Abstract
The ability of Listeria monocytogenes to grow at refrigeration temperatures is critical for transmission of this foodborne pathogen. We evaluated the contributions of different transcriptional regulators and two-component regulatory systems to L. monocytogenes cold adaptation and cold growth. L. monocytogenes parent strain 10403S and selected isogenic null mutants in genes encoding four alternative σ factors (sigB, sigH, sigC, and sigL), two regulators of σB (rsbT and rsbV), two negative regulators (ctsR and hrcA), and 15 two-component response regulators were grown in brain heart infusion broth at 4°C with (i) a high-concentration starting inoculum (108 CFU/ml), (ii) a low-concentration starting inoculum (102 CFU/ml), and (iii) a high-concentration starting inoculum of cold-adapted cells. With a starting inoculum of 108 CFU/ml, null mutants in genes encoding selected alternative σ factors (ΔsigH, ΔsigC, and ΔsigL), a negative regulator (ΔctsR), regulators of σB (ΔrsbT and ΔrsbV), and selected two-component response regulators (ΔlisR, Δlmo1172, and Δlmo1060) had significantly reduced growth (P < 0.05) compared with the parent strain after 12 days at 4°C. The growth defect for ΔsigL was limited and was not confirmed by optical density (OD600) measurement data. With a starting inoculum of 102 CFU/ml and after monitoring growth at 4°C over 84 days, only the ΔctsR strain had a consistent but limited growth defect; the other mutant strains had either no growth defects or limited growth defects apparent at only one or two of the nine sampling points evaluated during the 84-day growth period (ΔsigB, ΔsigC, and Δlmo1172). With a 108 CFU/ml starting inoculum of cold-adapted cells, none of the mutant strains that had a growth defect when inoculation was performed with cells pregrown at 37°C had reduced growth as compared with the parent strain after 12 days at 4°C, suggesting a specific defect in the ability of these mutant strains to adapt to 4°C after growth at 37°C. Our data indicate (i) selected σ factors and two-component regulators may contribute to cold adaptation even though two-component regulatory systems, alternative σ factors, and the negative regulators CtsR and HrcA appear have limited contributions to L. monocytogenes growth at 4°C in rich media, and (ii) inoculum concentration and pregrowth conditions affect the L. monocytogenes cold-growth phenotype.
Listeria monocytogenes has the ability to grow at temperatures as low as −0.4°C (25, 40). Growth of L. monocytogenes in refrigerated ready-to-eat (RTE) foods is critical to transmission of this foodborne pathogen because high numbers of bacteria are required to cause human disease. Consequently, considerable efforts have been focused on design and implementation of strategies to prevent L. monocytogenes growth in RTE foods (4, 33). Further characterization of molecular mechanisms that facilitate L. monocytogenes growth at low temperature is necessary to improve our ability to reduce or prevent L. monocytogenes growth in refrigerated RTE foods.
The alternative sigma factor σB is critical for the ability of L. monocytogenes to respond to a number of environmental stress conditions (e.g., low pH, high salt, and carbon starvation) (17, 28, 39, 41). L. monocytogenes σB regulates transcription of a number of stress response and virulence genes, including genes with possible roles in cold adaptation (e.g., ltrC and opuC) (10, 13, 28, 30, 41). At least some L. monocytogenes sigB null mutant strains previously showed reduced growth in defined medium at 8°C (3) and reduced survival in raw meat stored at 4°C (34). Regulation of σB activity is complex and involves at least seven regulators of σB (12, 13). In addition to σB, L. monocytogenes has three other alternative σ factors: σH, σC, and σL. Although the L. monocytogenes sigL gene (encoding σL) appears to have higher transcript levels in cells grown at low temperature (10°C) compared with cells grown at 37°C (32), the specific contributions of σH, σC, and σL to low-temperature growth of L. monocytogenes have not been examined.
The negative regulators CtsR and HrcA also regulate transcription under different stress conditions (20, 27, 29, 37), particularly during heat shock (21, 22, 27). The HrcA-dependent groEL gene has higher transcript levels in Bacillus subtilis grown at 15°C compared with that grown at 37°C, suggesting a possible role of HrcA-regulated genes in cold adaptation in gram-positive bacteria (7). Although L. monocytogenes ctsR mutants have shown enhanced survival under different stress conditions, including heat stress (27, 35), transcriptional profiling in B. subtilis showed higher ctsR transcript levels during growth at 15°C than during growth at 37°C (7), suggesting a role for CtsR in modulating transcription at low temperatures.
In L. monocytogenes, 16 two-component regulatory systems (TCRSs) have been identified (19, 43), including one (encoded by lmo0287 and lmo0288) that appears to be essential (26, 43). Phenotypic characterization of 15 TCRS RR in-frame deletion mutants in an L. monocytogenes EGD-e background indicated that none of these TCRS RR mutants contributed to growth during osmotic stress (9% NaCl) and oxidative stress (0.0025% H2O2); ΔdegU, ΔresD, ΔphoP, and ΔvirR strains had reduced growth during ethanol stress (43). In two other studies, a lisK null mutant had reduced osmotolerance and acid resistance (15, 38). Phenotypic characterization of five TCRS RR transposon mutant strains revealed impaired growth at 43.5°C for lisR and kdpE mutants (26). An L. monocytogenes strain with a deletion mutation in kdpE (encoding the response regulator KdpE) had growth characteristics in brain heart infusion (BHI) broth at 5°C similar to those of the EGD-e parent strain (6). No specific contributions of L. monocytogenes TCRSs to cold growth have been reported thus far.
In previous studies, researchers have evaluated some L. monocytogenes transcriptional regulators and TCRSs for contributions to cold growth through either mutant characterization (6) or transcriptional profiling (3, 32); however, there are no comprehensive data on the contributions of alternative σ factors, TCRSs, and negative regulators to L. monocytogenes cold adaptation and cold growth. We used a core set of 23 in-frame deletion mutant strains to evaluate the contributions of these different regulators to L. monocytogenes cold growth and cold adaptation.
MATERIALS AND METHODS
Bacterial strains
The L. monocytogenes serotype 1/2a strain 10403S (5) and isogenic deletion mutant strains in the 10403S genetic background were used throughout this study (Suppl. Table 1; all supplemental materials are available at http://www.foodscience.cornell.edu/cals/foodsci/research/labs/wiedmann/links/chan2007.cfm). Isogenic mutant strains used carried deletions in genes encoding (i) 15 of the 16 known L. monocytogenes two-component response regulators (TCRRs) (our laboratory and two others (26, 43) were unable to construct a deletion mutation in lmo0287, suggesting that the TCRR encoded by lmo0287 is essential); (ii) two negative regulators (CtsR and HrcA), (iii) four alternative σ factors, and (iv) RsbT and RsbV, two regulators of σB (12, 16). Isogenic strains with in-frame mutations in prfA (44), which encodes a major virulence gene regulator (9), and in opuC (1), which encodes an ABC transporter with a putative role in cold growth (2), also were used in selected experiments. A ΔctsRΔhrcA double mutant strain also was tested in selected experiments.
All isogenic in-frame deletion mutant strains have previously been constructed using splicing by overlap extension (SOE) PCR and standard allelic exchange mutagenesis (42) to generate a non-polar internal deletion within each gene of interest (see Suppl. Table 1 for SOE PCR primers used for mutant generation). For all mutant strains, allelic exchange mutagenesis has been confirmed through PCR and DNA sequencing to ensure in-frame deletions with no mutations in the sequences flanking the deletion.
For all mutant strains, their ability to grow at 37°C in BHI broth with shaking (250 rpm) was evaluated based on optical density at 600 nm (OD600) measurements. None of the mutant strains, except the ΔctsR and ΔctsRΔhrcA strains, showed evidence of growth defects at 37°C (compared with the parent strain). Reduced growth for the ΔctsR and ΔctsRΔhrcA strains was confirmed by cell enumeration; these two strains had limited growth defects at 37°C (<1 log CFU/ml difference between mutant and parent strain after 3, 5, and 8 h of growth at 37°C) (23).
Cold growth conditions
Prior to all cold growth experiments, L. monocytogenes 10403S and selected mutant strains were grown overnight (16 to 18 h) in BHI broth at 37°C with shaking (250 rpm) and then diluted 1:100 into fresh BHI broth and grown with shaking to log phase (OD600 = 0.4), unless otherwise stated. The cold growth capabilities of both parent and mutant strains were evaluated in BHI broth (without shaking) at 4°C in borosilicate glass tubes (Fisher Scientific, Pittsburgh, Pa.). For these experiments, three different inocula were used: (i) a high-concentration (108 CFU/ml) and (ii) a low-concentration (102 CFU/ml) starting inoculum of cells pregrown at 37°C and (iii) a high-concentration (108 CFU/ml) starting inoculum of cold-adapted cells. Cells were spiral plated on duplicate BHI agar plates using an Autoplate 4000 Automated Spiral Plater (Spiral Biotech, Inc., Norwood, Mass.). The plates were incubated at 37°C for 24 to 48 h, and colonies were enumerated using Q count (Spiral Biotech).
For high-inoculum experiments, log-phase cells of the L. monocytogenes parent strain and selected deletion mutant strains were used to inoculate BHI broth prechilled to 4°C to an OD600 of 0.15 ± 0.05, which is equivalent to 108 CFU/ml. On day 0 (immediately after inoculation) and on day 12, cells were enumerated after spiral plating, and OD600 values were determined; these measurements were performed in three independent experiments. In one experiment, bacterial numbers also were determined by spiral plating on days 3, 6, 9, and 12, and the OD600 was measured once a day for 12 days.
For low-inoculum experiments, log-phase L. monocytogenes cells were used to inoculate BHI broth prechilled to 4°C to achieve a starting inoculum of 102 CFU/ml. Cells were enumerated by spiral plating on BHI agar on day 0 (immediately after inoculation) and on days 7, 14, 21, 28, 35, 42, 56, 70, and 84. Three independent experiments were performed.
For growth experiments using a high-concentration inoculum of cold-adapted cells, bacteria that had been grown at 4°C for 43 days (as outlined for the low-inoculum experiment) were used to inoculate BHI broth prechilled to 4°C with a starting inoculum of 108 CFU/ml. Cells were enumerated by spiral plating on BHI agar on day 0 (immediately after inoculation) and on day 12. Three independent experiments were performed.
Statistical analyses
For all growth experiments at 4°C, bacterial numbers or growth and OD600 values (where available) for the L. monocytogenes parent strain (10403S) and deletion mutant strains were analyzed using the general linear model (GLM) in SAS v 9.1 (SAS Institute, Inc., Cary, N.C.). To allow blocking by experiments, a mixed-effects model with Tukey’s studentized range test (LS means) was used when the “experiment” variable was significant in the initial GLM analysis. For time points where the initial analysis indicated a significant effect of the “strain” variable, LS means was used to determine whether there was a significant difference in bacterial numbers or OD600 values between a given mutant strain and the L. monocytogenes parent strain. An α value of <0.05 was considered significant.
RESULTS
Growth of L. monocytogenes strain 10403S and selected deletion mutant strains at 4°C using a high-concentration inoculum
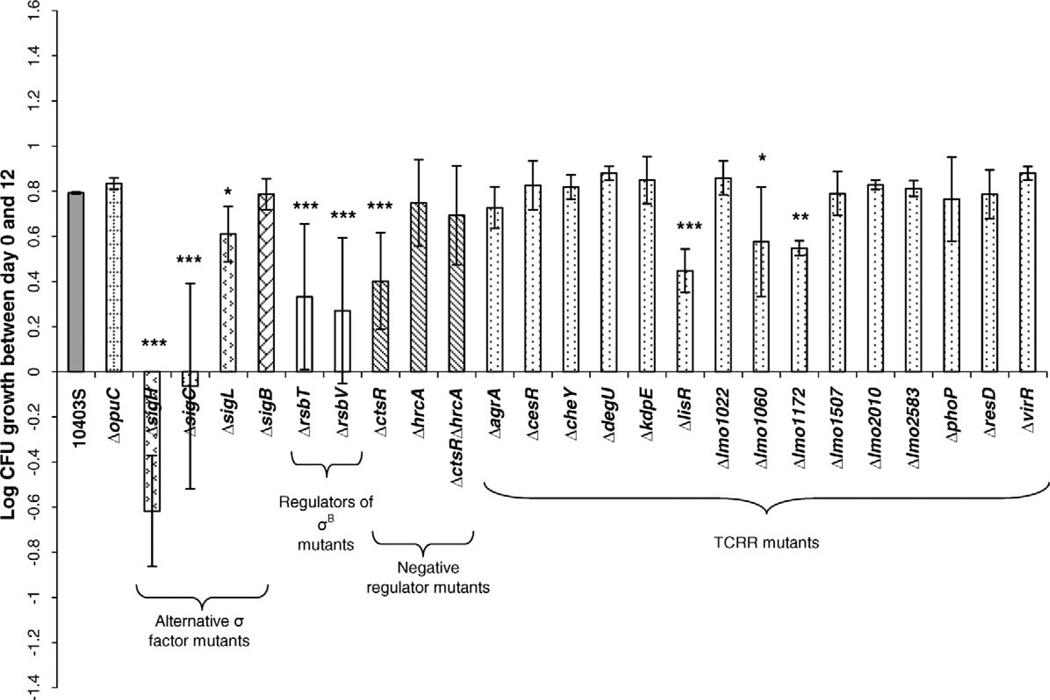
For initial characterization of the cold growth phenotype, L. monocytogenes mutant strains and the parent strain were inoculated into prechilled BHI broth at ~108 CFU/ml. Growth at 4°C was defined as the difference in bacterial numbers (log CFU per milliliter; Fig. 1) from day 0 to day 12 or as the difference in OD600 (Suppl. Fig. S1) from day 0 to day 12. Overall statistical analysis showed a significant effect of strain (parent and different mutant strains) on growth over 12 days at 4°C for both the bacterial numbers data (P < 0.0001; mixed effects model) and the OD600 data (P < 0.0001; mixed effects model). Based on the bacterial numbers data, three σ factor null mutant strains (ΔsigH, ΔsigC, and ΔsigL), the two Rsb mutant strains (ΔrsbT and ΔrsbV), three TCRR mutant strains (ΔlisR, Δlmo1172, and Δlmo1060), and the ΔctsR strain had significantly reduced growth at 4°C (P < 0.05; LS means) compared with the parent strain (Fig. 1). The same mutants (except for ΔsigL) also had reduced growth after 12 days at 4°C, based on the OD600 measurements. These mutants (except for ΔsigL) had growth defects at 4°C on days 3, 6, and 9 as determined by bacterial numbers data and OD600 measurements (Suppl. Fig. S2). Because the ΔsigL strain had borderline significantly reduced growth as assessed by bacterial numbers at day 12 (P = 0.018) (Fig. 1) and no clear patterns of reduced growth on days 3, 6, and 9 (Suppl. Fig. S2) or as indicated by the OD600 data (Suppl. Fig. S1), this mutant was considered to have limited evidence of reduced growth at 4°C. Although the ΔrsbT and ΔrsbV strains had significantly reduced growth over 12 days as compared with the parent strain (P < 0.0001), the ΔsigB strain did not have evidence of reduced growth (Fig. 1). Although the ΔctsR strain had reduced growth as compared with the parent strain (P < 0.001), growth of the ΔhrcA and ΔctsRΔhrcA strains did not differ significantly from that of the parent strain (Fig. 1). The ΔopuCA strain had no growth defect even though an opuC transposon mutant previously had reduced growth at 7°C in Pine’s medium containing carnitine (2).
FIGURE 1.
Growth in BHI broth after 12 days at 4°C of L. monocytogenes strain 10403S and null mutant strains with a starting inoculum of 108 CFU/ml. Log-phase cells (OD600 = 0.4) grown at 37°C were inoculated into prechilled (4°C) BHI broth to an OD600 of 0.15 ± 0.05 (equivalent to approximately 108 CFU/ml). Growth between days 0 and 12 (shown on the y axis) represent the increase in CFU per milliliter from day 0 to day 12. Data represent three independent experiments; error bars indicate standard deviations. LS means in a mixed effects model with experiment as a blocking variable was used to determine whether growth for a given mutant strain differed significantly from that for the L. monocytogenes parent strain (10403S). ***, **, and *, Growth of a given mutant strain differed (P < 0.0001, <0.01, or <0.05, respectively) from growth of the parent strain. Corresponding OD600 data are shown in Supplemental Figure S1.
Growth of L. monocytogenes 10403S and selected deletion mutant strains at 4°C using a low-concentration inoculum
To confirm the growth defects observed for selected mutant strains in the high-inoculum experiment at 4°C (and because L. monocytogenes typically contaminates refrigerated foods at low levels, and therefore growth is required to reach human infectious doses), we assessed the growth of selected L. monocytogenes mutant strains at 4°C over 84 days in experiments using a low-concentration starting inoculum (102 CFU/ml; Suppl. Fig. S3A). Strains tested in this experiment were (i) the L. monocytogenes parent strain (10403S), (ii) null mutants that had reduced growth at 4°C in the high-inoculum experiment (i.e., ΔsigC, ΔsigH, ΔsigL, ΔctsR, ΔrsbV, ΔlisR, Δlmo1060, and Δlmo1172), (iii) the ΔsigB strain, which did not have reduced growth at 4°C in the high-inoculum experiment but had previously been reported to have reduced growth at low temperatures in at least some types of media (3), and (iii) the ΔkdpE and ΔhrcA strains, which did not have reduced growth in the high-inoculum experiment (thus serving as negative controls). A ΔprfA strain also was included because PrfA, which positively regulates virulence genes in L. monocytogenes (9), has temperature-dependent expression with maximum activity at 37°C (24).
The average initial (day 0) inoculum in the low-inoculum experiments was 2.13 ± 0.12 log CFU/ml (average for all strains); at day 0, the strain variable was not significant (P > 0.2; mixed effects model), indicating a similar starting inoculum of approximately 102 CFU/ml for all strains. Separate analyses of variance (ANOVAs) for bacterial numbers data for different days of growth at 4°C revealed a significant effect of the strain on bacterial numbers on most of the days later in the experiment (i.e., days 28, 35, 42, 70, and 84) but not earlier (i.e., days 7, 14, and 21) (Suppl. Fig. S3). This finding indicates that none of the mutant strains tested had reduced growth at 4°C during log phase (i.e., days 7, 14, and 21; Suppl. Fig. S3), but growth of at least some mutant strains differed from that of the parent strain during stationary phase (i.e., after day 21; Suppl. Fig. S3). Specifically, ΔctsR had clear although limited reduction in stationary-phase survival at 4°C, and ΔctsR cell numbers were significantly lower than those for the parent strain at days 28, 35, 42, 70, and 84 (Suppl. Fig. S3). The ΔsigB strain had slightly (<1 log CFU/ml) but significantly lower numbers than the parent strain at days 35 and 42 (Suppl. Fig. S3); the Δlmo1172 and ΔsigC strains had slightly (<1 log CFU/ml) but also significantly lower numbers than the parent strain at day 42. The ΔrsbV and ΔsigL strains had slightly (<1 log CFU/ml) but significantly higher bacterial numbers than the parent strains at day 35 (9.2 log CFU/ml for both mutant strains and 9.0 log CFU/ml for the parent strain). Overall, our data indicate that none of the mutant strains tested (except for the ΔctsR strain, which also showed slightly reduced growth at 37°C) had sustained and consistent reductions in growth or survival at 4°C as compared with the parent strain when a low-concentration starting inoculum (102 CFU/ml) was used.
Growth of selected cold-adapted L. monocytogenes mutant strains at 4°C using a high-concentration inoculum
To further resolve the apparent discrepancies between the high-inoculum and low-inoculum experiments (i.e., a number of L. monocytogenes null mutant strains had reduced growth or survival in the high-inoculum experiment but not in the low-inoculum experiment), we repeated the high-inoculum experiment with cold-adapted L. monocytogenes (pregrown in BHI broth for 43 days at 4°C) as an inoculum. Strains tested in this experiment were (i) the parent strain (10403S), (ii) all mutant strains that had reduced growth in the high-inoculum experiment (i.e., ΔctsR, ΔsigC, ΔsigH, ΔsigL, ΔrsbV, ΔlisR, Δlmo1060, and Δlmo1172) except ΔrsbT, and (iii) ΔhrcA, which did not have a growth defect in the initial high-inoculum experiment (and thus served as a control). An ANOVA revealed no significant effect (P > 0.1; GLM) of the strain (parent strain or mutant strains) on growth (in log CFU per milliliter) (Suppl. Fig. S4), indicating that cold adaptation of L. monocytogenes results in similar growth among the parent and selected mutant strains at 4°C. These findings suggest a specific defect in the ability of these mutant strains to adapt to 4°C when they were pregrown at 37°C.
DISCUSSION
Characterization of a core set of 23 L. monocytogenes in-frame deletion mutant strains in genes encoding alternative σ factors, TCRSs, and negative regulators revealed that (i) selected σ factors, TCRRs, and negative regulators specifically contribute to L. monocytogenes cold adaptation but have limited contributions to cold growth at 4°C in rich medium and (ii) inoculum concentration and pregrowth conditions can affect L. monocytogenes cold growth and survival phenotypes.
Although strains with mutations in genes encoding TCRSs (i.e., strains ΔlisR, Δlmo1060, and Δlmo1172), in genes encoding alternative σ factors (i.e., strains ΔsigC and ΔsigH), and in genes encoding two regulators of σB activity (strains ΔrsbV and ΔrsbT) had reduced growth or survival at 4°C when BHI broth was inoculated with high bacterial numbers, these same mutant strains had very limited or no reductions in growth or survival at 4°C when BHI broth was inoculated with a low bacterial numbers (allowing for extended growth) or when BHI broth was inoculated with high numbers of cold-adapted cells. We thus conclude that selected TCRSs (i.e., LisR, Lmo1060, and Lmo1172) and the alternative σ factors σC and σH contribute to L. monocytogenes cold adaptation but make limited or no contributions to L. monocytogenes cold growth. Previous studies indicate that LisK is involved in osmotolerance (38), which further supports a role for LisRK in cold adaptation because a number of osmotolerance genes also have been linked to cold adaptation (e.g., opuC and gbu) (2, 31).
L. monocytogenes σC, which appears to contribute to cold adaptation based on the data from the present study, has previously been classified as a thermal resistance regulator (45), with transcription of the sigC operon induced during temperature upshift. However, sigC is present only in L. monocytogenes lineage II strains (e.g., strain 10403S), and lineage II strains are generally more common in RTE foods and are usually found at considerably higher levels in naturally contaminated RTE foods than are lineage I strains (14, 36), which lack a sigC gene. Thus, it is tempting to speculate that the action of σC in cold adaptation may contribute to the apparent enhanced ability of lineage II strains to grow in refrigerated RTE foods.
Although two regulators of σB activity (i.e., RsbV and RsbT) appear to contribute to L. monocytogenes cold adaptation, an L. monocytogenes ΔsigB strain pregrown at 37°C did not have evidence of reduced ability to adapt to 4°C in BHI broth. This observation is intriguing because the same ΔsigB, ΔrsbV, and ΔrsbT strains consistently had identical phenotypic characteristics under a number of other stress conditions (e.g., acid stress, oxidative stress, and carbon starvation) (12). Contributions of regulators of σB to cold adaptation are consistent with observations that the gene strings rsbR-rsbS-rsbT-rsbU and rsbV-rsbW-sigB-rsbX have higher transcript levels in L. monocytogenes (11) and B. subtilis (7), respectively, grown at low temperatures. Overall, these findings suggest that regulators of σB may affect L. monocytogenes cold adaptation through mechanisms other than regulation of σB activity. Under cold stress, RsbV and RsbT may activate σ factors other than σB. This hypothesis is consistent with evidence in B. subtilis that some regulators of σ factor activity can, under specific stress conditions, act promiscuously to contribute to regulation of σ factors other than their primary targets (8). Although in previous growth studies, reduced growth of a 10403S ΔsigB strain (compared with its parent strain) at 8°C in defined medium (3) and reduced survival of an L. monocytogenes serotype 4c ΔsigB strain in meat stored at 4°C (33) were reported, we did not find evidence for reduced growth of the ΔsigB strain in BHI broth at 4°C. Cold-growth differences for ΔsigB strains in defined medium and rich medium may indicate that nutrients (e.g., solutes) available in the medium may affect the contributions of L. monocytogenes σB to cold growth and cold adaptation.
Although some of the mutant strains tested in this study had reduced cold growth from a starting inoculum of 108 CFU/ml grown at 37°C, the ΔctsR strain was the only mutant that showed evidence for reduced cold growth or survival at 4°C regardless of starting inoculum (102 or 108 CFU/ml grown at 37°C). Because this mutant strain also had slightly reduced growth at 37°C, the effect of the ctsR null mutations on cold growth and survival may reflect a general growth defect.
Overall, our data clearly indicate that inoculum concentration and pregrowth conditions can affect cold-growth phenotypes. Among the L. monocytogenes mutant strains that had reduced growth at 4°C after inoculation into BHI broth at high numbers, most strains had limited or no evidence of growth defects in BHI broth at 4°C after inoculation with 102 CFU/ml and none had reduced growth in BHI broth at 4°C after inoculation with cold-adapted bacteria. Moorhead and Dykes (34) previously reported differences between the phenotypic characteristics of prechilled L. monocytogenes cells and cells grown at 30°C. Although these and other similar findings are not necessarily surprising, they reemphasize the importance of careful choice of pregrowth conditions for bacteria prior to phenotypic characterization, including the importance of using bacteria pregrown at different temperatures for experiments aimed at assessing cold adaptation and cold-growth phenotypes.
Various regulatory proteins play roles in L. monocytogenes cold adaptation, but null mutations in single genes encoding regulatory proteins have limited effects on cold growth and survival of cold-adapted L. monocytogenes in rich medium. However, the ability of L. monocytogenes to grow in most RTE foods at refrigeration temperatures may depend on some of the regulators tested here, because many refrigerated RTE foods impose multiple stresses (e.g., acid and osmotic stress) on L. monocytogenes and some of the regulatory proteins tested here (e.g., σB and LisRK) clearly contribute to acid and osmotolerance (15, 17, 18, 28, 38, 39, 42). Although evaluation of L. monocytogenes mutant strains in BHI broth can provide some initial insights into the importance of different regulatory proteins in L. monocytogenes cold growth and cold adaptation and may be representative for some RTE foods (e.g., milk), further characterization of the mutant strains in actual RTE foods is necessary.
ACKNOWLEDGMENTS
We thank Sherry E. Roof, Alphina J. Ho, and Matthew Stasiewicz for assistance with growth experiments and Karlyn Beer, Wan-Lin Su, Ute Schwab, Sara R. Milillo, and Courtney R. Lucas for assistance with mutant strain construction. We also thank G. M. Smith for the ΔopuC strain and N. Freitag for the ΔprfA strain. Y. C. Chan was supported by a U.S. Department of Agriculture (USDA) National Needs Fellowship grant (to K. J. Boor). This work also was supported in part by the National Institutes of Health (award no. RO1-AI052151-01A1 to K. J. Boor) and the USDA National Research Initiative (project no. 2005-35201-15330 to K. J. Boor).
REFERENCES
- 1.Angelidis AS, Smith GM. Three transporters mediate uptake of glycine betaine and carnitine by Listeria monocytogenes in response to hyperosmotic stress. Appl. Environ. Microbiol. 2003;69:1013–1022. doi: 10.1128/AEM.69.2.1013-1022.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelidis AS, Smith LT, Hoffman LM, Smith GM. Identification of OpuC as a chill-activated and osmotically activated carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 2002;68:2644–2650. doi: 10.1128/AEM.68.6.2644-2650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker LA, Evans SN, Hutkins RW, Benson AK. Role of σB in adaptation of Listeria monocytogenes to growth at low temperature. J. Bacteriol. 2000;182:7083–7087. doi: 10.1128/jb.182.24.7083-7087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedie GK, Samelis J, Sofos JN, Belk KE, Scanga JA, Smith GC. Antimicrobials in the formulation to control Listeria monocytogenes postprocessing contamination on frankfurters stored at 4°C in vacuum packages. J. Food Prot. 2001;64:1949–1955. doi: 10.4315/0362-028x-64.12.1949. [DOI] [PubMed] [Google Scholar]
- 5.Bishop DK, Hinrichs DJ. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 6.Brøndsted L, Kallipolitis BH, Ingmer H, Knöchel S. kdpE and a putative RsbQ homologue contribute to growth of Listeria monocytogenes at high osmolarity and low temperature. FEMS Microbiol. Lett. 2003;219:233–239. doi: 10.1016/S0378-1097(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 7.Budde I, Steil L, Scharf C, Völker U, Bremer E. Adaptation of Bacillus subtilis to growth at low temperature: a combined transcriptomic and proteomic appraisal. Microbiology. 2006;152:831–853. doi: 10.1099/mic.0.28530-0. [DOI] [PubMed] [Google Scholar]
- 8.Carniol K, Kim TJ, Price CW, Losick R. Insulation of the σF regulatory system in Bacillus subtilis. J. Bacteriol. 2004;186:4390–4394. doi: 10.1128/JB.186.13.4390-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty T, Leimeister-Wachter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan YC, Boor KJ, Wiedmann M. σB-dependent and -independent mechanisms contribute to transcription of Listeria monocytogenes cold stress genes during cold shock and cold growth. Appl. Environ. Microbiol. 2007;73:6010–6029. doi: 10.1128/AEM.00714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan YC, Raengpradub S, Boor KJ, Wiedmann M. Microarray-based characterization of the Listeria monocytogenes cold regulon in log- and stationary-phase cells. Appl. Environ. Microbiol. 2007;73:6484–6498. doi: 10.1128/AEM.00897-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturongakul S, Boor KJ. RsbT and RsbV contribute to σB-dependent survival under environmental, energy, and intracellular stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 2004;70:5349–5356. doi: 10.1128/AEM.70.9.5349-5356.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaturongakul S, Boor KJ. σB activation under environmental and energy stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 2006;72:5197–5203. doi: 10.1128/AEM.03058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Ross WH, Gray MJ, Wiedmann M, Whiting RC, Scott VN. Attributing risk to Listeria monocytogenes subgroups: dose response in relation to genetic lineages. J. Food Prot. 2006;69:335–344. doi: 10.4315/0362-028x-69.2.335. [DOI] [PubMed] [Google Scholar]
- 15.Cotter PD, Emerson N, Gahan CG, Hill C. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J. Bacteriol. 1999;181:6840–6843. doi: 10.1128/jb.181.21.6840-6843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira A, Gray M, Wiedmann M, Boor KJ. Comparative genomic analysis of the sigB operon in Listeria monocytogenes and in other gram-positive bacteria. Curr. Microbiol. 2004;48:39–46. doi: 10.1007/s00284-003-4020-x. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira A, O’Byrne CP, Boor KJ. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl. Environ. Microbiol. 2001;67:4454–4457. doi: 10.1128/AEM.67.10.4454-4457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira A, Sue D, O’Byrne CP, Boor KJ. Role of Listeria monocytogenes σB in survival of lethal acidic conditions and in the acquired acid tolerance response. Appl. Environ. Microbiol. 2003;69:2692–2698. doi: 10.1128/AEM.69.5.2692-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P, Chakraborty T, Charbit A, Chetouani F, Couve E, de Daruvar A, Dehoux P, Domann E, Dominguez-Bernal G, Duchaud E, Durant L, Dussurget O, Entian KD, Fsihi H, Garcia-del Portillo F, Garrido P, Gautier L, Goebel W, Gomez-Lopez N, Hain T, Hauf J, Jackson D, Jones LM, Kaerst U, Kreft J, Kuhn M, Kunst F, Kurapkat G, Madueno E, Maitournam A, Vicente JM, Ng E, Nedjari H, Nordsiek G, Novella S, de Pablos B, Perez-Diaz JC, Purcell R, Remmel B, Rose M, Schlueter T, Simoes N, Tierrez A, Vazquez-Boland JA, Voss H, Wehland J, Cossart P. Comparative genomics of Listeria species. Science. 2001;294:849–852. doi: 10.1126/science.1063447. [DOI] [PubMed] [Google Scholar]
- 20.Hartke A, Frère J, Boutibonnes P, Auffray Y. Differential induction of the chaperonin GroEL and the co-chaperonin GroES by heat, acid, and UV-irradiation in Lactococcus lactis subsp. lactis. Curr. Microbiol. 1997;34:23–26. doi: 10.1007/s002849900138. [DOI] [PubMed] [Google Scholar]
- 21.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 22.Helmann JD, Wu MF, Kobel PA, Gamo FJ, Wilson M, Morshedi MM, Navre M, Paddon C. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 2001;183:7318–7328. doi: 10.1128/JB.183.24.7318-7328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Y, Raengpradub S, Schwab U, Loss C, Wiedmann M, Boor KJ. Unpublished data. [Google Scholar]
- 24.Johansson J, Mandin P, Renzoni A, Chiaruttini C, Springer M, Cossart P. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell. 2002;110:551–561. doi: 10.1016/s0092-8674(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 25.Junttila JR, Niemela SI, Hirn J. Minimum growth temperatures of Listeria monocytogenes and non-haemolytic Listeria. J. Appl. Bacteriol. 1988;65:321–327. doi: 10.1111/j.1365-2672.1988.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 26.Kallipolitis BH, Ingmer H. Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol. Lett. 2001;204:111–115. doi: 10.1111/j.1574-6968.2001.tb10872.x. [DOI] [PubMed] [Google Scholar]
- 27.Karatzas KA, Wouters JA, Gahan CG, Hill C, Abee T, Bennik MH. The CtsR regulator of Listeria monocytogenes contains a variant glycine repeat region that affects piezotolerance, stress resistance, motility and virulence. Mol. Microbiol. 2003;49:1227–1238. doi: 10.1046/j.1365-2958.2003.03636.x. [DOI] [PubMed] [Google Scholar]
- 28.Kazmierczak MJ, Mithoe SC, Boor KJ, Wiedmann M. Listeria monocytogenes σB regulates stress response and virulence functions. J. Bacteriol. 2003;185:5722–5734. doi: 10.1128/JB.185.19.5722-5734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilstrup M, Jacobsen S, Hammer K, Vogensen FK. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl. Environ. Microbiol. 1997;63:1826–1837. doi: 10.1128/aem.63.5.1826-1837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H, Boor KJ, Marquis H. Listeria monocytogenes σB contributes to invasion of human intestinal epithelial cells. Infect. Immun. 2004;72:7374–7378. doi: 10.1128/IAI.72.12.7374-7378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko R, Smith LT, Smith GM. Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J. Bacteriol. 1994;176:426–431. doi: 10.1128/jb.176.2.426-431.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Graham JE, Bigelow L, Morse PD, II, Wilkinson BJ. Identification of Listeria monocytogenes genes expressed in response to growth at low temperature. Appl. Environ. Microbiol. 2002;68:1697–1705. doi: 10.1128/AEM.68.4.1697-1705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mbandi E, Shelef LA. Enhanced inhibition of Listeria monocytogenes and Salmonella Enteritidis in meat by combinations of sodium lactate and diacetate. J. Food Prot. 2001;64:640–644. doi: 10.4315/0362-028x-64.5.640. [DOI] [PubMed] [Google Scholar]
- 34.Moorhead SM, Dykes GA. Influence of the sigB gene on the cold stress survival and subsequent recovery of two Listeria monocytogenes serotypes. Int. J. Food Microbiol. 2004;91:63–72. doi: 10.1016/S0168-1605(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 35.Nair S, Derré I, Msadek T, Gaillot O, Berche P. CtsR controls class III heat shock gene expression in the human pathogen Listeria monocytogenes. Mol. Microbiol. 2000;35:800–811. doi: 10.1046/j.1365-2958.2000.01752.x. [DOI] [PubMed] [Google Scholar]
- 36.Piffaretti JC, Kressebuch H, Aeschbacher M, Bille J, Bannerman E, Musser JM, Selander RK, Rocourt J. Genetic characterization of clones of the bacterium Listeria monocytogenes causing epidemic disease. Proc. Natl. Acad. Sci. USA. 1989;86:3818–3822. doi: 10.1073/pnas.86.10.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salotra P, Singh DK, Seal KP, Krishna N, Jaffe H, Bhatnagar R. Expression of DnaK and GroEL homologs in Leuconostoc esenteroides in response to heat shock, cold shock or chemical stress. FEMS Microbiol. Lett. 1995;131:57–62. doi: 10.1111/j.1574-6968.1995.tb07754.x. [DOI] [PubMed] [Google Scholar]
- 38.Sleator RD, Hill C. A novel role for the LisRK two-component regulatory system in listerial osmotolerance. Clin. Microbiol. Infect. 2005;11:599–601. doi: 10.1111/j.1469-0691.2005.01176.x. [DOI] [PubMed] [Google Scholar]
- 39.Sue D, Fink D, Wiedmann M, Boor KJ. σB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology. 2004;150:3843–3855. doi: 10.1099/mic.0.27257-0. [DOI] [PubMed] [Google Scholar]
- 40.Walker SJ, Stringer MF. Growth of Listeria monocytogenes and Aeromonas hydrophila at chill temperatures. J. Appl. Bacteriol. 1987;63:R20. [Google Scholar]
- 41.Wemekamp-Kamphuis HH, Wouters JA, de Leeuw PP, Hain T, Chakraborty T, Abee T. Identification of sigma factor σB-controlled genes and their impact on acid stress, high hydrostatic pressure, and freeze survival in Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 2004;70:3457–3466. doi: 10.1128/AEM.70.6.3457-3466.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiedmann M, Arvik TJ, Hurley RJ, Boor KJ. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams T, Bauer S, Beier D, Kuhn M. Construction and characterization of Listeria monocytogenes mutants with in-frame deletions in the response regulator genes identified in the genome sequence. Infect. Immun. 2005;73:3152–3159. doi: 10.1128/IAI.73.5.3152-3159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong KK, Freitag NE. A novel mutation within the central Listeria monocytogenes regulator PrfA that results in constitutive expression of virulence gene products. J. Bacteriol. 2004;186:6265–6276. doi: 10.1128/JB.186.18.6265-6276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang C, Nietfeldt J, Zhang M, Benson AK. Functional consequences of genome evolution in Listeria monocytogenes: the lmo0423 and lmo0422 genes encode σC and LstR, a lineage II–specific heat shock system. J. Bacteriol. 2005;187:7243–7253. doi: 10.1128/JB.187.21.7243-7253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]