SUMMARY
Serial phlebotomy was performed on sixty children with sickle cell anaemia, stroke and transfusional iron overload randomized to hydroxycarbamide in the Stroke With Transfusions Changing to Hydroxyurea trial. There were 927 phlebotomy procedures with only 33 adverse events, all of which were grade 2. Among 23 children completing 30 months of study treatment, the net iron balance was favourable (−8.7 mg Fe/kg) with significant decrease in ferritin, although liver iron concentration remained unchanged. Therapeutic phlebotomy was safe and well-tolerated, with net iron removal in most children who completed 30 months of protocol-directed treatment.
Keywords: sickle cell, iron overload, phlebotomy, liver iron
INTRODUCTION
Stroke With Transfusions Changing to Hydroxyurea (SWiTCH, ClinicalTrials.gov NCT00122980), a National Heart, Lung and Blood Institute-sponsored Phase III multicentre trial, compared chronic blood transfusions with chelation (Standard Arm) to hydroxycarbamide (alos known as hydroxyurea) with phlebotomy (Alternative Arm) for reduction of recurrent stroke and improvement in iron overload management in children with sickle cell anaemia (SCA) and stroke (Ware et al, 2011). SWiTCH was terminated due to statistical futility and stroke rate differences (Ware & Helms, 2012). We describe the SWiTCH phlebotomy experience including feasibility, success rates, adverse events and effects on net iron balance and liver iron stores.
METHODS
Children between 5.0 and 18.9 years of age with SCA and previous stroke were eligible for enrollment if they had received ≥18 months of transfusions and had iron overload (Ware et al, 2011). Subjects with baseline liver iron concentration (LIC) ≥5 mg Fe/g dry weight liver were eligible for randomization; those on the Alternative Arm commenced hydroxycarbamide at 20 mg/kg/day while continuing transfusions for a short overlap period, to provide protection against recurrent stroke during hydroxycarbamide dose escalation to maximum tolerated dose (MTD). Transfusion volumes provided during this overlap period were progressively decreased every 8 weeks, while the hydroxycarbamide dose was increased by 5 mg/kg/day increments every 8 weeks to the MTD (Zimmerman et al, 2004; Heeney & Ware, 2008). During this overlap period, the subjects did not receive chelation therapy. Once hydroxycarbamide MTD was reached, transfusions were discontinued and the subjects started serial phlebotomy. Quantitative LIC (Mayo Laboratories, Rochester MN) was measured by liver biopsy at study entry and exit, and serum ferritin was monitored monthly.
Therapeutic phlebotomy was performed every 4±1 weeks. The initial procedure was performed at 5 ml/kg. If well tolerated, subsequent phlebotomy volumes were increased to 10 ml/kg, with a maximum volume of 500 ml removed at any procedure when Hb ≥80 g/l. For Hb concentration of 70-79 g/l, the phlebotomy volume was decreased to 5 ml/kg, and phlebotomy was not performed if Hb was <70 g/l or when protocol-defined haematological toxicities were present. Phlebotomy was performed over 30-45 min followed by replacement with an equal volume of normal saline.
Iron loading was calculated using the actual volume of each transfusion as follows: [(volume of transfusion in l) × (220 g Hb/l) × (3.4 mg Fe/g Hb)] / (subject's weight in kg). For phlebotomies, iron unloading was based on the actual blood volume removed and the subject's Hb on the day of phlebotomy: [(volume of phlebotomy in l) × (subject's Hb concentration in g/l) × (3.4 mg Fe/g Hb)] / (subject's weight in kg).
Descriptive statistics (mean, standard deviation, median and range) were performed for demographic variables, transfusion history, chelation history and baseline laboratory values. T-tests were used to examine the relationship of baseline LIC and serum ferritin levels and change in iron load. Associations between continuous variables were evaluated using the Spearman correlation coefficient. P-values less than 0.05 were considered statistically significant, no multiple comparisons adjustments were made and all analyses were conducted using SAS Version 9.2 (SAS, Cary, NC) and STATA Version 13.0 (STATA, College Station, TX).
RESULTS
Sixty-seven subjects (mean age 13.0±4.0 years; range 5.2-19.0 years; 61% male) were randomized to the Alternative Treatment Arm (Ware & Helms, 2012). The average duration of previous transfusion therapy was 7.4±3.8 years (median 7.6 years, range 1.5-15.5 years). Most children had previously received chelation: 47 (71%) with deferoxamine for 4.8±3.2 years and 57 (86%) with deferasirox for 1.5±0.8 years prior to study enrollment (Kwiatkowski et al, 2011).
Sixty of 67 subjects successfully escalated hydroxycarbamide to MTD and then initiated phlebotomy after receiving an average of 8±3 overlap transfusions. The remaining 7 did not transition to phlebotomy due either to hydroxycarbamide non-adherence (N=5), early study termination (N=1), or stroke recurrence during the overlap (N=1). A total of 23 subjects on the Alternative Treatment Arm (38% of the 60 receiving phlebotomy) completed the prescribed 30 months and received an exit liver biopsy; among the remaining 37, 26 were still on treatment when SWiTCH was closed, 6 had an adjudicated recurrent stroke and 4 others withdrew. The last subject never discontinued transfusions due to poor hydroxycarbamide adherence; her data were excluded from iron loading/unloading analyses because she never received phlebotomy.
A total of 927 therapeutic phlebotomy procedures were performed on 60 subjects (mean 15±8, median 16, range 1-28 procedures per subject). Of those, 909 (98%) were performed at protocol-directed volumes including 741 at 10 ml/kg or 500 ml, 108 at 5 ml/kg for Hb 70-79 g/l, and 59 at 5 ml/kg per protocol for the first scheduled phlebotomy. Only 26 (3%) of phlebotomy procedures stopped before the scheduled volume was removed, typically from loss of venous access. Phlebotomy removed an average of 127±74 ml/kg blood, representing 8.5 ml/kg of blood removed per procedure. The 23 children who completed 30 months of study treatment received an average of 23 phlebotomy procedures, removing 193.3±46.7 ml/kg blood. There were 119 off-protocol transfusions administered during the phlebotomy phase, including 52 given to 5 children with medication non-compliance and 37 during the exit window, most commonly before general anaesthesia for end-of-study liver biopsy or brain magnetic resonance imaging/angiogram.
There were 33 adverse events (AEs, 3.5% prevalence) associated with phlebotomy reported in 12 subjects. All 33 AEs were Grade 2 and self-limited, with no serious adverse events (SAE). The most common AE was hypotension (9 events in 5 subjects) followed by dizziness (3), syncope (3), headache (2) and weakness (2).
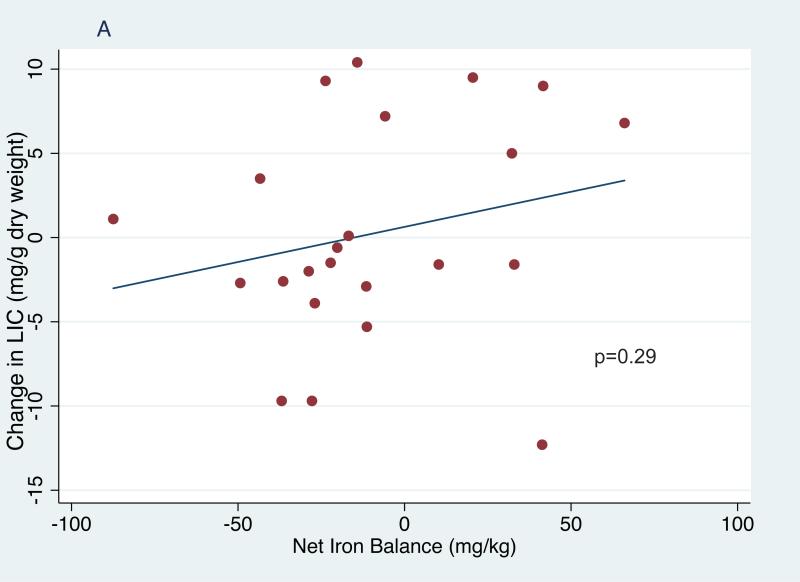
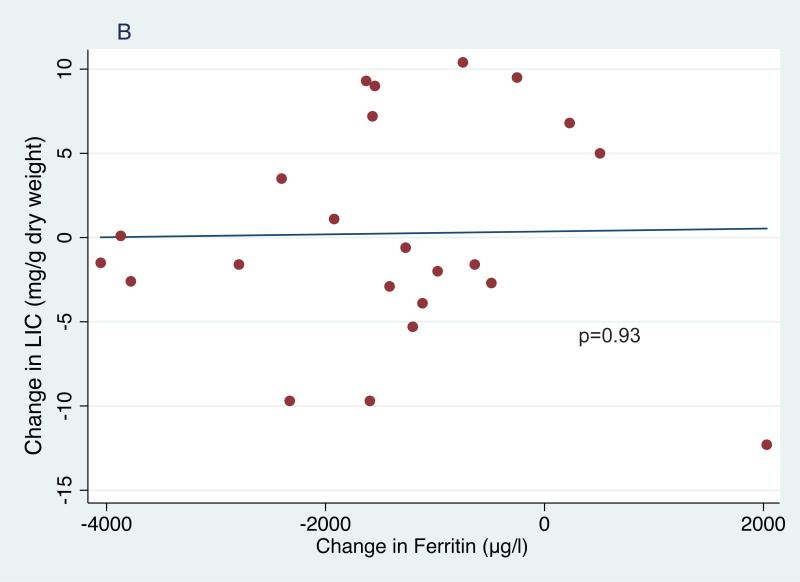
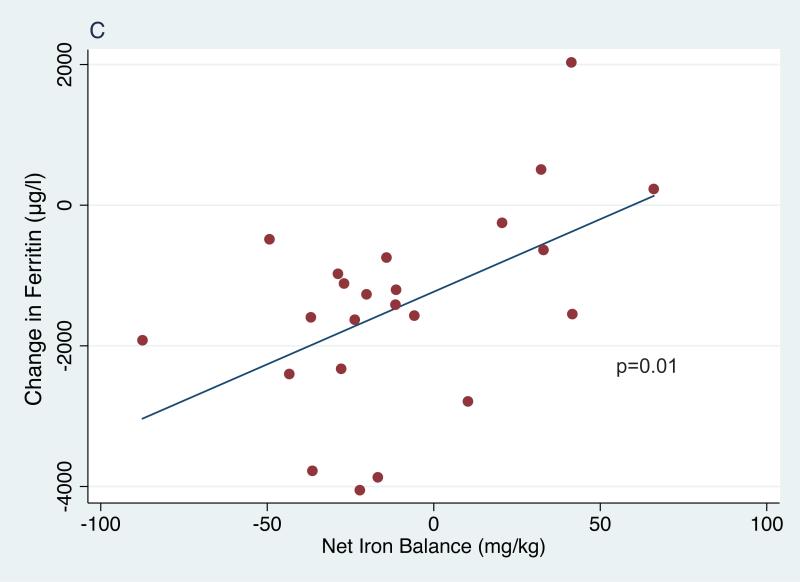
In the entire cohort of children receiving phlebotomy, serum ferritin decreased significantly from 3272±1587 μg/l at study entry to 2772±1564 μg/l at phlebotomy initiation (p=0.007) and then further to 2097±1631 μg/l at study exit (p<.001 entry to exit, p<0.001 first phlebotomy to exit,Table I). In the 23 subjects who completed treatment, serum ferritin decreased significantly from 3462±1387 μg/l at entry to 2793±1595 μg/l when starting phlebotomy (p=0.01) and then further to 2036±1838 μg/l at exit (p<0.0001 entry to exit, p=0.001 first phlebotomy to exit, Table I). In this smaller cohort, average net iron balance was −8.7 mg Fe/kg (range −83.4 to +67.5 mg Fe/kg); the highly positive values reflect 2 subjects who restarted chronic transfusions for non-adherence to hydroxycarbamide. However, their overall average LIC was unchanged, from an entry value of 18.0±9.1 mg Fe/g dry weight liver (median 15.9 range 6.4-42.4) to 18.2±10.8 mg Fe/g at exit (median: 17.2, range: 2.5-38.2, p=0.86). These inconsistencies between iron balance and change in LIC raise concerns about the accuracy and validity of liver biopsy as a marker of total body iron burden. There was no association between net change in LIC and calculated net iron change (Figure 1A) or change in ferritin (Figure 1B). However, changes in the ferritin levels correlated with net iron balance (p=0.01; Figure 1C). Among univariate models examined with regression analyses (age, sex, duration of transfusions, ferritin and LIC), only baseline ferritin was significantly associated with change in LIC (p=0.04).
TABLE I.
Iron loading and unloading in 23 subjects who completed 30 months of study treatment.
| Transfusion volume (ml/kg) | Phlebotomy volume (ml/kg) | Iron Load (mg/kg) | Iron Unload (mg/kg) | Net Iron Balance (mg/kg) | Entry Ferritin (μg/l) | Exit Ferritin (μg/l) | Entry LIC (mg/g dwl) | Exit LIC (mg/g dwl) | Delta LIC (mg/g dwl) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54.2 | 205.6 | 40.5 | 60.7 | −20.2 | 1779 | 511 | 15.9 | 15.3 | −0.6 |
| 2 | 29.5 | 188.5 | 22.1 | 65.5 | −43.4 | 3484 | 1083 | 12.2 | 15.7 | 3.5 |
| 3 | 47.6 | 208.1 | 35.6 | 62.6 | −27.0 | 1467 | 353 | 6.4 | 2.5 | −3.9 |
| 4 | 57.4 | 203.0 | 43.0 | 70.8 | −27.8 | 2789 | 462 | 14.6 | 4.9 | −9.7 |
| 5 | 54.2 | 172.5 | 40.5 | 54.7 | −14.2 | 4105 | 3360 | 27.8 | 38.2 | 10.4 |
| 6 | 73.3 | 127.4 | 54.8 | 34.3 | 20.5 | 3064 | 2813 | 23.5 | 33 | 9.5 |
| 7 | 42.7 | 143.4 | 32.0 | 43.3 | −11.3 | 3568 | 2365 | 23.2 | 17.9 | −5.3 |
| 8 | 53.7 | 200.4 | 39.2 | 62.9 | −23.7 | 2797 | 1168 | 22.4 | 31.7 | 9.3 |
| 9 | 22.1 | 204.4 | 16.6 | 65.9 | −49.3 | 1091 | 606 | 12.5 | 9.8 | −2.7 |
| 10 | 61.2 | 161.6 | 45.8 | 51.6 | −5.8 | 6133 | 4562 | 27.1 | 34.3 | 7.2 |
| 11 | 96.8 | 117.2 | 67.8 | 35.5 | 32.2 | 4908 | 5415 | 13.4 | 18.4 | 5 |
| 12 | 58.7 | 210.0 | 43.9 | 66.1 | −22.2 | 5318 | 1265 | 11.4 | 9.9 | −1.5 |
| 13 | 61.2 | 245.2 | 45.8 | 74.6 | −28.8 | 1651 | 675 | 8.9 | 6.9 | −2 |
| 14 | 100.5 | 153.3 | 75.2 | 42.2 | 33.0 | 3346 | 2709 | 21.1 | 19.5 | −1.6 |
| 15 | 50.2 | 222.6 | 37.5 | 74.0 | −36.4 | 4092 | 314 | 7.5 | 4.9 | −2.6 |
| 16 | 115.4 | 143.6 | 86.3 | 44.7 | 41.6 | 4381 | 2832 | 8.2 | 17.2 | 9 |
| 17 | 77.5 | 216.0 | 58.0 | 69.5 | −11.5 | 3676 | 2261 | 33.5 | 30.6 | −2.9 |
| 18 | 94.5 | 199.6 | 70.7 | 60.4 | 10.3 | 3935 | 1144 | 9.4 | 7.8 | −1.6 |
| 19 | 60.4 | 224.2 | 48.6 | 65.4 | −16.8 | 4338 | 468 | 10 | 10.1 | 0.1 |
| 20 | 20.6 | 269.4 | 15.4 | 102.9 | −87.5 | 2448 | 527 | 22 | 23.1 | 1.1 |
| 21 | 116.7 | 146.3 | 87.3 | 46.0 | 41.3 | 3106 | 5136 | 42.4 | 30.1 | −12.3 |
| 22 | 71.0 | 240.0 | 36.4 | 73.3 | −36.9 | 2048 | 453 | 19.7 | 10 | −9.7 |
| 23 | 144.3 | 138.7 | 107.9 | 41.8 | 66.1 | 6108 | 6338 | 19.8 | 26.6 | 6.8 |
LC, liver iron concentration; dwl, dry weight of liver
Figure 1.
Correlations of net iron balance with change in liver iron concentration (LIC) (1A), change in ferritin and LIC (1B) and change in net iron balance with change in ferritin (1C).
DISCUSSION
SWiTCH provides a large prospective data set concerning the feasibility, safety and benefits of serial phlebotomy for removing transfusional iron burden in children with SCA. Our experience suggests phlebotomy is both safe and feasible, even when performed in anaemic children with previous stroke. Almost all scheduled phlebotomy procedures occurred at the prescribed volume; the rate of AE was quite low with no SAE.
The effects of repeated phlebotomy on iron burden were reflected in significantly lower serum ferritin (Figure 1C). The most important question, and also the primary reason for premature termination of SWiTCH, is why serial phlebotomy did not significantly decrease LIC in children randomized to the Alternative Treatment Arm. The actual overlap period, plus occasional unscheduled transfusions, added 49 mg Fe/kg, substantially more iron loading than the predicted 35 mg/kg. Moreover, actual phlebotomy procedures removed only 60 mg Fe/kg among 23 children who completed the phlebotomy phase, substantially less than the 82 mg/kg iron unloading predicted by the protocol. Additional contributors to reduced iron unloading include age and weight of the SWiTCH subjects, which limited their phlebotomy volumes to 500 ml. Together, these help explain why net iron unloading in the Alternative Arm was less than predicted, but do not explain why negative iron balance among subjects completing the study was not accompanied by LIC reduction.
Phlebotomy for transfusional iron overload can use an intensive schedule (Angelucci et al 1997, Franchini et al, 2004), and with longer duration, can normalize iron stores in children with SCA and stroke (Ware et al, 1999; Ware et al, 2004), and then maintain low iron burden (Greenway et al, 2011). The lack of observed LIC change probably relates to the inherent inaccuracy of biopsy-guided LIC measurements, probably due to sampling errors, because no significant associations were observed between net iron balance and LIC change. The SWiTCH experience documents that therapeutic phlebotomy is well-tolerated in children with SCA, stroke and iron overload. Serial blood removal was feasible and safe, and the data suggest this approach can be effective treatment for selected patients with transfusional haemosiderosis who discontinue transfusions, particularly if phlebotomy is provided for an extended period of time.
ACKNOWLEDGEMENTS
We thank the SWITCH personnel at the participating clinical centres listed in the Appendix, as well as the children and their families for their commitment. The SWiTCH Clinical Trial was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health (grants U01-HL078787 to REW and U01-HL078987 to Ronald W. Helms at Rho) and Novartis Inc. for donation of deferasirox for study participants on the Standard Treatment Arm.
APPENDIX
SWiTCH Trial investigators and key contributors include the following: Baylor College of Medicine, Houston, TX (Brigitta U. Mueller, MD, Bogdan Dinu, MD); Boston Children's Hospital, Boston, MA (Matthew Heeney, MD, Meredith Anderson, Amber Smith, Tiffany Kang); Children's Hospital of Philadelphia, Philadelphia, PA (Janet L. Kwiatkowski, MD, MSCE, Kim Smith-Whitley, MD, PhD, Jeffrey Olson, MSPH, Vanessa Nixon, LaVerne Murphy); Children's Hospital of Pittsburgh at UPMC, Pittsburgh, PA (Lakshmanan Krishnamurti, MD, Regina McCollum, BSN, RN); Children's Memorial Hospital, Chicago, IL (Alexis Thompson, MD, MPH, Jillian Prado, Kelly Verel, CCRC, Ashley Brummel); Children's Mercy Hospital, Kansas, MO (Gerald Woods, MD, Julie Routhieaux, RN, PCNS, Anne Mehrhof, RN, MSN, CCRC, Kristin Stegenga, PhD, RN); Children's National Medical Center, Washington, DC (Lori Luchtman-Jones, MD, Caterina Minniti, MD, Sheronda D. Brown, MBA, MHCM, Tracy Boswell, MBA, Ryan Burnett); Children's Hospital Medical Center, Cincinnati, OH (Karen Kalinyak, MD, Laurie Vanderah, RN, Monique Lumpkin, MSW, Tammy Nordheim, BSN, RN, OCN, CCRP); Cohen Children's Medical Center of NY, New Hyde Park, NY (Sharon Singh, MD, Ellen Muir, RN); Columbia University, New York, NY (Margaret T. Lee, MD, Genia Billote, RN, MPH, MFA); East Carolina University, Greenville, NC (Charles Daeschner, MD, Diana Wyn Gordon, RN, MSN, CPON, Cynthia Brown, CCRP); Eastern Virginia Medical School, Norfolk, VA (William Owen, MD, Anthony Villella, MD, Terri Forsyth, PNP, Annette Slade, PNP, Lorrie Coggsdale, RN); Emory University/Children's Healthcare of Atlanta, Atlanta, GA (Clark Brown, MD, PhD, Ifeyinwa Osunkwo, MD, Peter Lane, MD, Brian Philbrook, MD, Terrell Faircloth, Korin Cherry, Elizabeth Record, CPNP, DNP, Eldrida Carter Randall, Ann Martha Felder, James Rhodes, PharmD); Cincinnati Medical College of Wisconsin, Milwaukee, WI (J. Paul Scott, MD, Danielle Jirovec, Lindsey Nelson); Medical University of South Carolina, Charleston, SC (Sherron M. Jackson, MD, Mary Ellen Cavalier, MD, Lisa Kuisel, RN, Jessica Peterson, RN, Betsy Rackoff); Nemours Children's Clinic, Jacksonville, FL (Cynthia Gauger, MD, Mary Warde, RN ,BSN, CCRC), Nemours Children's Clinic, Orlando, FL (Ramamoorthy Nagasubramanian, MD, Leslie Natal, Dawn Cook, RN, BSN,); St. Joseph's Children's Hospital, Paterson, NJ (Rafael Barilari, MD, JoAnne Neville, CCRP); St. Jude Children's Research Hospital, Memphis, TN (Banu Aygun, MD, Winfred C. Wang, MD, Eileen Hansbury, PA-C, Jennifer Larkin, Laura Martino, Lane Faughnan); SUNY-Downstate Medical Center, Brooklyn, NY (Scott Miller, MD, Kathy Rey, PAC, Lezlie Woods, RN); The Children's Hospital at Montefiore, Bronx, NY (Catherine Driscoll, MD, Lakeisha N. Nicholls, MA, RN, CPNP); The University of Texas Southwestern, Dallas, TX (Zora R. Rogers, M.D., Leah Adix, CCRP, Roxana Mars, RN, Brad Cook, RN, Jennifer Marshall, RN); University of Alabama, Birmingham, AL (Lee Hilliard, MD, Jeanine Dumas, Lindley Webb, Annelle Reed, MSN, CRNP); University of Miami, Miami, FL (Ofelia Alvarez, MD, Tally Hustace, ARNP, Patrice Williams, RN, Mary Donovan, RN); University of Mississippi Medical Center, Jackson, MS (Rathi Iyer, MD, Mary Gail Smith, MD, Stephanie Pepper, RN, Glenda Thomas, RN, Gloria Bishop, RN, Cindy Kendig, RN, Teresa Walker, RN); Vanderbilt University, Nashville, TN (Elizabeth Yang, MD, PhD, Lesley Ann Owen, RN, BSN, MSN); Wayne State University, Detroit, MI (Sharada Sarnaik, MD, Mary Murphy, Cynthia Burnett, Clarissa Shavers, PhD)
Consultants and Supporting Staff:
Robert Adams, MD, MS; Steven Pavlakis, MD; E. Steve Roach, MD; Corinne Hilbert; Judy Luden; Melanie Bonner, PhD; Alan Cohen, MD; Kathleen Helton, MD; Noah Sabin, MD; Jeff Creasy, MD; Zoltan Patay, MD, PhD; Fred Laningham, MD; Fred Hoffer, MD; Thad Howard, MS; Abdullah Kutlar, MD; Niren Patel, MBBS; Naomi Luban, MD; Beth McCarville, MD; Nicole Mortier, MHS, PA-C; Julie Richardson, PharmD, BCPS; Pamela Sylvestre, MD
Statistics and Data Management Center (SDMC):
Ronald W. Helms, PhD; Nancy Yovetich, PhD; Kevin Clark, MS; Julian Garro, MS; Allison Fowlkes; Wendy McBane; Megan Hsu; Danielle Boulet; Beth Olen; Ann Flaherty, CCRP; Jamie Spencer, CCRP; Christopher Woods; Karyn Mumma; Jennifer Stasiak
Medical Coordinating Center Supporting Staff:
Russell E. Ware, MD PhD; Joyce Banton, CCRP; Paul Eddlemon; Christina Radcliffe, CPhT, CCRP; William Schultz MHS, PA-C
Footnotes
A complete list of the members of the SWiTCH Trial appears in Appendix 1.
COMPETING INTERESTS: The authors have no competing interests
- BA, NAM, WHS, ARC,OA, ZRR, JLK, STM, PS, RI, PAL, REW and the SWiTCH Trial investigators performed the research
- REW, NAM, WHS, BA designed the research study
- BA, NAM, KK, AL, REW analysed the data
- BA, REW, NAM, KK, AL, WHS, ARC,OA, ZRR, JLK, STM, PS, RI, PAL wrote the paper.
REFERENCES
- Angelucci E, Muretto P, Lucarelli G, Ripalti M, Baronciani D, Erer B, Galimberti M, Giardini C, Gaziev D, Polchi P. Phlebotomy to reduce iron overload in patients cured of thalassemia by bone marrow transplantation. Italian Cooperative Group for Phlebotomy Treatment of Transplanted Thalassemia Patients. Blood. 1997;90:994–998. [PubMed] [Google Scholar]
- Franchini M, Gandini G, Veneri D, de Matteis G, Federici F, Solero P, Aprili G. Efficacy and safety of phlebotomy to reduce transfusional iron overload in adult, long-term survivors of acute leukemia. Transfusion. 2004;44:833–837. doi: 10.1111/j.1537-2995.2004.03264.x. [DOI] [PubMed] [Google Scholar]
- Greenway A, Ware RE, Thornburg CD. Long-term results using hydroxyurea/phlebotomy for reducing secondary stroke risk in children with sickle cell anemia and iron overload. American Journal of Hematology. 2011;86:357–361. doi: 10.1002/ajh.21986. [DOI] [PubMed] [Google Scholar]
- Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Pediatric Clinics of North America. 2008;55:483–501. doi: 10.1016/j.pcl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski JL, Cohen AR, Garro J, Alvarez O, Nagasubramanian R, Sarnaik S, Thompson A, Woods GM, Schultz W, Mortier N, Lane P, Mueller B, Yovetich N, Ware RE, for the SWiTCH Study Investigators Transfusional iron overload in children with sickle cell anemia on chronic transfusion therapy for secondary stroke prevention. American Journal of Hematology. 2012;87:221–223. doi: 10.1002/ajh.22228. [DOI] [PubMed] [Google Scholar]
- Ware RE, Helms RW. Stroke with transfusions changing to hydroxyurea (SWiTCH). Blood. 2012;119:3925–3932. doi: 10.1182/blood-2011-11-392340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware RE, Zimmerman SA, Schultz WH. Hydroxyurea as an alternative to blood transfusions for the prevention of recurrent stroke in children with sickle cell disease. Blood. 1999;94:3022–6. [PubMed] [Google Scholar]
- Ware RE, Zimmerman SA, Sylvestre PB, Mortier NA, Davis JS, Treem WR, Schultz WH. Prevention of secondary stroke and resolution of transfusional iron overload in children with sikle cell anemia using hydroxyurea and phlebotomy. The Journal of Pediatrics. 2004;145:346–352. doi: 10.1016/j.jpeds.2004.04.058. [DOI] [PubMed] [Google Scholar]
- Ware RE, Schultz WH, Yovetich N, Mortier NA, Alvarez O, Hilliard L, Iyer RV, Miller ST, Rogers ZR, Scott JP, Waclawiw M, Helms RW. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH): a phase III randomized clinical trial for treatment of children with sickle cell anemia, stroke, and iron overload. Pediatric Blood Cancer. 2011;57:1011–1017. doi: 10.1002/pbc.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, Ware RE. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103:2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]