Abstract
Studies on the evolution of female preference and male color polymorphism frequently focus on single species since traits and preferences are thought to co-evolve. The guppy, Poecilia reticulata, has long been a premier model for such studies because female preferences and orange coloration are well known to covary, especially in upstream/downstream pairs of populations. However, focused single species studies lack the explanatory power of the comparative method, which requires detailed knowledge of multiple species with known evolutionary relationships. Here we describe a red color polymorphism in Poecilia picta, a close relative to guppies. We show that this polymorphism is restricted to males and is maintained in natural populations of mainland South America. Using tests of female preference we show female P. picta are not more attracted to red males, despite preferences for red/orange in closely related species, such as P. reticulata and P. parae. Male color patterns in these closely related species are different from P. picta in that they occur in discrete patches and are frequently Y chromosome-linked. P. reticulata have an almost infinite number of male patterns, while P. parae males occur in discrete morphs. We show the red male polymorphism in P. picta extends continuously throughout the body and is not a Y-linked trait despite the theoretical prediction that sexually-selected characters should often be linked to the heterogametic sex chromosome. The presence/absence of red male coloration of P. picta described here makes this an ideal system for phylogenetic comparisons that could reveal the evolutionary forces maintaining mate choice and color polymorphisms in this speciose group.
Introduction
Understanding the forces maintaining within species color polymorphisms has important implications across the fields of both ecology and evolutionary biology. Variation in coloration patterns are often associated with differences in behavior such as mating strategies (e.g. Poecilia parae [1,2], bluegill sunfish [3], side-blotched lizards [4]), microhabitat choice [5], and thermal regulation (e.g. land snails [6], and spittle bugs [7]). It has been suggested that these differences can lead to speciation across populations in parapatry, peripatry and even (although more controversially) sympatry (reviewed in [8]). Currently, most studies of color polymorphism focus on one species at a time, thus these studies lack the power of a phylogenetic approach when asking questions about the effects of color polymorphism on diversification. Wielding the power of phylogenetic analyses requires studying several species with well-characterized traits in a clade for which evolutionary relationships are well known [9].
The family Poeciliidae is a speciose group of small tropical freshwater fishes that has been well studied for the last 100 years and for which phylogenetic relationships are well known [10]. Sex specific color polymorphisms are especially well characterized for 2 species of Poeciliid, Poecilia parae [1], and Poecilia reticulata (reviewed in [11]). In both P. reticulata and P. parae males show color polymorphisms that play a large role in female mate choice (P. reticulata: [11,12], P. parae [13]) but attract unwanted attention from predators [13, 14, 15]. When there are sex-specific benefits to coloration, but costs to all carriers, this creates sexual conflict over the expression of color polymorphism [16]. This conflict is frequently resolved through linkage to heterogametic sex chromosomes [16, 17]. Male coloration in both P. reticulata and P. parae follow this pattern and have strong Y-linked components [1,18].
The guppy, Poecilia reticulata, is one of the premier models for studying the balance between sexual and natural selection (reviewed in [11, 19]). Males are much more colorful than females, and bear such an immense variety of colors and patterns that many populations have no two males that look identical [11]. Why this is so has been the subject of intense study for nearly one hundred years but these studies have largely lacked the strength of phylogenetic approaches.
Poecilia parae, a close relative of P. reticulata, also possesses extreme male color polymorphism [1] which has been shown to represent a balance between natural and sexual selection [2]. Interestingly, P. parae is often found in sympatry with guppies, yet male polymorphism is restricted to one of five discrete morphs: four color morphs (displaying body and tail stripes of red, yellow or blue, outlined in black, or only a multi-color tail stripe) and one juvenile-like morph [1], in contrast to the almost infinite number of male coloration patterns observed in guppies.
Another closely related species of Poeciliid is frequently found in sympatry with both P. reticulata and P. parae in northeast South America, including Guyana, Venezuela, and Suriname: Poecilia picta, also known as the swamp guppy. P. picta and P. parae are part of a clade that comprises the sister taxon to the guppy [20], within the subgenus Lebistes sensu Rosen and Bailey 1963 [21]. This sister taxon has been described as the genus Micropoecilia [22], but this is inconsistent with the standard taxonomy of the genus Poecilia as described by Rosen and Bailey [21]. Sexual color dimorphism has been described in P. picta from South America and Trinidad with males possessing an orange and black stripe on the top of their caudal fin and a spot on their dorsal fin [23]. Female mate choice appears to play an important role in P. picta as males display for females and females either accept or reject them [23, 24]. While orange plays a dramatic role in female mate choice in P. reticulata (reviewed in [11, 19]) conspicuous orange coloration on the caudal fin does not appear to play a role in female preference in P. picta [24].
Here we report for the first time a striking red and a gold color sex-specific polymorphism in male P. picta from the mainland of South America that has persisted across a series of field surveys spanning 11 years. Previous studies of P. picta from the island of Trinidad suggest the absence of this color polymorphism in that island population. This red coloration can extend over most of the body of the male (Fig 1). In order to compare this to P. reticulata and P. parae male coloration, we investigate the inheritance pattern of the red morph through inbred lines maintained in the laboratory. Since natural selection via predation against red/orange morphs is countered by sexual selection via female mate choice in both of the close relatives, P. reticulata and P. parae, we used dichotomous choice tests to look for a female preference for the red color morph in P. picta.
Fig 1. Standard (top), fully red (mid) and partially red (bottom) morph of P. picta from Georgetown, Guyana.

Materials and Methods
Field surveys
Poecilia picta has been reported to occur in Guyana, French Guiana, Brazil and Trinidad and Tobago [21, 25, 26]. We have anecdotally observed the presence of the red morph within P. picta populations in Suriname, Guyana and Venezuela but not in Trinidad on collecting trips from 1983 to 2011. To quantify the frequency of the red morph, we searched for P. picta near the coast in ditches draining into rivers in four separate sets of field surveys in Guyana (June–August 1999; November 2000; January–February 2002; July 2010) and one field survey in Venezuela. In Guyana, P. picta were only found in mud-bottomed ditches of a variety of sizes which served as sewerage outlets. We considered different drainage systems into a river as different sampling sites. Adult males and females were captured with dip nets or a 3 x 1.5 m seine net, and male color pattern was scored. At the end of each sampling period, fish were either released or taken into captivity.
Investigation of the genetic basis of the red color morph
Male and female P. picta were transported to Simon Fraser University, Canada from Georgetown, Guyana. We tested whether the loci underlying the red color morph conform to predictions of Y-linkage in 5 inbred lines founded by offspring of wild caught field-inseminated females. Two lines were started with red males and red male offspring were used as sires for the next generation, and the same for three lines of standard males. Full sibling crosses were set up in each generation for 2–4 generations per inbred line. In each generation, the number of red vs. standard males was scored at sexual maturity to assess whether the red phenotype is inherited as a Y-linked characteristic.
Dichotomous female preference tests
Dichotomous choice tests were conducted in Georgetown, Guyana, using the same experimental apparatus and design as used for P. parae in Lindholm et al. [1]. In short, the social preferences of test females for red or standard morphs were assessed in two identical glass aquaria.
The test P. picta females were placed in a central compartment (23 x 30 cm) and could see two conspecific males in adjacent compartments (each 11 x 15 cm) at one end of the aquarium, and at the other end of the aquarium was a compartment (11 x 30 cm) which housed two conspecific females. Each aquarium contained 20 liters of rainwater and a tablespoon of sea salt. Tan gravel covered the bottom of the tanks. The walls of the tanks were covered with translucent waxed paper, except for the glass of the companion female compartment, through which the behavior of the test female and males was observed. Lighting was provided by natural daylight and an Aquari-lux FL-20 full-spectrum aquarium light suspended 37 cm above the water surface and centered between the two tanks, which were placed 11 cm apart.
At the start of a choice test, a test female, a red male and an opponent standard male were placed into their respective compartments. Red males used in the choice tests were slightly larger than standard males (mean difference of 0.3 ± 0.03 cm, paired t-test, t = 2.32, df = 24, P = 0.030) and showed strong red coloration. The red male was placed into the right compartment (with respect to the observer) in half of the trials. Opaque and clear glass partitions divided the two male compartments, and also the male compartments and the test female compartment. After a 10 minute acclimation period, the opaque partitions were removed, and male and female behavior was observed for 10 minutes. Removing the opaque partition between males was important because it allowed the males to see each other, which increased male courtship (as in guppies P. reticulata [27]). The opaque partitions were then replaced, and the males were switched between compartments. After another acclimation period, the opaque partitions were again removed and the fish were observed for an additional 10 minutes. The second observation period controlled for any side preferences of the female.
Fish behaviors were measured by the observer seated approximately 0.5 m away from the end of the tank housing the companion females. Event recorder software written by FB was used to record the number of seconds that the test female spent orientated toward each male against the glass of his compartment. The duration of attentiveness (defined as orientation to the female) was measured for each male. Trials were considered successful if the female was orientated towards at least one male in each of the two observation periods, if the female swam calmly in the tank, and if both males showed interest in the female. 39 trials were conducted, of which 25 were successful. Eighteen of these 25 females were tested within 48 hours of parturition, two were tested later in the reproductive cycle, and the remaining 5 females did not give birth in the laboratory. These trials tested female preference between 20 different red males paired with 18 different standard males. All fish used in the trials were wild-caught as adults. At the end of the study, animals were euthanized using MS-222. We used G*Power 3.1.9.2 for power analysis [28], whereas the base package of R 3.1.0 [29] was used for all other analyses.
Ethics information
The Environmental Protection Agency of Guyana (permit #’s: 010301 BR 003, 120710 BR 135), Ministry of Agriculture of Guyana, Animal Husbandry and Fisheries of Suriname, Republica de Venezuela, Ministerio de Agricultura y Cría Servicio Autónomo de los Recursos Pesqueros y Acuícolas (permit #’s 0301, 0497) and the Trinidad Ministry of Housing and the Environment (permit # 001453) provided research and collecting permits. All fieldwork was done on publicly owned land and no protected species were sampled. Research was approved and animals were housed following animal protocol # 982B-06 granted by the Simon Fraser University Animal Care Committee.
Results
Distribution of red color morph in natural populations from northeast South America
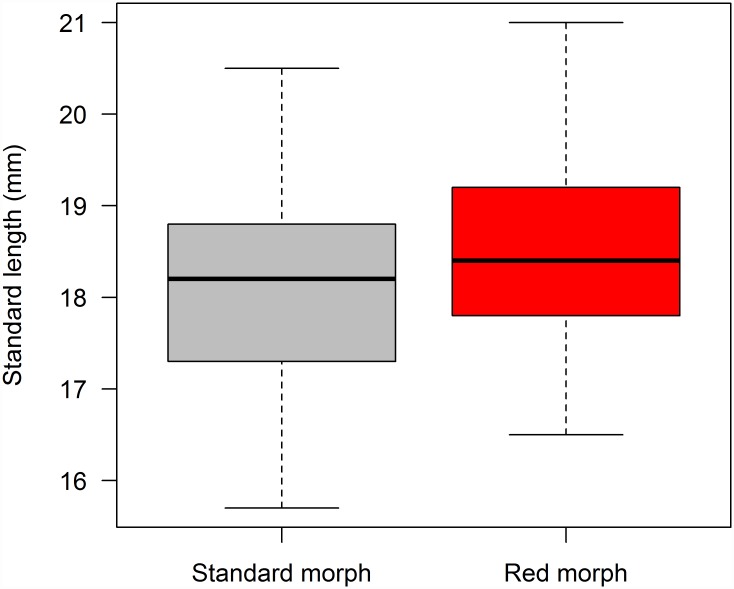
Natural populations of Poecilia picta were observed on collecting trips to Venezuela, Guyana, Suriname and Trinidad, from 1983 through 2011. All P. picta males had black spots on their bodies and dorsal and tail fins ornamented with black and orange or yellow (Fig 1). The Venezuelan populations that were observed are either sympatric with the Cumana guppy in Cumana, or sympatric with standard guppies (e.g., Cano Pedernales and Tucupita in the Orinoco Delta, or Pozo Azufre, Estado Sucre) [30, 31]. The other populations that are sympatric with P. reticulata and that we observed to contain the red color morph are from Guyana (Georgetown, Demerara River, and New Amsterdam) and Suriname (Corentyne River population). Extent of red coloration varied, as 68.8% of males classified as red showed red color from the base of the tail to the snout (excluding black spots), as in Fig 1. The remainder were classified as partially red, showing red color on approximately half to one-third of the body; we combined all males showing any red coloration on the body when we report frequency of the red male morph in Table 1. Red males were larger in standard length than standard males (mean red males 18.6 ± 0.01 SE, n = 69, mean standard males 18.05 ± SE 0.01, n = 156, two sample t test, t = 3.43, df = 223, p = 0.0007, Fig 2;S1 Table). A few individuals from Guyana, 2 from the West Demerara, and 3 from West Berbice, showed full gold coloration instead of red.
Table 1. Survey of P. picta color morph frequency in Guyana and Venezuela.
| Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | Site | N | W | Year | Red | Standard | Gold | % Red |
| Guyana | ||||||||
| Essequibo | Tuschen | 6° 52.728 | 58° 20.991 | 2010 | 8 | 19 | 0 | 29.6 |
| West Demerara | Pouderoyen | 6° 47.797 | 58° 11.112 | 2002 | 12 | 75 | 1 | 13.6 |
| 2010 | 0 | 24 | 0 | 0.0 | ||||
| All | 12 | 99 | 1 | 10.7 | ||||
| Good Fortune | 6° 47.148 | 58° 11.497 | 2002 | 21 | 95 | 0 | 18.1 | |
| Patentia | 6° 41.472 | 58° 11.858 | 2002 | 5 | 57 | 1 | 7.9 | |
| Georgetown | Seawall | 6° 49.632 | 58° 07.346 | 1999 | 8 | 89 | 0 | 8.2 |
| 2000 | 1 | 9 | 0 | 10.0 | ||||
| 2010 | 0 | 23 | 0 | 0.0 | ||||
| All | 9 | 121 | 0 | 6.9 | ||||
| Botanical Gardens | 6° 48.332 | 58° 08.720 | 1999 | 103 | 395 | 0 | 20.7 | |
| 2000 | 20 | 139 | 0 | 12.6 | ||||
| 2002 | 3 | 9 | 0 | 25.0 | ||||
| 2010 | 4 | 53 | 0 | 7.0 | ||||
| All | 130 | 596 | 0 | 12.6 | ||||
| Turkeyen | 6° 49.067 | 58° 06.764 | 2010 | 1 | 34 | 0 | 2.9 | |
| All | All | 140 | 751 | 0 | 15.7 | |||
| East Demerara | Great Diamond | 6° 43.312 | 58° 11.532 | 2010 | 4 | 90 | 0 | 4.3 |
| Timeri | 6° 31.643 | 58° 15.062 | 2010 | 4 | 53 | 0 | 7.0 | |
| West Berbice | Rossignol | 6° 16.347 | 57° 32.511 | 2002 | 5 | 16 | 3 | 20.8 |
| Guyana | All | All | 199 | 1180 | 5 | 14.4 | ||
| Venezuela | ||||||||
| Orinoco Delta | Tucipita | 9° 3.417 | 62° 2.983 | 199 | 2 | 12 | 0 | 14.3 |
Fig 2. Box and whisker plot of standard length of standard and red males, indicating median value, upper and lower quartiles and minimum and maximum values.
Although some collections exhibited no red color morphs at some times, all regions observed on the mainland have exhibited the red color morph at some time point. We surveyed several of these populations intensively enough to report a frequency for the red color morph (N = 10 or more, Table 1). The frequency of the red color morph in the set of populations from Guyana compared to all other morphs ranged from 0% to 29.6%, with the proportion of red morph across all Guyanese collections being 199 of 1384, or 14.4%. This is similar to our largest collection from Venezuela, in which the red morph occurred in 2 of 14 adult males (14.3%). Overall, there is significant geographic variation in frequencies of the red morph (proportion test, χ 2 = 21.64, df = 8, p = 0.006).
Intriguingly, the red morph does not seem to occur on the South American “land bridge” island of Trinidad. We examined Poecilia picta from 3 populations spanning the island of Trinidad; Icacos Point in the far southwest, Tompire River in the northeast, and Caroni swamp in the western part of the Caroni drainage, and have never observed the red phenotype in any males. Reports of P. picta from Trinidad in the sexual selection literature also do not mention this red phenotype [32, 33, 34].
Inheritance
In two to four generations of inbreeding, we found that lines started by males of the standard color morph never produced red morph male offspring, whereas lines started by red males produced both red and standard color offspring (Table 2). Male color patterns were stable, as we observed no males changing color morph during their lifespan. We can conclude from these breeding experiments so far that red coloration is not Y-linked, as opposed to many of the genes for male coloration in the guppy P. reticulata and male color morph in the closely related Poecilia parae.
Table 2. Inheritance results from inbred lines established by either red or standard morph males of P. picta.
| Generation | Offspring Phenotype | Inbred Line | ||||
|---|---|---|---|---|---|---|
| Red 1 | Red 2 | Standard 1 | Standard 2 | Standard 3 | ||
| 1 | Red males | 14 | 18 | 0 | 0 | 0 |
| Standard males | 14 | 21 | 5 | 2 | 7 | |
| Females | 21 | 61 | 7 | 11 | 3 | |
| 2 | Red males | 7 | 3 | 0 | 0 | 0 |
| Standard males | 9 | 4 | 8 | 2 | 3 | |
| Females | 15 | 9 | 9 | 1 | 7 | |
| 3 | Red males | 7 | 16 | 0 | - | 0 |
| Standard males | 4 | 9 | 4 | - | 6 | |
| Females | 8 | 31 | 14 | - | 10 | |
| 4 | Red males | 0 | - | 0 | - | - |
| Standard males | 3 | - | 24 | - | - | |
| Females | 3 | - | 29 | - | - | |
Female Preference
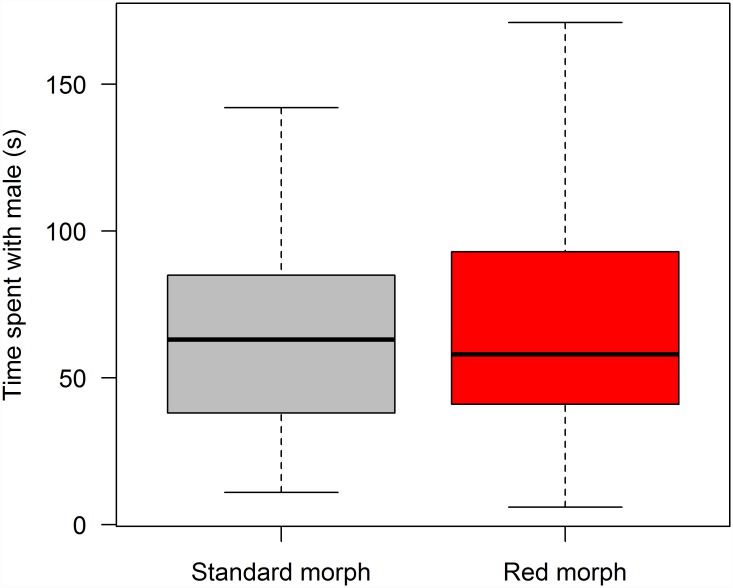
Females showed no preference for either morph in paired choice tests with a red vs. standard male (Fig 3; paired Wilcoxon signed rank test, n = 25, V = 160, p = 0.96, S2 Table). Restricting the dataset to females within 1 day of parturition, as non-virgin female P. reticulata are most sexually active close to parturition [23], did not change this result (n = 18, V = 85.5, p = 1.00). Accounting for reuse of male pairs in four cases by averaging the results of the paired trials also did not change this result (paired Wilcoxon signed rank test, n = 21, V = 108, p = 0.96). There was no difference between display times of males within a trial (median for red males 961 s and standard males 960 s, Wilcoxon test V = 131.5, p = 0.41). Focal females spent significantly more time with conspecific females than with the test males (paired Wilcoxon signed rank test, n = 25, V = 24, p<0.001), suggesting that choosiness, the effort that females put in to assess males [35], may have been low. We assessed the power of this study to detect differences in preference function, the relative ranking of males [35]. For comparison we used a well-cited study [36] of female choice between male guppies from a high carotenoid treatment resulting in bright red/orange spots and low carotenoid treatment leading to dull red/orange spots using a similar apparatus to ours (albeit without using conspecific females). Using the software G*power 3.1 [28], the effect size in this study was calculated to be 1.048, with a preference for highly red/orange males. With 25 trials, our study has a 99% power to detect such a difference, 76.6% power to detect an effect size of 0.5, and 24.3% power to detect a small effect size of 0.2.
Fig 3. Duration of association with males in seconds (mean ± SE) in female preference tests.
Discussion
We describe for the first time a male specific color polymorphism in Poecilia picta that persists at a moderate frequency in nature. This polymorphism consists of a presence or absence of red, or in a few instances gold, coloration that runs through the body. Unlike in the closely related species P. reticulata and P. parae the red morph of P. picta does not appear to be preferred by females. Our work adds P. picta as another member of Poeciliidae to possess sex specific male color polymorphism, but sets it apart in terms of the forces maintaining the polymorphism and its inheritance pattern. We first discuss the potential mechanisms that could explain the presence/absence of the red/gold morph polymorphism across populations. We then discuss the unique characteristics of the color polymorphism in P. picta, and suggest ways in which studies utilizing this male color-polymorphism will further the fields of ecology and evolutionary biology.
Red male coloration is common among fishes and red male coloration plays a large role in the life history of species closely related to P. picta including the sister species P. reticulata [11, 19] and P. parae [1, 2]. Red coloration is well known to cause individuals in shallow freshwater habitats to be more conspicuous to predators leading to strong natural selection against red phenotypes [37]. Indeed, both P. reticulata and P. parae are well known to experience strong natural selection acting against red/orange male coloration (P. reticulata [11, 19, 37, 38], P. parae [2, 39]). In terms of evolutionary dynamics, we consider orange and red coloration functionally equivalent, in that their perception is controlled by the long-wave sensitive family of opsins and the frequencies in the spectrum are very close. Both P. reticulata and P. parae are roughly the same size and shape and occupy similar ecological niches as P. picta [21, 23], especially in the Guyanese and Venezuelan populations where the 3 species occur in sympatry and where the red morph is found in similar frequencies. Therefore it would be expected that P. picta also experiences strong natural selection acting against red male coloration. In both P. reticulata and P. parae the adverse effects of natural selection are opposed, and even overcome, by sexual selection in which females prefer to mate with red/orange males (P. reticulata [40, 41], P. parae [2, 39]). The results of our study show that female P. picta are not strongly attracted to red morph males, yet the red morph is maintained both across populations and within populations across time. This suggests that natural selection acting against red male morphs may be opposed by another form of selection in P. picta selecting for the red male morph. Indeed, we show the frequency of red male phenotype varies across populations and within populations over time. Ultimately this distribution of morph frequencies would represent a balance among opposing selection pressures and migration among populations.
Over the last 80 years, populations of P. picta in Trinidad and Tobago have been the focus of studies on sexual selection [42], mate recognition [32, 33], heterospecific insemination [34], life history [43], parasite resistance [44] and ecological niches [45]. Despite the extensive body of work on Trinidadian populations of P. picta the red male morph has never been noted in Trinidad and Tobago. This supports the idea that there are forces working against the red male phenotype. There remain two likely processes that could explain the maintenance of the red male phenotype in P. picta: (1) intrasexual selection or (2) correlated characters. We explore these possible processes below.
Sexual selection can occur either intersexually (usually due to mate choice by females for attractive males) or intrasexually (usually due to competition between males) [46]. Our results reveal intersexual selection is not acting strongly on the red male phenotype in P. picta; however it is possible that male-male competition could result in the red males having higher reproductive success than the non-red males. If red males indeed have higher fitness than non-red males it would at first seem surprising that there are no red males in the P. picta populations on the islands of Trinidad and Tobago. However, Kaneshiro proposes that male sexual signals can be lost in island populations, which could explain the lack of red on the islands of Trinidad and Tobago [47, 48]. Alternatively, the relative fitness gain through male-male competition could be density dependent as seen in a wide range of taxa and models (reviewed in [49]). If this is the case then variation in density across populations could lead to a loss of red morph when the relative fitness gained through male-male competition no longer exceeded natural selection acting against the red phenotype.
While intrasexual selection could explain the maintenance of the red male polymorphism in P. picta, another possible process could be at play: the red phenotype may be correlated to a character that is not involved in sexual selection. A growing body of literature across a wide range of taxa shows that color polymorphisms can be maintained as correlated characters (reviewed in [50]). If this is the case then the variation in red male morph frequency across populations could be due to differences in selective pressure for the unknown character to which the red morph is linked.
Understanding the patterns, implications, and ubiquity of mechanisms underlying the maintenance of genetic polymorphisms in nature has been a long-standing question in the fields of ecology and evolution, and the forces maintaining these polymorphisms were succinctly summarized in Ford’s seminal book in 1965 [51]. Color polymorphisms have been shown to be capable of maintaining genetic diversity within individual species across a wide range of taxa (reviewed in [8]). Yet these taxa are largely disparate and their evolutionary relationships uncertain, making phylogenetic approaches difficult. Poeciliidae is a well studied family with species known to maintain high genetic diversity [52], often through frequency dependent selection based on color polymorphisms [2, 12]. By adding P. picta as another member of Poeciliidae with male color polymorphism, future work will be able to wield the power of phylogenetic analyses to answer long standing questions such as ‘does color polymorphism coincide with faster rates of speciation?’.
The lack of female preference for red male coloration in P. picta is perhaps surprising as guppies (P. reticulata) and P. parae, close relatives, exhibit strong female preferences for red/orange male coloration. It should be noted that males and females were not allowed to interact with each other in the choice tests in this study, and courtship in P. picta involves the male circling the head of the female; therefore the lack of preference in our tests could be an artifact of the study design. However, a separate test for female preference for the orange stripe on the caudal fin of P. picta males, which did allow for male/female interactions, did not show preference for orange signals [24], supporting the lack of female preference for orange and red coloration in P. picta. It has been proposed that female attraction to red/orange male coloration arose due to a pre-existing bias in guppies [53]. If such a pre-existing bias does indeed underlie female mate choice for red in guppies then closely related species could also demonstrate such female preferences [24]. P. parae females are also attracted to red conspecific males [1]. However, P. picta is one of the closest relatives to guppies [20, 54], and yet does not demonstrate female preferences for red male coloration. This lends support to alternative hypotheses of female preference for red coloration in guppies, such as sensory exploitation [55].
Despite theoretical predictions that suggest the inheritance of a sex specific coloration should be linked to the heterogametic sex, we found the inheritance pattern of the red morph in P. picta not to be Y-linked. This contrasts with multiple studies of Poeciliids, reviewed in [17], that show that not only most color patterns, but also male size and courtship patterns, tend to be linked to the heterogametic sex chromosome. The red morph in P. picta is also distinct in that it does not appear as a discrete color patch (Fig 1), as do most of the male coloration patterns in Poeciliids. This difference in appearance and inheritance suggests that this red male color polymorphism has evolved independently of the other types of red/orange coloration in guppies and close relatives.
The other phylogenetic contrast that our results for P. picta sets up involves the distribution of male coloration patterns in guppies and its sister clade [17]. Two species in the sister clade to the guppy have been studied, P. parae and now P. picta, and they both exhibit discrete male morphs: 5 in P. parae, and the red, standard and a rare gold morph in P. picta. The inheritance of the 5 morphs in P. parae is controlled by a single Y-linked locus, while the inheritance of the red morph in P. picta is not Y-linked. Further work will be needed to resolve the details of the inheritance pattern. This contrasts very clearly with P. reticulata, in which the male coloration patterns are controlled by as many as 30–40 loci, which produce highly polymorphic patterns such that almost every male within a population can be distinct [17]. This contrast could be very informative for the forces initiating and maintaining color polymorphisms in nature.
In summary, our work shows there to be a male specific color polymorphism that occurs in natural populations of P. picta that is not Y-linked and does not seem to play a role in female mate choice. It remains for further study to determine the precise mechanisms responsible for maintaining the red color polymorphism in nature. However, the presence of a non Y-linked male color polymorphism should prove useful in further studies investigating color polymorphism, sexual conflict, genetic diversity, and even female mate preference of closely related species.
Supporting Information
(TXT)
(TXT)
Acknowledgments
We thank the Environmental Protection Agency of Guyana, Ministry of Agriculture of Guyana, Animal Husbandry and Fisheries of Suriname, Ministerio de Agricultura y Cría Servicio Autónomo de los Recursos Pesqueros y Acuícolas of Venezuela and the Trinidad Ministry of Housing and the Environment for permits. Indarjit Ramdass of the University of Guyana, Godfrey Bourne and personnel of the Ceiba Biological Centre, Jan Mol of the Anton de Kom University of Suriname and Center for Agricultural Research of Suriname, and personnel at the Centre for the Study of Biological Diversity in Guyana, the National Zoological Park of Guyana, and Guyana Aquarium Traders provided valuable support in South America. We thank Michael Krützen, Sahid Cantallops Peña, and Frances Margaret Walker Breden for assistance with field work in Guyana, the Smithsonian Institution for housing and logistical support in Guyana, Heather Alexander and Carlos Augusto Figueiredo for assistance in Suriname, and David Reznick for a collection from the Tompire River, Trinidad.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Natural Sciences and Engineering Council of Canada (www.nserc-crsng.gc.ca) provided funding through a postdoctoral fellowship to AL, and an operating grant to FB (#163318). Additional funding was provided by the University of New South Wales through a Vice-Chancellor's Postdoctoral Fellowship (AL; https://research.unsw.edu.au/unsw-internal-funding-opportunities#VC_Postdoc_Anchor), The Field Museum of Natural History (FB; www.fieldmuseum.org) and the National Geographic Society (FB; www.nationalgeographic.com). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lindholm AK, Brooks R, Breden F (2004) Extreme polymorphism in a Y-linked sexually selected trait. Heredity, 92, 156–162. [DOI] [PubMed] [Google Scholar]
- 2. Hurtado-Gonzales JL, Uy JAC (2009) Alternative mating strategies may favour the persistence of a genetically based colour polymorphism in a pentamorphic fish. Animal Behaviour, 77, 1187–1194. [Google Scholar]
- 3. Gross MR, Charnov EL (1980) Alternative male life histories in bluegill sunfish. Proceedings of the National Academy of Sciences, 77, 6937–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sinervo B, Lively CM (1996) The rock-paper-scissors game and the evolution of alternative male strategies. Nature, 380, 240–243. [Google Scholar]
- 5. Ravigné V, Olivieri I, Dieckmann U (2004) Implications of habitat choice for protected polymorphisms. Evolutionary Ecology Research, 6, 125–145. [Google Scholar]
- 6. Hazel WN, Johnson MS (1990) Microhabitat choice and polymorphism in the land snail Theba pisana (Müller). Heredity, 65, 449–454. [Google Scholar]
- 7. Berry AJ, Willmer PG (1986) Temperature and the colour polymorphism of Philaenus spumarius (Homoptera: Aphrophoridae). Ecological Entomology, 11, 251–259. [Google Scholar]
- 8. Gray SM, McKinnon JS (2007) Linking color polymorphism maintenance and speciation. Trends In Ecology & Evolution, 22, 71–79. [DOI] [PubMed] [Google Scholar]
- 9. Morlon H (2014) Phylogenetic approaches for studying diversification (Mooers A, Ed,). Ecology Letters, 17, 508–525. 10.1111/ele.12251 [DOI] [PubMed] [Google Scholar]
- 10. Evans JP, Pilastro A, Schlupp I (Eds.) (2011) Ecology and evolution of Poeciliid fishes. The University of Chicago Press; Chicago, IL. [Google Scholar]
- 11. Houde AE (1997) Sex, color, and mate choice in guppies. Princeton University Press; Princeton, NJ. [Google Scholar]
- 12. Hughes KA, Houde AE, Price AC, Rodd FH (2013) Mating advantage for rare males in wild guppy populations. Nature, 503, 108–110. 10.1038/nature12717 [DOI] [PubMed] [Google Scholar]
- 13. Hurtado-Gonzales JL, Baldassarre DT, Uy JAC (2010) Interaction between female mating preferences and predation may explain the maintenance of rare males in the pentamorphic fish Poecilia parae . Journal Of Evolutionary Biology, 23, 1293–1301. 10.1111/j.1420-9101.2010.01995.x [DOI] [PubMed] [Google Scholar]
- 14. Endler JA (1995) Multiple trait coevolution and environmental gradients in guppies. Trends in Ecology & Evolution 10, 22–29. 10.1186/s13071-015-1124-7 [DOI] [PubMed] [Google Scholar]
- 15. Godin JGJ., McDonough HE (2003) Predator preference for brightly colored males in the guppy: a viability cost for a sexually selected trait. Behavioral Ecology, 14, 194–200. [Google Scholar]
- 16. Fisher RA (1931) The evolution of dominance. Biological Reviews, 6, 345–368. [Google Scholar]
- 17. Lindholm A, Breden F (2002) Sex chromosomes and sexual selection in poeciliid fishes. American Naturalist, 160, S214–S224. 10.1086/342898 [DOI] [PubMed] [Google Scholar]
- 18. Houde AE (1992) Sex-linked heritability of a sexually selected character in a natural population of Poecilia reticulata (Pisces: Poeciliidae) (guppies). Heredity, 69, 229–235. [Google Scholar]
- 19. Magurran AE (2005) Evolutionary Ecology: The Trinidadian Guppy. Oxford University Press, New York. [Google Scholar]
- 20. Breden F, Ptacek MB, Rashed M, Taphorn D, Figueiredo CA (1999) Molecular phylogeny of a live-bearing fish genus Poecilia (Poeciliidae: Cyprinidontiformes). Molecular Phylogenetics and Evolution, 12, 95–104. [DOI] [PubMed] [Google Scholar]
- 21. Rosen DE, Bailey RM (1963) The Poeciliid fishes (Cyprinodontiformes), their structure, zoogeography, and systematics. Bulletin of the America Museum of Natural History, 126, 1–176. [Google Scholar]
- 22. Meyer MK (1993) Reinstatement of Micropoecilia Hubbs, 1926, with a redescription of M. bifurca (Eigenmann, 1909) from Northeast South America (Teleostei, Cyprinodontiformes: Poeciliidae). Zoologische Abhandlungen Staatliches Museum für Tierkunde Dresden 47, 121–130. [Google Scholar]
- 23. Liley R (1966) Ethological isolating mechanisms in four sympatric species of poeciliid fishes. Behaviour Supplement 13, 1–197 [Google Scholar]
- 24. Breden F, Bertrand M (1999) A test for female attraction to male orange coloration in Poecilia picta . Environmental Biology Of Fishes, 55, 449–453. [Google Scholar]
- 25. Keith P, Le Bail P-Y, Planquette P (2000) Atlas des Poissons d’Eau Douce de Guyane. Tome 2, fascicule I. Batrachoidiformes, Mugiliformes, Beloniformes, Cyprinodontiformes, Synbranchiformes, Perciformes, Pleuronectiformes, Tetradontiformes. Patrimoines naturels (M.N.H.N/S.P.N) 43, 1–286. [Google Scholar]
- 26. Lucinda PHF, Reis RE (2005) Systematics of the subfamily Poeciliinae Bonaparte (Cyprinodontiformes: Poeciliidae), with an emphasis on the tribe Cnesterodontini Hubbs. Neotropical Ichthyology 3, 1–60. [Google Scholar]
- 27. Farr JA (1976) Social facilitation of male sexual behavior, intrasexual competition, and sexual selection in the guppy, Poecilia reticulata (Pisces: Poeciliidae). Evolution, 30, 707–717. [DOI] [PubMed] [Google Scholar]
- 28. Faul F, Erdfelder E, Lang AG, Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- 29. R Development Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 30. Alexander HJ, Breden F (2004) Sexual isolation and extreme morphological divergence in the Cumana guppy: a possible case of incipient speciation. Journal Of Evolutionary Biology, 17, 1238–1254. [DOI] [PubMed] [Google Scholar]
- 31. Winemiller KO, Leslie M, Roche R (1990) Phenotypic variation in male guppies from natural inland populations: an additional test of Haskins’ sexual selection/predation hypothesis. Environmental Biology of Fishes, 29, 179–191. [Google Scholar]
- 32. Magurran AE, Ramnarine IW (2004) Learned mate recognition and reproductive isolation in guppies. Animal Behaviour, 67, 1077–1082. [Google Scholar]
- 33. Magurran AE, Ramnarine I (2005) Evolution of mate discrimination in a fish. Current Biology, 15, R867 [DOI] [PubMed] [Google Scholar]
- 34. Russell S, Ramnarine IW, Mahabir R, Magurran AE (2006) Genetic detection of sperm from forced copulations between sympatric populations of Poecilia reticulata and Poecilia picta . Biological Journal Of The Linnean Society, 88, 397–402. [Google Scholar]
- 35. Jennions MD, Petrie M (1997) Variation in mate choice and mating preferences: A review of causes and consequences. Biol Rev, 72, 283–327. [DOI] [PubMed] [Google Scholar]
- 36. Kodric-Brown A (1989) Dietary carotenoids and male mating success in the guppy: an environmental component to female choice. Behav Ecol Sociobiol 25:393–401. Springer-Verlag. [Google Scholar]
- 37. Endler JA (1991) Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vision Research, 31, 587–608. [DOI] [PubMed] [Google Scholar]
- 38. Haskins CP, Haskins EF, McLaughlin JJA, Hewitt RE (1961) Polymorphism and population structure in Lebistes reticulatus, an ecological study In: Vertebrate Speciation (ed Blair WF), pp. 320–395. [Google Scholar]
- 39. Hurtado-Gonzales JL, Loew ER, Uy JAC (2014) Variation in the visual habitat may mediate the maintenance of color polymorphism in a poeciliid fish. Plos One, 9, e101497 10.1371/journal.pone.0101497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Endler JA (1980) Natural selection on color patterns in Poecilia reticulata . Evolution, 34, 76–91. [DOI] [PubMed] [Google Scholar]
- 41. Lindholm AK, Head ML, Brooks RC, Rollins LA, Ingleby FC, Zajitschek SR (2014) Causes of male sexual trait divergence in introduced populations of guppies. Journal Of Evolutionary Biology, 27, 437–448. 10.1111/jeb.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haskins CP, Haskins EF (1949) The role of sexual selection as an isolating mechanism in three species of poeciliid fishes. Evolution, 3, 160–169. [DOI] [PubMed] [Google Scholar]
- 43. Reznick DN, Miles DB, Winslow S (1992) Life history of Poecilia picta (Poeciliidae) from the island of Trinidad. Copeia, 3, 782–790. [Google Scholar]
- 44. Dargent F, Torres-Dowdall J, Scott ME, Ramnarine I, Fussmann GF (2013) Can mixed-species groups reduce individual parasite load? A field test with two closely related poeciliid fishes (Poecilia reticulata and Poecilia picta). Plos One, 8, e56789 10.1371/journal.pone.0056789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Torres-Dowdall J, Dargent F, Handelsman CA, Ramnarine IW, Ghalambor CK (2013) Ecological correlates of the distribution limits of two poeciliid species along a salinity gradient. Biological Journal of the Linnean Society, 108, 709–805. [Google Scholar]
- 46. Andersson MB (1994) Sexual selection. Princeton University Press, Princeton, New Jersey. [Google Scholar]
- 47. Kaneshiro KY (1976) Ethological isolation and phylogeny in the Planitibia subgroup of Hawaiian Drosophila. Evolution, 30, 740–745. [DOI] [PubMed] [Google Scholar]
- 48. Kaneshiro KY (1980) Sexual isolation, speciation and the direction of evolution. Evolution, 34, 437–444. [DOI] [PubMed] [Google Scholar]
- 49. Kokko H, Rankin DJ (2006) Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philosophical Transactions Of The Royal Society B-Biological Sciences, 361, 319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McKinnon JS, Pierotti MER (2010) Colour polymorphism and correlated characters: genetic mechanisms and evolution. Molecular Ecology, 19, 5101–5125. 10.1111/j.1365-294X.2010.04846.x [DOI] [PubMed] [Google Scholar]
- 51. Ford EB (1965) Genetic polymorphism. London: Faber & Faber Ltd. [Google Scholar]
- 52. Breden F, Lindholm A (2011) Genetic variation in natural populations In: Ecology and Evolution of Poeciliid Fishes (eds. Evans Jonathan, Pilastro Andrea, and Schlupp Ingo) University of Chicago Press, Chicago. [Google Scholar]
- 53. Rodd FH, Hughes KA, Grether GF, Baril CT (2002) A possible non-sexual origin of mate preference: are male guppies mimicking fruit? Proceedings Of The Royal Society Of London Series B-Biological Sciences, 269, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hamilton A (2001) Phylogeny of Limia (Teleostei: Poeciliidae) based on NADH dehydrogenase subunit 2 sequences. Molecular Phylogenetics And Evolution, 19, 277–289. [DOI] [PubMed] [Google Scholar]
- 55. Sandkam BA, Young CM, Breden F (2015) Beauty in the eyes of the beholders: colour vision is tuned to mate preference in the Trinidadian guppy (Poecilia reticulata). Molecular Ecology, 24, 596–609. 10.1111/mec.13058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TXT)
(TXT)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.