Abstract
Obesity is associated with a range of health outcomes that are of clinical and public health significance, including cancer. Herein, we summarize epidemiologic and preclinical evidence for an association between obesity and increased risk of breast and prostate cancer incidence and mortality. Moreover, we describe data from observational studies of weight change in humans and from calorie restriction studies in mouse models which support a potential role for weight loss in counteracting tumor-promoting properties of obesity in breast and prostate cancers. Given that weight loss is challenging to achieve and maintain, we also consider evidence linking treatments for obesity-associated co-morbidities, including metformin, statins and non-steroidal anti-inflammatory drugs, with reduced breast and prostate cancer incidence and mortality. Finally, we highlight several challenges that should be considered when conducting epidemiologic and preclinical research in the area of obesity and cancer, including the measurement of obesity in population-based studies, the timing of obesity and weight change in relation to tumor latency and cancer diagnosis, and the heterogeneous nature of obesity and its associated co-morbidities. Given that obesity is a complex trait, comprised of behavioral, epidemiologic and molecular/metabolic factors, we argue that a transdisciplinary approach is the key to understanding the mechanisms linking obesity and cancer. As such, this review highlights the critical need to integrate evidence from both epidemiologic and preclinical studies to gain insight into both biologic and non-biologic mechanisms contributing to the obesity-cancer link.
Keywords: aspirin, breast cancer, cholesterol, epidemiology, insulin, prostate cancer, mechanisms, metformin, mouse models, NSAIDs, obesity, screening, statins, transdisciplinary, weight loss
1. Introduction
Cancer is predicted to overtake heart disease as the leading cause of death across all age groups in the US by 2030, translating to a 45% increase in the number of cancer diagnoses in the next 15 years (American Society of Clinical Oncology 2014). Global obesity prevalence has been increasing by approximately half a body mass index (BMI) unit per decade over the past three decades, resulting in over 600 million adults worldwide with a BMI of 30 kg/m2 or greater (Finucane, et al. 2011; Stevens, et al. 2012). With more than one in three US adults classified as obese, the prevalence of obesity in the US is currently the highest in the Western world (Finucane et al. 2011; Ogden, et al. 2014).
Obesity is associated with increased risk of a variety of different cancer types (World Cancer Research Fund 2007). Of these obesity-associated cancer types, almost 13% of incident cases worldwide, and approximately 20% of incident cases in Europe and North America, are attributable to obesity (Arnold, et al. 2014). Furthermore, it is estimated that one quarter of these cases could have been avoided had the worldwide obesity prevalence not approximately doubled since 1980 (Arnold, et al. 2014). Overweight and obesity also drive cancer progression, and have been estimated to account for 14% of all cancer deaths in men and 20% in women in the United States (Calle, et al. 2003), while approximately 6% of cancer deaths around the same time period in Europe were attributable to obesity (Banegas, et al. 2003).
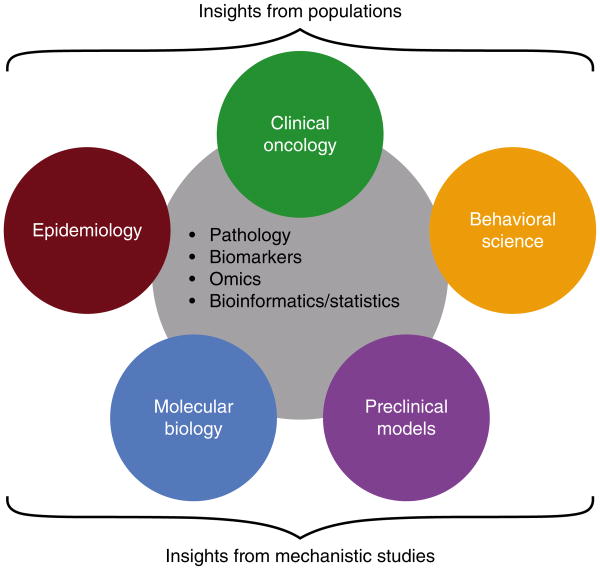
Cancers of the breast and prostate are among the most commonly diagnosed and among the leading causes of cancer deaths in women and men, respectively, both in the US and worldwide (Jemal, et al. 2011; Siegel, et al. 2013). Therefore, understanding mechanisms linking obesity and risk of these common tumor types will be important for cancer prevention efforts worldwide. In addition, of over 11 million US individuals living with cancer, survivors of breast cancer constitute the largest group (22%), followed by survivors of prostate cancer (19%) (Centers for Disease Control & Prevention 2011). Therefore, understanding mechanisms linking obesity and cancer progression in these common tumor types has great importance for a large proportion of cancer survivors, and will likely also benefit survivors of other obesity-associated cancer types. Molecular mechanisms linking obesity and cancer have been reviewed in depth elsewhere (Allott, et al. 2013b; Ford, et al. 2013a; Lashinger, et al. 2014a). As such, this review adopts a transdisciplinary approach, summarizing findings from both epidemiologic and preclinical studies (Figure 1), in addition to examining evidence for the modifiable nature of obesity and related co-morbidities through weight loss and pharmacologic interventions in both humans and mouse models. Finally, we consider how a transdisciplinary approach can minimize the weaknesses and maximize the strengths of each discipline, enabling a deeper understanding of mechanisms linking obesity and cancer.
Figure 1.
Using a transdisciplinary approach to study mechanisms linking obesity and cancer.
2. Transdisciplinary insights into associations between obesity and cancer
i. Obesity and breast cancer
The association between obesity and breast cancer risk is complex, varying by menopausal status and by breast cancer subtype. Obesity is associated with reduced breast cancer incidence in premenopausal women (World Cancer Research Fund 2007), but increased breast cancer incidence in postmenopausal women (Munsell, et al. 2014; World Cancer Research Fund 2007), although the association with postmenopausal breast cancer is attenuated in women using hormone replacement therapy (Munsell et al. 2014). While these contrasting associations by menopausal status are consistently reported, differences in these associations by hormone receptor status are less well understood. Within postmenopausal breast cancers, there is a suggestion that the association with obesity is strongest among hormone receptor-positive cases (Althuis, et al. 2004; Canchola, et al. 2012; Rosenberg, et al. 2006; Suzuki, et al. 2009), although other studies did not find differences in these associations by breast cancer subtype (Millikan, et al. 2008; Phipps, et al. 2008; Yang, et al. 2011). Several studies have reported that, although obesity is a protective factor for total premenopausal breast cancer, it is associated with increased risk of triple negative and basal-like disease in premenopausal women (Gaudet, et al. 2011; Millikan et al. 2008; Yang et al. 2011). Interestingly, some evidence suggests that obesity may be a risk factor for basal-like breast cancer regardless of menopausal status (Millikan et al. 2008), suggesting a role for non-hormonal mechanisms in basal-like breast cancer pathogenesis.
While the association between obesity and breast cancer risk differs by menopausal status, obesity is associated with increased risk of breast cancer recurrence and mortality in both pre and postmenopausal women (Chan, et al. 2014; Niraula, et al. 2012). While several studies have suggested that this association may be stronger among women with hormone receptor-positive tumors (Azrad and Demark-Wahnefried 2014; Jiralerspong, et al. 2013; Sparano, et al. 2012; Tait, et al. 2014), others reported no difference in the association between obesity and breast cancer-specific mortality by subtype (Niraula et al. 2012; Phipps et al. 2008). However, partial availability of subtype data and lower numbers of patients with rarer breast cancer subtypes is a limitation for many studies (Althuis et al. 2004). Given evidence for etiologic heterogeneity of breast cancer, understanding the obesity-breast cancer link in humans requires large, well-annotated studies with sufficient power to conduct stratified analysis both by menopausal status and subtype.
The recognition of breast cancer as a heterogeneous disease has led to the characterization of mouse models which reflect human breast cancer subtypes (Herschkowitz, et al. 2007). One particular challenge of preclinical models of breast cancer is that estrogen receptor (ER) is weakly expressed in most mouse mammary tumors, particularly in genetically-engineered mice, and thus murine models of hormone receptor-positive breast cancer may not be fully representative of human luminal breast cancer (Borowsky 2011; Herschkowitz et al. 2007). Nonetheless, diet-induced obesity has been demonstrated to drive tumor growth in a variety of mouse models with both luminal (Ford, et al. 2013b; Pape-Ansorge, et al. 2002) and basal-like tumor characteristics (Dogan, et al. 2007; Dunlap, et al. 2012; Giles, et al. 2012; Hakkak, et al. 2007; Nogueira, et al. 2012; Sundaram, et al. 2013), lending support to epidemiologic observations. In contrast, there is little preclinical support for links between obesity and luminal B or HER-2 breast cancer subtypes (Cleary, et al. 2004; Ford, et al. 2013b), although the degree to which these models are representative of these human subtypes is unclear. Finally, one study showed that diet-induced obesity enhanced the growth of luminal-like tumors in ovariectomized mice, but not in mice with intact ovaries (Nunez, et al. 2008), suggesting that the relationship between obesity and postmenopausal luminal breast cancer should be tested in ovariectomized mice in order to model the human postmenopausal environment. However, given epidemiologic evidence supporting an association between obesity and basal-like breast cancer regardless of menopausal status, the relevance of ovariectomization to study the impact of diet-induced obesity on basal-like breast cancer in mouse models is less clear.
ii. Obesity and prostate cancer
Although individual studies are conflicted regarding the association between obesity and prostate cancer risk, a number of large meta-analyses have reported that obesity is associated with a modestly elevated total prostate cancer incidence (Bergstrom, et al. 2001; Hu, et al. 2014; MacInnis and English 2006; Renehan, et al. 2008). One meta-analysis demonstrated that findings from the individual contributing studies differed by geographic region (Renehan et al. 2008), thereby offering insight into these somewhat conflicted results. Prostate specific antigen (PSA) levels are reduced in obese men via hemodilution (Banez, et al. 2007), thereby lowering the likelihood of a PSA-driven biopsy and giving rise to an obesity-associated detection bias. This bias becomes apparent when comparing the results of US studies where PSA screening is widespread, with the results of European studies where PSA screening is less common (Renehan et al. 2008). In the US, where prostate biopsies are largely driven by PSA screening, obese men have a reduced chance of undergoing biopsy compared to normal weight men, leading to the detection of fewer cancers in obese individuals and biasing the association between obesity and prostate cancer towards the null. In countries with lower PSA screening rates, such as Europe and Australia, this detection bias is reduced and meta-analysis of studies from these regions demonstrates a positive association between obesity and prostate cancer risk (Renehan et al. 2008). These data highlight the importance of considering how the association between obesity and prostate cancer risk is impacted by mode of cancer detection, particularly in the context of changing PSA screening recommendations in the US (Moyer and U.S. Preventative Services Task Force 2012).
While the association between obesity and total prostate cancer is complicated by obesity-associated detection bias, there is consistent and convincing evidence for an association between obesity and elevated risk of aggressive prostate cancer (Rodriguez, et al. 2007; Zhang, et al. 2015). Furthermore, multiple large studies, both before and after the introduction of widespread PSA screening in the US, demonstrated an association between obesity and increased prostate cancer-specific mortality (Andersson, et al. 1997; Cao and Ma 2011; Rodriguez, et al. 2001; Wright, et al. 2007; Zhang et al. 2015), indicating that obesity-associated detection bias does not completely explain the association between obesity and prostate cancer, but that biologic mechanisms must also play a role.
Consistent with epidemiologic evidence for an association between obesity and tumor aggressiveness and progression, tumor growth in mouse models of prostate cancer has been shown to be responsive to obesity. A number of studies have demonstrated a role for diet-induced obesity in promoting prostate tumor growth in the transgenic adenocarcinoma of the prostate (TRAMP) mouse model (Bonorden, et al. 2012; Llaverias, et al. 2010). In addition, diet-induced obesity reduced tumor latency in the Hi-Myc mouse model, via increased Akt/mTOR signaling (Blando, et al. 2011; Kobayashi, et al. 2008), and promoted tumor progression in a transgenic mouse model with PTEN haploinsufficiency, via increased inflammatory and insulin signaling pathways (Liu, et al. 2015). These transgenic models may have relevance to human prostate cancer given that Myc copy number is amplified in up to one third of human prostate cancers (Ellwood-Yen, et al. 2003) and PTEN loss is the most common genetic alteration in human prostate cancer (Wang, et al. 2003).
3. Non-biologic mechanisms contributing to the obesity - cancer link
i. Obesity and cancer screening
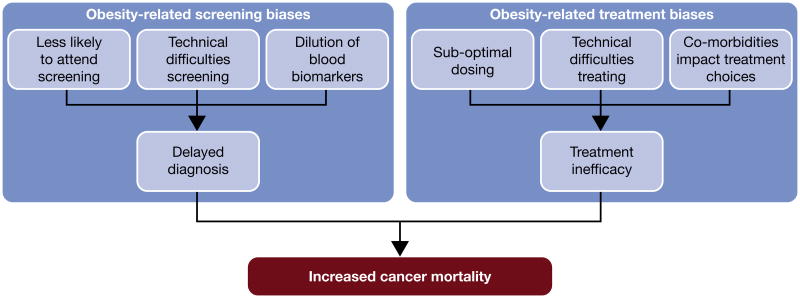
Evidence suggests that obesity-associated screening and detection biases may act to delay cancer diagnosis, thereby increasing cancer-specific mortality in obese patients (Figure 2). Obese individuals may be less likely to participate in screening programs which could contribute to delayed cancer diagnosis, an association shown to be modified by the type of screening test, by race and by gender (Fagan, et al. 2011). Indeed, mammography screening rates are reduced by as much as 10% in obese (BMI ≥ 30 kg/m2) and 20% in morbidly obese (BMI ≥ 40 kg/m2) women (Maruthur, et al. 2009) and while the reasons for reduced participation in cancer screening are not completely understood, they may include modesty, pain, and/or competing healthcare demands (Friedman, et al. 2012).
Figure 2.
Non-biologic mechanisms linking obesity and cancer mortality.
In contrast to associations with breast cancer screening, an inverse association between obesity and PSA screening frequency has been reported in prostate cancer, with obese men being screened more frequently than their normal weight counterparts (Scales, et al. 2007). However, obesity is associated with reduced likelihood of prostate cancer diagnosis in a screened population despite higher PSA screening frequency in obese men, suggesting that obesity may decrease screening effectiveness in some cancer types. In obese prostate cancer patients, their larger body size and bigger prostate make conducting a digital rectal exam more challenging (Chu, et al. 2011), and a large prostate may reduce the likelihood of finding the cancer at biopsy (Freedland, et al. 2006). This is also true for other cancer types; mammography is anecdotally more difficult in obese patients (Amy, et al. 2006), potentially delaying cancer diagnosis even in screened obese individuals. Indeed, obesity has been associated with higher stage at breast cancer diagnosis (Cui, et al. 2002), suggesting that obese women may be diagnosed later in the course of their disease. However, another study reported this finding regardless of mammography screening frequency, suggesting that the association between obesity and high stage breast cancer may not simply be a result of lower screening rates in this population (Kerlikowske, et al. 2008), but that biologic mechanisms must also play a role.
Finally, the larger blood volume in obese individuals has been associated with biomarker hemodilution, as has been suggested in PSA-detected prostate cancer (Banez et al. 2007). Several studies have estimated that PSA levels are reduced by approximately 15% in cancer-free morbidly obese (BMI ≥ 35 kg/m2) men, while PSA mass (i.e. the absolute quantity of PSA in the blood) is not associated with obesity status (Grubb, et al. 2009; Rundle and Neugut 2009). Lower biomarker levels in obesity reduce the likelihood of reaching biopsy thresholds, potentially contributing to delayed diagnosis in obese individuals.
ii. Obesity and cancer treatment
In addition to biologic mechanisms [reviewed in (Lashinger, et al. 2014b)], non-biologic factors also contribute to reduced treatment efficacy in obese patients (Figure 2). There is considerable evidence to suggest that obese cancer patients are undertreated with systemic therapies (Lyman and Sparreboom 2013), and this may negatively impact cancer outcomes. Traditionally, chemotherapy dosing is based upon the body surface area (BSA) of the patient. However, there is uncertainty regarding dosing of obese patients and evidence that dose reduction occurs in this population, either because an idealized body weight is used to calculate BSA, or because BSA is arbitrarily capped due to toxicity concerns (Lyman, et al. 2003). Current clinical guidelines for chemotherapy dosing in obese patients indicate that weight-based chemotherapy doses should be given and that dose should not be reduced for obese patients (Griggs, et al. 2012). As such, it is recommended that any modifications to dose due to toxicity concerns or presence of co-morbidities should be made independent of the patient's obesity status (Lyman and Sparreboom 2013).
In prostate cancer, obesity is associated with increased daily prostate shift, rendering external beam radiation treatment more technically challenging, and potentially contributing to increased rates of treatment failure in obese patients (Merrick, et al. 2007). Technical difficulties applying the adequate radiation dose to the correct area in obese patients has also been suggested in breast cancer (Carmichael and Bates 2004). In addition to these potential difficulties with radiation therapy, obese patients may also be less likely to make good surgical candidates. In prostate cancer, obesity has been shown to be associated with capsular incision, reflecting a less-than-ideal operation (Freedland, et al. 2005). However, even when focusing solely on patients with organ-confined disease and negative surgical margins, obesity remains associated with biochemical recurrence following radical prostatectomy, implying that obesity is associated with disease progression in prostate cancer through mechanisms other than how it may affect surgical technique (Freedland, et al. 2004).
Obesity and related co-morbidities are associated with increased risk of adverse treatment effects, which may impact the treatment plan (Schmitz, et al. 2013). In breast cancer, obesity has been associated with a higher risk of lymphedema, in addition to other treatment-related side effects (Togawa, et al. 2014). Obese, diabetic breast cancer patients undergoing chemotherapy have increased likelihood of side effects including infection and chemotherapy-related toxicity (Srokowski, et al. 2009). In addition, cytotoxic therapy has been associated with treatment-related weight gain and metabolic syndrome in breast cancer patients (Bicakli, et al. 2014; Makari-Judson, et al. 2014), potentially exacerbating these aforementioned side effects. In prostate cancer, obesity is associated with weight gain and increased risk of diabetes following androgen deprivation therapy (Keto, et al. 2011; Tsai, et al. 2014), particularly among older men (Morgans, et al. 2014). Given that more men diagnosed with prostate cancer die from cardiovascular disease than from prostate cancer (Allott et al. 2013b), this relationship has important implications for prostate cancer survivors.
Finally, excess body weight has been linked to altered metabolism of cytotoxic drugs and therapies, potentially via a number of mechanisms including altered levels of circulating growth factors, hormones and cytokines (Litton, et al. 2008; Rodvold, et al. 1988). Despite evidence that aromatase inhibitors may not be as efficacious in obese breast cancer patients (Azrad and Demark-Wahnefried 2014; Ioannides, et al. 2014; Wolters, et al. 2012), standard doses are given irrespective of BSA or body size (Goodwin and Pritchard 2010). In contrast, response to tamoxifen has not been shown to differ by obesity status (Wolters et al. 2012), and no differences in ER-positive breast cancer recurrence rates by obesity status have been reported following tamoxifen treatment (Dignam, et al. 2003). Finally, one trial suggested that obese breast cancer patients treated with chemotherapy had reduced disease-free survival relative to normal weight patients, even after controlling for other prognostic factors (de Azambuja, et al. 2010). In prostate cancer, obesity at the time of androgen deprivation therapy is associated with increased risk of castrate-resistant prostate cancer, metastasis, and prostate cancer-specific mortality (Keto et al. 2011). Although the exact explanation for this is unclear, one study found that testosterone levels in obese men on androgen deprivation therapy were higher, suggesting inadequate testosterone suppression (Smith 2007). It has been hypothesized that this may be because the amount of gonadotropin-releasing hormone analogue given is the same regardless of BSA, and thus obese men may be under dosed. However, while these non-biological factors must be considered, they cannot completely explain the association between obesity and cancer mortality, and biologic mechanisms also contribute (Lashinger et al. 2014b).
4. Obesity and related co-morbidities as modifiable lifestyle factors
i. Weight gain and loss
Evidence for the association between obesity and cancer is substantiated by studies showing that weight change can impact both risk and survival for obesity-associated cancers (Figure 3). Bariatric procedures to cause weight loss have been associated with reduced risk of obesity-associated cancer types and a 40-50% decrease in cancer-specific mortality across cancer types (Adams, et al. 2009; Sjostrom, et al. 2007), suggesting that weight loss may be an effective chemopreventive strategy in obese individuals (Ashrafian, et al. 2011). Interestingly, one prospective intervention trial suggested that bariatric procedures may be more effective at decreasing cancer risk in women, compared to men (Sjostrom, et al. 2009), although these results should be interpreted with caution given the smaller sample size of males and mean follow-up time of only one decade, which may not be a long enough latency period for certain cancer types to manifest (Renehan 2009). Aside from these studies of bariatric patients, the majority of evidence supporting a role for weight change in breast cancer incidence and mortality comes from secondary analyses of observational studies. Of note, the most common pattern of weight change over time is consistent weight gain throughout adulthood (Harvie, et al. 2005), and therefore statistical power to study the impact of weight loss is often limited.
Figure 3.
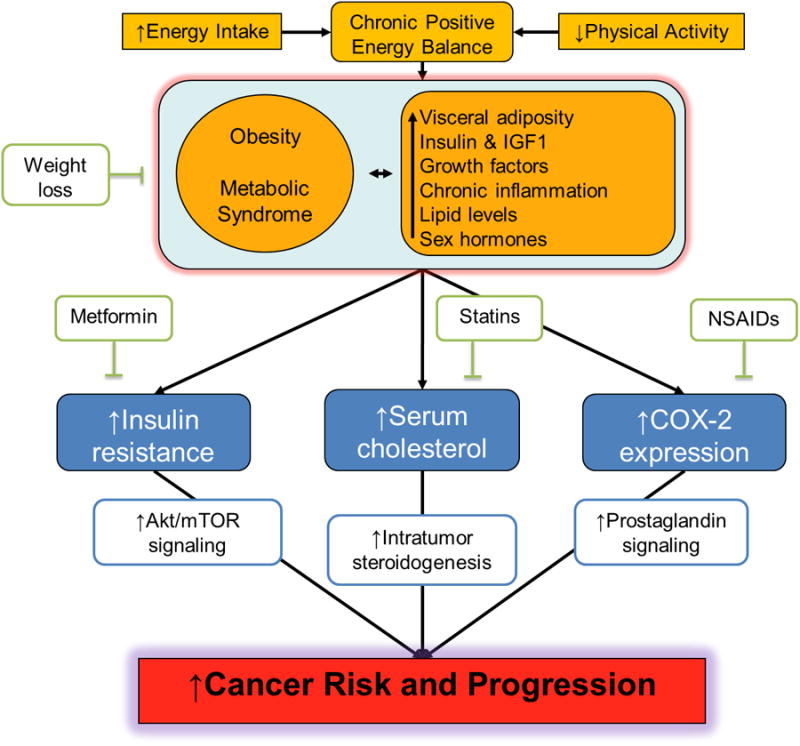
Putative mechanisms linking obesity with cancer risk and progression: lessons from studies of chemopreventive agents
Adult weight gain is associated with increased risk of postmenopausal breast cancer (Eliassen, et al. 2006), with a suggestion of a stronger association for hormone receptor-positive breast cancer (Eliassen et al. 2006; Vrieling, et al. 2010). Furthermore, adult weight gain prior to diagnosis is associated with increased mortality from postmenopausal breast cancer (Cleveland, et al. 2007). Conversely, weight loss during adulthood, whether before or after menopause, has been associated with decreased risk of postmenopausal breast cancer (Eliassen et al. 2006; Harvie et al. 2005), again with the strongest inverse associations for hormone receptor-positive breast cancer (Eliassen et al. 2006). While clinical trials to formally examine the impact of weight loss on breast cancer-specific mortality have not yet been conducted, two trials in breast cancer survivors reported that weight loss of >10% of body mass can be achieved in this population (Befort, et al. 2012; Goodwin, et al. 2014). Furthermore, a weight loss intervention in cancer-free postmenopausal women suggested that 10% weight loss is sufficient to positively impact biomarker levels associated with breast cancer in both serum and benign breast tissue (Fabian, et al. 2013), indicating that such an intervention may be both feasible and worthwhile (Irwin 2014).
Evidence from mouse models of breast cancer showing that weight loss impacts tumor growth supports these epidemiologic data. Weight loss can be achieved in mouse models by calorie restriction (CR), a 20-40% reduction in total energy intake relative to a control group fed ad libitum, arguably one of the most potent dietary regimens for suppressing carcinogenesis (Hursting, et al. 2010). Despite the suggestion of stronger epidemiologic associations between weight change and hormone receptor-positive breast cancer, CR slows tumor growth in mouse models regardless of tumor subtype (Dunlap et al. 2012; Ford et al. 2013b; Mizuno, et al. 2013; Nogueira et al. 2012; Pape-Ansorge et al. 2002), providing rationale to study the impact of weight loss across breast cancer subtypes in humans. Mechanistic insights from these models have highlighted Akt/mTOR signaling and the insulin/insulin-like growth factor (IGF) 1 axis as two pro-tumor pathways upregulated by obesity and reversed by CR (Hursting et al. 2010). Interestingly, a protective effect of intermittent CR on tumor growth has also been reported (Rogozina, et al. 2013), and this dietary regimen may be more feasible and appealing to humans. While the majority of preclinical studies randomize mice to CR from the outset, two preclinical studies have specifically tested the impact of diet-induced obesity followed by weight loss, by incorporating a diet switch during the intervention period. Interestingly, these studies reported contrasting findings, with one reporting that weight loss reversed the tumor-promoting effect of obesity (Sundaram, et al. 2014b), and the other reporting the weight loss did not impact obesity-fueled tumor growth (De Angel, et al. 2013). Key differences between studies included the degree of obesity attained by the mice and the timing and duration of weight loss, in addition to the use of two different mouse models of basal-like breast cancer, one xenograft (De Angel et al. 2013) and the other transgenic (Sundaram et al. 2014b). Discrepant findings such as these may shed light on mechanisms linking weight loss and breast cancer, in addition to informing future study design in both preclinical mouse models and humans.
Retrospective analyses of large cohort studies have also revealed inverse associations between weight loss and prostate cancer incidence and mortality. Relative to weight maintenance, adult weight gain is associated with increased risk of aggressive prostate cancer (Bassett, et al. 2011), while adult weight loss is associated with reduced risk of aggressive prostate cancer (Rodriguez et al. 2007). Furthermore, some large prospective cohort studies have reported that weight gain in adulthood is associated with increased prostate cancer-specific mortality (Bassett et al. 2011; Wright et al. 2007), although others reported no association between adult weight change and prostate cancer-specific mortality (Chamberlain, et al. 2011; Moller, et al. 2013). Studies focused on weight change within the decade of prostate cancer diagnosis report more consistent findings, with weight gain in the five year time period preceding diagnosis associated with increased risk of recurrence (Joshu, et al. 2011; Whitley, et al. 2011), and weight gain in the five year time period following diagnosis associated with increased risk of prostate cancer-specific mortality (Bonn, et al. 2014).
While evidence for a role for weight loss in counteracting tumor growth in mouse models of prostate cancer is relatively sparse, a tumor-inhibitory effect of CR has been demonstrated via reduced insulin/IGF1 levels and increased tumor apoptosis in a xenograft mouse model (Galet, et al. 2013), although this effect has not been reported in all xenograft models (Buschemeyer, et al. 2010; Thomas, et al. 2010). In addition, one study demonstrated that CR slowed progression to adenocarcinoma in the Hi-Myc transgenic mouse model of prostate cancer, via reduced prostate inflammation and inhibition of Akt/mTOR signaling (Blando et al. 2011). CR has also been demonstrated to impact tumor growth in the TRAMP model, with intermittent CR having a greater impact than chronic CR (Bonorden, et al. 2009), although a role for intermittent CR has not been supported by other prostate cancer studies (Buschemeyer et al. 2010; Thomas et al. 2010).
Given the challenges surrounding compliance with CR and maintenance of weight loss in humans, alternative strategies are also worth exploring. Intermittent CR is one such strategy, and while one randomized weight loss trial in women showed that both intermittent and chronic CR caused weight loss and improved insulin sensitivity and cytokine profile (Harvie, et al. 2011), avoiding weight gain may remain the most sensible approach both for general health in addition to chemoprevention (Thompson and McTiernan 2011). Alternatively, understanding the mechanisms by which CR increases tumor latency and slows tumor progression may help to identify CR mimetics with chemopreventive properties [reviewed in (Hursting et al. 2010)].
ii. Pharmaceuticals
a. Type II diabetes and metformin
Type II diabetes currently affects approximately 15% of the adult population in the US, and the prevalence of this obesity-associated co-morbidity is on the increase (Boyle, et al. 2010). Epidemiologic data support an association between diabetes and increased risk of certain cancer types, including breast cancer (De Bruijn, et al. 2013; Giovannucci, et al. 2010; Larsson, et al. 2007; Tsilidis, et al. 2015b). Although not all studies have reported subtype-specific differences in this association (Campos-Gomez, et al. 2014), there is some evidence that the association between diabetes and breast cancer risk may be stronger in postmenopausal women (Larsson et al. 2007; Michels, et al. 2003) and for hormone receptor-positive breast cancer (Michels et al. 2003). In addition, a meta-analysis of clinical trials and prospective cohort studies reported that diabetes is associated with increased breast cancer-specific mortality, although subtype was not examined (De Bruijn et al. 2013). In direct contrast to the positive association with breast cancer risk, there is an inverse association between diabetes and total prostate cancer incidence (Kasper and Giovannucci 2006; Zhang, et al. 2012). Temporal analysis has demonstrated that longer duration of diabetes is more protective for total prostate cancer risk (Kasper, et al. 2009; Tsilidis, et al. 2015a), suggesting that the metabolic and hormonal environment of advanced/end stage diabetes, characterized by reduced bioavailable testosterone and low insulin, is consistent with protection from total prostate cancer incidence (Allott, et al. 2013a; Kasper and Giovannucci 2006). However, longer duration of diabetes in obese men was associated with increased risk of metastasis following radical prostatectomy (Wu, et al. 2013), and diabetes has been associated with increased risk of prostate cancer-specific mortality (Cai, et al. 2014), suggesting that the low insulin environment of advanced diabetes may select for more aggressive prostate cancers which can survive in this environment (Allott et al. 2013a). Therefore, while associations with diabetes are contrasting for breast and prostate cancer incidence, associations with cancer progression and mortality are consistent for both tumor types.
Relative to other anti-diabetic therapies, metformin has been associated with reduced total cancer incidence in some (Decensi, et al. 2010; Evans, et al. 2005), but not all studies (Tsilidis, et al. 2014), and with decreased cancer-specific mortality (Currie, et al. 2012, Lega, et al 2014). In line with these findings, evidence supporting a role for metformin in breast cancer is somewhat mixed. A meta-analysis reported that metformin use was associated with reduced breast cancer incidence and mortality (Zhang, et al. 2013), but other studies failed to demonstrate a protective effect of metformin on breast cancer incidence (Franciosi, et al. 2013; Kowall, et al. 2015; Tsilidis, et al. 2014) or mortality (Lega, et al. 2014). Furthermore, there is inconsistent evidence for differences in these associations by breast cancer subtype, with one study reporting a larger proportion of progesterone receptor-positive tumors in metformin users versus non-users (Berstein, et al. 2011), while another study did not find any difference in the frequency of hormone receptor-positive tumors by metformin use (Besic, et al. 2014). A trial in which women were randomized to metformin one month prior to breast cancer surgery found that metformin use reduced Ki67 expression in HER2-positive resected tumors (DeCensi, et al. 2014), while another study reported that metformin use was associated with reduced HER2-positive breast cancer-specific mortality (He, et al. 2012). In support of these epidemiologic data, preclinical data show that metformin suppressed overexpression of the HER2 protein via Akt/mTOR pathway inhibition (Vazquez-Martin, et al. 2009), and delayed the onset of adenocarcinoma in a transgenic mouse model of HER2-positive breast cancer (Anisimov, et al. 2010). However, metformin has also been shown to slow tumor growth in an ER-positive mouse model of breast cancer, via suppression of obesity-associated adipokine levels and reduced Akt/mTOR pathway activation (Fuentes-Mattei, et al. 2014), suggesting that the potential chemopreventive effect of metformin may not be limited to HER2-positive tumors. Finally, a preclinical study reported that metformin does not impact tumor growth in nondiabetic rat and mouse models (Thompson, et al. 2015), a finding supported by epidemiologic data that metformin impacts Ki67 tumor expression in insulin resistant but not in insulin sensitive women (DeCensi et al. 2014).
In prostate cancer, although some studies report inconsistent findings (Azoulay, et al. 2011; Currie, et al. 2009; Franciosi, et al. 2013; Murtola, et al. 2008; Wright and Stanford 2009), mounting evidence supports an inverse association between metformin use and prostate cancer risk in men with diabetes (Clyne 2014; Preston, et al. 2014; Yu, et al. 2014a). Fewer studies have examined the association between metformin use and prostate cancer recurrence and mortality, with somewhat mixed results. While several individual studies reported null findings (Allott et al. 2013a; Kaushik, et al. 2013; Patel, et al. 2010), a meta-analysis demonstrated that metformin use was associated with reduced risk of recurrence following primary therapy (Yu et al. 2014a), and there is evidence to support an inverse association between metformin use and prostate cancer-specific mortality (Bensimon, et al. 2014; Margel, et al. 2013), although this has not been reported by all studies (Lega, et al. 2014). Further supporting a chemopreventive role for metformin in prostate cancer, metformin slowed tumor growth in a xenograft mouse model of prostate cancer (Ben Sahra, et al. 2008), in addition to reducing IGF1 levels and modestly counteracting the tumor-promoting properties of a high fat diet in a small study using the TRAMP model (Xu, et al. 2014). Finally, metformin reduced mouse prostatic intraepithelial neoplasia (mPIN) development and slowed transition to adenocarcinoma in the Hi-Myc transgenic mouse model of prostate cancer, via down regulation of Myc gene expression (Akinyeke, et al. 2013).
While data from both preclinical and population-based studies support an antineoplastic role for metformin, translating findings from laboratory studies to humans is rendered more challenging by high metformin doses commonly used in animal and cell-based models, often exceeding recommended therapeutic doses in humans (Ben Sahra, et al. 2010). In addition, the progressive nature of diabetes and the various medications used to control it makes population-based research into the impact of metformin on cancer risk and mortality somewhat challenging. Time-related biases are a consideration in observational studies of drug use and may lead to overestimation of the inverse association between metformin and cancer (Suissa and Azoulay 2012). However, a meta-analysis of prospective studies which controlled for obesity status and which were not subject to time-related biases concluded that metformin was associated with a modest reduction in overall cancer risk (Gandini, et al. 2014). Whether metformin should be considered as a chemopreventive agent for individuals without diabetes is another issue. Preclinical studies have demonstrated direct action of metformin on cancer cells through AMPK activation and mTOR signaling (Hardie and Alessi 2013; Zhou, et al. 2001), suggesting that metformin may also have chemopreventive properties in individuals without insulin resistance or diabetes. However, a body of evidence in both population and preclinical mouse models suggests that metformin impacts tumor growth only in the context of insulin resistance, obesity, and/or diabetes, indicating that improving insulin sensitivity may be a key indirect mechanism by which metformin impacts cancer growth (Bonanni at al. 2012; DeCensi et al. 2014; Thompson et al. 2015). This proposed mechanism is further supported by evidence linking elevated levels of C-peptide (a surrogate for insulin levels) with breast and prostate cancer-specific mortality (Goodwin, et al. 2012; Ma, et al. 2008). Therefore, while evidence suggests that metformin may be most beneficial for preventing cancer growth in insulin resistant individuals via indirect mechanisms, future studies should explore potential direct mechanisms using preclinical models and metformin doses which reflect those used in humans.
b. Inflammation and non-steroidal anti-inflammatory drugs
Chronic, low-grade inflammation is a hallmark of obesity, and has been proposed as a mechanistic link between obesity and cancer (Colotta, et al. 2009). Metabolism of arachidonic acid, a major component of animal fat, by cyclooxygenase (COX) generates prostaglandin and other eicosanoids, a group of biologically active lipids that play a key role in inflammation. Obesity is associated with elevated levels of COX-2 expression and heightened prostaglandin signaling (Subbaramaiah, et al. 2012), suggesting a targetable inflammatory mechanism linking obesity and cancer. The anti-inflammatory properties of non-steroidal anti-inflammatory drugs (NSAIDs) are mediated via COX inhibition which, in turn, reduces prostaglandin levels (Wang and Dubois 2010). While the strongest evidence for a role for NSAIDs in chemoprevention comes from inverse associations with adenoma and colorectal cancer (Baron, et al. 2003; Rostom, et al. 2007), there is also support for a role for NSAIDs in other cancer types (Wang and Dubois 2010).
While normal breast tissue expresses low levels of COX-2, approximately 40% of invasive breast cancers overexpress this enzyme, and elevated levels are associated with increased risk of breast cancer-specific mortality (Ristimaki, et al. 2002). The inverse association between NSAID use and breast cancer incidence does not appear to differ significantly by drug type, with similar effect estimates reported for both aspirin and non-aspirin NSAIDs (de Pedro, et al. 2015). However, a randomized trial of low-dose aspirin failed to demonstrate an inverse association for breast cancer incidence (Cook, et al. 2005), potentially suggesting that a higher dose may be required for chemoprevention. Some studies report that the protective association between NSAID use and breast cancer is limited to hormone receptor-positive disease (de Pedro et al. 2015; Terry, et al. 2004), and others have reported a stronger inverse association between NSAID use and breast cancer-specific mortality in hormone receptor-positive cases (Allott, et al. 2014d). Given that COX-2-mediated prostaglandin production promotes estrogen biosynthesis via up regulation of the aromatase pathway (Zhao, et al. 1996), there is biologic rationale to support a role for NSAIDs in suppressing hormone receptor-positive breast cancer incidence and progression. Indeed, NSAID use is associated with reduced serum estradiol levels in women with breast cancer (Hudson, et al. 2008), which may impact the growth of estrogen-responsive tumors. Finally, in further support of a true biologic association between NSAID use and breast cancer, there is evidence that longer duration of NSAID use is more protective for both breast cancer incidence (Terry et al. 2004) and breast cancer-specific mortality (Allott et al. 2014d).
Studies in breast cancer mouse models support a role for COX-2 in promoting tumor growth (Liu, et al. 2001; Lyons, et al. 2011) and for selective COX-2 inhibitors, including celecoxib, in inhibiting tumor growth (Chang, et al. 2004). One study showed that celecoxib was most protective in HER2-expressing mouse models of breast cancer, via reduction of prostaglandin levels (Howe, et al. 2002), indicating a potential role for COX-2 inhibition in preventing HER2-positive breast cancer. However, while COX-2 overexpression has been reported in HER2-positive breast cancers in women (Ristimaki et al. 2002; Subbaramaiah, et al. 2002), the impact of NSAID use in this tumor subtype has not been widely examined, perhaps due to the relatively low frequency of HER2-enriched breast cancers in the human population. Finally, a study in a rat model of breast cancer showed that NSAIDs were only effective in hormone-responsive tumors (Woditschka, et al. 2008), supporting epidemiologic literature reporting stronger associations between NSAID use and hormone receptor-positive breast cancer risk and mortality.
Similar to breast cancer, there is evidence for overexpression of COX-2 in prostate cancer, relative to non-cancerous tissue or benign prostatic hyperplasia (Yoshimura, et al. 2000), and elevated expression is associated with increased prostate cancer-specific mortality (Khor, et al. 2007). A meta-analysis reported an inverse association between NSAID use and aggressive prostate cancer risk which was strongest amongst aspirin users, particularly longer duration aspirin users (Liu, et al. 2014). However, a randomized trial of celecoxib for 4-6 weeks in patients with localized prostate cancer had no impact on prostaglandin levels in the prostate (Antonarakis, et al. 2009), potentially suggesting an exposure period of insufficient length to impact prostate cancer biology. Interestingly, the association between aspirin use and aggressive prostate cancer incidence appears to differ by geographic region, with a significant protective effect evident in North American, but not in European studies (Liu et al. 2014; Wang, et al. 2014). Given that anti-inflammatory agents such as NSAIDs can lower PSA levels (Chang, et al. 2010), this geographic disparity in results may be attributable in part to differences in screening practices. However, one North American study where prostate cancer screening was largely independent of PSA testing reported that aspirin and NSAID use was associated with reduced risk of aggressive disease (Vidal, et al. 2014), supporting a true biologic association between NSAID use and prostate cancer. Finally, a number of studies in the TRAMP mouse model of prostate cancer support a role for COX inhibition in prostate cancer chemoprevention, reporting that celecoxib reduced tumor size (Gupta, et al. 2004), caused regression of mPIN lesions (Narayanan, et al. 2004) and inhibited the development of adenocarcinoma in a dose-dependent fashion (Narayanan, et al. 2006).
One limitation of many observational studies of NSAID use is incomplete capture of NSAID use information, with many studies focusing solely on aspirin or ibuprofen use, or on prescription NSAIDs only (Allott et al. 2014d; de Pedro et al. 2015). Incomplete NSAID use data may attenuate the association between NSAID use and cancer, since “unexposed” individuals may have taken other types of NSAIDs that were not ascertained during data collection, especially given the widespread use of these drugs. In addition, confounding by indication is an important consideration in observational studies of NSAID use, as users may be more likely to have chronic conditions such as arthritis for which they are taking NSAIDs (Allott et al. 2014d). Finally, although epidemiologic and preclinical evidence supports a role for COX inhibition in chemoprevention, the clinical utility of selective COX-2 inhibitors is limited due to cardiovascular and gastrointestinal side effects (Wang and Dubois 2010). As such, understanding the mechanisms linking NSAID use and cancer will result in the identification of additional biomarkers and therapeutic targets, potentially enabling the development of therapeutic agents with fewer side effects than existing COX inhibitors.
c. Hypercholesterolemia and statins
Hypercholesterolemia, an obesity-associated co-morbidity, affects approximately 20% of the US adult population (Fryar, et al. 2012). Cholesterol is an essential plasma membrane component of animal cells and plays a crucial role in maintaining membrane fluidity, in addition to regulating intracellular signaling processes (Krycer and Brown 2013). The role of cholesterol as the precursor for endogenous sex steroid biosynthesis suggests its potential importance in both breast and prostate cancer, two hormone-dependent tumor types.
Dietary cholesterol intake is associated with increased risk of breast cancer, with evidence for a dose-dependent effect across increasing quartiles of cholesterol intake (Hu, et al. 2012). Moreover, a large prospective study of over one million adults in Korea showed that serum cholesterol levels were associated with increased risk of breast cancer (Kitahara, et al. 2011). Consistent with the role of cholesterol as a precursor for sex steroid biosynthesis, studies which stratified by hormone receptor status reported that elevated serum cholesterol levels were associated with increased risk of hormone receptor-positive disease (Fagherazzi, et al. 2010) and that high dietary cholesterol intake was associated with increased risk of ER-positive, but not ER-negative disease (Jakovljevic, et al. 2002). Finally, a recent study demonstrated that 27-hydroxycholesterol (27-HC), the most abundant cholesterol metabolite in the circulation and an endogenous selective estrogen receptor modulator (SERM), promoted ER-positive breast cancer progression in multiple mouse models (Nelson, et al. 2013), suggesting a mechanistic link between cholesterol and breast cancer.
Relative to other organs of the body, normal prostate epithelial cells have high cholesterol content and these levels increase further during progression to prostate cancer (Krycer and Brown 2013), suggesting that cholesterol accumulation may be advantageous to prostate cancer progression. In support of this hypothesis, hypermethylation of the cholesterol efflux transporter ABCA1, an epigenetic silencing mechanism leading to accumulation of intratumoral cholesterol, has been associated with increased risk of aggressive prostate cancer (Lee, et al. 2013). There is a suggestion that elevated cholesterol may be associated with increased risk of aggressive prostate cancer (Farwell, et al. 2011; Mondul, et al. 2011; Platz, et al. 2008; Platz, et al. 2009; Shafique, et al. 2012) but not total prostate cancer (Mondul, et al. 2010; Platz et al. 2008), although other studies have reported no association between cholesterol or its sub-fractions and prostate cancer risk (Jacobs, et al. 2012; Martin, et al. 2009). While studies examining prostate cancer progression are few, positive associations between elevated serum cholesterol levels and risk of prostate cancer recurrence (Allott, et al. 2014b) and mortality (Batty, et al. 2011) have been reported. In support of these epidemiologic associations, cholesterol has been shown to promote prostate cancer cell line growth in vitro and in vivo (Zhuang, et al. 2002), while reduction of serum cholesterol levels lowered intratumoral androgen levels and slowed tumor growth in xenograft mouse models of human prostate cancer (Mostaghel, et al. 2012), supporting the hypothesis that steroid biosynthesis may be an important mechanism linking cholesterol and prostate cancer.
Statins are cost-effective and widely prescribed cholesterol-lowering drugs with proven benefits for cardiovascular disease prevention (Ridker and Cook 2013), and are used by approximately one in every four adults in the US population (Gu Q 2014). Statin use has been associated with reduced total cancer risk (Farwell, et al. 2008) and lower cancer-specific mortality (Nielsen, et al. 2012) via a number of potential mechanisms including mevalonate pathway inhibition (Pelton, et al. 2012), reduced inflammation and angiogenesis (Pelton et al. 2012) and lower serum cholesterol levels (Demierre, et al. 2005). Given that the bioavailability of statins in the circulation is low (Roy, et al. 2011), their cholesterol-lowering properties may be the most relevant for breast and prostate cancers.
Despite biologic rationale for a role of statins in breast cancer chemoprevention, the majority of evidence supports no association between statin use and total breast cancer risk (Bonovas, et al. 2005; Undela, et al. 2012), and studies examining this association by breast cancer subtype are few. While one study reported reduced rates of ER-negative breast cancers among statin users (Kumar, et al. 2008), another found a null association overall and no evidence of effect modification by hormone receptor status (Desai, et al. 2013). However, epidemiologic evidence supports an inverse association between statin use and risk of breast cancer recurrence and mortality. Statin use after a breast cancer diagnosis has been associated with reduced risk of recurrence, with a stronger association for lipophilic statins (Ahern, et al. 2011; Kwan, et al. 2008). Furthermore, a study of over 30,000 breast cancer cases in the Finnish Cancer Registry showed an inverse effect of both pre and post-diagnosis statin use on breast cancer-specific mortality, with evidence for a dose and time-dependent effect among pre-diagnosis users (Murtola, et al. 2014). Another study in the UK Cancer Registry also reported a weak protective effect of statin use on breast cancer-specific mortality, with some evidence that simvastatin use showed the strongest association (Cardwell, et al. 2015). Studies stratifying by hormone receptor status are required, as this information has been lacking in previous studies (Cardwell et al. 2015; Murtola et al. 2014).
In prostate cancer, while the preponderance of evidence does not support an association between statin use and total prostate cancer risk (Bansal, et al. 2012; Bonovas, et al. 2008; Browning and Martin 2007; Dale, et al. 2006; Kuoppala, et al. 2008), an inverse association between statin use and risk of aggressive disease has been consistently reported (Bansal et al. 2012; Bonovas et al. 2008). Regarding prostate cancer outcomes after treatment, three meta-analyses have reported a null association between statin use and risk of recurrence (Mass, et al. 2012; Park, et al. 2013; Scosyrev, et al. 2013). However, the studies contributing to these meta-analyses were few and most examined statin use at the time of prostate cancer treatment. Given the widespread use of statins, it is likely that many nonusers became statin users after treatment, which may bias the results of these previous studies towards the null. A retrospective analysis which minimized misclassification of statin users by excluding prevalent users at the time of prostate cancer treatment and capturing statin use throughout the follow up period showed that post-diagnosis statin use was associated with reduced risk of recurrence (Allott, et al. 2014a), suggesting a true protective association between statin use and prostate cancer. Finally, an analysis of almost 12,000 men with localized prostate cancer showed that post-diagnostic statin use was associated with reduced prostate cancer-specific mortality, with evidence for a stronger protective effect amongst those who had also used statins before diagnosis (Yu, et al. 2014b). Given that cardiovascular disease and cancer are the two most common causes of mortality in the US (American Society of Clinical 2014), understanding the potential role of cholesterol reduction for cancer prevention will have important public health impact.
5. Transdisciplinary challenges facing obesity and cancer research
Obesity is a complex and heterogeneous exposure, giving rise to a number of considerations and challenges for obesity and cancer research, particularly in translating findings from preclinical to population-based research, and vice versa.
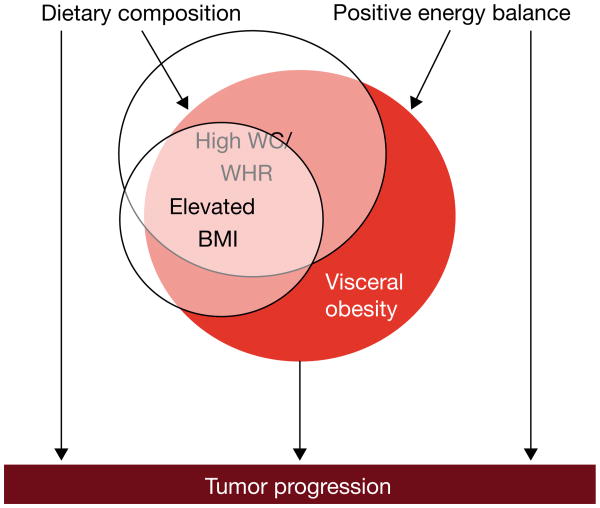
First, inter-individual variation in adipose tissue distribution leads to challenges in defining obesity status for population-based research. BMI is a well-validated surrogate of overall obesity with the important strength of being widely used, thus enabling inter-study comparisons (Allott et al. 2013b). However, visceral obesity, accumulation of adipose tissue within the abdominal cavity, is a risk factor for cardiovascular disease (Neeland, et al. 2013) and certain types of cancer, including breast and prostate (Doyle, et al. 2012; Pischon, et al. 2008). Population-based studies rely on waist circumference and waist-to-hip ratio as surrogates of visceral obesity, given that computed tomography (CT), the gold standard method for direct quantification of visceral fat area (VFA), is not feasible for population-based research. While these surrogate measures correlate more strongly with VFA than BMI does (Allott, et al. 2014c), it is important to consider that they do not distinguish between subcutaneous and visceral adipose tissue at waist level (Figure 4), potentially giving rise to some misclassification of visceral obesity status. Despite this limitation, waist circumference and waist-to-hip ratio offer advantages over BMI for estimating visceral obesity, given that these measures are less influenced by lean body mass. Of note, the prevalence of visceral obesity (measured by VFA or waist circumference) in the US population is higher than that of overall obesity (measured by BMI), with approximately 45% of men and women classified as viscerally obese by VFA (Pou, et al. 2009), and approximately 40% of men and 60% of women classified as viscerally obese by waist circumference (Li, et al. 2007), while only one third of individuals are classified as obese by BMI (Ogden, et al. 2014). As such, defining obesity using BMI underestimates the prevalence of visceral obesity in the population. Moreover, roughly 20% of men and 10% of women with BMI ≥30 kg/m2 do not have elevated VFA, while approximately 10% of men and women with BMI <30 kg/m2 have elevated VFA (Pou et al. 2009). Misclassification of visceral obesity status contaminates the “unexposed” (i.e. non-obese) group with “exposed” individuals (i.e. viscerally obese) and vice versa, with the likely result of biasing associations between obesity and cancer towards the null (Figure 5).
Figure 4.
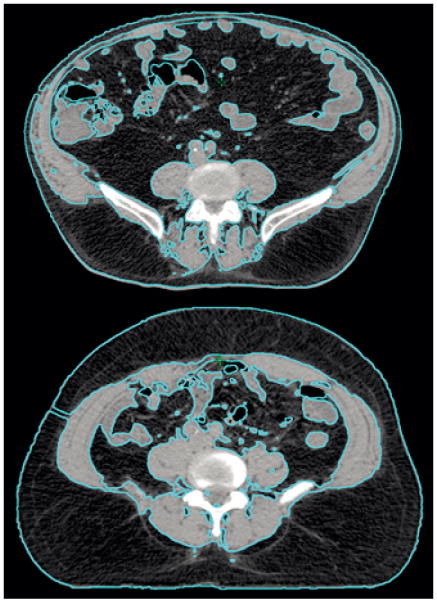
Computed tomography (CT) scans of two individuals with similar waist circumference (WC) but different amounts of visceral and subcutaneous fat area, illustrating the potential for misclassification when using WC as a surrogate of visceral obesity.
Figure 5.
Challenges associated with studying obesity: defining the exposure, and potential confounders.
Second, adipose tissue is a unique organ in its ability to expand and contract throughout the life course of the individual, and the relevant timing or “window of susceptibility” for obesity to impact tumor growth is unknown. Furthermore, population-based studies that examine weight change over the life course are usually limited to self-reported obesity status, and often require individuals to recollect their body weight many decades in the past. However, while self-reported weight is known to be systematically underreported, it remains predictive of obesity-associated co-morbidities and is not outperformed by corrected measures, indicating that it may be sufficient for population-based obesity and cancer research (Dutton and McLaren 2014). Another consideration is that population-based studies of weight change across the life course are limited both by relatively rare occurrences of weight loss and by unknown reasons for weight loss. As such, preclinical models provide an opportunity to evaluate the impact of diet-induced obesity and weight loss at different “windows of susceptibility” over the life course of the mouse, for example, post pregnancy (Sundaram, et al. 2014a). Careful design of preclinical studies with a consideration for timing of weight change in relation to tumor development will help to inform research questions in the population-based setting.
Finally, obesity is a heterogeneous phenotype, and it may be useful to consider it a disease comprised of distinct subtypes (Field, et al. 2013). For example, individuals with elevated BMI may be further stratified according to visceral obesity, diabetes or metabolic syndrome status. This research approach may help us to determine, for example, whether high cholesterol or insulin resistance rather than obesity per se are the driving forces behind the obesity-cancer link. Large, population-based studies with sufficient power for stratified analysis by various components of obesity are required for this approach. In addition, while pharmacoepidemiologic studies provide insight into potential chemopreventive properties of a number of medications which are widely used in the treatment of obesity-associated co-morbidities, these studies are subject to confounding by indication, in addition to other biases inherent in retrospective, observational studies of drug use. As such, results from population-based studies should be interpreted alongside preclinical findings, and alongside randomized controlled clinical trials.
6. Conclusions
The obesity-cancer link is of public health interest given the pervasiveness of both conditions, and the potentially modifiable nature of obesity. Although calorie restriction and weight loss are some of the most effective approaches for inhibiting tumor growth in animal models, weight loss is challenging for humans to achieve and maintain. Furthermore, observational data supporting a role for weight loss in cancer incidence and mortality in humans are lacking, and neither have clinical trials been conducted. While some epidemiologic and preclinical data suggest a chemopreventive role for agents targeting obesity-associated comorbidities, including metformin, NSAIDs and statins, not all studies support these findings and further research is needed. Given that preclinical models may be limited by their relevance to human cancers and observational studies are limited by non-randomized design, integrating findings from different disciplines using a transdisciplinary approach may help us to gain insight into mechanisms linking obesity and cancer. An improved understanding of the mechanisms contributing to the obesity - cancer link will be important for cancer prevention and treatment efforts. In addition, given that the top five causes of mortality in the US are heart disease, stroke, diabetes, kidney disease and cancer (Schmitz et al. 2013), targeting obesity is relevant not only to improve cancer-specific outcomes but also to impact all-cause mortality among cancer survivors.
Acknowledgments
Funding: This work was supported by the National Cancer Institute (R35 CA197627; SDH), the Breast Cancer Research Foundation (SDH), and the University Cancer Research Fund of North Carolina (EHA)
Footnotes
Declaration of Interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
References
- Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, Halverson RC, Simper SC, Hopkins PN, Hunt SC. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17:796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sorensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103:1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyeke T, Matsumura S, Wang X, Wu Y, Schalfer ED, Saxena A, Yan W, Logan SK, Li X. Metformin targets c-MYC oncogene to prevent prostate cancer. Carcinogenesis. 2013;34:2823–2832. doi: 10.1093/carcin/bgt307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott EH, Abern MR, Gerber L, Keto CJ, Aronson WJ, Terris MK, Kane CJ, Amling CL, Cooperberg MR, Moorman PG, et al. Metformin does not affect risk of biochemical recurrence following radical prostatectomy: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2013a;16:391–397. doi: 10.1038/pcan.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, Amling CL, Freedland SJ. Postoperative statin use and risk of biochemical recurrence following radical prostatectomy: Results from the SEARCH database. BJU Int. 2014a;114:661–666. doi: 10.1111/bju.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, Amling CL, Freedland SJ. Serum Lipid Profile and Risk of Prostate Cancer Recurrence: Results from the SEARCH Database. Cancer Epidemiol Biomarkers Prev. 2014b;23:2349–2356. doi: 10.1158/1055-9965.EPI-14-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott EH, Howard LE, Song HJ, Sourbeer KN, Koontz BF, Salama JK, Freedland SJ. Racial differences in adipose tissue distribution and risk of aggressive prostate cancer among men undergoing radiotherapy. Cancer Epidemiol Biomarkers Prev. 2014c;23:2404–2412. doi: 10.1158/1055-9965.EPI-14-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013b;63:800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott EH, Tse CK, Olshan AF, Carey LA, Moorman PG, Troester MA. Non-steroidal anti-inflammatory drug use, hormone receptor status, and breast cancer-specific mortality in the Carolina Breast Cancer Study. Breast Cancer Res Treat. 2014d;147:415–421. doi: 10.1007/s10549-014-3099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–1568. [PubMed] [Google Scholar]
- American Society of Clinical Oncology. The state of cancer care in America, 2014: a report by the American Society of Clinical Oncology. J Oncol Pract. 2014;10:119–142. doi: 10.1200/JOP.2014.001386. [DOI] [PubMed] [Google Scholar]
- Amy NK, Aalborg A, Lyons P, Keranen L. Barriers to routine gynecological cancer screening for White and African-American obese women. Int J Obes (Lond) 2006;30:147–155. doi: 10.1038/sj.ijo.0803105. [DOI] [PubMed] [Google Scholar]
- Andersson SO, Wolk A, Bergstrom R, Adami HO, Engholm G, Englund A, Nyren O. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–389. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV, Karkach AS, Romanyukha AA. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–197. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- Antonarakis ES, Heath EI, Walczak JR, Nelson WG, Fedor H, De Marzo AM, Zahurak ML, Piantadosi S, Dannenberg AJ, Gurganus RT, et al. Phase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkers. J Clin Oncol. 2009;27:4986–4993. doi: 10.1200/JCO.2009.21.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, Ferlay J, Miranda JJ, Romieu I, Dikshit R, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2014;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian H, Ahmed K, Rowland SP, Patel VM, Gooderham NJ, Holmes E, Darzi A, Athanasiou T. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer. 2011;117:1788–1799. doi: 10.1002/cncr.25738. [DOI] [PubMed] [Google Scholar]
- Azoulay L, Dell'Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer Epidemiol Biomarkers Prev. 2011;20:337–344. doi: 10.1158/1055-9965.EPI-10-0940. [DOI] [PubMed] [Google Scholar]
- Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: A review of the recent literature. Curr Nutr Rep. 2014;3:9–15. doi: 10.1007/s13668-013-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banegas JR, Lopez-Garcia E, Gutierrez-Fisac JL, Guallar-Castillon P, Rodriguez-Artalejo F. A simple estimate of mortality attributable to excess weight in the European Union. Eur J Clin Nutr. 2003;57:201–208. doi: 10.1038/sj.ejcn.1601538. [DOI] [PubMed] [Google Scholar]
- Banez LL, Hamilton RJ, Partin AW, Vollmer RT, Sun L, Rodriguez C, Wang Y, Terris MK, Aronson WJ, Presti JC, Jr, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–2280. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- Bansal D, Undela K, D'Cruz S, Schifano F. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One. 2012;7:e46691. doi: 10.1371/journal.pone.0046691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- Bassett JK, Severi G, Baglietto L, Macinnis RJ, Hoang HN, Hopper JL, English DR, Giles GG. Weight change and prostate cancer incidence and mortality. Int J Cancer. 2011;131:1711–1719. doi: 10.1002/ijc.27414. [DOI] [PubMed] [Google Scholar]
- Batty GD, Kivimaki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: forty years of follow-up in the Whitehall study. Cancer Causes Control. 2011;22:311–318. doi: 10.1007/s10552-010-9691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befort CA, Klemp JR, Austin HL, Perri MG, Schmitz KH, Sullivan DK, Fabian CJ. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132:631–639. doi: 10.1007/s10549-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- Bensimon L, Yin H, Suissa S, Pollak MN, Azoulay L. The use of metformin in patients with prostate cancer and the risk of death. Cancer Epidemiol Biomarkers Prev. 2014;23:2111–2118. doi: 10.1158/1055-9965.EPI-14-0056. [DOI] [PubMed] [Google Scholar]
- Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. Int J Cancer. 2001;91:421–430. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Berstein LM, Boyarkina MP, Tsyrlina EV, Turkevich EA, Semiglazov VF. More favorable progesterone receptor phenotype of breast cancer in diabetics treated with metformin. Med Oncol. 2011;28:1260–1263. doi: 10.1007/s12032-010-9572-6. [DOI] [PubMed] [Google Scholar]
- Besic N, Satej N, Ratosa I, Horvat AG, Marinko T, Gazic B, Petric R. Long-term use of metformin and the molecular subtype in invasive breast carcinoma patients - a retrospective study of clinical and tumor characteristics. BMC Cancer. 2014;14:298. doi: 10.1186/1471-2407-14-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicakli DH, Varol U, Degirmenci M, Tunali D, Cakar B, Durusoy R, Karaca B, Ali Sanli U, Uslu R. Adjuvant chemotherapy may contribute to an increased risk for metabolic syndrome in patients with breast cancer. J Oncol Pharm Pract. 2014 doi: 10.1177/1078155214551315. [DOI] [PubMed] [Google Scholar]
- Blando J, Moore T, Hursting S, Jiang G, Saha A, Beltran L, Shen J, Repass J, Strom S, DiGiovanni J. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer Prev Res (Phila) 2011;4:2002–2014. doi: 10.1158/1940-6207.CAPR-11-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanni B, Puntoni M, Cazzaniga M, Pruneri G, Serrano D, Guerrieri-Gonzaga A, Gennari A, Trabacca MS, Galimberti V, Veronesi P, Johansson H, Aristarco V, Bassi F, Luini A, Lazzeroni M, Varricchio C, Viale G, Bruzzi P, Decensi A. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol. 2012;30:2593–2600. doi: 10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- Bonn SE, Wiklund F, Sjolander A, Szulkin R, Stattin P, Holmberg E, Gronberg H, Balter K. Body mass index and weight change in men with prostate cancer: progression and mortality. Cancer Causes Control. 2014;25:933–943. doi: 10.1007/s10552-014-0393-3. [DOI] [PubMed] [Google Scholar]
- Bonorden MJ, Grossmann ME, Ewing SA, Rogozina OP, Ray A, Nkhata KJ, Liao DJ, Grande JP, Cleary MP. Growth and Progression of TRAMP Prostate Tumors in Relationship to Diet and Obesity. Prostate Cancer. 2012;2012 doi: 10.1155/2012/543970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grande JP, Lokshin A, Cleary MP. Cross-sectional analysis of intermittent versus chronic caloric restriction in the TRAMP mouse. Prostate. 2009;69:317–326. doi: 10.1002/pros.20878. [DOI] [PubMed] [Google Scholar]
- Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer. 2008;123:899–904. doi: 10.1002/ijc.23550. [DOI] [PubMed] [Google Scholar]
- Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23:8606–8612. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]
- Borowsky AD. Choosing a mouse model: experimental biology in context--the utility and limitations of mouse models of breast cancer. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120:833–843. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- Buschemeyer WC, 3rd, Klink JC, Mavropoulos JC, Poulton SH, Demark-Wahnefried W, Hursting SD, Cohen P, Hwang D, Johnson TL, Freedland SJ. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate. 2010;70:1037–1043. doi: 10.1002/pros.21136. [DOI] [PubMed] [Google Scholar]
- Cai H, Xu Z, Xu T, Yu B, Zou Q. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: a meta-analysis of 11 cohort studies. Diabetes Metab Res Rev. 2014 doi: 10.1002/dmrr.2582. [DOI] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Campos-Gomez S, Valero V, Flores-Arredondo JH, Isassi-Chapa A, Rangel-Rodriguez I, Hortobagyi GN, Gonzalez-Angulo AM. Breast cancer subtype and baseline characteristics from diabetic breast cancer patients are not different from nondiabetics. Breast J. 2014;20:434–436. doi: 10.1111/tbj.12294. [DOI] [PubMed] [Google Scholar]
- Canchola AJ, Anton-Culver H, Bernstein L, Clarke CA, Henderson K, Ma H, Ursin G, Horn-Ross PL. Body size and the risk of postmenopausal breast cancer subtypes in the California Teachers Study cohort. Cancer Causes Control. 2012;23:473–485. doi: 10.1007/s10552-012-9897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin use after diagnosis of breast cancer and survival: a population-based cohort study. Epidemiology. 2015;26:68–78. doi: 10.1097/EDE.0000000000000189. [DOI] [PubMed] [Google Scholar]
- Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast. 2004;13:85–92. doi: 10.1016/j.breast.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control & Prevention. Cancer survivors--United States, 2007. MMWR Morb Mortal Wkly Rep. 2011;60:269–272. [PubMed] [Google Scholar]
- Chamberlain C, Romundstad P, Vatten L, Gunnell D, Martin RM. The association of weight gain during adulthood with prostate cancer incidence and survival: a population-based cohort. Int J Cancer. 2011;129:1199–1206. doi: 10.1002/ijc.25739. [DOI] [PubMed] [Google Scholar]
- Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SL, Harshman LC, Presti JC., Jr Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol. 2010;28:3951–3957. doi: 10.1200/JCO.2009.27.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DI, De Nunzio C, Gerber L, Thomas JA, 2nd, Calloway EE, Albisinni S, Senocak C, McKeever MG, Moreira DM, Tubaro A, et al. Predictive value of digital rectal examination for prostate cancer detection is modified by obesity. Prostate Cancer Prostatic Dis. 2011;14:346–353. doi: 10.1038/pcan.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MP, Grande JP, Juneja SC, Maihle NJ. Diet-induced obesity and mammary tumor development in MMTV-neu female mice. Nutr Cancer. 2004;50:174–180. doi: 10.1207/s15327914nc5002_7. [DOI] [PubMed] [Google Scholar]
- Cleveland RJ, Eng SM, Abrahamson PE, Britton JA, Teitelbaum SL, Neugut AI, Gammon MD. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1803–1811. doi: 10.1158/1055-9965.EPI-06-0889. [DOI] [PubMed] [Google Scholar]
- Clyne M. Prostate cancer: metformin--the new wonder drug? Nat Rev Urol. 2014;11:366. doi: 10.1038/nrurol.2014.136. [DOI] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- Cui Y, Whiteman MK, Flaws JA, Langenberg P, Tkaczuk KH, Bush TL. Body mass and stage of breast cancer at diagnosis. Int J Cancer. 2002;98:279–283. doi: 10.1002/ijc.10209. [DOI] [PubMed] [Google Scholar]
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- De Angel RE, Conti CJ, Wheatley KE, Brenner AJ, Otto G, Degraffenried LA, Hursting SD. The enhancing effects of obesity on mammary tumor growth and Akt/mTOR pathway activation persist after weight loss and are reversed by RAD001. Mol Carcinog. 2013;52:446–458. doi: 10.1002/mc.21878. [DOI] [PubMed] [Google Scholar]
- de Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown JP, Vicente M, Giuliani R, Nordenskjold B, Gutierez J, Andersson M, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02-98 trial. Breast Cancer Res Treat. 2010;119:145–153. doi: 10.1007/s10549-009-0512-0. [DOI] [PubMed] [Google Scholar]
- De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100:1421–1429. doi: 10.1002/bjs.9229. [DOI] [PubMed] [Google Scholar]
- de Pedro M, Baeza S, Escudero MT, Dierssen-Sotos T, Gomez-Acebo I, Pollan M, Llorca J. Effect of COX-2 inhibitors and other non-steroidal inflammatory drugs on breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2015;149:525–536. doi: 10.1007/s10549-015-3267-9. [DOI] [PubMed] [Google Scholar]
- DeCensi A, Puntoni M, Gandini S, Guerrieri-Gonzaga A, Johansson HA, Cazzaniga M, Pruneri G, Serrano D, Schwab M, Hofmann U, et al. Differential effects of metformin on breast cancer proliferation according to markers of insulin resistance and tumor subtype in a randomized presurgical trial. Breast Cancer Res Treat. 2014;148:81–90. doi: 10.1007/s10549-014-3141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- Desai P, Chlebowski R, Cauley JA, Manson JE, Wu C, Martin LW, Jay A, Bock C, Cote M, Petrucelli N, et al. Prospective analysis of association between statin use and breast cancer risk in the women's health initiative. Cancer Epidemiol Biomarkers Prev. 2013;22:1868–1876. doi: 10.1158/1055-9965.EPI-13-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst. 2003;95:1467–1476. doi: 10.1093/jnci/djg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan S, Hu X, Zhang Y, Maihle NJ, Grande JP, Cleary MP. Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-alpha mice. Breast Cancer Res. 2007;9:R91. doi: 10.1186/bcr1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc. 2012;71:181–189. doi: 10.1017/S002966511100320X. [DOI] [PubMed] [Google Scholar]
- Dunlap SM, Chiao LJ, Nogueira L, Usary J, Perou CM, Varticovski L, Hursting SD. Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prevention Research. 2012a;5:930–942. doi: 10.1158/1940-6207.CAPR-12-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton DJ, McLaren L. The usefulness of “corrected” body mass index vs. self-reported body mass index: comparing the population distributions, sensitivity, specificity, and predictive utility of three correction equations using Canadian population-based data. BMC Public Health. 2014;14:430. doi: 10.1186/1471-2458-14-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian CJ, Kimler BF, Donnelly JE, Sullivan DK, Klemp JR, Petroff BK, Phillips TA, Metheny T, Aversman S, Yeh HW, et al. Favorable modulation of benign breast tissue and serum risk biomarkers is associated with > 10 % weight loss in postmenopausal women. Breast Cancer Res Treat. 2013;142:119–132. doi: 10.1007/s10549-013-2730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan HB, Wender R, Myers RE, Petrelli N. Obesity and Cancer Screening according to Race and Gender. J Obes. 2011;2011:218250. doi: 10.1155/2011/218250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagherazzi G, Fabre A, Boutron-Ruault MC, Clavel-Chapelon F. Serum cholesterol level, use of a cholesterol-lowering drug, and breast cancer: results from the prospective E3N cohort. Eur J Cancer Prev. 2010;19:120–125. doi: 10.1097/CEJ.0b013e3283354918. [DOI] [PubMed] [Google Scholar]
- Farwell WR, D'Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011;103:885–892. doi: 10.1093/jnci/djr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, Gaziano JM. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100:134–139. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
- Field AE, Camargo CA, Jr, Ogino S. The merits of subtyping obesity: one size does not fit all. JAMA. 2013;310:2147–2148. doi: 10.1001/jama.2013.281501. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford NA, Lashinger LM, Allott EH, Hursting SD. Mechanistic targets and phytochemical strategies for breaking the obesity-cancer link. Front Oncol. 2013a;3:209. doi: 10.3389/fonc.2013.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford NA, Nunez NP, Holcomb VB, Hursting SD. IGF1 dependence of dietary energy balance effects on murine Met1 mammary tumor progression, epithelial-to-mesenchymal transition, and chemokine expression. Endocr Relat Cancer. 2013b;20:39–51. doi: 10.1530/ERC-12-0329. [DOI] [PubMed] [Google Scholar]
- Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLos One. 2013;8:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland SJ, Grubb KA, Yiu SK, Nielsen ME, Mangold LA, Isaacs WB, Epstein JI, Partin AW. Obesity and capsular incision at the time of open retropubic radical prostatectomy. J Urol. 2005;174:1798–1801. doi: 10.1097/01.ju.0000177077.53037.72. [DOI] [PubMed] [Google Scholar]
- Freedland SJ, Platz EA, Presti JC, Jr, Aronson WJ, Amling CL, Kane CJ, Terris MK. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006;175:500–504. doi: 10.1016/S0022-5347(05)00162-X. [DOI] [PubMed] [Google Scholar]
- Freedland SJ, Terris MK, Presti JC, Jr, Amling CL, Kane CJ, Trock B, Aronson WJ. Obesity and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. J Urol. 2004;172:520–524. doi: 10.1097/01.ju.0000135302.58378.ae. [DOI] [PubMed] [Google Scholar]
- Friedman AM, Hemler JR, Rossetti E, Clemow LP, Ferrante JM. Obese women's barriers to mammography and pap smear: the possible role of personality. Obesity (Silver Spring) 2012;20:1611–1617. doi: 10.1038/oby.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar CD, Chen TC, Li X. NCHS Data Brief. 2012. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999-2010; pp. 1–8. [PubMed] [Google Scholar]
- Fuentes-Mattei E, Velazquez-Torres G, Phan L, Zhang F, Chou PC, Shin JH, Choi HH, Chen JS, Zhao R, Chen J, et al. Effects of obesity on transcriptomic changes and cancer hallmarks in estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galet C, Gray A, Said JW, Castor B, Wan J, Beltran PJ, Calzone FJ, Elashoff D, Cohen P, Aronson WJ. Effects of Calorie Restriction and IGF-1 Receptor Blockade on the Progression of 22Rv1 Prostate Cancer Xenografts. Int J Mol Sci. 2013;14:13782–13795. doi: 10.3390/ijms140713782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7:867–885. doi: 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, Gammon MD, Douglas Thompson W, Bernstein JL. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130:587–597. doi: 10.1007/s10549-011-1616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles ED, Wellberg EA, Astling DP, Anderson SM, Thor AD, Jindal S, Tan AC, Schedin PS, Maclean PS. Obesity and overfeeding affecting both tumor and systemic metabolism activates the progesterone receptor to contribute to postmenopausal breast cancer. Cancer Res. 2012;72:6490–6501. doi: 10.1158/0008-5472.CAN-12-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Taylor SK, Hood N. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30:164–171. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Pritchard KI. Obesity and hormone therapy in breast cancer: an unfinished puzzle. J Clin Oncol. 2010;28:3405–3407. doi: 10.1200/JCO.2010.29.5113. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, Blackburn GL, Findlay B, Gralow JR, Mukherjee S, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32:2231–2239. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, Morrison VA, Pini TM, Runowicz CD, Rosner GL, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30:1553–1561. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- Grubb RL, 3rd, Black A, Izmirlian G, Hickey TP, Pinsky PF, Mabie JE, Riley TL, Ragard LR, Prorok PC, Berg CD, et al. Serum prostate-specific antigen hemodilution among obese men undergoing screening in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2009;18:748–751. doi: 10.1158/1055-9965.EPI-08-0938. [DOI] [PubMed] [Google Scholar]
- Gu Q, P RR, Burt VL, Kit BK. NCHS data brief. 2014. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012; pp. 1–8. [PubMed] [Google Scholar]
- Gupta S, Adhami VM, Subbarayan M, MacLennan GT, Lewin JS, Hafeli UO, Fu P, Mukhtar H. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64:3334–3343. doi: 10.1158/0008-5472.can-03-2422. [DOI] [PubMed] [Google Scholar]
- Hakkak R, MacLeod S, Shaaf S, Holley AW, Simpson P, Fuchs G, Jo CH, Kieber-Emmons T, Korourian S. Obesity increases the incidence of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in an ovariectomized Zucker rat model. Int J Oncol. 2007;30:557–563. [PubMed] [Google Scholar]
- Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link – ten years after. BMC Biol. 2013;11:36. doi: 10.1186/1741-7007-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Howell A, Vierkant RA, Kumar N, Cerhan JR, Kelemen LE, Folsom AR, Sellers TA. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women's health study. Cancer Epidemiol Biomarkers Prev. 2005;14:656–661. doi: 10.1158/1055-9965.EPI-04-0001. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee MH, Yeung SC. Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2+ breast cancer. Ann Oncol. 2012;23:1771–1780. doi: 10.1093/annonc/mdr534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe LR, Subbaramaiah K, Patel J, Masferrer JL, Deora A, Hudis C, Thaler HT, Muller WJ, Du B, Brown AM, et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62:5405–5407. [PubMed] [Google Scholar]
- Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L Canadian Cancer Registries Epidemiology Research Group. Dietary cholesterol intake and cancer. Ann Oncol. 2012;23:491–500. doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- Hu MB, Liu SH, Jiang HW, Bai PD, Ding Q. Obesity affects the biopsy-mediated detection of prostate cancer, particularly high-grade prostate cancer: a dose-response meta-analysis of 29,464 patients. PLoS One. 2014;9:e106677. doi: 10.1371/journal.pone.0106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AG, Gierach GL, Modugno F, Simpson J, Wilson JW, Evans RW, Vogel VG, Weissfeld JL. Nonsteroidal anti-inflammatory drug use and serum total estradiol in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:680–687. doi: 10.1158/1055-9965.EPI-07-2739. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83–89. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- Ioannides SJ, Barlow PL, Elwood JM, Porter D. Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: a systematic review. Breast Cancer Res Treat. 2014;147:237–248. doi: 10.1007/s10549-014-3091-7. [DOI] [PubMed] [Google Scholar]
- Irwin ML. Weight loss interventions and breast cancer survival: the time is now. J Clin Oncol. 2014;32:2197–2199. doi: 10.1200/JCO.2014.56.4583. [DOI] [PubMed] [Google Scholar]
- Jacobs EJ, Stevens VL, Newton CC, Gapstur SM. Plasma total, LDL, and HDL cholesterol and risk of aggressive prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Causes Control. 2012;23:1289–1296. doi: 10.1007/s10552-012-0006-y. [DOI] [PubMed] [Google Scholar]
- Jakovljevic J, Touillaud MS, Bondy ML, Singletary SE, Pillow PC, Chang S. Dietary intake of selected fatty acids, cholesterol and carotenoids and estrogen receptor status in premenopausal breast cancer patients. Breast Cancer Res Treat. 2002;75:5–14. doi: 10.1023/a:1016588629495. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24:2506–2514. doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshu CE, Mondul AM, Menke A, Meinhold C, Han M, Humphreys EB, Freedland SJ, Walsh PC, Platz EA. Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila) 2011;4:544–551. doi: 10.1158/1940-6207.CAPR-10-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009;124:1398–1403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik D, Karnes RJ, Eisenberg MS, Rangel LJ, Carlson RE, Bergstralh EJ. Effect of metformin on prostate cancer outcomes after radical prostatectomy. Urol Oncol. 2013;32:43e1–7. doi: 10.1016/j.urolonc.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlikowske K, Walker R, Miglioretti DL, Desai A, Ballard-Barbash R, Buist DS. Obesity, mammography use and accuracy, and advanced breast cancer risk. J Natl Cancer Inst. 2008;100:1724–1733. doi: 10.1093/jnci/djn388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keto CJ, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL, Freedland SJ. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2011;110:492–498. doi: 10.1111/j.1464-410X.2011.10754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor LY, Bae K, Pollack A, Hammond ME, Grignon DJ, Venkatesan VM, Rosenthal SA, Ritter MA, Sandler HM, Hanks GE, et al. COX-2 expression predicts prostate-cancer outcome: analysis of data from the RTOG 92-02 trial. Lancet Oncol. 2007;8:912–920. doi: 10.1016/S1470-2045(07)70280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–1598. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Barnard RJ, Said J, Hong-Gonzalez J, Corman DM, Ku M, Doan NB, Gui D, Elashoff D, Cohen P, et al. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res. 2008;68:3066–3073. doi: 10.1158/0008-5472.CAN-07-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall B, Stang A, Rathmann W, Kostev K. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Saf. 2015;24:865–874. doi: 10.1002/pds.3823. [DOI] [PubMed] [Google Scholar]
- Krycer JR, Brown AJ. Cholesterol accumulation in prostate cancer: a classic observation from a modern perspective. Biochim Biophys Acta. 2013;1835:219–229. doi: 10.1016/j.bbcan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Kumar AS, Benz CC, Shim V, Minami CA, Moore DH, Esserman LJ. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomarkers Prev. 2008;17:1028–1033. doi: 10.1158/1055-9965.EPI-07-0726. [DOI] [PubMed] [Google Scholar]
- Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: A systematic review and meta-analysis. Eur J Cancer. 2008;44:2122–2132. doi: 10.1016/j.ejca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109:573–579. doi: 10.1007/s10549-007-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- Lashinger LM, Ford NA, Hursting SD. Interacting inflammatory and growth factor signals underlie the obesity-cancer link. J Nutr. 2014a;144:109–113. doi: 10.3945/jn.113.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashinger LM, Rossi EL, Hursting SD. Obesity and resistance to cancer chemotherapy: interacting roles of inflammation and metabolic dysregulation. Clin Pharmacol Ther. 2014b;96:458–463. doi: 10.1038/clpt.2014.136. [DOI] [PubMed] [Google Scholar]
- Lee BH, Taylor MG, Robinet P, Smith JD, Schweitzer J, Sehayek E, Falzarano SM, Magi-Galluzzi C, Klein EA, Ting AH. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Res. 2013;73:1211–1218. doi: 10.1158/0008-5472.CAN-12-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lega IC, Shah PS, Margel D, Beyene J, Rochon PA, Lipscombe LL. The effect of metformin on mortality following cancer among patients with diabetes. Cancer Epidemiol Biomarkers Prev. 2014;23:1974–1984. doi: 10.1158/1055-9965.EPI-14-0327. [DOI] [PubMed] [Google Scholar]
- Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007;15:216–224. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- Litton JK, Gonzalez-Angulo AM, Warneke CL, Buzdar AU, Kau SW, Bondy M, Mahabir S, Hortobagyi GN, Brewster AM. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J Clin Oncol. 2008;26:4072–4077. doi: 10.1200/JCO.2007.14.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- Liu J, Ramakrishnan SK, Khuder SS, Kaw MK, Muturi HT, Lester SG, Lee SJ, Fedorova LV, Kim AJ, Mohamed IE, et al. High-calorie diet exacerbates prostate neoplasia in mice with haploinsufficiency of Pten tumor suppressor gene. Mol Metab. 2015;4:186–198. doi: 10.1016/j.molmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen JQ, Xie L, Wang J, Li T, He Y, Gao Y, Qin X, Li S. Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. BMC Med. 2014;12:55. doi: 10.1186/1741-7015-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaverias G, Danilo C, Wang Y, Witkiewicz AK, Daumer K, Lisanti MP, Frank PG. A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am J Pathol. 2010;177:3180–3191. doi: 10.2353/ajpath.2010.100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21:4524–4531. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- Lyman GH, Sparreboom A. Chemotherapy dosing in overweight and obese patients with cancer. Nat Rev Clin Oncol. 2013;10:451–459. doi: 10.1038/nrclinonc.2013.108. [DOI] [PubMed] [Google Scholar]
- Lyons TR, O'Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan AC, Schedin P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, Gaziano JM, Pollak M, Stampfer MJ. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9:1039–1047. doi: 10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- Makari-Judson G, Braun B, Jerry DJ, Mertens WC. Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J Clin Oncol. 2014;5:272–282. doi: 10.5306/wjco.v5.i3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, Fleshner N. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J Clin Oncol. 2013;31:3069–3075. doi: 10.1200/JCO.2012.46.7043. [DOI] [PubMed] [Google Scholar]
- Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control. 2009;20:1181–1192. doi: 10.1007/s10552-009-9319-x. [DOI] [PubMed] [Google Scholar]
- Maruthur NM, Bolen S, Brancati FL, Clark JM. Obesity and mammography: a systematic review and meta-analysis. J Gen Intern Med. 2009;24:665–677. doi: 10.1007/s11606-009-0939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass AY, Agalliu I, Laze J, Lepor H. Preoperative statin therapy is not associated with biochemical recurrence after radical prostatectomy: our experience and meta-analysis. J Urol. 2012;188:786–791. doi: 10.1016/j.juro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Merrick GS, Galbreath RW, Butler WM, Wallner KE, Allen ZA, Adamovich E. Obesity is not predictive of overall survival following permanent prostate brachytherapy. Am J Clin Oncol. 2007;30:588–596. doi: 10.1097/COC.0b013e318068b506. [DOI] [PubMed] [Google Scholar]
- Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, Manson JE Nurses' Health S. Type 2 diabetes and subsequent incidence of breast cancer in the Nurses' Health Study. Diabetes Care. 2003;26:1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno NK, Rogozina OP, Seppanen CM, Liao DJ, Cleary MP, Grossmann ME. Combination of intermittent calorie restriction and eicosapentaenoic acid for inhibition of mammary tumors. Cancer Prevention Research. 2013;6:540–547. doi: 10.1158/1940-6207.CAPR-13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller E, Adami HO, Mucci LA, Lundholm C, Bellocco R, Johansson JE, Gronberg H, Balter K. Lifetime body size and prostate cancer risk in a population-based case-control study in Sweden. Cancer Causes Control. 2013;24:2143–2155. doi: 10.1007/s10552-013-0291-0. [DOI] [PubMed] [Google Scholar]
- Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21:61–68. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control. 2011;22:1545–1552. doi: 10.1007/s10552-011-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans AK, Fan KH, Koyama T, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Stanford JL, Stroup AM, Resnick MJ, et al. Influence of Age on Incident Diabetes and Cardiovascular Disease Among Prostate Cancer Survivors Receiving Androgen Deprivation Therapy. J Urol. 2014;193:1226–1231. doi: 10.1016/j.juro.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One. 2012;7:e30062. doi: 10.1371/journal.pone.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer VA U.S. Preventative Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–136. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol. 2008;168:925–931. doi: 10.1093/aje/kwn190. [DOI] [PubMed] [Google Scholar]
- Murtola TJ, Visvanathan K, Artama M, Vainio H, Pukkala E. Statin use and breast cancer survival: a nationwide cohort study from Finland. PLoS One. 2014;9:e110231. doi: 10.1371/journal.pone.0110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan BA, Narayanan NK, Pittman B, Reddy BS. Regression of mouse prostatic intraepithelial neoplasia by nonsteroidal anti-inflammatory drugs in the transgenic adenocarcinoma mouse prostate model. Clin Cancer Res. 2004;10:7727–7737. doi: 10.1158/1078-0432.CCR-04-0732. [DOI] [PubMed] [Google Scholar]
- Narayanan BA, Narayanan NK, Pttman B, Reddy BS. Adenocarcina of the mouse prostate growth inhibition by celecoxib: downregulation of transcription factors involved in COX-2 inhibition. Prostate. 2006;66:257–265. doi: 10.1002/pros.20331. [DOI] [PubMed] [Google Scholar]
- Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013;21:E439–447. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat. 2012;134:769–781. doi: 10.1007/s10549-012-2073-x. [DOI] [PubMed] [Google Scholar]
- Nogueira LM, Dunlap SM, Ford NA, Hursting SD. Calorie restriction and rapamycin inhibit MMTV-Wnt-1 mammary tumor growth in a mouse model of postmenopausal obesity. Endocr Relat Cancer. 2012;19:57–68. doi: 10.1530/ERC-11-0213. [DOI] [PubMed] [Google Scholar]
- Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, Barrett JC, Hursting SD. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008;60:534–541. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape-Ansorge KA, Grande JP, Christensen TA, Maihle NJ, Cleary MP. Effect of moderate caloric restriction and/or weight cycling on mammary tumor incidence and latency in MMTV-Neu female mice. Nutr Cancer. 2002;44:162–168. doi: 10.1207/S15327914NC4402_07. [DOI] [PubMed] [Google Scholar]
- Park HS, Schoenfeld JD, Mailhot RB, Shive M, Hartman RI, Ogembo R, Mucci LA. Statins and prostate cancer recurrence following radical prostatectomy or radiotherapy: a systematic review and meta-analysis. Ann Oncol. 2013;24:1427–1434. doi: 10.1093/annonc/mdt077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T, Hruby G, Badani K, Abate-Shen C, McKiernan JM. Clinical outcomes after radical prostatectomy in diabetic patients treated with metformin. Urology. 2010;76:1240–1244. doi: 10.1016/j.urology.2010.03.059. [DOI] [PubMed] [Google Scholar]
- Pelton K, Freeman MR, Solomon KR. Cholesterol and prostate cancer. Curr Opin Pharmacol. 2012;12:751–759. doi: 10.1016/j.coph.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Body size and risk of luminal, HER2-overexpressing, and triple-negative breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2078–2086. doi: 10.1158/1055-9965.EPI-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischon T, Boeing H, Weikert S, Allen N, Key T, Johnsen NF, Tjonneland A, Severinsen MT, Overvad K, Rohrmann S, et al. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2008;17:3252–3261. doi: 10.1158/1055-9965.EPI-08-0609. [DOI] [PubMed] [Google Scholar]
- Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008;123:1693–1698. doi: 10.1002/ijc.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, Neuhouser ML, Klein EA, Thompson IM, Jr, Kristal AR. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2807–2813. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou KM, Massaro JM, Hoffmann U, Lieb K, Vasan RS, O'Donnell CJ, Fox CS. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32:481–485. doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston MA, Riis AH, Ehrenstein V, Breau RH, Batista JL, Olumi AF, Mucci LA, Adami HO, Sorensen HT. Metformin use and prostate cancer risk. Eur Urol. 2014;66:1012–1020. doi: 10.1016/j.eururo.2014.04.027. [DOI] [PubMed] [Google Scholar]
- Renehan AG. Bariatric surgery, weight reduction, and cancer prevention. Lancet Oncol. 2009;10:640–641. doi: 10.1016/S1470-2045(09)70170-6. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- Robien K, Demark-Wahnefried W, Rock CL. Evidence-based nutrition guidelines for cancer survivors: current guidelines, knowledge gaps, and future research directions. J Am Diet Assoc. 2011;111:368–375. doi: 10.1016/j.jada.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, Thun MJ, Calle EE. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:63–69. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–353. [PubMed] [Google Scholar]
- Rodvold KA, Rushing DA, Tewksbury DA. Doxorubicin clearance in the obese. J Clin Oncol. 1988;6:1321–1327. doi: 10.1200/JCO.1988.6.8.1321. [DOI] [PubMed] [Google Scholar]
- Rogozina OP, Nkhata KJ, Nagle EJ, Grande JP, Cleary MP. The protective effect of intermittent calorie restriction on mammary tumorigenesis is not compromised by consumption of a high fat diet during refeeding. Breast Cancer Res Treat. 2013;138:395–406. doi: 10.1007/s10549-013-2464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg LU, Einarsdottir K, Friman EI, Wedren S, Dickman PW, Hall P, Magnusson C. Risk factors for hormone receptor-defined breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:2482–2488. doi: 10.1158/1055-9965.EPI-06-0489. [DOI] [PubMed] [Google Scholar]
- Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, Sampson M, Moher D, Force USPST. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- Roy M, Kung HJ, Ghosh PM. Statins and prostate cancer: role of cholesterol inhibition vs. prevention of small GTP-binding proteins. Am J Cancer Res. 2011;1:542–561. [PMC free article] [PubMed] [Google Scholar]
- Rundle AG, Neugut AI. Modeling the effects of obesity and weight gain on PSA velocity. Prostate. 2009;69:1573–1578. doi: 10.1002/pros.21005. [DOI] [PubMed] [Google Scholar]
- Scales CD, Jr, Curtis LH, Norris RD, Schulman KA, Dahm P, Moul JW. Relationship between body mass index and prostate cancer screening in the United States. J Urol. 2007;177:493–498. doi: 10.1016/j.juro.2006.09.059. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, Dean LT, Drake BF. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst. 2013;105:1344–1354. doi: 10.1093/jnci/djt223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scosyrev E, Tobis S, Donsky H, Wu G, Joseph J, Rashid H, Messing E. Statin use and the risk of biochemical recurrence of prostate cancer after definitive local therapy: a meta-analysis of eight cohort studies. BJU Int. 2013;111:E71–77. doi: 10.1111/j.1464-410X.2012.11527.x. [DOI] [PubMed] [Google Scholar]
- Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years' follow up. BMC Cancer. 2012;12:25. doi: 10.1186/1471-2407-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B, Dahlgren S, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007;13:241–245. doi: 10.1158/1078-0432.CCR-06-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW, Jr, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118:5937–5946. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, Bahalim AN, McIntire RK, Gutierrez HR, Cowan M, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, Hudis CA, Dannenberg AJ. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002;277:18649–18657. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- Suissa S, Azoulay L. Metformin and the Risk of Cancer: Time-related biases in observational studies. Diabetes Care. 2012;35:2665–2673. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, Freemerman AJ, Galanko JA, McNaughton KK, Bendt KM, Darr DB, Troester MA, Makowski L. Obesity-mediated regulation of HGF/c-Met is associated with reduced basal-like breast cancer latency in parous mice. PLoS One. 2014a;9:e111394. doi: 10.1371/journal.pone.0111394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, Freemerman AJ, Johnson AR, Milner JJ, McNaughton KK, Galanko JA, Bendt KM, Darr DB, Perou CM, Troester MA, et al. Role of HGF in obesity-associated tumorigenesis: C3(1)-TAg mice as a model for human basal-like breast cancer. Breast Cancer Res Treat. 2013;142:489–503. doi: 10.1007/s10549-013-2741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S, Le TL, Essaid L, Freemerman AJ, Huang MJ, Galanko JA, McNaughton KK, Bendt KM, Darr DB, Troester MA, et al. Weight Loss Reversed Obesity-Induced HGF/c-Met Pathway and Basal-Like Breast Cancer Progression. Front Oncol. 2014b;4:175. doi: 10.3389/fonc.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer. 2009;124:698–712. doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- Tait S, Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Body mass index, diabetes, and triple-negative breast cancer prognosis. Breast Cancer Res Treat. 2014;146:189–197. doi: 10.1007/s10549-014-3002-y. [DOI] [PubMed] [Google Scholar]
- Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, Subbaramaiah K, Dannenberg AJ, Neugut AI. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;291:2433–2440. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- Thomas JA, 2nd, Antonelli JA, Lloyd JC, Masko EM, Poulton SH, Phillips TE, Pollak M, Freedland SJ. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis. 2010;13:350–355. doi: 10.1038/pcan.2010.24. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, McTiernan A. Weight cycling and cancer: weighing the evidence of intermittent caloric restriction and cancer risk. Cancer Prev Res (Phila) 2011;4:1736–1742. doi: 10.1158/1940-6207.CAPR-11-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MD, Grubbs CJ, Bode AM, Reid JM, McGovern R, Bernard PS, Stijleman IJ, Green JE, Bennett C, Juliana MM, et al. Lack of effect of metformin on mammary carcinogenesis in nondiabetic rat and mouse models. Cancer Prev Res (Phila) 2015;8:231–239. doi: 10.1158/1940-6207.CAPR-14-0181-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa K, Ma H, Sullivan-Halley J, Neuhouser ML, Imayama I, Baumgartner KB, Smith AW, Alfano CM, McTiernan A, Ballard-Barbash R, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res. 2014;16:414. doi: 10.1186/s13058-014-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H, Keating NL, Van Den Eeden SK, Haque R, Cassidy-Bushrow AE, Yood MU, Smith MR, Potosky AL. Risk of Diabetes among Patients Receiving Primary Androgen Deprivation Therapy for Clinically Localized Prostate Cancer. J Urol. 2014;193:1956–1962. doi: 10.1016/j.juro.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilidis KK, Allen NE, Appleby PN, Rohrmann S, Nothlings U, Arriola L, Gunter MJ, Chajes V, Rinaldi S, Romieu I, et al. Diabetes mellitus and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2015a;136:372–381. doi: 10.1002/ijc.28989. [DOI] [PubMed] [Google Scholar]
- Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, Sacerdote C, Ashby D, Vineis P, Tzoulaki I, et al. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care. 2014;37:2522–2532. doi: 10.2337/dc14-0584. [DOI] [PubMed] [Google Scholar]
- Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015b;350 doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- Undela K, Srikanth V, Bansal D. Statin use and risk of breast cancer: a meta-analysis of observational studies. Breast Cancer Res Treat. 2012;135:261–269. doi: 10.1007/s10549-012-2154-x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8:88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]
- Vidal AC, Howard LE, Moreira DM, Castro-Santamaria R, Adriole GL, Freedland SJ. Aspirin, NSAID and risk of prostate cancer: Results from the REDUCE study. Clin Cancer Res. 2014;15:756–762. doi: 10.1158/1078-0432.CCR-14-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling A, Buck K, Kaaks R, Chang-Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat. 2010;123:641–649. doi: 10.1007/s10549-010-1116-4. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Lin YW, Wu J, Zhu Y, Xu XL, Xu X, Liang Z, Hu ZH, Li SQ, Zheng XY, et al. Meta-analysis of nonsteroidal anti-inflammatory drug intake and prostate cancer risk. World J Surg Oncol. 2014;12:304. doi: 10.1186/1477-7819-12-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley BM, Moreira DM, Thomas JA, Aronson WJ, Terris MK, Presti JC, Jr, Kane CJ, Amling CL, Freedland SJ. Preoperative weight change and risk of adverse outcome following radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. Prostate Cancer Prostatic Dis. 2011;14:361–366. doi: 10.1038/pcan.2011.42. [DOI] [PubMed] [Google Scholar]
- Woditschka S, Haag JD, Mau B, Lubet RA, Gould MN. Chemopreventive effects of celecoxib are limited to hormonally responsive mammary carcinomas in the neu-induced retroviral rat model. Breast Cancer Res. 2008;10:R18. doi: 10.1186/bcr1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters R, Schwentner L, Regierer A, Wischnewsky M, Kreienberg R, Wockel A. Endocrine therapy in obese patients with primary breast cancer: another piece of evidence in an unfinished puzzle. Breast Cancer Res Treat. 2012;131:925–931. doi: 10.1007/s10549-011-1874-7. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund / American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- Wu C, Aronson WJ, Terris MK, Presti JC, Jr, Kane CJ, Amling CL, Freedland SJ. Diabetes predicts metastasis after radical prostatectomy in obese men: results from the SEARCH database. BJU Int. 2013;111:E310–318. doi: 10.1111/j.1464-410X.2012.11687.x. [DOI] [PubMed] [Google Scholar]
- Xu H, Hu MB, Bai PD, Zhu WH, Ding Q, Jiang HW. Will metformin postpone high-fat diet promotion of TRAMP mouse prostate cancer development and progression? Int Urol Nephrol. 2014;46:2327–2334. doi: 10.1007/s11255-014-0823-x. [DOI] [PubMed] [Google Scholar]
- Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, Yoshimura N, Hla T, Wada S. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89:589–596. [PubMed] [Google Scholar]
- Yu H, Yin L, Jiang X, Sun X, Wu J, Tian H, Gao X, He X. Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta-analysis of epidemiological observational studies. PLoS One. 2014a;9:e116327. doi: 10.1371/journal.pone.0116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu O, Eberg M, Benayoun S, Aprikian A, Batist G, Suissa S, Azoulay L. Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol. 2014b;32:5–11. doi: 10.1200/JCO.2013.49.4757. [DOI] [PubMed] [Google Scholar]
- Zhang F, Yang Y, Skrip L, Hu D, Wang Y, Wong C, Qiu J, Lei H. Diabetes mellitus and risk of prostate cancer: an updated meta-analysis based on 12 case-control and 25 cohort studies. Acta Diabetol. 2012;49:S235–246. doi: 10.1007/s00592-012-0439-5. [DOI] [PubMed] [Google Scholar]
- Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37:207–218. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou G, Sun B, Zhao G, Liu D, Sun J, Liu C, Guo H. Impact of obesity upon prostate cancer-associated mortality: A meta-analysis of 17 cohort studies. Oncol Lett. 2015;9:1307–1312. doi: 10.3892/ol.2014.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]