and survival period in ovarian cancer in our study.
Pancreatic cancer has one of the lowest response rates to chemotherapy. Maithel et al. examined ERCC1 expression by immunohistochemical method in 95 patients who had previously had pancreaticoduodenectomy operation. There was an inverse relationship between ERCC1 expression and survival. The median survival period in patients with low ERCC1 expression was 18 months and it was 9 months in patients with high ERCC1 expression (p = 0.01) [20]. The survival rate in our study is consistent with this study; however we did not find any statistically significant relationship. The median survival of the patients with low level of ERCC1 expression was 18 months while it was 12 months in those with high level of expression. We did not find a statistically significant relationship between ERCC1 and the response to the therapy. We found that 71% of patients with low level of ERCC1 expression responded to therapy while 29% remained unresponsive. In the study performed by Kim et al., there was no significant relationship between RRM1 expression and survival [17]. In contrast to the aforementioned study, we found a significant relationship between RRM1 and survival. We found that patients with low level RRM1 expression have longer survival period compared to those with high-level expression. A high level of both ERCC1 and RRM1 in operated pancreas carcinoma patients who had had therapy previously was found to be proportional with the survival (p = 0.006). However, in follow up it was observed that patients with low level RRM1 expression responded to therapy. In our study, we found that 82% of the patients with low-level RRM1 expression responded to therapy while 18% remained unresponsive. On the other hand, 17% of the patients with high level of RRM1 expression responded to the therapy (p = 0.018). All of the pancreas cancer patients in our study received gemcitabine-based therapy. Our study was in parallel with similar studies in which there was a significant relationship between RRM1 and gemcitabine as well as response to the therapy.
In conclusion, the results of the current study suggest an association between ERCC1 in lung and ovarian cancers, and RRM1 in lung and pancreatic cancers. ERCC1 show the patients who would benefit from cisplatin which can be determined by immunohistochemistry, alone or in combination with RRM1. We also demonstrated that both expressions should be evaluated prior to therapy.
The authors would like to thank Yasar Sertdemir for statistical advice.
The authors declare no conflict of interest.
References
- 1.Akita H, Zheng Z, Takeda Y, et al. Significance of RRM1 and ERCC1 expression in resectable pancreatic adenocarcinoma. Oncogene. 2009;28:2903–9. doi: 10.1038/onc.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bepler G, Kusmartseva I, Sharma S, Gautam A, Cantor A, Sharma A, Simon G. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol. 2006;24:4731–7. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 3.Bergman AM, Eijk PP, Ruiz van Haperen VW, et al. In vivo induction of resistance to gemcitabine results in increased expression of ribonucleotide reductase subunit M1 as the major determinant. Cancer Res. 2005;65:9510–6. doi: 10.1158/0008-5472.CAN-05-0989. [DOI] [PubMed] [Google Scholar]
- 4.Booton R, Ward T, Ashcroft L, Morris J, Heighway J, Thatcher N. ERRC1 mRNA expression is not associated with response and survival after platinum-based chemotherapy regimens in advanced non-small cell lung cancer. J Clin Oncol. 2007;2:902–6. doi: 10.1097/JTO.0b013e318155a637. [DOI] [PubMed] [Google Scholar]
- 5.Codegoni AM, Broggini M, Pitelli MR, Pantarotto M, Torri V, Mangioni C, D'Incalci M. Expression of genes of potential importance in the response to chemotherapy and DNA repair in patients with ovarian cancer. Gynecol Oncol. 1997;65:130–7. doi: 10.1006/gyno.1996.4609. [DOI] [PubMed] [Google Scholar]
- 6.Davidson JD, Ma L, Flagella M, Geeganage S, Gelbert LM, Slapak CA. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small-cell lung cancer cell lines. Cancer Res. 2004;64:3761–6. doi: 10.1158/0008-5472.CAN-03-3363. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Furuta T, Ueda T, Aune G, Sarasin A, Kraemer KH, Pommier Y. Transcription-coupled nucleotide excision repair as determinant of cisplatin sensitivity of human cells. Cancer Res. 2002;62:4899–902. [PubMed] [Google Scholar]
- 9.Ferrandina G, Mey V, Nannizzi S, et al. Expression of nucleoside transporters, deoxycitidine kinase, ribonucleotide reductase regulatory subunits, and gemcitabine catabolic enzymes in primary ovarian cancer. Cancer Chemother Pharmacol. 2010;65:679–86. doi: 10.1007/s00280-009-1073-y. [DOI] [PubMed] [Google Scholar]
- 10.Heinemann V, Xu YZ, Chubb S, Sen A, Hertel LW, Grindey GB, Plunkett W. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2’,2’-difluorodeoxycytidine. Mol Pharmacol. 1990;38:567–72. [PubMed] [Google Scholar]
- 11.Weberpals J, Garbuio K, O'Brien A, Clark-Knowles K, Doucette S, Antoniouk O, Goss G, Dimitroulakos J. The DNA repair proteins BRCA1 and ERCC1 as predictive markers in sporadic ovarian cancer. Int J Cancer. 2009;124:806–15. doi: 10.1002/ijc.23987. [DOI] [PubMed] [Google Scholar]
- 12.Lord RV, Brabender J, Gandara D, et al. Low level ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8:2286–91. [PubMed] [Google Scholar]
- 13.Dabholkar M, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J Clin Invest. 1994;94:703–8. doi: 10.1172/JCI117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17(Suppl 5):7–12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 15.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 16.Perez RP. Cellular and molecular determinants of cisplatin resistance. Eur J Cancer. 1998;34:1535–42. doi: 10.1016/s0959-8049(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 17.Kim R, Tan A, Lai KK, Jiang J, Wang Y, Rybicki LA, Liu X. Prognostic roles of human equilibrative transporter 1 (hENT-1) and ribonucleoside reductase subunit M1 (RRM1) in resected pancreatic cancer. Cancer. 2011;117:3126–34. doi: 10.1002/cncr.25883. [DOI] [PubMed] [Google Scholar]
- 18.Richardson A, Kaye SB. Drug resistance in ovarian cancer: the emerging importance of gene transcription and spatio-temporal regulation of resistance. Drug Resist Updat. 2005;8:311–21. doi: 10.1016/j.drup.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Rosell R, Felip E, Taron M, et al. Gene expression as a predictive marker of outcome in stage IIB–IIIA–IIIB non-small-cell lung cancer after induction gemcitabine-based chemotherapy followed by resectional surgery. Clin Cancer Res. 2004;10(12 Pt 2):4215–9. doi: 10.1158/1078-0432.CCR-040006. [DOI] [PubMed] [Google Scholar]
- 20.Maithel SK, Coban I, Kneuertz PJ, et al. Differential expression of ERCC1 in pancreas adenocarcinoma: high tumor expression is associated with earlier recurrence and shortened survival after resection. Ann Surg Oncol. 2011;18:2699–705. doi: 10.1245/s10434-011-1610-x. [DOI] [PubMed] [Google Scholar]
- 21.Scheil-Bertram S, Tylus-Schaaf P, du Bois A, Harter P, Oppitz M, Ewald-Riegler N, Fisseler-Eckhoff A. Excision repair cross-complementation group 1 protein overexpression as a predictor of poor survival for high-grade serous ovarian adenocarcinoma. Gynecol Oncol. 2010;119:325–31. doi: 10.1016/j.ygyno.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Mojgan H, Massoud H, Ahmad E. ERCC1 intron 1 was associated with breast cancer risk. Arch Med Sci. 2012;8:655–8. doi: 10.5114/aoms.2012.30289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (verion 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Eldeeb H, Reza S. Northampton outcome for first and second line chemotherapy in non small cell lung cancer: 5 years data. Contemp Oncol (Pozn) 2012;16:420–3. doi: 10.5114/wo.2012.31772. [DOI] [PMC free article] [PubMed] [Google Scholar]