Abstract
Scavenger receptors constitute a large family of evolutionally conserved protein molecules that are structurally and functionally diverse. Although scavenger receptors were originally identified based on their capacity to scavenge modified lipoproteins, these molecules have been shown to recognize and bind to a broad spectrum of ligands, including modified and unmodified host-derived molecules or microbial components. As a major subset of innate pattern recognition receptors, scavenger receptors are mainly expressed on myeloid cells and function in a wide range of biological processes, such as endocytosis, adhesion, lipid transport, antigen presentation, and pathogen clearance. In addition to playing a crucial role in maintenance of host homeostasis, scavenger receptors have been implicated in the pathogenesis of a number of diseases, e.g., atherosclerosis, neurodegeneration, or metabolic disorders. Emerging evidence has begun to reveal these receptor molecules as important regulators of tumor behavior and host immune responses to cancer. This review summarizes our current understanding on the newly identified, distinct functions of scavenger receptors in cancer biology and immunology. The potential of scavenger receptors as diagnostic biomarkers and novel targets for therapeutic interventions to treat malignancies is also highlighted.
1. INTRODUCTION
In late 1970s, Michael Brown and Joseph Goldstein initially identified scavenger receptors in macrophages and described their activity in the uptake of modified low-density lipoprotein (LDL), i.e., acetylated LDL (acLDL) (Goldstein, Ho, Basu, & Brown, 1979). Monty Krieger’s group first cloned the scavenger receptors, i.e., prototype class A scavenger receptors, in 1990 (Kodama et al., 1990). With additional scavenger receptor family members identified, scavenger receptors are currently categorized into 10 classes (A–J) based on their sequence similarity or shared structural features. However, there is no or little sequence homology between different classes of scavenger receptors (Krieger, 1997; Whelan, Meehan, Golding, McConkey, & Bowdish, 2012). There are currently no known mammalian class C scavenger receptors, and the class C scavenger receptors have only been described in Drosophila melanogaster (Krieger, 1997). To address the inconsistencies and confusion of multiple names of scavenger receptors being used in the literature, a unified nomenclature system was recently proposed to describe the different classes of mammalian scavenger receptors (Prabhudas et al., 2014). It is now appreciated that these structurally heterogeneous scavenger receptors recognize a broad spectrum of ligands, including microbial pathogens or pathogen-derived molecular patterns (PAMPs), e.g., lipopolysaccharide (LPS) and lipoteichoic acid (LTA), as well as host-derived self-molecules or damage-associated molecular patterns, e.g., stress/heat shock proteins (HSPs), lipoproteins (Canton, Neculai, & Grinstein, 2013; Greaves & Gordon, 2009; Krieger et al., 1993; Pluddemann, Neyen, & Gordon, 2007). Based on their broad ligand-binding specificities during interaction with conserved microbial structures or endogenous self-molecules, scavenger receptors are considered to be an important subclass of the pattern recognition receptors (PRRs) in innate immunity (Gordon, 2002; Krieger, 1997). Scavenger receptors were recently defined as “cell surface receptors that typically bind multiple ligands and promote the removal of non-self or altered-self targets” (Prabhudas et al., 2014). These receptors often function by mechanisms that include adhesion, endocytosis, phagocytosis, transport, and signaling that ultimately lead to the elimination of degraded or harmful substances (Prabhudas et al., 2014).
Due to their property initially identified in the uptake of modified LDL, e.g., acLDL and oxidized LDL (oxLDL), by macrophages, the proatherogenic role of the scavenger receptors in atherosclerosis has been studied extensively (Kzhyshkowska, Neyen, & Gordon, 2012). However, their precise contribution to this disease remains unclear. Given their ability to recognize such a large repertoire of ligands, it is anticipated that scavenger receptors are critically involved in the maintenance of host homeostasis as well as in the pathogenesis of multiple diseases, e.g., type 2 diabetes mellitus (Kennedy & Kashyap, 2011), Alzheimer’s disease (El Khoury et al., 2003; Wilkinson & El Khoury, 2012). The functional versatility of scavenger receptors in various diseases were recently discussed in several reviews by us or other researchers (Armengol et al., 2013; Canton et al., 2013; Kelley, Ozment, Li, Schweitzer, & Williams, 2014; Yu, Zuo, Subjeck, & Wang, 2012). Over the last few years, there is emerging evidence indicating that scavenger receptors act as an important regulator of tumor progression and host immune response to cancer (Neyen et al., 2013; Wang, Facciponte, Chen, Subjeck, & Repasky, 2007; Yi et al., 2011). Certain scavenger receptors have been exploited as diagnostic or prognostic markers in cancer of various types. In this review, we highlight recent insights into these previously under-appreciated functions of the mammalian scavenger receptors in cancer biology and immunology. We will also discuss the feasibility of developing scavenger receptor-targeted therapeutic strategies for cancer treatment.
2. SCAVENGER RECEPTORS IN CANCER IMMUNOBIOLOGY
2.1 Class A Scavenger Receptor
2.1.1 Scavenger Receptor Class A
Scavenger receptor class A (SR-A), also called SCARA1/SR-A1, macrophage scavenger receptor 1 (MSR1) or CD204, was the first scavenger receptor cloned and represents the prototypic member of class A scavenger receptors (Kodama et al., 1990). A signature for all the class A scavenger receptors, including SR-A, is a distinct collagenous domain (Kodama et al., 1990; Rohrer, Freeman, Kodama, Penman, & Krieger, 1990). SR-A is a phagocytic PRR expressed primarily on tissue macrophages and dendritic cells (DCs). SR-A recognizes a diverse array of “self” and “nonself” ligands, including modified LDLs (acLDL, oxLDL), Gram-positive and Gram-negative bacteria, molecules of microbial origin or PAMPs (e.g., LPS, double strand RNA, unmethylated bacterial CpG DNA), hepatitis C virus, HSPs, proteoglycans, and β-amyloid (Pluddemann et al., 2007). The role of SR-A in atherosclerosis has been extensively studied because it was the first receptor identified for modified lipoproteins that are pertinent to the development of vascular disease (Kzhyshkowska et al., 2012; Suzuki et al., 1997). However, controversies still exist with regard to the precise contribution of SR-A to atherosclerosis (Kuchibhotla et al., 2008; Moore et al., 2005). SR-A is also involved in the maintenance of tissue homeostasis by clearance of modified self-components and apoptotic cells, host defense against invading microorganisms, and disease pathogenesis, such as neurodegeneration (Canton et al., 2013; El Khoury et al., 1996; Frenkel et al., 2013; Gordon, 2002; Kelley et al., 2014; Suzuki et al., 1997; Yu et al., 2012). However, accumulating evidence is now revealing important roles for SR-A in cancer.
Tumor-associated macrophages (TAMs) are a major stromal component in the tumor microenvironment (TME) (Lewis & Pollard, 2006). TAMs not only support survival and growth of tumors but also contribute to tumor metastasis, and immune evasion (Guo, Buranych, Sarkar, Fisher, & Wang, 2013; Lewis & Pollard, 2006; Qian & Pollard, 2010). Highly plastic macrophages mainly originate from circulating monocytes infiltrating peripheral tissues and acquire distinct characteristics as a result of environmental cues (Murdoch, Muthana, Coffelt, & Lewis, 2008). Macrophages can be generally categorized into two populations with distinct functional phenotypes: classically activated or M1 macrophages with proinflammatory and tumor-suppressive features, alternatively activated or M2 macrophages with anti-inflammatory, proangiogenic, immunosuppressive, and tumor-promoting activities. Macrophage polarization is increasingly recognized as an important pathogenic factor in inflammatory and neoplastic diseases (Labonte, Tosello-Trampont, & Hahn, 2014; Sica et al., 2008). It has been well documented that TAMs often show an M2-like phenotype and contribute to cancer cell proliferation, invasion and metastasis, tumor angiogenesis, matrix remodeling, and immune suppression (Guo, Buranych, et al., 2013). Immunohistochemistry studies in lung cancer (Ohtaki et al., 2010), liver cancer (Yeung et al., 2014), T cell leukemia/lymphoma (Saito et al., 2014), esophageal squamous cell carcinoma (Shigeoka et al., 2013), and pancreatic cancer (Yoshikawa et al., 2012) demonstrate that SR-A expression in tumor tissues is restricted to TAMs and that high level of SR-A is associated with a more aggressive cancer phenotype. Furthermore, an increased number of SR-A+ TAMs has been shown to correlate with histological grade and poor prognosis in numerous cancers (Hirayama et al., 2012; Hou, Chao, Tung, Wang, & Shan, 2014; Kawamura, Komohara, Takaishi, Katabuchi, & Takeya, 2009; Kurahara et al., 2011; Sugimoto et al., 2014; Yoshikawa et al., 2012).
Although accumulating evidence points to a potential role of SR-A in TAM polarization toward an M2-like phenotype, the underlying mechanism remains elusive. Mer tyrosine kinase (MerTK), the major apoptotic cell receptor on macrophages, was recently shown to have a central role in M2 polarization of macrophages and favor an anti-inflammatory, immunosuppressive microenvironment (Graham, DeRyckere, Davies, & Earp, 2014; Zizzo, Hilliard, Monestier, & Cohen, 2012). Indeed, SR-A was reported to be required for the optimized function of MerTK during engulfment of apoptotic cells and subsequent resolution of inflammation (Todt, Hu, & Curtis, 2008). While the detailed interplay between SR-A and MerTK in ingesting of apoptotic cells is unclear, it is reasonable to believe that abundant apoptotic tumor cells in the TME together with elevated expression of SR-A and MerTK are critically involved in the programing of TAMs and immunosuppressive mechanisms in the TME.
Elevation of SR-A in TAMs implicates its potential involvement in their functions. However, studies of the functional regulation of TAMs by SR-A during tumor growth and progression are very limited. SR-A was recently reported to inhibit the production of tumoricidal molecules by TAMs, e.g., nitric oxide, interferon (IFN)-β, and IFN-γ, which caused outgrowth of EL4 lymphoma (Komohara et al., 2009). SR-A-mediated inhibition of IFN-β secretion seems to be consistent with a previous work showing that SR-A suppressed the TLR4-mediated interferon regulatory factor (IRF)-3/IFN-β pathway in endoplasmic reticulum (ER)-stressed macrophages (Seimon, Obstfeld, Moore, Golenbock, & Tabas, 2006). Using SR-A-deficient mice, Neyen et al. demonstrated that SR-A on TAMs was necessary for invasion and metastasis of ovarian and pancreatic tumors (Neyen et al., 2013). In this study, several potential SR-A ligands (e.g., Galectin-1, Fibronectin, Vimentin, and Matrilin-2) present in the supernatants of tumor cell-macrophage coculture were identified (Neyen et al., 2013). While the effect of SR-A engagement by these putative ligands on tumor progression was not examined, these molecules have been implicated in mesenchymal–epithelial transition (EMT) and are known to promote tumor cell invasiveness (Bacigalupo et al., 2014; Shankar et al., 2010; Thiery, 2002). However, tumor-suppressive activities of SR-A were also reported. SR-A can inhibit Lewis lung carcinoma in mice via modulating proangiogenic factors, e.g., vascular endothelial growth factor (VEGF) and matrix metalloproteinase-9 (MMP9) (Ben et al., 2012). SR-A in leukemia stem cell of chronic myeloid leukemia (CML) has been suggested as a tumor suppressor gene. In this study, SR-A deletion accelerated CML development and increased the self-renewal and differentiation capacity of leukemia stem cells through PI3K-AKT-GSK-3β and β-Catenin pathways (Chen et al., 2011). Although the discrepancies on SR-A action in these studies have not been addressed, SR-A appears to be of significance in the development of novel therapeutic strategies directed to target SR-A+ TAMs for therapeutic benefits.
Single-nucleotide polymorphisms are a type of genetic variation associated with population diversity, genome evolution, and disease susceptibility. Epidemiology and pathological studies have established an intimate link between chronic inflammation and cancer development (McLean & El-Omar, 2009). The polymorphisms of multiple genes involved in regulation of inflammatory response have been associated with higher cancer risk (Caruso et al., 2009). Sra gene is located on chromosome 8p22, a genetic region that is associated with multiple tumor susceptibility phenotypes (Low et al., 2011). Genetic variations of Sra have been associated with the increased susceptibility of prostate cancer (Miller et al., 2003; Xu et al., 2002), although the conclusion was queried by other studies (Rennert et al., 2008; Seppala et al., 2003; Wang et al., 2003). Several common and rare missense polymorphisms variants in the Sra gene were also associated with a high risk of prostate cancer (Xu et al., 2002). Significant differences in the spectrum of mutations and sequence variants in the Sra gene have been found among various racial groups and populations (Hsing et al., 2007; Rennert et al., 2008; Sun, Turner, Xu, Gronberg, & Isaacs, 2007). A T+25C polymorphism located in the 5′ untranslated region of the Sra gene was recently suggested to increase lung cancer risk via down-regulating SR-A expression (Ben et al., 2012). While more work is clearly required to validate these findings, it is possible that screening of Sra polymorphisms may help identify populations at risk of cancer prior to disease onset.
The identification of SR-A as a negative regulator of antitumor immune response provides new insight into the functional diversity of this innate PRR (Wang et al., 2007). This finding was derived from our research in understanding the immunogenicity of HSPs or molecular chaperones as immunostimulatory adjuvants in cancer vaccination therapy (Murshid, Gong, & Calderwood, 2008; Srivastava, 2002; Wang, Facciponte, & Subjeck, 2006; Wang & Subjeck, 2013). A primary feature of chaperone molecules in cancer immunotherapy is their capacity to deliver polypeptide antigens efficiently to specialized antigen-presenting cells (APCs), such as DCs, and introduce these antigenic targets into the major histocompatibility complex (MHC) class I pathway for cross-presentation, resulting in priming CD8+ cytotoxic T lymphocytes (CTLs). Given the superior antigen cross-presenting capacity of HSPs, there has been an intensive search for dedicated receptors or binding structures on APCs that specifically recognize HSPs. Surprisingly, SR-A, with a promiscuous binding feature, was found to bind a number of HSPs, including Hsp110, Grp94, and Grp170 (Berwin et al., 2003; Facciponte, Wang, & Subjeck, 2007). Although SR-A clearly participated in binding and uptake of these HSPs, the exact contribution of the SR-A to antitumor immunity generated by HSP-based vaccines was not addressed.
We first examined the impact of SR-A on vaccination-induced anti-tumor immune response using SR-A-deficient mice (Wang et al., 2007). We made a striking observation that, in contrast to our initial prediction that SR-A-deficiency should reduce HSP vaccine activity, lack of SR-A was found to profoundly enhance a protective antitumor immune response upon administration of HSP-based chaperone vaccines (Wang et al., 2007). Additionally, SR-A absence restored the immunogenicity of poorly immunogenic murine melanoma and lung tumor cells, as indicated by improved antitumor efficacy after immunization with irradiated tumor cells as cell-based vaccines. Although an early study reported that SR-A knockout animals showed no defect in clearance of apoptotic cells (Komohara et al., 2005; Platt, Suzuki, Kodama, & Gordon, 2000), it is evident that immune tolerance to dying tumor cells was broken in the absence of SR-A. Our finding indeed was supported by a study from the Karlsson group, which suggested that class A scavenger receptors function as regulators of immune tolerance to apoptotic cells (Wermeling et al., 2007). The SR-A absence enhanced antitumor immune response was dependent on a CTL response, because depletion of CD8+ T cells or phagocytic cells (presumably APCs) abolished the antitumor activity of vaccines in SRA−/− mice. Furthermore, we demonstrated that SRA−/− mice similarly developed enhanced immune response upon vaccination with Freund’s complete adjuvant or LPS, suggesting that SR-A dampens the immunostimulatory activities of adjuvants of both mammalian and non-mammalian origins and that in these contexts SR-A represents an immune suppressor of antigen/tumor-specific T cell responses (Qian et al., 2011; Wang et al., 2007).
Monophosphoryl lipid A (MPL) is a chemically modified low toxic LPS that targets TLR4 signaling and has been tested in multiple vaccine trials (Baldridge et al., 2004). We showed that SR-A attenuated antitumor CTL response elicited by ovalbumin (OVA)-MPL immunization (Yi et al., 2009). Furthermore, the lack of SR-A appeared to render DCs more responsive to LPS stimulation as indicated by elevation of costimulatory molecules and production of proinflammatory factors, e.g., tumor necrosis factor α (TNF-α). SR-A-altered immunogenicity of DCs in response to TLR4 activation correlates with a previously suggested role of SR-A in limiting DC maturation and activation (Becker, Cotena, Gordon, & Platt, 2006). Our subsequent studies revealed that SR-A downregulated the activation TLR4-NF-κB signaling pathway in DCs by directly interacting with the TNF receptor-associated factor 6 (TRAF6), resulting in inhibition of TRAF6 dimerization and ubiquitination (Yu et al., 2011). Intriguingly, the attenuation of NF-κB activity by SR-A appeared to be independent of its ligand-binding domain, suggesting that the signaling-regulatory feature of SR-A may be uncoupled from its endocytic functions. These results not only elucidate a novel mechanism by which SR-A restricts TLR4 activation and consequent inflammatory response but also provide molecular basis of SR-A-mediated suppression of functional activation of APCs in TLR4-targeting vaccination-induced CTL response. In addition to CD8+ T cells, SR-A can also limit the activation of CD4+ T cells by inhibiting the function of DCs treated with anti-CD40 antibodies and IFN-γ (Yi et al., 2012).
In light of the fact that the immunogenicity of dying tumor cells increases in SR-A−/− mice, we examined the regulatory effect of SR-A in cross-presentation of cell-associated antigen using OVA-expressing RM1 prostate tumor model. While SR-A deficiency does not significantly influence the phagocytic ability of DCs, SR-A−/− DCs displayed increased capacity in cross-presenting OVA antigen from dying RM1 cells and generated a much more potent antigen-specific T cell response compared with wild-type DCs, which resulted in improved tumor inhibition in both prophylactic and therapeutic settings (Guo, Yi, Yu, Hu, et al., 2012).
The immunosuppressive activity of SR-A in antitumor immunity was also supported by independent studies from other groups. Inhibition of SR-A by fucoidan, a sulfated polysaccharide from the Fucus species and other brown algae that is capable of blocking SR-A function (Tateno, Ogawa, Muramoto, Kamiya, & Saneyoshi, 2002), can enhance the cross-presentation of NY-ESO-1, a cancer-testis antigen, leading to an increased CTL activation against NY-ESO-1 positive cancers (Hu et al., 2010). Herber et al. demonstrated that, in tumor-bearing mice and in cancer patients, SR-A-mediated excessive lipid uptake and accumulation in DCs endowed them with tolerogenic properties, which caused a profound defect in DC function and impaired antitumor immunity (Herber et al., 2010). This study provides an additional insight into the dysfunctional APCs in tumor-bearing host and a new mechanism by which SR-A downregulates the immunogenicity of APCs.
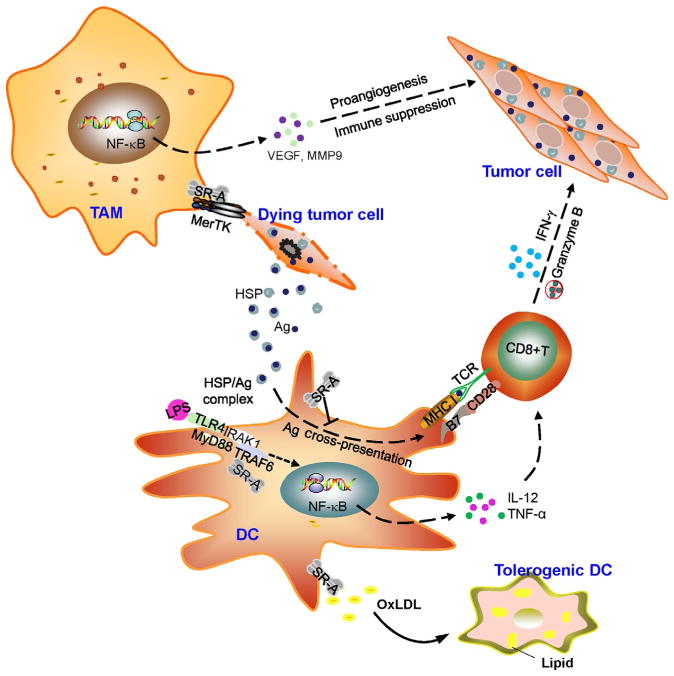
APCs, particularly DCs and macrophages, are sentinels distributed throughout the body. These cells play a central role not only in maintaining immune tolerance to self-antigens but also direct effective immune responses to eliminate “danger,” such as invading pathogens (Banchereau & Steinman, 1998). These findings also raise an intriguing question as to whether SR-A may be involved in tumor-induced immune tolerance during tumor growth and progression. DCs are known to capture antigen from live tumor cells via a “nibbling” process that is dependent on cell–cell contact, and this has been shown to be mediated by SR-A (Harshyne, Zimmer, Watkins, & Barratt-Boyes, 2003). SR-A was responsible for the acquisition and cross-presentation of tumor antigen gp100 from melanoma cells to T cells; however, this study did not address the potential significance of this SR-A activity in vivo. It is likely that SR-A-mediated transfer of live cell-associated antigen by DCs may lead to immune tolerance under a steady state or immune escape during tumor development. It is conceivable that the immunoregulatory effect of SR-A may depend on the context of their ligation and other receptors engaged simultaneously, as well as factors released from cells. Recently, we showed that SR-A is essential for maintenance of tissue homeostasis in a mouse model of immune-mediated liver injury (Zuo et al., 2013). Genetic SR-A ablation highly sensitized mice to concanavalin A (Con A)-induced hepatitis. Mechanistic studies revealed that SR-A on myeloid cells mobilized in response to tissue injury functions as a negative regulator limiting the activation of T cells that produce cytotoxic cytokine IFN-γ. This result is analogous to our finding of SR-A in suppressing the effector function of tumor-specific T cells in cancer immunotherapy. Together, our studies of SR-A in cancer- and immune-mediated tissue injury establish SR-A on myeloid cells, including DCs, as a key regulator involved in host immune homeostasis and tumor-induced immune tolerance (Fig. 1).
Figure 1.
A schematic representation of tumor-promoting functions of scavenger receptor class A (SR-A). The increased expression of SR-A may interact with and engage Mer tyrosine kinase (MerTK) signaling during interaction with dying tumor cells in the tumor microenvironment (TME), which facilitates the polarization of tumor-associated macrophages (TAMs) toward an M2-like phenotype. Proangiogenic and immunosuppressive activity of TAMs contributes to tumor progression and invasion, metastasis. The SR-A also inhibits the antigen-cross-presenting functions and activation of on dendritic cells (DCs) in response to vaccines, such as antigen (Ag) carried by heat shock proteins (HSPs) or formulated with toll-like receptor (TLR) agonists (e.g., LPS). It is executed by SR-A interference of activation of TNF receptor-associated factor 6 (TRAF6) in DCs, which leads to downregulation of TLR4-NF-κB signaling and production of immunostimulatory cytokines (e.g., TNF-α, IL-12) that are crucial for expansion and tumoricidal activity of CD8+ cytotoxic T lymphocytes (CTLs). Additionally, SR-A-mediated excessive lipid accumulation can skew DCs differentiation toward a tolerogenic phenotype in the TME, which suppresses the functions of effector T cells and promotes cancer escape from the immune attack.
2.1.2 Macrophage Receptor with Collagenous Structure
Macrophage receptor with collagenous structure (MARCO also referred to as SR-A6) is considered a dominant receptor for unopsonized particles and bacteria in the lung (Elomaa et al., 1995; van der Laan et al., 1999), due to its restricted expression in distinct populations of macrophages in lung, spleen, and lymph node. Most studies have been centered on the role of MARCO in the host defense against respiratory tract infections and pneumoconiosis induced by crystalline silica or cigarette smoke (Thakur, Hamilton, & Holian, 2008; Thomsen, Nordestgaard, Kobzik, & Dahl, 2013). However, several lines of evidence recently implicated MARCO as a regulator of DC function and antitumor immunity. An early study reported that MARCO was involved in actin cytoskeleton rearrangements during the phenotypic and functional maturation of DCs (Granucci et al., 2003). It was shown that MARCO was highly elevated in DCs upon pulsing with mouse tumor lysate (Matsushita, Komine, Grolleau-Julius, Pilon-Thomas, & Mule, 2010). Intriguingly, treatment of tumor lysate-pulsed DCs with anti-MARCO antibodies promoted these cells to traffic to draining lymph nodes and facilitated the induction of tumor-reactive IFN-γ producing T cells for tumor inhibition (Matsushita et al., 2010). Komine et al. recently found that MARCO−/− DCs displayed increased motility in response to the chemokine CCL-21 in vivo and migrated more efficiently after injection into mice (Komine, Kuhn, Matsushita, Mule, & Pilon-Thomas, 2013). Immunization with tumor lysate-pulsed DCs derived from MARCO−/− mice, compared to WT counterparts, significantly enhanced T cell-mediated antitumor immune responses and prolonged the survival of B16 melanoma-bearing mice (Komine et al., 2013). These observations are generally in line with our results of SR-A function in DC-induced antitumor immunity. Since MARCO is also expressed on human monocyte-derived DCs (Matsushita et al., 2010), the strategies that target MARCO on these cells may improve the therapeutic efficacy DC-based anticancer vaccination.
2.1.3 Scavenger Receptor Class A, Member 3 and Member 5 (SCARA3 and SCARA5)
Unlike SR-A and MARCO that are primarily expressed in myeloid cells, two other class A scavenger receptor, SCARA3 (also referred to as cellular stress response 1 [CSR1], SR-A3) and SCARA5 (SR-A5), can be detected in a variety of tissues or cells (Whelan et al., 2012). The methylation of the Scara3 gene promoter, and to a lesser extent downregulation of SCARA3 protein expression, has been associated with a high rate of prostate cancer metastasis. Forced overexpression of SCARA3 in prostate cancer cell lines (DU145 and PC3) resulted in inhibition of tumor growth and invasion, suggesting that SCARA3 may be a potent tumor suppressor gene in prostate cancer (Yu et al., 2006). However, analysis of clinical samples from 351 multiple myeloma (MM) patients showed an inverse correlation between the level of Scara3 gene, disease progression and favorable clinical outcome (Brown et al., 2013). SCARA3 expression in MM can be upregulated by ionizing radiation (IR) or chemotherapeutic drugs, which is believed to protect cells from oxidative stress by scavenging IR- or drug-generated reactive oxygen species, and therefore contribute to the resistance of MM to cancer therapeutics (Brown et al., 2013). Genomic analyses suggested that SCARA3 overexpression in ovarian carcinoma and primary peritoneal carcinoma correlates with disease progression and recurrence (Bock, Nymoen, Brenne, Kaern, & Davidson, 2012; Han, Tokino, & Nakamura, 1998). While the discrepancies on SCARA3 association with cancer progression or therapeutic resistance is unclear, future studies should examine the protein levels of SCARA3 in patient samples using immunohistochemistry, since the studies involving ovarian cancer or MM only analyzed the mRNA levels of SCARA3.
SCARA5 is unable to endocytose-modified LDLs, but it can mediate the scavenging of serum ferritin from cell surface or iron delivery (Li et al., 2009). SCARA5 expression was frequently downregulated in various cancer cell lines and tumor samples (Yan et al., 2012). Promoter hypermethylation and allelic imbalance-induced SCARA5 suppression has been detected in patient specimens with hepatocellular carcinoma (HCC) (Huang, Zheng, et al., 2010). Forced SCARA5 overexpression in HCC cell line can reverse its malignant phenotype in vitro and suppress its tumorigenesis and metastasis through the focal adhesion kinase (FAK)–Src-p130Cas signaling pathway (Huang, Zheng, et al., 2010). Mechanistic studies demonstrated that DNA methyltransferase 1 is responsible for the Scara5 promoter silence by physically associating with Snail1, a transcription factor that can directly bind to the E-box elements in Scara5 promoter. Snail1-mediated SCARA5 suppression in tumor cells is essential for EMT-induced cell migration, a crucial event in cancer metastasis (Liu et al., 2013; Son & Moon, 2010). Yan et al. further demonstrated that systemic delivery of SCARA5 by using the Scara5-cationic liposome complex markedly inhibited subcutaneous glioma tumors and spontaneous lung tumors in mice (Yan et al., 2012). Upregulation of SCARA5 also resulted in the inactivation of signal transducer and activator of transcription 3 (STAT3), as well as downregulation of STAT3-regulated genes that have been implicated in tumor progression and metastasis, including cyclinB1, cyclinD1, AKT, survivin, VEGF-A, and MMP9 (Yan et al., 2012). These studies provide a rationale for using SCARA5 as a therapeutic agent for potential cancer control.
2.2 Class B Scavenger Receptor
2.2.1 Thrombospondin Receptor CD36
CD36 (also referred to as SR-B2), initially identified as a receptor for thrombospondin (TSP), is the prototype class B scavenger receptor (Asch, Barnwell, Silverstein, & Nachman, 1987). As one of the most widely studied scavenger receptors, CD36 plays an important role in the recognition and endocytic uptake of oxidized phospholipids, modified LDL, apoptotic cells, or amyloid proteins (Stewart et al., 2010), and is involved in the regulation of many aspects of inflammatory processes in atherosclerosis and Alzheimer’s disease (Endemann et al., 1993; Philips, Rubin, & Perrimon, 2005; Ren, Silverstein, Allen, & Savill, 1995; Sun et al., 2006). Since TSP-1/2, the ligands of CD36, are the potent endogenous inhibitors of angiogenesis, the role of CD36 in controlling tumor neovascularization has been studied (Hale et al., 2012). On endothelial cells, the interaction between CD36 and TSP-1/2 initiated sequential intracellular signaling cascades involving phosphorylation of nonreceptor tyrosine kinase Fyn, the mitogen-activated protein kinase (MAPK) p38 and c-Jun N-terminal kinase, which resulted in the activation of proapoptotic signals such as caspase 3 cleavage, induction of Fas/Fas ligand and TNF-α (Jimenez et al., 2000; Simantov, Febbraio, & Silverstein, 2005; Volpert et al., 2002). Genetic depletion of Cd36 gene in mice enhanced the growth of syngeneic tumors, associated with increased tumoral vascularity (Hale et al., 2012). Given the importance of TSP-mediated suppression of tumor angiogenesis, a TSP-2 and IgG-Fc fusion protein (N-TSP2-Fc) was administrated to mice-bearing human breast tumor xenografts (MDA-MB-435, MDA-MB-231). N-TSP2-Fc significantly inhibited the growth and metastasis of breast tumors in a CD36-dependent manner (Koch et al., 2011).
Low-CD36 expression has been associated with higher metastasis grade or worse prognosis in colon cancer, breast cancer, and ovarian cancer (Rachidi, Qin, Sun, Zheng, & Li, 2013; Uray, Liang, & Hyder, 2004). It was shown that the highly aggressive breast tumor MDA-MB-231 expresses much less CD36 than the less aggressive MCF-7 and T47-D cells (Uray et al., 2004). This may be explained by the binding capacity of CD36 for collagen in extracellular matrix (ECM). Low expression of CD36 may reduce tumor cell adhesion to ECM, thus causing increased cell mobility and metastatic potential (Uray et al., 2004). Paradoxically, the expression level of CD36 in glioblastoma was found to negatively correlate with patient prognosis (Hale et al., 2014). Interestingly, CD36 appeared to be enriched on a subset of glioblastoma cancer stem cells (CSCs). CD36 reduction resulted in concomitant loss of self-renewal and tumor initiation capacity in these cells (Hale et al., 2014), suggesting that the selectively enhanced expression of scavenger receptors, such as CD36, may provide survival, and metabolic advantages in CSCs.
CD36 has been called “fatty acid translocase” due to its abundant presence in cell types involved in fatty acid metabolism, such as adipocytes, hepatocytes, cardiomyocytes, and intestinal enterocytes (Coburn et al., 2000; Drover et al., 2008; Zhou et al., 2008). CD36-mediated binding and transportation of long-chain fatty acids may facilitate intracellular lipid accumulation, a hallmark of aggressive cancer cells (Nieva et al., 2012). Surprisingly, a recent study showed that repressed CD36 expression in fibroblast from mammary tissue is responsible for pathologic changes in mammary gland hyperplasia and breast cancer, including impaired adipocyte differentiation, excessive ECM deposition, and is associated with increased risk of aggressive breast cancer in patients (DeFilippis et al., 2012). This finding underscores a crucial role of the pro-oncogenic tissue state via repression of CD36 in supporting tumorigenesis and suggests that strategies to modulate CD36 may help prevent the progression of breast cancer in women who are at high risk. While the mechanism underlying tumorigenesis promoted by the repression of CD36 is not completely understood, it is clear from steatohepatitis and obesity models that aberrant fatty acid levels can upregulate CD36 expression and affect the activity of CD36 in nonadipose cells, e.g., cardiomyocytes and vascular smooth muscle cells (Angin et al., 2012; Lau, Chu, & Weiss, 2004). Further studies are necessary to define whether dysregulated lipid metabolism may alter the expression of CD36 and its function during cancer development.
2.2.2 Scavenger Receptor Class B, Member 1 (SR-BI)
Scavenger Receptor Class B, Member 1 SR-BI (also known as SCARB1 or SR-B1) was the first identified receptor for high-density lipoprotein (HDL). SR-BI and CD36 share considerable sequence homology and mediate the transport of modified LDL, native HDL, and very low-density lipoprotein (VLDL). SR-BI is ubiquitously expressed in multiple tissues, but it is more densely expressed in organs involved in cholesterol metabolism, e.g., liver, adrenal, and gonad (Landschulz, Pathak, Rigotti, Krieger, & Hobbs, 1996; Nakagawa-Toyama et al., 2005). HDL is one of the major carriers for cholesterol, which is an important regulator of cancer development (Cruz, Mo, McConathy, Sabnis, & Lacko, 2013). Epidemiologic studies suggest that low levels of HDL cholesterol and HDL particles are risk markers of cancer development and prognosis (Kotani et al., 2013; Vilchez, Martinez-Ruiz, Sancho-Rodriguez, Martinez-Hernandez, & Noguera-Velasco, 2014). As the primary receptor responsible for the selective internalization of cholesteryl ester from HDL molecules, the role of SR-BI in cancer development recently received increasing attention.
Abundant expression of SR-BI, which participates in selective uptake of HDL-cholesteryl ester (HDL-CE), has been observed in human choriocarcinoma cells, malignant human epithelial cells, prostate cancer cells, breast cancer cells, and hepatoma cells (Graf, Roswell, & Smart, 2001; Mooberry, Nair, Paranjape, McConathy, & Lacko, 2010; Wadsack, Hirschmugl, et al., 2003; Wadsack, Hrzenjak, et al., 2003). In breast cancer, SR-BI protein levels were found to be significantly elevated in malignant tissues compared with surrounding histologically disease-free tissues (Cao et al., 2004). Overexpression of SR-BI protected breast cancer MCF-7 cells against TNF-α-induced apoptosis, whereas expression of the extracellular domain of SR-BI (amino acids 1–464) significantly inhibited the cell proliferation (Cao et al., 2004). This truncated SR-BI retained the HDL uptake capacity, but lacked the C-terminal intracellular region that is believed to regulate HDL signaling, indicating that SR-BI may be able to define the growth behavior of breast cancer cells in the presence of HDL. Another study revealed that SR-BI-mediated AKT signaling was required for sustained proliferation, mobility, and invasiveness of breast cancer cells in response to stimulation of HDL-CE (Danilo et al., 2013).
Cholesterol is the precursor of bioactive steroid hormones, such as androgen. Prostate cancer patients who have received androgen deprivation therapy commonly develop recurrence that is more aggressive and castration resistant. SR-BI expression was significantly elevated upon progression to castration-resistance in the LNCaP xenograft model, suggesting an essential role that SR-BI may play in regulation of cholesterol uptake during prostate cancer development and progression (Leon et al., 2010). Recently, small-interfering RNA (siRNA)-mediated downregulation of SR-BI was reported to effectively inhibit the production of prostate-specific antigen, and the viability of prostate cancer cells, implicating a possible therapeutic effect of SR-BI in the treatment of the castration-resistant disease (Twiddy, Cox, & Wasan, 2012).
2.3 Class D Scavenger Receptor
CD68, also known as macrosialin in mice, is the only known member of the class D scavenger receptors. CD68 is a glycosylated type I membrane protein that belongs to the lysosome-associated membrane protein family of molecules (Song, Lee, & Schindler, 2011). CD68 is predominantly expressed in late endosomes and lysosomes of macrophages, but is also found on the surface of DCs and osteoclasts (Jiang et al., 1998; Ramprasad et al., 1995). CD68 has been widely used as a pan-macrophage marker (Holness & Simmons, 1993). Increased macrophage (CD68+) index is associated with high vascularity and nodal metastasis, as well as reduced overall survival in human breast cancer (Leek et al., 1996). In a large cohort study involving 1322 patients with breast cancer, higher numbers of CD68+ macrophages predicted worse breast cancer-specific survival and a shorter disease-free interval (Mahmoud et al., 2012). A multicenter study also suggested that patients with lower CD68+ TAMs level showed improved metastasis-free survival (Jezequel et al., 2012), which further supports the predictive value of CD68+ TAMs in human breast cancer. Recently, multivariate analyses showed that the number of CD68+ cells was also an independent and significant factor for poor prognosis in patients with myxoid liposarcoma (Nabeshima et al., 2015).
Angiogenesis is a critical event in tumor growth and metastasis. The high density of CD68+ TAMs is accompanied with high stromal and serum levels of VEGF. There is an association between expressions of CD68 and Ras in the angiogenesis of breast cancer (Li et al., 2015). Upregulation of VEGF in macrophages by IR is believed to decrease the antitumor efficacy of radiotherapy (Meng et al., 2010), suggesting that the CD68+ TAMs may also be an indicator for the outcome of cancer treatment, such as radiotherapy. Despite its wide use as a predictor for cancer prognosis, the functions of CD68 in TAMs, the major inflammatory component of the tumor stroma, are largely unknown.
2.4 Class E Scavenger Receptor
2.4.1 Lectin-Like Oxidized LDL Receptor 1
Lectin-like oxidized LDL receptor 1 (LOX-1), also called oxidized low-density lipoprotein receptor 1 (OLR1), is a major class E scavenger receptor for oxLDL, advanced glycation end products (AGEs), bacteria, and apoptotic cells (Huysamen & Brown, 2009; Oka et al., 1998; Sawamura et al., 1997; Shimaoka et al., 2001). LOX-1 is primarily expressed in endothelial cells, cardiomyocytes, smooth muscle cells, B cells, macrophages, DCs, and platelets (Delneste et al., 2002; Dunn et al., 2008; Huysamen & Brown, 2009; Jeannin et al., 2005; Joo et al., 2014; Nickel et al., 2009). LOX-1 has been implicated in multiple physiological and pathophysiological processes, e.g., lipid metabolism, cholesterol biosynthesis, and atherogenesis (Huysamen & Brown, 2009; Mehta et al., 2007). Recent studies identified LOX-1 as a possible link between obesity, dyslipidemia, and cancer (Hirsch et al., 2010; Khaidakov et al., 2011). Hirsch and colleagues conducted a study to identify the cancer cell-specific transcriptional signature by comparing the oncogene Src or telomerase/Ras transformed mammary epithelial cells (MCF10A) or primary fibroblasts with their parental nontransformed cells (Hirsch et al., 2010). This signature revealed several common genes related to metabolic disorders, inflammation and carcinogenesis, indicating the importance of lipid metabolism in cellular transformation. Interestingly, upregulation of LOX-1 in the transformed cells was shown to contribute to the cellular transformation and maintenance of the transformed state by stimulating inflammatory signaling (e.g., IL-6, IL-8, and IL-1β) and hypoxia-regulated pathways (VEGF, HIF-1α, carbonic anhydrase 9) in an NF-κB-dependent manner. LOX-1 knockdown or blockade in transformed cells impaired their anchorage-independent growth, cell migration as well as invasion. In addition to supporting tumor growth in mouse xenograft models, LOX-1 was shown to be highly expressed in the patient specimens with late stage metastatic breast cancer and prostate cancer. These results provide supporting evidence that the activation of LOX-1 pathway may be a major event in tumorigenesis.
This hypothesis was tested further by Khaidakov et al. using a LOX-1 deficient mouse model (Khaidakov et al., 2011). LOX-1 abrogation resulted in broad suppression of NF-κB-targeted genes that are related to cell proliferation, migration, apoptosis, angiogenesis, and immune defense. Deficiency of LOX-1 also caused profound inhibition of rate-limiting enzymes involved in lipogenesis. Overexpression of LOX1 activated an NF-κB-dependent anti-apoptosis pathway (BCL2, BCL2A1, and TNFAIP3) as well as lipogenesis in MCF10A normal mammary epithelial cells and HCC1143 breast cancer cells, suggesting that LOX-1 acts as an oncogene promoting tumorigenesis (Khaidakov et al., 2011). Supporting evidence also came from a study showing that upregulation of endothelial LOX-1 by TNF-α facilitated the adhesion and trans-endothelial migration of MDA-MB-231 breast cancer cells (Liang, Zhang, & Fu, 2007).
Several studies have documented the involvement of LOX-1 in host immune responses, especially T cell immunity (Delneste et al., 2002; Jeannin et al., 2005; Joo et al., 2014; Parlato et al., 2010). LOX-1 on DCs can capture PAMPs and collaborate with TLR2 to activate DCs for enhanced cellular responses (Jeannin et al., 2005). In addition, LOX-1 on mouse or human DCs functions as a receptor for Hsp60 (Xie et al., 2010) and Hsp70 (Delneste et al., 2002; Theriault, Adachi, & Calderwood, 2006). LOX-1 was shown to mediate the delivery of Hsp60-fused antigen into the MHC class I presentation pathway (Xie et al., 2010). Anti-LOX-1-neutralizing antibodies can inhibit Hsp70 binding to DCs, Hsp70-induced antigen cross-presentation, as well as subsequent antigen-specific CD8+ T cell response for tumor inhibition (Delneste et al., 2002). Furthermore, human monocyte-derived DCs capture antigens for cross-presentation via LOX-1 and a LOX-1-dependent pathway is essential for IFN-α to render DCs fully competent for cross-priming CD8+ effector T cells (Parlato et al., 2010). A recent study highlighted combined expression of LOX-1 on DCs and B cells in supporting humoral responses (Joo et al., 2014). While LOX-1 signaling on DCs promotes B cell differentiation via the production of the TNF superfamily ligands APRIL and BAFF, LOX-1 signaling on B cells upregulates C–C chemokine receptor type 7 (CCR7), promoting cellular migration toward lymphoid tissues. Together, these findings suggest that LOX-1 may be exploited for the rational design of novel cancer vaccines to augment both cellular and humoral immunity.
2.4.2 β-Glucan Receptor Dectin-1
β-Glucan, a naturally derived polysaccharide present in the cell walls of plants, bacteria, and fungi including mushrooms, has been shown to stimulate the function of innate immune cells, e.g., macrophages, DCs, granulocytes, natural killer (NK) cells, and augment adaptive immune responses that inhibit tumor growth and metastasis (Lee & Kim, 2014; Yoon, Koppula, & Lee, 2013). β-Glucan has been approved as an immunoadjuvant therapeutic for cancer treatment in some countries (Ina, Kataoka, & Ando, 2013; Wang, Bi, Zou, & Gu, 2012). A recent study has shown that β-Glucan exerts multiple antitumor effects in a Dectin-1-dependent manner (Aleem, 2013). Increased tumor growth and lung metastasis was observed in Dectin-1−/− mice with subcutaneous B16 melanomas as compared with WT mice (Chiba et al., 2014).
Dectin-1, a type II transmembrane protein with a C-type lectin-like carbohydrate recognition domain, is the major β-Glucan receptor on myeloid DCs, macrophages, monocytes, and B cells (Agrawal, Gupta, & Agrawal, 2010). Dectin-1 has been shown to induce NF-κB-targeted inflammatory cytokines in cooperation with TLR2/TLR6 upon β-Glucan stimulation (Moreira et al., 2011; Yadav & Schorey, 2006). Unlike other scavenger receptors that lack conserved signaling motif, Dectin-1 in DCs can directly trigger production of inflammatory cytokines (TNF-α, IL-6, IL-2, IL-23) through its cytoplasmic domain containing an immunoreceptor tyrosine-based activation motif (ITAM), phosphorylation of downstream kinase Syk, and adaptor protein caspase recruitment domain 9 (CARD9), thus inducing the Th17 and Th1 responses (Carter, Thompson, Reid, Wong, & Tough, 2006b; LeibundGut-Landmann et al., 2007; Strasser et al., 2012; Taylor et al., 2007). In addition to inducing the differentiation of naïve CD4 T cells into Th17 cells, Dectin-1 is able to directly trigger the secretion of IL-17 from TCRγδ T cells, which are essential for antifungal immunity (Martin, Hirota, Cua, Stockinger, & Veldhoen, 2009).
Dectin-1 engagement can also drive CD8+ T cell responses. Curdlan, a selective β-Glucan agonist and a ligand for Dectin-1, is a potent adjuvant to prime the expansion and differentiation of CTL precursors in vitro and in vivo (Leibundgut-Landmann, Osorio, Brown, & Reis e Sousa, 2008). Antibody-mediated Dectin-1 ligation induces CTL, CD4+ T cell, and antibody responses, which protected mice against melanoma challenge (Leibundgut-Landmann et al., 2008). A recent study showed that Dectin-1 contributes to NK cell-mediated tumor killing (Chiba et al., 2014). Upon recognition of the N-Glycan structures that are highly expressed on tumor cells, Dectin-1-Syk pathway triggers nuclear translocation of IRF5 and induction of genes (e.g., Fam26f) that are known to enhance the tumoricidal activity of NK cells. Therefore, the ability of Dectin-1 to mobilize both innate and adaptive components of the host immune system makes it a promising target for cancer immunotherapy.
2.5 Class F Scavenger Receptor
Scavenger receptor expressed by endothelial cells-I (SREC-I), also named scavenger receptor class F, member 1 (SCARF1), was identified as an endothelial receptor for modified LDL (Adachi, Tsujimoto, Arai, & Inoue, 1997). A recent study showed that SREC-I, similar to CED-1, was involved in the clearance of apoptotic cells (Ramirez-Ortiz et al., 2013). SREC-I is also expressed in phagocytic cells (e.g., macrophages, DCs) and functions to bind immunostimulatory HSPs, including calreticulin (Berwin, Delneste, Lovingood, Post, & Pizzo, 2004), Hsp70 (Theriault et al., 2006), Hsp90 (Murshid, Gong, & Calderwood, 2010), Hsp110, and Grp170 (Facciponte et al., 2007). The early study showed that Hsp70 isolated from tumor-DC fusions (Hsp70.PC-F) were highly immunogenic and induced potent antitumor immunity (Enomoto et al., 2006). Although SREC-I is present at relatively low levels in murine bone marrow-derived DCs, its expression is elevated upon exposure to the Hsp70 vaccine (Gong et al., 2009). This study also demonstrated that SREC-I together with TLRs is required for T cell responses generated by Hsp70.PC-F vaccine, indicating a positive cross-talk between these two sets of PRRs (Gong et al., 2009). Indeed, a tightly orchestrated cooperation between signaling and endocytic PRRs involving SREC-I, SR-A, and TLR2 during the recognition of the hepatitis C virus by DCs was recently reported (Beauvillain et al., 2010).
Molecular studies revealed that SREC-I-mediated internalization of Hsp90–OVA peptide complexes through a Cdc42-regulated, dynamin-independent endocytic pathway. These Hsp90 complexes were transported from the GPI-anchored protein-enriched early endosomal compartment to recycling endosomes. Peptides that did not require additional processing were loaded directly onto MHC class I in endosomes, whereas extended peptides were targeted for cytosomal processing by aminopeptidases and proteases (Murshid et al., 2010). The requirement of cytosomal processing pathway for generating antigenic peptide epitopes from extended polypeptides carried by HSPs is consistent with our recent study of Grp170-enhanced cross-presentation of melanoma antigen gp100 (Wang, Chang, et al., 2013). We demonstrated that Grp170 efficiently facilitated the gp100 protein access to the ER. The interaction of gp100 with molecular components involved in ER-associated protein dislocation and/or degradation, strengthened by Grp170-based chaperoning, resulted in cytosolic translocation of tumor antigen for ubiquitination and proteasome-dependent processing (Wang, Chang, et al., 2013). However, whether SREC-I is involved in transporting or directing of internalized Grp170-gp100 complex to the ER, and required for resultant activation of tumor antigen-specific CTLs remains to be determined.
2.6 Class G Scavenger Receptor
Scavenger receptor for phosphatidylserine and oxidized lipoprotein (SR-PSOX), the single family member of class G scavenger receptor, not only binds phosphatidylserine and oxidized lipoprotein but also functions as a chemokine named CXC chemokine ligand (CXCL) 16, which can recruit NKT cells, T cells via interacting with the orphan G-protein coupled chemokine receptor CXCR6 (Geissmann et al., 2005; Kim et al., 2001; Matloubian, David, Engel, Ryan, & Cyster, 2000; Shimaoka et al., 2000). The transmembrane form of SR-PSOX/CXCL16 (TM-CXCL16) is mainly expressed on macrophages, DCs, monocytes, B cells, liver sinusoidal endothelial cells, and various types of tumor cells, where it acts as an adhesion molecule to promote the activation of antigen-specific, primary and secondary T cell responses (Heydtmann et al., 2005; Matsumura & Demaria, 2010; Matsumura et al., 2008). SR-PSOX/CXCL16 was shown to facilitate interaction between DCs and CD8+ T cells, and to guide T cell movements in the splenic red pulp (Matloubian et al., 2000). Additionally, binding and scavenging of both Gram-negative and Gram-positive bacteria by the membrane-anchored form of CXL16 on macrophages or DCs are dependent on its chemokine domain, indicating an important role of TM-CXCL16 in the host defense involving both innate and adaptive responses (Shimaoka et al., 2003). There also exists a soluble form of SR-PSOX/CXCL16 (sCXCL16) with similar chemotactic activity, which is generated by constitutive or inducible cleavage through the activation of cell surface disintegrin-like metalloproteinases ADAM10 and ADAM17 (Abel et al., 2004; Pupovac, Foster, & Sluyter, 2013; Schramme, Abdel-Bakky, Kampfer-Kolb, Pfeilschifter, & Gutwein, 2008). Shedding of SR-PSOX/CXCL16 from cell surface can be triggered by the treatment with TNF-α, IL-1β, IFN-γ, phorbol 12-myristate 13-acetate (PMA), extracellular ATP, or radiation (Gutwein et al., 2009; Matsumura & Demaria, 2010; Pupovac et al., 2013).
SR-PSOX/CXCL16 and its binding partner CXCR6, as well as its processing enzyme ADAM10 and ADAM17, are overexpressed in many tumor cells, including prostate, breast, colorectal, gastric, liver, ovarian, pancreatic, cervical, lung cancer, glial tumors, and Ewing sarcoma (Gooden et al., 2014; Hattermann, Held-Feindt, Ludwig, & Mentlein, 2013; Huang, Zhang, Cui, Zhao, & Zheng, 2013; Na et al., 2014; Wente et al., 2008). Increasing evidence supports the involvement of sCXCL16 in tumor progression, e.g., promoting proliferation, migration, and invasiveness of CXCR6+ cancer cells (Lu et al., 2008; Schramme et al., 2008; Xing et al., 2012). The elevated level of sCXCL16 in serum has been considered an independent predictor for poor survival in ovarian cancer patients and those with colorectal cancer who have developed the recurrence of liver metastases (Gooden et al., 2014; Matsushita et al., 2012). Independent studies also support the association of SR-PSOX/CXCL16 with poor prognosis in various cancers. Analysis of 354 prostate cancer samples revealed that SR-PSOX/CXCL16 expression was significantly increased in patients with perineural invasion, lymph node and bone metastasis, large tumor burden, and high pathological disease stage (Ha et al., 2011). In 92 patient samples of epithelial ovarian cancer, expression of SR-PSOX/CXCL16 and CXCR6 was significantly related to lymph node metastasis and reduced median survival (Guo, Cui, Zhang, & Huang, 2011). Studies using 461 specimens from 12 different cancers indicated that the elevation of SR-PSOX/CXCL16 and CXCR6 on both tumor cells and adjacent T cells may facilitate the formation of proinflammatory TME via recruiting tumor-infiltrating leukocytes, which enhances tumor cell proliferation and invasiveness (Darash-Yahana et al., 2009). The involvement of sCXCL16 in promoting neutrophil infiltration, angiogenesis, and formation of tumor-promoting inflammatory environment has also been shown in HCC patients (Gao et al., 2012).
A feed-forward signaling loop between tumor cells and mesenchymal stem cells (MSCs), which can be triggered by the engagement of CXCL16-CXCR6, was recently proposed (Chaturvedi, Gilkes, Takano, & Semenza, 2014; Jung et al., 2013). Tumor cell-produced sCXCL16 recruits CXCR6+ MSCs to the tumor site. CXCL12 secreted from these recruited MSCs will in turn bind to its receptor CXCR4 on tumor cells, resulting in enhanced cancer invasiveness and metastasis (Chaturvedi et al., 2014; Jung et al., 2013). Another study showed that activation of CXCL16-CXCR6 axis enhanced prostate cancer progression and metastasis, which was associated with the upregulation of proangiogenic factor IL-8 and VEGF that depended on the CXCR6/AKT/mTOR signaling pathway (Wang, Lu, Koch, Zhang, & Taichman, 2008).
A different finding was made in studies that involved 104 patient samples of renal cell carcinoma (RCC) and 58 samples of colorectal carcinoma (CRC) (Gutwein et al., 2009; Hojo et al., 2007). In these two studies, TM-CXCL16 correlated inversely with tumor stage and a high level of tumoral TM-CXCL16 appeared to be a prognostic marker for long-term survival. Immunohistochemistry and flow cytometry analyses showed that SR-PSOX/CXCL16 was located predominantly in the membrane and cytosol of RCC cell lines (Gutwein et al., 2009). In line with these clinical data, overexpression of TM-CXCL16 in breast cancer MDA-MB-231 cells suppressed their invasiveness in vitro and tumorigenesis in vivo (Fang et al., 2014). IR can upregulate the expression of TM-CXCL16 and promote the shedding of sCXCL16, which results in improved therapeutic efficacy by recruiting CD8+CXCR6+ effector T cells (Matsumura & Demaria, 2010; Matsumura et al., 2008). These results clearly demonstrate the different functions of SR-PSOX/CXCL16 in various cancers or therapeutic settings. Understanding the context-dependent activities of SR-PSOX/ CXCL16 is essential for developing therapeutics to block cancer progression and invasion.
2.7 Class H Scavenger Receptor
Stabilin-1, also referred to as Fasciclin, epidermal growth factor-like and lamin-type epidermal growth factor-like, and link domain-containing scavenger receptor-1 (FEEL-1), or common lymphatic endothelial and vascular endothelial receptor-1 (CLEVER-1), and stabilin-2/FEEL-2 are the current known class H scavenger receptor members (Adachi & Tsujimoto, 2002; Irjala et al., 2003; Politz et al., 2002). Stabilin-1 and stabilin-2, which bind several ligands, including oxLDL, acLDL, hyaluronan (HA), heparin, and matricellular protein such as secreted protein acidic and rich in cysteine (SPARC), play important roles in clearance of these “unwanted” self-substances (Qian et al., 2009; Rost & Sumanas, 2014; Workman & Sage, 2011). Stabilin-1 was also reported to bind Hsp70–peptide complexes and mediate its internalization (Theriault et al., 2006). However, its role in modulating Hsp70 vaccine-induced antitumor immune response is unknown. Stabilin-1 and stabilin-2 are constitutively present on tissue resident macrophages, lymphatic endothelial cells, and sinusoidal endothelial cells in bone marrow and liver (Qian et al., 2009; Shetty et al., 2011). However, their expression changes on vasculature at the sites of chronic inflammation or tumor, where they mediate the trafficking of lymphocytes, granulocytes, and monocytes to the inflamed tissues (David et al., 2012; Karikoski et al., 2009).
An early study suggested stabilin-1 as a multifunctional scavenger receptor that could link endocytic clearance, intracellular sorting, and transcytosis in macrophages (Kzhyshkowska & Krusell, 2009). SPARC, a nonstructural glycoprotein crucial for angiogenesis, wound healing, and tissue remodeling, plays a significant role in altering cancer cell activity and remodeling the TME (Nagaraju, Dontula, El-Rayes, & Lakka, 2014). Given that stabilin-1+ macrophages can actively uptake and degrade circulating SPARC, it is conceivable that aberrant clearance of SPARC may contribute to tumor progression and metastasis (Nagaraju et al., 2014). Indeed, the expression of stabilin-1 in TAMs decreased during the progression of glioma and melanoma (David et al., 2012). Stabilin-1 on liver sinusoidal endothelial cells was shown to preferentially facilitate the recruitment of CD4+FoxP3+ regulatory T cells (Treg) to the liver tissue with HCC, which could disable the immune effector functions and facilitate tumor escape (Shetty et al., 2011).
The role of stabilin-1 in tumor growth was further validated using cell-specific stabilin-1 knockout mice (Karikoski et al., 2014). Elevation of stabilin-1 in tumor vasculature enhanced the binding of immunosuppressive leukocytes to the intratumoral blood vessels and promoted tumor cell trafficking via the lymphatics. Growth of primary tumors, not of metastases, was inhibited in mice that lacked stabilin-1 in macrophages or in vascular endothelium. The absence of functional stabilin-1 resulted in diminished tumor infiltration by immunosuppressive leukocytes and suppressed tumor progression, suggesting that stabilin-1 represents a novel target for overcoming immune evasion and blocking lymphatic spread of cancer (Karikoski et al., 2014).
HA, a major component of the ECM, is highly increased in tumor progression, which correlates with high tumor grade and poor prognosis (Karbownik & Nowak, 2013). The binding of HA to its receptor activates signaling pathways that stimulate cell proliferation, invasiveness, multidrug resistance, and EMT in many tumors (Workman & Sage, 2011). Antibody blockade or genetic ablation of stabilin-2, which disrupted HA–stabilin-2 interaction, led to elevated circulating HA levels and prevented tumor metastasis (Sironen et al., 2011), suggesting that functional inhibition of stabilin-2 may be a potential approach to suppressing tumor progression. In addition, stabilin-2 expression levels have been linked to the outcome of cancer patients. Tissue microarray analysis of 296 samples from patients with HCC showed that loss of stabilin-2 expression in peritumorous liver tissue correlated with increased survival (Geraud et al., 2013). Based on its differential expression in prostate cancer patients versus healthy controls, stabilin-2 has been identified as a potential early diagnostic biomarker for detecting indolent and advanced prostate cancer (Neuhaus et al., 2013).
2.8 Class I Scavenger Receptor
CD163 is the prototype class I scavenger receptor for haptoglobin–hemoglobin (Hp–Hb) complexes. CD163 is exclusively expressed on monocytes/macrophages or hematopoietic malignancies with monocytic/ histiocytic differentiation (Nguyen et al., 2005). CD163 expression is tightly regulated by inflammatory responses, where anti-inflammatory signals (e.g., IL-10, glucocorticoid) induce CD163 expression, but proinflammatory signals (e.g., LPS, TNF-α, IFN-γ) suppress CD163 synthesis (Buechler et al., 2000). Studies of CD163 regulation in multiple tumor models indicate that the high level of CD163 is a feature of macrophages undergoing differentiation toward the “alternatively activated” M2 phenotype (Edin et al., 2012; Fujimura, Kambayashi, Furudate, Kakizaki, & Aiba, 2013; Gordon & Martinez, 2010; Komohara, Ohnishi, Kuratsu, & Takeya, 2008; Tiainen et al., 2014; van Dongen et al., 2010). CD163-involved macrophage polarization may be related to its function of scavenging Hp–Hb complexes. Release of Hb into plasma is a phenomenon occurred during physiologic or pathologic intravascular hemolysis, e.g., inflammation and hemorrhage in the tumor. Free form of Hb in the circulation forms complexes with plasma glycoprotein Hp, which results in the high-affinity interaction of CD163 with Hp–Hb complexes in a calcium-dependent manner (Madsen et al., 2004). The binding of the complexes to CD163+ TAMs stimulates the induction of stress-responsive hemo oxygenase-1 (HO-1), a hemedetoxification enzyme that is also involved in macrophage polarization toward an M2 phenotype and important for production of anti-inflammatory cytokine IL-10 (Naito, Takagi, & Higashimura, 2014; Sierra-Filardi, Vega, Sanchez-Mateos, Corbi, & Puig-Kroger, 2010; Weis, Weigert, von Knethen, & Brune, 2009). Thus, the CD163–HO-1–IL-10 axis may be an important contributor to the formation of immunosuppressive TME.
CD68 and CD163 are often used to identify macrophages in tissue sections (Kong et al., 2013). Compared to CD68 that is commonly used as a pan-macrophage marker (Holness & Simmons, 1993), CD163 is regarded as a specific monocyte/macrophage marker for M2 macrophages (Ambarus et al., 2012; Lau et al., 2004; Qian & Pollard, 2010). The presence of CD163+ macrophages was suggested to have a stronger association with less favorable clinicopathological features than CD68+ macrophages (Medrek, Ponten, Jirstrom, & Leandersson, 2012). Numerous studies demonstrate that elevated CD163 expression correlates with advanced cancer stages, unfavorable prognosis, early distant recurrence, and reduced patient survival in various types of cancer, which include melanoma (Jensen et al., 2009), meningioma (Kanno et al., 2013), breast cancer (Mansfield, Heikkila, von Smitten, Vakkila, & Leidenius, 2012; Shabo, Stal, Olsson, Dore, & Svanvik, 2008; Tiainen et al., 2014), colorectal cancer (Edin et al., 2012; Shabo, Olsson, Elkarim, Sun, & Svanvik, 2014), oral squamous cell carcinoma (He, Bao, et al., 2014; Wang et al., 2014), ovarian carcinoma (Reinartz et al., 2014), HCC (Kong et al., 2013), angiosarcoma (Fujimura et al., 2013), glioma (Komohara et al., 2008), and gastrointestinal stromal tumors (van Dongen et al., 2010), and hematopoietic malignancies, such as T cell leukemia/lymphoma (Komohara et al., 2013), acute myeloid leukemia (Garcia, Gardner, & Reichard, 2008), and classical Hodgkin lymphoma (Klein et al., 2014; Koh, Park, Yoon, Suh, & Huh, 2014). A recent study showed that relapse of head and neck cancer after chemoradiotherapy also correlated with CD163+ macrophages in primary tumor and CD11b+ myeloid cells in recurrences (Balermpas et al., 2014).
Several studies reported that the tumor cell itself in breast cancer, rectal cancer, bladder cancer, and meningioma expresses CD163 and that the CD163 levels are associated with metastatic grade, early recurrence, and reduced patient survival (Kanno et al., 2013; Maniecki et al., 2012; Shabo, Olsson, Sun, & Svanvik, 2009; Shabo et al., 2008). It was found that IR-induced CD163 expression on tumor cells rendered these cells more resistant to radiotherapy (Shabo et al., 2008). Two mechanisms have been proposed to explain how tumor cells express macrophage surface markers such as CD163, which include the heterotypic cell fusion of cancer cells with TAMs or a generic molecular exchange between those cells via exosome-mediated transfer (Shabo & Svanvik, 2011). CD163+ tumor cells are also suggested to constitute a subpopulation of cancer cells, which are associated with EMT and increased metastatic activity induced by TAMs. Upregulation of granulocyte colony-stimulating factor (G-CSF) is believed to be responsible for suppressed apoptosis and enhanced proliferation in CD163+ tumor cells (Kanno et al., 2013).
CD163 was recently identified as a receptor for TNF-like weak inducer of apoptosis (TWEAK), a member of the TNF superfamily that is involved in proinflammatory responses, proangiogenesis, and tissue remodeling (Bover et al., 2007; Michaelson & Burkly, 2009; Moreno et al., 2009). In tumor cells, binding of TWEAK to its receptor FGF-inducible molecule 14 (Fn14) results in stimulation of tumor cell proliferation, migration and invasion, as well as NF-κB signaling and gene expression that promotes tumor growth, angiogenesis, and immune suppression (Cheng, Whitsett, Tran, & Winkles, 2014; Yin et al., 2014, 2013). On macrophages, TWEAK selectively binds to the scavenger receptor cysteine-rich domain of the CD163. CD163-mediated TWEAK scavenging by macrophages contributes to its degradation and sequestration in TME, which may prevent TWEAK from exerting its tumor-promoting functions, which suggests a potential antitumor benefit of TWEAK–CD163 interaction in macrophages (Bover et al., 2007).
CD163 not only exists as a membrane-bound form, but also is present as a soluble form (sCD163) in plasma and other tissue fluids. ADAM17 was shown to cleave CD163 ectodomain, thereby downregulating the surface expression of CD163 (Etzerodt, Maniecki, Moller, Moller, & Moestrup, 2010; Etzerodt et al., 2014). Intriguingly, sCD163 has the abilities to inhibit T cell proliferation (Hogger & Sorg, 2001) and to promote recognition and phagocytosis of Staphylococcus aureus (Kneidl et al., 2012). Circulating sCD163 level has been suggested to be a prognostic biomarker for cancer patients with poor outcome and may reflect increased activity of CD163+ TAMs (Andersen, Abildgaard, Maniecki, Moller, & Andersen, 2014; Jones et al., 2013; No, Moon, Kim, & Kim, 2013; Sugaya et al., 2012). The role of sCD163 in cancer is poorly understood. It is possible that sCD163 may also be involved in TAM polarization by competing for binding to Hp–Hb complexes.
2.9 Class J Scavenger Receptor
Receptor for advanced glycation endproducts (RAGE), the only member of the class J SR group, also belongs to the immunoglobulin (IgG) superfamily. RAGE has a broad spectrum of ligands, including advanced glycation end-products, prototype of high mobility group (HMGB) family proteins (e.g., HMGB1, amphoterin), glycosaminoglycan, amyloid A peptides, members of the S100/calgranulin protein family, collagen I and IV, β-amyloid, integrin Mac-1, and complement C3 (Yamagishi, Matsui, & Fukami, 2015). Among six isoforms of RAGE mRNA splicing variants identified so far (Hudson, Carter, et al., 2008), only two functionally relevant isoforms have been well studied. These include a full-transmembrane form, which can initiate signaling through its intracellular domain, and a truncated, endogenous secretory form of RAGE (esRAGE), which can function as a decoy receptor (Cheng et al., 2005). The ectodomain of RAGE can be proteolytically cleaved off by MMP9 and/or ADAM-10 to generate another soluble form of RAGE (sRAGE) with the similar decoy activity (Allmen, Koch, Fritz, & Legler, 2008; Raucci et al., 2008). Reduced or silenced esRAGE expression has been found in nonsmall cell lung carcinoma patients with significantly lower survival rate (Kobayashi et al., 2007). A meta-analysis of cancer patients, including breast, pancreatic, liver, lung, and colorectal cancers, showed that sRAGE level was inversely associated with the significant risk of cancer, indicating a protective role of circulating sRAGE in the development of cancer (He, Zhang, et al., 2014).
More than 30 gene polymorphisms in RAGE have been identified in multiple cancers, including renal caner, oral cancer, breast cancer, colorectal cancer, lung cancer, ovarian cancer, pancreatic cancer, nasopharyngeal carcinoma, and gastric cancer (Cheng et al., 2005; Chocholaty et al., 2014; Gu et al., 2008; Krechler et al., 2010; Pan et al., 2013; Qian, Sun, Zhang, Ke, & Zhu, 2014; Su, Chien, Lin, Chen, & Yang, 2015; Zhang et al., 2013; Zhou, Deng, Li, Yin, & Ye, 2014). A comprehensive meta-analysis suggested that the 82G/S polymorphism (3374 cancer cases vs. 3757 controls) in the RAGE promoter region is associated with a significantly increased risk of cancer, where −374T/A polymorphism (2936 cancer cases vs. 3,338 control) is associated with a reduced risk of cancer (Xia et al., 2015). Therefore, the polymorphisms of RAGE may be used as a potential marker for early screening or diagnosis of certain types of cancers.
Co-overexpression of RAGE and its ligands are commonly found in many types of cancer and are associated with cancer progression and poor outcome (Moser et al., 2014; Yamagishi et al., 2015; Zhao et al., 2014). Accumulating evidence suggests that the multiligand-RAGE axis plays crucial roles in tumorigenesis, e.g., enhancing tumor angiogenesis and hypoxia resistance, promoting tumor cell proliferation and invasion, orchestrating the immunosuppressive TME (Chen et al., 2014; Kang et al., 2014; Nasser et al., 2015). An array of signaling pathways have been linked with the tumor-promoting activities of RAGE, including the activation of MAPKs, PI3K/Akt, NF-κB, Jak/STAT, Src kinase, and Rho GTPases Diaphanous-1 (Dukic-Stefanovic, Gasic-Milenkovic, Deuther-Conrad, & Munch, 2003; Hofmann et al., 1999; Hudson, Kalea, et al., 2008; Kim et al., 2008; Palumbo et al., 2009; Reddy et al., 2006; Toure et al., 2008). Both Erk1 and Erk2 have been identified as direct RAGE-binding partners (Ishihara, Tsutsumi, Kawane, Nakajima, & Kasaoka, 2003). HMGB1, S100A4, S100A7-mediated RAGE-Erk1/2 activation were shown to inhibit tumor cell autophagy and apoptosis, promote tumor cell proliferation, increase cell mobility and invasiveness (Dahlmann et al., 2014; Nasser et al., 2015; Yamagishi et al., 2015; Zhang, Wu, Zhang, Han, & Lin, 2015). HMGB1 interaction with RAGE were also reported to provoke the proliferation of lung cancer cells and inhibit their apoptosis by regulating Bax and Bcl-2 levels through PI3K/Akt signaling pathway (Xu et al., 2014). In oncogene Kras mutation-driven pancreatic cancer, hypoxia induced RAGE expression in an NF-κB-dependent but HIF-1α-independent manner. Consequently, overexpressed RAGE directly interacted with KRAS, further sustaining KRAS downstream signaling pathways (Raf-Mek-Erk and PI3K-Akt) and driving tumor progression (Kang et al., 2014).
In studies that evaluated the functional significance of RAGE in cancer development, it was shown that RAGE-deficient mice displayed reduced tumor growth, metastasis, and tumor vascularization, as well as decreased infiltration of M2 TAMs and tumor-associated inflammation (Medrek et al., 2012). In both mouse glioma and breast cancer models, systemic blockade of RAGE by administration of RAGE-neutralizing antibodies or soluble RAGE resulted in profound inhibition of tumor growth and metastasis (Chen et al., 2014; Nasser et al., 2015). However, an antitumor effect of RAGE was recently reported in embryonal rhabdomyosarcoma (ERMS) (Chiappalupi, Riuzzi, Fulle, Donato, & Sorci, 2014). RAGE is expressed in muscle tissue during embryonic development and can maintain myogenesis via a Cdc42-Rac1-MKK6-p38-dependent and myogenin-dependent repression of Pax7, a critical transcription factor for self-renewal and biogenesis of muscle satellite cells (Riuzzi, Sorci, Sagheddu, & Donato, 2012). Highly expressed Pax7 was found to promote the migration and invasiveness of ERMS cells and in turn cause reduction of RAGE. Over-expression of RAGE in human ERMS cells can downregulate Pax7 and reduce metastasis. Additional studies are needed to define the distinct effects of RAGE on cancers of different types, which should be considered during the design of RAGE-targeted antitumor therapeutics.
3. SCAVENGER RECEPTORS IN CANCER THERAPY
3.1 Scavenger Receptor-Based Delivery of Antineoplastic Drugs
To date, systemic delivery of anticancer therapeutics to targeted cancer cells and tissues remains a major challenge. Barriers to effective targeted drug delivery include systemic toxicity or side effects, poor solubility and biostability, and high entrapment and first-pass metabolism by the liver. Recently, a variety of biomimetic lipoprotein nanoparticles carrying therapeutic agents to target cancer cells that highly express certain scavenger receptors have been tested in preclinical applications. HDL consists of apolipoproteins A–I and lipophilic components (e.g., cholesteryl ester, cholesterol, and phospholipids). HDL-based nanoparticles (8–11 nm in diameter) are ideal drug carriers because their shielded hydrophobic core is suitable for accommodating lipophilic drugs. These nanoparticles may be selectively targeted to tumor cells based on the expression of their high-affinity receptor SR-BI (Ng, Lovell, & Zheng, 2011). HDL particles are completely biodegradable and do not trigger any immune response, since all components of HDL are considered self and already present in the human body. Due to their small size, these nanoparticles can easily escape the removal by the reticuloendothelial system. Importantly, the overexpression of the major HDL receptor SR-BI on cancer cells potentiates the binding and uptake of HDL-CE, a nutrient for rapid dividing tumor cells (Ng et al., 2011).
HDL can capture exogenous and endogenous circulating microRNA (miRNA) and deliver them to SR-BI+ target cells to induce differential gene expression (Vickers, Palmisano, Shoucri, Shamburek, & Remaley, 2011). Therefore, recombinant HDL has been exploited to deliver siRNA that targets key molecules involved in cancer growth and metastasis. RNAi-HDL nanoparticles that directly deliver siRNA for VEGF or VEGFR2 to SR-BI-overexpressed tumor endothelial cells strongly inhibit neovascularization and tumor growth in mice established with orthotopic Lewis lung or MCF-7 breast cancer (Ding et al., 2014; Tripathy, Vinokour, McMahon, Volpert, & Thaxton, 2014). RNAi-HDL nanoparticle targeting signal transducer and activator transcription 3 (STAT3) or FAK resulted in the suppression of ovarian and colorectal cancers (Shahzad et al., 2011). Recombinant HDL nanoparticles have also been used to deliver chemotherapeutic drugs, e.g., paclitaxel or α-tocopheryl-succinate, to SR-BI+ cancers (Hrzenjak et al., 2004; Mooberry et al., 2010; Shin et al., 2012). It was recently reported that a highly biocompatible HDL-mimicking peptide-phospholipid scaffold nanocarrier not only can serve as a cargo to deliver therapeutic drugs to SR-BI+ nasopharyngeal carcinoma but also can exert direct antitumor efficacy in vivo (Zheng et al., 2013).
SR-A has also been suggested as a targeting molecule for nanoparticle-based drug delivery. SR-A can efficiently mediate opsonin-independent internalization of dextran-coated superparamagnetic iron oxide (SPIO) nanoparticles via its positively charged extracellular collagenous domain (Chao et al., 2013, 2012). SPIO-based delivery platform may be used for early detection of metastases in cancer patients or used as a carrier to deliver chemotherapeutics into tumors (Chiang, Tseng, Liao, & Chen, 2015; Jafari et al., 2015). SR-A was shown to specifically express on the immunosuppressive vascular leukocytes (VCLs) in mouse and human ovarian cancer. Administration of toxin-conjugated anti-SR-A antibodies to mice with peritoneal ovarian tumors resulted in selective depletion of these SR-A-expressing cell population and effectively reduced tumor burden and ascites accumulation (Bak, Walters, Takeya, Conejo-Garcia, & Berwin, 2007).
3.2 Scavenger Receptors and Immune Modulation Therapy
Given the immunosuppressive activity of SR-A in vaccination-generated antitumor response, manipulation of SR-A expression or blockade of SR-A function is expected to enhance the potency of cancer vaccines. Large stress/HSP, such as Hsp110 and Grp170, when complexed with tumor antigen (e.g., Gp100) have demonstrated superior antitumor efficacy primarily through enhancing antigen cross-presentation and activation of tumor-specific CTLs (Wang, Chang, et al., 2013; Wang et al., 2007; Wang & Subjeck, 2013; Wang et al., 2010). The promising preclinical results of this recombinant chaperone vaccine (i.e., Hsp110-gp100) have led to an ongoing Phase I melanoma clinical trial at Roswell Park Cancer Institute. Due to the presence of various HSP-binding receptors on APCs, including scavenger receptors, these HSP-based vaccines are believed to selectively target endogenous DCs. Hence, downregulation of SR-A in DCs may be used in conjunction with HSP vaccines for improved antitumor immunity (Qian et al., 2011). We recently evaluated the feasibility of reducing SR-A expression levels to enhance the potency of DC-based vaccine in pre-clinical cancer models (Yi et al., 2011; Yu & Wang, 2012). We demonstrated that lentivirus-mediated delivery of a short hairpin RNA (shRNA) for SR-A resulted in efficient downregulation of SR-A in DCs. SR-A-silenced DCs carrying tumor antigen gp100 were much more immunogenic than mock-modified DCs in provoking an antigen-specific CD8+ CTL response. Tumor-specific CTLs elicited by SR-A-silenced DC-gp100 vaccine exhibited increased effector functions (e.g., cytokine production and tumoricidal activity), which markedly inhibited established melanoma and metastases (Yi et al., 2011). In the setting of combined radiotherapy and in situ DC vaccine therapy, we showed that intratumoral administration of unmodified DCs failed to synergize with radiotherapy (Guo, Yi, Yu, Zuo, et al., 2012). However, administration of SR-A-silenced DCs, when combined with local radiotherapy, profoundly suppressed mouse prostate cancers (e.g., RM1 and TRAMP-C2) and distant metastases, and prolonged the lifespan of tumor-bearing animals, which depended on CD8+ cells and IFN-γ (Guo, Yi, Yu, Zuo, et al., 2012). Therefore, these studies provide preclinical evidence supporting the principle of silencing SR-A in DCs as a means to break tolerance against tumor-associated antigens.
In addition to the genetic approach for downregulating SR-A expression, small molecule inhibitors (SMIs) may also be exploited for potential therapeutic benefits. Sennoside B, a small molecule from screening of bio-active SMI libraries, was reported to effectively reduce SR-A-mediated antigen transfer and inhibit T cell proliferation in vitro and in vivo (Raycroft, Harvey, Bruck, & Mamula, 2012). However, the effect of this compound on vaccine-induced antitumor immunity has not been examined. An apolipoprotein A–I mimetic peptide, which can compete with a range of SR-A ligands, was shown to block SR-A-mediated adhesion (Navab, Anantharamaiah, Reddy, Van Lenten, & Fogelman, 2008) and inhibit tumor growth and metastasis in mice-bearing ovarian and pancreatic cancers (Neyen et al., 2013).
It has been well established that DCs function as the essential link between the innate and adaptive arms of the immune system (Todt et al., 2008). Various vaccination and immunotherapeutic strategies aim to target DCs because of their pivotal role in adaptive immunity (Guo, Manjili, et al., 2013). While our studies provide a rationale for selectively blocking immunosuppressive SR-A to improve DC-based cancer vaccines, other scavenger receptors on DCs provide an opportunity to target antigens preferentially to DCs for processing and presentation. Anti-Dectin-1/2 antibodies conjugated to OVA antigen induced significant expansion of T cells in the draining lymph nodes of mice and IFN-γ production by T cells (Carter, Thompson, Reid, Wong, & Tough, 2006a, 2006b). In addition, β1,3-d-glucan conjugated to OVA, which can be rapidly phagocytosed by DCs possibly via Dectin-1/2, provoked DC maturation and stimulated >100-fold more efficiently OVA-specific OT-I and OT-II T cells (Huang, Ostroff, Lee, Specht, & Levitz, 2010), suggesting that antigen targeted to Dectin-1/2 on DCs may facilitate efficient antigen capture and concurrently induce DC activation. Anti-LOX-1 antibodies have also been successfully to target antigens to DCs to induce antigen-specific CTL response (Li et al., 2012) and antitumor immunity (Delneste et al., 2002), indicating that scavenger receptor targeting of tumor antigens is a promising strategy for cancer immunotherapy.
4. CONCLUDING REMARKS
The large repertoire of ligands recognized by scavenger receptors defines their broad range of functions in many physiologic and pathologic conditions, e.g., lipid metabolism, tissue homeostasis, inflammation, atherosclerosis, and autoimmune disorders. Recently, these innate PRRs are emerging as important regulators of tumorigenesis, cancer invasion, and the antitumor immune response (Table 1). However, the extent and the mechanism of their involvement in cancer biology and immunology have not yet been fully appreciated because research focused on these scavenger receptors in these areas is in its infancy. The overexpression of certain SRs (e.g., SR-A, CD163) on TAMs and their use as cancer diagnostic or prognosis markers strongly suggest their potential involvement in the functional regulation of TAMs during tumor progression, including tumor cell proliferation and mobility, tumor angiogenesis, and immune suppression. However, the molecular underpinnings of the distinct actions of specific scavenger receptors in these processes are largely unknown. The recent discovery of the immunosuppressive activity of SR-A in vaccination-induced T cell activation and antitumor immune response was striking, given its established role in DC-mediated capture of immunostimulatory HSPs and associated tumor antigens. Our proof-of-principle studies demonstrated the feasibility of SR-A blockade to improve the antitumor efficacy of DC vaccines. In contrast, antigen targeting to certain scavenger receptors, such as LOX-1, may lead to enhanced T cell response and tumor eradication, further stressing the functional versatility and complexity of these scavenger receptors. More work is needed to determine the relative contribution of scavenger receptors to the functional activation of APCs and resultant anti-tumor immunity. Furthermore, additional studies are necessary to elucidate the molecular basis of how the ligand-specific interaction and various coreceptors may dictate or influence the outcome of engaging these scavenger receptors in homeostatic state and pathological settings. A better understating of the immunobiology of scavenger receptors will facilitate the development of rational approaches to target functionally distinct receptors for drug delivery, diagnostic imaging, antigen capture and processing, and antagonizing immune suppression, which are expected to lead to improved outcomes in cancer treatment.
Table 1.
Diverse Functions of Scavenger Receptors in Cancer
| Name | Family | Expression | Roles | References |
|---|---|---|---|---|
| SR-A | Class A | Myeloid cells and LSEC | Inhibition of NO, IFN-β and IFN-γ production by TAMs Required for tumor cell invasion Inhibits DC activation and antigen cross-presentation Induction of tolerogenic DCs Regulation of tumor angiogenesis Suppression of leukemia stem cells |
Komohara et al. (2009) Neyen et al. (2013) Wang et al. (2007), Yi et al. (2009), Yi et al. (2011), Guo, Yi, Yu, Hu, et al. (2012), and Guo, Yi, Yu, Zuo, et al. (2012) Herber et al. (2010) Ben et al. (2012) Chen et al. (2011) |
| MARCO | Class A | Myeloid cells | Modulation of DC-based cancer vaccine | Matsushita et al. (2010) and Komine et al. (2013) |
| SCARA3 | Class A | Ubiquitous expression | Tumor suppressor gene in prostate cancer Multiple myeloma therapeutic resistance |
Yu et al. (2006) Brown et al. (2013) |
| SCARA5 | Class A | Selected ECs | Inhibition of tumorigenesis and metastasis Tumor suppressor gene |
Huang, Zheng, et al. (2010) Liu et al. (2013) and Yan et al. (2012) |
| CD36 | Class B | Phagocytic cells and ECs | Inhibition of tumor angiogenesis Biomarkers |
Jimenez et al. (2000), Simantov et al. (2005), Volpert et al. (2002) DeFilippis et al. (2012), Hale et al. (2014), Rachidi et al. (2013), and Uray et al. (2004) |
| SR-BI | Class B | Ubiquitous expression | Protection of tumor cells from apoptosis Mediates invasiveness of breast cancer Required for prostate cancer tumor development |
Cao et al. (2004) Danilo et al. (2013) Leon et al. (2010) and Twiddy et al. (2012) |
| CD68 | Class D | Macrophages, DCs, and osteoclast | Biomarkers | Jezequel et al. (2012), Leek et al. (1996), Mahmoud et al. (2012), and Nabeshima et al. (2015) |
| LOX-1 | Class E | ECs, smooth muscle cells, macrophages, and neutrophils | Participation in cancer cell transformation Activation of NF-κB-dependent tumorigenic pathway Cross-presentation of HSP- associated antigen |
Hirsch et al. (2010) Khaidakov et al. (2011) Delneste et al. (2002) |
| Dectin-1 | Class E | Myeloid cells, B cells, and monocytes | Induction of CTLs Contributes to tumoricidal activity of NK cells |
Leibundgut-Landmann et al. (2008) Chiba et al. (2014) |
| SREC-I | Class F | Myeloid cells and ECs | Cross-presentation of HSP- associated antigen | Gong et al. (2009) |
| SR- PSOX/ CXCL16 | Class G | Myeloid cells, B cells, monocytes, LSECs, and some tumor cells | sCXCL16 facilitates tumor progression TM-CXCL16 inhibits tumorigenesis Biomarkers |
Chaturvedi et al. (2014), Jung et al. (2013), Lu et al. (2008), Shimaoka et al. (2003), Xing et al. (2012) and Wang et al. (2008) Fang et al. (2014) Gooden et al. (2014), Hattermann et al. (2013), Huang et al. (2013), Na et al. (2014), and Wente et al. (2008) |
| Stabilin-1 | Class H | Macrophage, lymphatic, and sinusoidal ECs | Scavenging circulating SPARC Recruitment of Tregs Cancer immune evasion and metastasis |
Nagaraju et al. (2014) Shetty et al. (2011) Karikoski et al. (2014) |
| Stabilin-2 | Class H | Macrophage, lymphatic, and sinusoidal ECs | Scavenging circulating HA Biomarkers |
Sironen et al. (2011) Geraud et al. (2013) and Neuhaus et al. (2013) |
| CD163 | Class I | Monocytes, macrophages, and some tumor cells | Promoting immunosuppressive TME Scavenging circulating TWEAK Biomarkers |
Sierra-Filardi et al. (2010) Bover et al. (2007) Andersen et al. (2014), Edin et al. (2012), Fujimura et al. (2013), Garcia et al. (2008) and He, Bao, et al. (2014) |
| RAGE | Class J | Myeloid cells, neutrophils, and T and B lymphocytes | Promoting tumorigenesis Biomarkers |
Chen et al. (2014), Kang et al. (2014), Nasser et al. (2015), Wang, Chang, et al. (2013), and Xu et al. (2014) Kang et al. (2014), Kobayashi et al. (2007), and He, Zhang, et al. (2014) |
CTL, cytotoxic T lymphocyte; DC, dendritic cell; EC, endothelial cells; HA, hyaluronan; HSP, heat shock protein; IFN-β, interferon-β; LOX-1, lectin-like oxidized low-density lipoprotein (LDL) receptor-1; LSEC, liver sinusoidal endothelial cells; MARCO, macrophage receptor with collagenous structure; NK, natural killer; NO, nitric oxide; RAGE, receptor for advanced glycation endproducts; SR-A, scavenger receptor class A; SREC-I, scavenger receptor expressed by endothelial cell-I; SR-PSOX, scavenger receptor for phosphatidylserine and oxidized lipoprotein; SCARA3, scavenger receptor class A member 3; SPARC, secreted protein acidic and rich in cysteine; TAM, tumor-associated macrophages; TM-CXCL16, transmembrane form of CXC chemokine ligand 16; TME, tumor microenvironment, TWEAK, TNF-like weak inducer of apoptosis; Treg, regulatory T cell.
Acknowledgments
The present study was supported in part by National Institutes of Health (NIH) Grants CA175033, CA154708, CA099326, Department of Defense (DOD) W81XWH-11-1-0481, W81XWH-13-1-0162, the National Foundation for Cancer Research and NCI Cancer Center Support Grant to VCU Massey Cancer Center P30CA16059. X-Y.W. is the Harrison Endowed Scholar in Cancer Research in the VCU Massey Cancer Center. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Footnotes
Competing Interests: None declared.
References
- Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. Journal of Immunology. 2004;172:6362–6372. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- Adachi H, Tsujimoto M. FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. The Journal of Biological Chemistry. 2002;277:34264–34270. doi: 10.1074/jbc.M204277200. [DOI] [PubMed] [Google Scholar]
- Adachi H, Tsujimoto M, Arai H, Inoue K. Expression cloning of a novel scavenger receptor from human endothelial cells. The Journal of Biological Chemistry. 1997;272:31217–31220. doi: 10.1074/jbc.272.50.31217. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Gupta S, Agrawal A. Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS One. 2010;5:e13418. doi: 10.1371/journal.pone.0013418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem E. beta-Glucans and their applications in cancer therapy: Focus on human studies. Anti-Cancer Agents in Medicinal Chemistry. 2013;13:709–719. doi: 10.2174/1871520611313050005. [DOI] [PubMed] [Google Scholar]
- Allmen EU, Koch M, Fritz G, Legler DF. V domain of RAGE interacts with AGEs on prostate carcinoma cells. Prostate. 2008;68:748–758. doi: 10.1002/pros.20736. [DOI] [PubMed] [Google Scholar]
- Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. Journal of Immunological Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Andersen MN, Abildgaard N, Maniecki MB, Moller HJ, Andersen NF. Monocyte/macrophage-derived soluble CD163: A novel biomarker in multiple myeloma. European Journal of Haematology. 2014;93:41–47. doi: 10.1111/ejh.12296. [DOI] [PubMed] [Google Scholar]
- Angin Y, Steinbusch LK, Simons PJ, Greulich S, Hoebers NT, Douma K, et al. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. The Biochemical Journal. 2012;448:43–53. doi: 10.1042/BJ20120060. [DOI] [PubMed] [Google Scholar]
- Armengol C, Bartoli R, Sanjurjo L, Serra I, Amezaga N, Sala M, et al. Role of scavenger receptors in the pathophysiology of chronic liver diseases. Critical Reviews in Immunology. 2013;33:57–96. [PubMed] [Google Scholar]
- Asch AS, Barnwell J, Silverstein RL, Nachman RL. Isolation of the thrombospondin membrane receptor. The Journal of Clinical Investigation. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo ML, Manzi M, Espelt MV, Gentilini LD, Compagno D, Laderach DJ, et al. Galectin-1 triggers epithelial-mesenchymal transition in human hepatocellular carcinoma cells. Journal of Cellular Physiology. 2014;230:1298–1309. doi: 10.1002/jcp.24865. [DOI] [PubMed] [Google Scholar]
- Bak SP, Walters JJ, Takeya M, Conejo-Garcia JR, Berwin BL. Scavenger receptor-A-targeted leukocyte depletion inhibits peritoneal ovarian tumor progression. Cancer Research. 2007;67:4783–4789. doi: 10.1158/0008-5472.CAN-06-4410. [DOI] [PubMed] [Google Scholar]
- Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, et al. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opinion on Biological Therapy. 2004;4:1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- Balermpas P, Rodel F, Liberz R, Oppermann J, Wagenblast J, Ghanaati S, et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. British Journal of Cancer. 2014;111:1509–1518. doi: 10.1038/bjc.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Beauvillain C, Meloni F, Sirard JC, Blanchard S, Jarry U, Scotet M, et al. The scavenger receptors SRA-1 and SREC-I cooperate with TLR2 in the recognition of the hepatitis C virus non-structural protein 3 by dendritic cells. Journal of Hepatology. 2010;52:644–651. doi: 10.1016/j.jhep.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Becker M, Cotena A, Gordon S, Platt N. Expression of the class A macrophage scavenger receptor on specific subpopulations of murine dendritic cells limits their endotoxin response. European Journal of Immunology. 2006;36:950–960. doi: 10.1002/eji.200535660. [DOI] [PubMed] [Google Scholar]
- Ben J, Jin G, Zhang Y, Ma B, Bai H, Chen J, et al. Class A scavenger receptor deficiency exacerbates lung tumorigenesis by cultivating a procarcinogenic microenvironment in humans and mice. American Journal of Respiratory and Critical Care Medicine. 2012;186:763–772. doi: 10.1164/rccm.201204-0592OC. [DOI] [PubMed] [Google Scholar]
- Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV. SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. The Journal of Biological Chemistry. 2004;279:51250–51257. doi: 10.1074/jbc.M406202200. [DOI] [PubMed] [Google Scholar]
- Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, et al. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. The EMBO Journal. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock AJ, Nymoen DA, Brenne K, Kaern J, Davidson B. SCARA3 mRNA is overexpressed in ovarian carcinoma compared with breast carcinoma effusions. Human Pathology. 2012;43:669–674. doi: 10.1016/j.humpath.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Bover LC, Cardo-Vila M, Kuniyasu A, Sun J, Rangel R, Takeya M, et al. A previously unrecognized protein-protein interaction between TWEAK and CD163: Potential biological implications. Journal of Immunology. 2007;178:8183–8194. doi: 10.4049/jimmunol.178.12.8183. [DOI] [PubMed] [Google Scholar]
- Brown CO, Schibler J, Fitzgerald MP, Singh N, Salem K, Zhan F, et al. Scavenger receptor class A member 3 (SCARA3) in disease progression and therapy resistance in multiple myeloma. Leukemia Research. 2013;37:963–969. doi: 10.1016/j.leukres.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. Journal of Leukocyte Biology. 2000;67:97–103. [PubMed] [Google Scholar]
- Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nature Reviews Immunology. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- Cao WM, Murao K, Imachi H, Yu X, Abe H, Yamauchi A, et al. A mutant high-density lipoprotein receptor inhibits proliferation of human breast cancer cells. Cancer Research. 2004;64:1515–1521. doi: 10.1158/0008-5472.can-03-0675. [DOI] [PubMed] [Google Scholar]
- Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Induction of CD8+ T cell responses through targeting of antigen to Dectin-2. Cellular Immunology. 2006a;239:87–91. doi: 10.1016/j.cellimm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. Journal of Immunology. 2006b;177:2276–2284. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- Caruso C, Balistreri CR, Candore G, Carruba G, Colonna-Romano G, Di Bona D, et al. Polymorphisms of pro-inflammatory genes and prostate cancer risk: A pharmacogenomic approach. Cancer Immunology, Immunotherapy. 2009;58:1919–1933. doi: 10.1007/s00262-009-0658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Karmali PP, Mukthavaram R, Kesari S, Kouznetsova VL, Tsigelny IF, et al. Direct recognition of superparamagnetic nanocrystals by macrophage scavenger receptor SR-AI. ACS Nano. 2013;7:4289–4298. doi: 10.1021/nn400769e. [DOI] [PubMed] [Google Scholar]
- Chao Y, Makale M, Karmali PP, Sharikov Y, Tsigelny I, Merkulov S, et al. Recognition of dextran-superparamagnetic iron oxide nanoparticle conjugates (Feridex) via macrophage scavenger receptor charged domains. Bioconjugate Chemistry. 2012;23:1003–1009. doi: 10.1021/bc200685a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2120–E2129. doi: 10.1073/pnas.1406655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sullivan C, Peng C, Shan Y, Hu Y, Li D, et al. A tumor suppressor function of the Msr1 gene in leukemia stem cells of chronic myeloid leukemia. Blood. 2011;118:390–400. doi: 10.1182/blood-2010-11-316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang L, Zhang IY, Liang J, Wang H, Ouyang M, et al. RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Research. 2014;74:7285–7297. doi: 10.1158/0008-5472.CAN-14-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Tsuneyama K, Kominami R, Shinohara H, Sakurai S, Yonekura H, et al. Expression profiling of endogenous secretory receptor for advanced glycation end products in human organs. Modern Pathology. 2005;18:1385–1396. doi: 10.1038/modpathol.3800450. [DOI] [PubMed] [Google Scholar]
- Cheng E, Whitsett TG, Tran NL, Winkles JA. The TWEAK receptor Fn14 is a Src-inducible protein and a positive regulator of Src-driven cell invasion. Molecular Cancer Research. 2014;13:575–583. doi: 10.1158/1541-7786.MCR-14-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CS, Tseng YH, Liao BJ, Chen SY. Magnetically targeted nanocapsules for PAA-cisplatin-conjugated cores in PVA/SPIO shells via surfactant-free emulsion for reduced nephrotoxicity and enhanced lung cancer therapy. Advanced Healthcare Materials. 2015;4:1066–1075. doi: 10.1002/adhm.201400794. http://dx.doi.org/10.1002/adhm.201400794. [DOI] [PubMed] [Google Scholar]
- Chiappalupi S, Riuzzi F, Fulle S, Donato R, Sorci G. Defective RAGE activity in embryonal rhabdomyosarcoma cells results in high PAX7 levels that sustain migration and invasiveness. Carcinogenesis. 2014;35:2382–2392. doi: 10.1093/carcin/bgu176. [DOI] [PubMed] [Google Scholar]
- Chiba S, Ikushima H, Ueki H, Yanai H, Kimura Y, Hangai S, et al. Recognition of tumor cells by Dectin-1 orchestrates innate immune cells for anti-tumor responses. eLife. 2014;3:e04177. doi: 10.7554/eLife.04177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocholaty M, Jachymova M, Schmidt M, Havlova K, Krepelova A, Zima T, et al. Polymorphisms of the receptor for advanced glycation end-products and glyoxalase I in patients with renal cancer. Tumour Biology. 2014;36:2121–2126. doi: 10.1007/s13277-014-2821-0. [DOI] [PubMed] [Google Scholar]
- Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. The Journal of Biological Chemistry. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: A review of scientific findings, relevant to future cancer therapeutics. Frontiers in Pharmacology. 2013;4:119. doi: 10.3389/fphar.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlmann M, Okhrimenko A, Marcinkowski P, Osterland M, Herrmann P, Smith J, et al. RAGE mediates S100A4-induced cell motility via MAPK/ERK and hypoxia signaling and is a prognostic biomarker for human colorectal cancer metastasis. Oncotarget. 2014;5:3220–3233. doi: 10.18632/oncotarget.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilo C, Gutierrez-Pajares JL, Mainieri MA, Mercier I, Lisanti MP, Frank PG. Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Research. 2013;15:R87. doi: 10.1186/bcr3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darash-Yahana M, Gillespie JW, Hewitt SM, Chen YY, Maeda S, Stein I, et al. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS One. 2009;4:e6695. doi: 10.1371/journal.pone.0006695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C, Nance JP, Hubbard J, Hsu M, Binder D, Wilson EH. Stabilin-1 expression in tumor associated macrophages. Brain Research. 2012;1481:71–78. doi: 10.1016/j.brainres.2012.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen YY, Fontenay GV, et al. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discovery. 2012;2:826–839. doi: 10.1158/2159-8290.CD-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- Ding Y, Wang Y, Zhou J, Gu X, Wang W, Liu C, et al. Direct cytosolic siRNA delivery by reconstituted high density lipoprotein for target-specific therapy of tumor angiogenesis. Biomaterials. 2014;35:7214–7227. doi: 10.1016/j.biomaterials.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Drover VA, Nguyen DV, Bastie CC, Darlington YF, Abumrad NA, Pessin JE, et al. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. The Journal of Biological Chemistry. 2008;283:13108–13115. doi: 10.1074/jbc.M708086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukic-Stefanovic S, Gasic-Milenkovic J, Deuther-Conrad W, Munch G. Signal transduction pathways in mouse microglia N-11 cells activated by advanced glycation endproducts (AGEs) Journal of Neurochemistry. 2003;87:44–55. doi: 10.1046/j.1471-4159.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- Dunn S, Vohra RS, Murphy JE, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. The lectin-like oxidized low-density-lipoprotein receptor: A pro-inflammatory factor in vascular disease. The Biochemical Journal. 2008;409:349–355. doi: 10.1042/BJ20071196. [DOI] [PubMed] [Google Scholar]
- Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, et al. CD36 mediates the innate host response to beta-amyloid. The Journal of Experimental Medicine. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, et al. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. The Journal of Biological Chemistry. 1993;268:11811–11816. [PubMed] [Google Scholar]
- Enomoto Y, Bharti A, Khaleque AA, Song B, Liu C, Apostolopoulos V, et al. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell-tumor fusion cells. Journal of Immunology. 2006;177:5946–5955. doi: 10.4049/jimmunol.177.9.5946. [DOI] [PubMed] [Google Scholar]
- Etzerodt A, Maniecki MB, Moller K, Moller HJ, Moestrup SK. Tumor necrosis factor alpha-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. Journal of Leukocyte Biology. 2010;88:1201–1205. doi: 10.1189/jlb.0410235. [DOI] [PubMed] [Google Scholar]
- Etzerodt A, Rasmussen MR, Svendsen P, Chalaris A, Schwarz J, Galea I, et al. Structural basis for inflammation-driven shedding of CD163 ectodomain and tumor necrosis factor-alpha in macrophages. The Journal of Biological Chemistry. 2014;289:778–788. doi: 10.1074/jbc.M113.520213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciponte JG, Wang XY, Subjeck JR. Hsp110 and Grp170, members of the Hsp70 superfamily, bind to scavenger receptor-A and scavenger receptor expressed by endothelial cells-I. European Journal of Immunology. 2007;37:2268–2279. doi: 10.1002/eji.200737127. [DOI] [PubMed] [Google Scholar]
- Fang Y, Henderson FC, Jr, Yi Q, Lei Q, Li Y, Chen N. Chemokine CXCL16 expression suppresses migration and invasiveness and induces apoptosis in breast cancer cells. Mediators of Inflammation. 2014;2014:478641. doi: 10.1155/2014/478641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, et al. Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nature Communications. 2013;4:2030. doi: 10.1038/ncomms3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Kambayashi Y, Furudate S, Kakizaki A, Aiba S. Immunomodulatory effect of bisphosphonate Risedronate sodium on CD163+ arginase 1+ M2 macrophages: The development of a possible supportive therapy for angiosarcoma. Clinical & Developmental Immunology. 2013;2013:325412. doi: 10.1155/2013/325412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH, Sun J, et al. CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Research. 2012;72:3546–3556. doi: 10.1158/0008-5472.CAN-11-4032. [DOI] [PubMed] [Google Scholar]
- Garcia C, Gardner D, Reichard KK. CD163: A specific immunohistochemical marker for acute myeloid leukemia with monocytic differentiation. Applied Immunohistochemistry & Molecular Morphology. 2008;16:417–421. doi: 10.1097/PAI.0b013e31815db477. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biology. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraud C, Mogler C, Runge A, Evdokimov K, Lu S, Schledzewski K, et al. Endothelial transdifferentiation in hepatocellular carcinoma: Loss of Stabilin-2 expression in peri-tumorous liver correlates with increased survival. Liver International. 2013;33:1428–1440. doi: 10.1111/liv.12262. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Zhu B, Murshid A, Adachi H, Song B, Lee A, et al. T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells-1. Journal of Immunology. 2009;183:3092–3098. doi: 10.4049/jimmunol.0901235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooden MJ, Wiersma VR, Boerma A, Leffers N, Boezen HM, ten Hoor KA, et al. Elevated serum CXCL16 is an independent predictor of poor survival in ovarian cancer and may reflect pro-metastatic ADAM protease activity. British Journal of Cancer. 2014;110:1535–1544. doi: 10.1038/bjc.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Pattern recognition receptors: Doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Graf GA, Roswell KL, Smart EJ. 17beta-Estradiol promotes the up-regulation of SR-BII in HepG2 cells and in rat livers. Journal of Lipid Research. 2001;42:1444–1449. [PubMed] [Google Scholar]
- Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: Phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nature Reviews Cancer. 2014;14:769–785. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- Granucci F, Petralia F, Urbano M, Citterio S, Di Tota F, Santambrogio L, et al. The scavenger receptor MARCO mediates cytoskeleton rearrangements in dendritic cells and microglia. Blood. 2003;102:2940–2947. doi: 10.1182/blood-2002-12-3651. [DOI] [PubMed] [Google Scholar]
- Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: Current knowledge and future challenges. Journal of Lipid Research. 2009;50(Suppl):S282–S286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Yang L, Sun Q, Zhou B, Tang N, Cong R, et al. Gly82Ser polymorphism of the receptor for advanced glycation end products is associated with an increased risk of gastric cancer in a Chinese population. Clinical Cancer Research. 2008;14:3627–3632. doi: 10.1158/1078-0432.CCR-07-4808. [DOI] [PubMed] [Google Scholar]
- Guo C, Buranych A, Sarkar D, Fisher PB, Wang XY. The role of tumor-associated macrophages in tumor vascularization. Vascular Cell. 2013;5:20. doi: 10.1186/2045-824X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Cui ZM, Zhang J, Huang Y. Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node metastasis in epithelial ovarian carcinoma. Chinese Journal of Cancer. 2011;30:336–343. doi: 10.5732/cjc.010.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: Past, present, and future. Advances in Cancer Research. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Yi H, Yu X, Hu F, Zuo D, Subjeck JR, et al. Absence of scavenger receptor A promotes dendritic cell-mediated cross-presentation of cell-associated antigen and antitumor immune response. Immunology and Cell Biology. 2012;90:101–108. doi: 10.1038/icb.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Yi H, Yu X, Zuo D, Qian J, Yang G, et al. In situ vaccination with CD204 gene-silenced dendritic cell, not unmodified dendritic cell, enhances radiation therapy of prostate cancer. Molecular Cancer Therapeutics. 2012;11:2331–2341. doi: 10.1158/1535-7163.MCT-12-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutwein P, Schramme A, Sinke N, Abdel-Bakky MS, Voss B, Obermuller N, et al. Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. European Journal of Cancer. 2009;45:478–489. doi: 10.1016/j.ejca.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Ha HK, Lee W, Park HJ, Lee SD, Lee JZ, Chung MK. Clinical significance of CXCL16/CXCR6 expression in patients with prostate cancer. Molecular Medicine Reports. 2011;4:419–424. doi: 10.3892/mmr.2011.446. [DOI] [PubMed] [Google Scholar]
- Hale JS, Li M, Sinyuk M, Jahnen-Dechent W, Lathia JD, Silverstein RL. Context dependent role of the CD36–thrombospondin–histidine-rich glycoprotein axis in tumor angiogenesis and growth. PLoS One. 2012;7:e40033. doi: 10.1371/journal.pone.0040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Otvos B, Sinyuk M, Alvarado AG, Hitomi M, Stoltz K, et al. Cancer stem cell-specific scavenger receptor 36 drives glioblastoma progression. Stem Cells. 2014;32:1746–1758. doi: 10.1002/stem.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HJ, Tokino T, Nakamura Y. CSR, a scavenger receptor-like protein with a protective role against cellular damage caused by UV irradiation and oxidative stress. Human Molecular Genetics. 1998;7:1039–1046. doi: 10.1093/hmg/7.6.1039. [DOI] [PubMed] [Google Scholar]
- Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. Journal of Immunology. 2003;170:2302–2309. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- Hattermann K, Held-Feindt J, Ludwig A, Mentlein R. The CXCL16-CXCR6 chemokine axis in glial tumors. Journal of Neuroimmunology. 2013;260:47–54. doi: 10.1016/j.jneuroim.2013.04.006. [DOI] [PubMed] [Google Scholar]
- He L, Bao H, Xue J, Zheng L, Zhang Q, Sun L, et al. Circulating soluble advanced glycation end product is inversely associated with the significant risk of developing cancer: Evidence from a meta-analysis. Tumour Biology. 2014;35:8749–8755. doi: 10.1007/s13277-014-2122-7. [DOI] [PubMed] [Google Scholar]
- He KF, Zhang L, Huang CF, Ma SR, Wang YF, Wang WM, et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. BioMed Research International. 2014;2014:838632. doi: 10.1155/2014/838632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nature Medicine. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. Journal of Immunology. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- Hirayama S, Ishii G, Nagai K, Ono S, Kojima M, Yamauchi C, et al. Prognostic impact of CD204-positive macrophages in lung squamous cell carcinoma: Possible contribution of Cd204-positive macrophages to the tumor-promoting microenvironment. Journal of Thoracic Oncology. 2012;7:1790–1797. doi: 10.1097/JTO.0b013e3182745968. [DOI] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, Joshi A, Zhang Y, Jaeger SA, Bulyk M, et al. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell. 2010;17:348–361. doi: 10.1016/j.ccr.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Hogger P, Sorg C. Soluble CD163 inhibits phorbol ester-induced lymphocyte proliferation. Biochemical and Biophysical Research Communications. 2001;288:841–843. doi: 10.1006/bbrc.2001.5845. [DOI] [PubMed] [Google Scholar]
- Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Research. 2007;67:4725–4731. doi: 10.1158/0008-5472.CAN-06-3424. [DOI] [PubMed] [Google Scholar]
- Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- Hou YC, Chao YJ, Tung HL, Wang HC, Shan YS. Coexpression of CD44-positive/CD133-positive cancer stem cells and CD204-positive tumor-associated macrophages is a predictor of survival in pancreatic ductal adenocarcinoma. Cancer. 2014;120:2766–2777. doi: 10.1002/cncr.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrzenjak A, Reicher H, Wintersperger A, Steinecker-Frohnwieser B, Sedlmayr P, Schmidt H, et al. Inhibition of lung carcinoma cell growth by high density lipoprotein-associated alpha-tocopheryl-succinate. Cellular and Molecular Life Sciences. 2004;61:1520–1531. doi: 10.1007/s00018-004-4101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing AW, Sakoda LC, Chen J, Chokkalingam AP, Sesterhenn I, Gao YT, et al. MSR1 variants and the risks of prostate cancer and benign prostatic hyperplasia: A population-based study in China. Carcinogenesis. 2007;28:2530–2536. doi: 10.1093/carcin/bgm196. [DOI] [PubMed] [Google Scholar]
- Hu Y, Cheng SC, Chan KT, Ke Y, Xue B, Sin FW, et al. Fucoidin enhances dendritic cell-mediated T-cell cytotoxicity against NY-ESO-1 expressing human cancer cells. Biochemical and Biophysical Research Communications. 2010;392:329–334. doi: 10.1016/j.bbrc.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio. 2010;1:e00164–10. doi: 10.1128/mBio.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhang J, Cui ZM, Zhao J, Zheng Y. Expression of the CXCL12/ CXCR4 and CXCL16/CXCR6 axes in cervical intraepithelial neoplasia and cervical cancer. Chinese Journal of Cancer. 2013;32:289–296. doi: 10.5732/cjc.012.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zheng DL, Qin FS, Cheng N, Chen H, Wan BB, et al. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. The Journal of Clinical Investigation. 2010;120:223–241. doi: 10.1172/JCI38012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, et al. Identification, classification, and expression of RAGE gene splice variants. FASEB Journal. 2008;22:1572–1580. doi: 10.1096/fj.07-9909com. [DOI] [PubMed] [Google Scholar]
- Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V, et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. The Journal of Biological Chemistry. 2008;283:34457–34468. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huysamen C, Brown GD. The fungal pattern recognition receptor, Dectin-1, and the associated cluster of C-type lectin-like receptors. FEMS Microbiology Letters. 2009;290:121–128. doi: 10.1111/j.1574-6968.2008.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ina K, Kataoka T, Ando T. The use of lentinan for treating gastric cancer. Anti-Cancer Agents in Medicinal Chemistry. 2013;13:681–688. doi: 10.2174/1871520611313050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irjala H, Elima K, Johansson EL, Merinen M, Kontula K, Alanen K, et al. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. European Journal of Immunology. 2003;33:815–824. doi: 10.1002/eji.200323859. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Tsutsumi K, Kawane S, Nakajima M, Kasaoka T. The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Letters. 2003;550:107–113. doi: 10.1016/s0014-5793(03)00846-9. [DOI] [PubMed] [Google Scholar]
- Jafari A, Salouti M, Shayesteh SF, Heidari Z, Rajabi AB, Boustani K, et al. Synthesis and characterization of Bombesin-superparamagnetic iron oxide nanoparticles as a targeted contrast agent for imaging of breast cancer using MRI. Nanotechnology. 2015;26:075101. doi: 10.1088/0957-4484/26/7/075101. [DOI] [PubMed] [Google Scholar]
- Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, Sjoegren P, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. Journal of Clinical Oncology. 2009;27:3330–3337. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- Jezequel P, Campion L, Spyratos F, Loussouarn D, Campone M, Guerin-Charbonnel C, et al. Validation of tumor-associated macrophage ferritin light chain as a prognostic biomarker in node-negative breast cancer tumors: A multicentric 2004 national PHRC study. International Journal of Cancer. 2012;131:426–437. doi: 10.1002/ijc.26397. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Shih DM, Xia YR, Lusis AJ, de Beer FC, de Villiers WJ, et al. Structure, organization, and chromosomal mapping of the gene encoding macrosialin, a macrophage-restricted protein. Genomics. 1998;50:199–205. doi: 10.1006/geno.1998.5327. [DOI] [PubMed] [Google Scholar]
- Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nature Medicine. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- Jones K, Vari F, Keane C, Crooks P, Nourse JP, Seymour LA, et al. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clinical Cancer Research. 2013;19:731–742. doi: 10.1158/1078-0432.CCR-12-2693. [DOI] [PubMed] [Google Scholar]
- Joo H, Li D, Dullaers M, Kim TW, Duluc D, Upchurch K, et al. C-type lectin-like receptor LOX-1 promotes dendritic cell-mediated class-switched B cell responses. Immunity. 2014;41:592–604. doi: 10.1016/j.immuni.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nature Communications. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Hou W, Zhang Q, Chen R, Lee YJ, Bartlett DL, et al. RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell Death & Disease. 2014;5:e1480. doi: 10.1038/cddis.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno H, Nishihara H, Wang L, Yuzawa S, Kobayashi H, Tsuda M, et al. Expression of CD163 prevents apoptosis through the production of granulocyte colony-stimulating factor in meningioma. Neuro-Oncology. 2013;15:853–864. doi: 10.1093/neuonc/not028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbownik MS, Nowak JZ. Hyaluronan: Towards novel anti-cancer therapeutics. Pharmacological Reports. 2013;65:1056–1074. doi: 10.1016/s1734-1140(13)71465-8. [DOI] [PubMed] [Google Scholar]
- Karikoski M, Irjala H, Maksimow M, Miiluniemi M, Granfors K, Hernesniemi S, et al. Clever-1/Stabilin-1 regulates lymphocyte migration within lymphatics and leukocyte entrance to sites of inflammation. European Journal of Immunology. 2009;39:3477–3487. doi: 10.1002/eji.200939896. [DOI] [PubMed] [Google Scholar]
- Karikoski M, Marttila-Ichihara F, Elima K, Rantakari P, Hollmen M, Kelkka T, et al. Clever-1/stabilin-1 controls cancer growth and metastasis. Clinical Cancer Research. 2014;20:6452–6464. doi: 10.1158/1078-0432.CCR-14-1236. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathology International. 2009;59:300–305. doi: 10.1111/j.1440-1827.2009.02369.x. [DOI] [PubMed] [Google Scholar]
- Kelley JL, Ozment TR, Li C, Schweitzer JB, Williams DL. Scavenger receptor-A (CD204): A two-edged sword in health and disease. Critical Reviews in Immunology. 2014;34:241–261. doi: 10.1615/critrevimmunol.2014010267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DJ, Kashyap SR. Pathogenic role of scavenger receptor CD36 in the metabolic syndrome and diabetes. Metabolic Syndrome and Related Disorders. 2011;9:239–245. doi: 10.1089/met.2011.0003. [DOI] [PubMed] [Google Scholar]
- Khaidakov M, Mitra S, Kang BY, Wang X, Kadlubar S, Novelli G, et al. Oxidized LDL receptor 1 (OLR1) as a possible link between obesity, dyslipidemia and cancer. PLoS One. 2011;6:e20277. doi: 10.1371/journal.pone.0020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Kunkel EJ, Boisvert J, Johnston B, Campbell JJ, Genovese MC, et al. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. The Journal of Clinical Investigation. 2001;107:595–601. doi: 10.1172/JCI11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Park HK, Yoon JS, Kim SJ, Kim ES, Ahn KS, et al. Advanced glycation end product (AGE)-induced proliferation of HEL cells via receptor for AGE-related signal pathways. International Journal of Oncology. 2008;33:493–501. [PubMed] [Google Scholar]
- Klein JL, Nguyen TT, Bien-Willner GA, Chen L, Foyil KV, Bartlett NL, et al. CD163 immunohistochemistry is superior to CD68 in predicting outcome in classical Hodgkin lymphoma. American Journal of Clinical Pathology. 2014;141:381–387. doi: 10.1309/AJCP61TLMXLSLJYS. [DOI] [PubMed] [Google Scholar]
- Kneidl J, Loffler B, Erat MC, Kalinka J, Peters G, Roth J, et al. Soluble CD163 promotes recognition, phagocytosis and killing of Staphylococcus aureus via binding of specific fibronectin peptides. Cellular Microbiology. 2012;14:914–936. doi: 10.1111/j.1462-5822.2012.01766.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Kubo H, Suzuki T, Ishizawa K, Yamada M, He M, et al. Endogenous secretory receptor for advanced glycation end products in non-small cell lung carcinoma. American Journal of Respiratory and Critical Care Medicine. 2007;175:184–189. doi: 10.1164/rccm.200602-212OC. [DOI] [PubMed] [Google Scholar]
- Koch M, Hussein F, Woeste A, Grundker C, Frontzek K, Emons G, et al. CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Research and Treatment. 2011;128:337–346. doi: 10.1007/s10549-010-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990;343:531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- Koh YW, Park CS, Yoon DH, Suh C, Huh J. CD163 expression was associated with angiogenesis and shortened survival in patients with uniformly treated classical Hodgkin lymphoma. PLoS One. 2014;9:e87066. doi: 10.1371/journal.pone.0087066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine H, Kuhn L, Matsushita N, Mule JJ, Pilon-Thomas S. Examination of MARCO activity on dendritic cell phenotype and function using a gene knockout mouse. PLoS One. 2013;8:e67795. doi: 10.1371/journal.pone.0067795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komohara Y, Niino D, Saito Y, Ohnishi K, Horlad H, Ohshima K, et al. Clinical significance of CD163(+) tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Science. 2013;104:945–951. doi: 10.1111/cas.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. The Journal of Pathology. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- Komohara Y, Takemura K, Lei XF, Sakashita N, Harada M, Suzuki H, et al. Delayed growth of EL4 lymphoma in SR-A-deficient mice is due to upregulation of nitric oxide and interferon-gamma production by tumor-associated macrophages. Cancer Science. 2009;100:2160–2166. doi: 10.1111/j.1349-7006.2009.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komohara Y, Terasaki Y, Kaikita K, Suzuki H, Kodama T, Takeya M. Clearance of apoptotic cells is not impaired in mouse embryos deficient in class A scavenger receptor types I and II (CD204) Developmental Dynamics. 2005;232:67–74. doi: 10.1002/dvdy.20206. [DOI] [PubMed] [Google Scholar]
- Kong LQ, Zhu XD, Xu HX, Zhang JB, Lu L, Wang WQ, et al. The clinical significance of the CD163+ and CD68+ macrophages in patients with hepatocellular carcinoma. PLoS One. 2013;8:e59771. doi: 10.1371/journal.pone.0059771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani K, Sekine Y, Ishikawa S, Ikpot IZ, Suzuki K, Remaley AT. High-density lipoprotein and prostate cancer: An overview. Journal of Epidemiology/Japan Epidemiological Association. 2013;23:313–319. doi: 10.2188/jea.JE20130006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krechler T, Jachymova M, Mestek O, Zak A, Zima T, Kalousova M. Soluble receptor for advanced glycation end-products (sRAGE) and polymorphisms of RAGE and glyoxalase I genes in patients with pancreas cancer. Clinical Biochemistry. 2010;43:882–886. doi: 10.1016/j.clinbiochem.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Krieger M. The other side of scavenger receptors: Pattern recognition for host defense. Current Opinion in Lipidology. 1997;8:275–280. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Krieger M, Acton S, Ashkenas J, Pearson A, Penman M, Resnick D. Molecular flypaper, host defense, and atherosclerosis. Structure, binding properties, and functions of macrophage scavenger receptors. The Journal of Biological Chemistry. 1993;268:4569–4572. [PubMed] [Google Scholar]
- Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovascular Research. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. The Journal of Surgical Research. 2011;167:e211–e219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Kzhyshkowska J, Krusell L. Cross-talk between endocytic clearance and secretion in macrophages. Immunobiology. 2009;214:576–593. doi: 10.1016/j.imbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Labonte AC, Tosello-Trampont AC, Hahn YS. The role of macrophage polarization in infectious and inflammatory diseases. Molecules and Cells. 2014;37:275–285. doi: 10.14348/molcells.2014.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz KT, Pathak RK, Rigotti A, Krieger M, Hobbs HH. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. The Journal of Clinical Investigation. 1996;98:984–995. doi: 10.1172/JCI118883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SK, Chu PG, Weiss LM. CD163: A specific marker of macrophages in paraffin-embedded tissue samples. American Journal of Clinical Pathology. 2004;122:794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- Lee DH, Kim HW. Innate immunity induced by fungal beta-glucans via dectin-1 signaling pathway. International Journal of Medicinal Mushrooms. 2014;16:1–16. doi: 10.1615/intjmedmushr.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Research. 1996;56:4625–4629. [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nature Immunology. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–4980. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70:390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Research. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- Li W, Liang RR, Zhou C, Wu MY, Lian L, Yuan GF, et al. The association between expressions of Ras and CD68 in the angiogenesis of breast cancers. Cancer Cell International. 2015;15:17. doi: 10.1186/s12935-015-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Developmental Cell. 2009;16:35–46. doi: 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. The Journal of Experimental Medicine. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhang P, Fu J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Letters. 2007;258:31–37. doi: 10.1016/j.canlet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Liu J, Hu G, Chen D, Gong AY, Soori GS, Dobleman TJ, et al. Suppression of SCARA5 by Snail1 is essential for EMT-associated cell migration of A549 cells. Oncogenesis. 2013;2:e73. doi: 10.1038/oncsis.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low JS, Tao Q, Ng KM, Goh HK, Shu XS, Woo WL, et al. A novel isoform of the 8p22 tumor suppressor gene DLC1 suppresses tumor growth and is frequently silenced in multiple common tumors. Oncogene. 2011;30:1923–1935. doi: 10.1038/onc.2010.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wang J, Xu Y, Koch AE, Cai Z, Chen X, et al. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Molecular Cancer Research. 2008;6:546–554. doi: 10.1158/1541-7786.MCR-07-0277. [DOI] [PubMed] [Google Scholar]
- Madsen M, Moller HJ, Nielsen MJ, Jacobsen C, Graversen JH, van den Berg T, et al. Molecular characterization of the haptoglobin.hemoglobin receptor CD163. Ligand binding properties of the scavenger receptor cysteine-rich domain region. The Journal of Biological Chemistry. 2004;279:51561–51567. doi: 10.1074/jbc.M409629200. [DOI] [PubMed] [Google Scholar]
- Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. Journal of Clinical Pathology. 2012;65:159–163. doi: 10.1136/jclinpath-2011-200355. [DOI] [PubMed] [Google Scholar]
- Maniecki MB, Etzerodt A, Ulhoi BP, Steiniche T, Borre M, Dyrskjot L, et al. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. International Journal of Cancer. 2012;131:2320–2331. doi: 10.1002/ijc.27506. [DOI] [PubMed] [Google Scholar]
- Mansfield AS, Heikkila P, von Smitten K, Vakkila J, Leidenius M. The presence of sinusoidal CD163(+) macrophages in lymph nodes is associated with favorable nodal status in patients with breast cancer. Virchows Archiv. 2012;461:639–646. doi: 10.1007/s00428-012-1338-4. [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nature Immunology. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Demaria S. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiation Research. 2010;173:418–425. doi: 10.1667/RR1860.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. Journal of Immunology. 2008;181:3099–3107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita N, Komine H, Grolleau-Julius A, Pilon-Thomas S, Mule JJ. Targeting MARCO can lead to enhanced dendritic cell motility and anti-melanoma activity. Cancer Immunology, Immunotherapy. 2010;59:875–884. doi: 10.1007/s00262-009-0813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Toiyama Y, Tanaka K, Saigusa S, Hiro J, Uchida K, et al. Soluble CXCL16 in preoperative serum is a novel prognostic marker and predicts recurrence of liver metastases in colorectal cancer patients. Annals of Surgical Oncology. 2012;19(Suppl 3):S518–S527. doi: 10.1245/s10434-011-1993-8. [DOI] [PubMed] [Google Scholar]
- McLean MH, El-Omar EM. Genetic aspects of inflammation. Current Opinion in Pharmacology. 2009;9:370–374. doi: 10.1016/j.coph.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circulation Research. 2007;100:1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- Meng Y, Beckett MA, Liang H, Mauceri HJ, van Rooijen N, Cohen KS, et al. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Research. 2010;70:1534–1543. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson JS, Burkly LC. Therapeutic targeting of TWEAK/Fnl4 in cancer: Exploiting the intrinsic tumor cell killing capacity of the pathway. Results and Problems in Cell Differentiation. 2009;49:145–160. doi: 10.1007/400_2008_18. [DOI] [PubMed] [Google Scholar]
- Miller DC, Zheng SL, Dunn RL, Sarma AV, Montie JE, Lange EM, et al. Germ-line mutations of the macrophage scavenger receptor 1 gene: Association with prostate cancer risk in African-American men. Cancer Research. 2003;63:3486–3489. [PubMed] [Google Scholar]
- Mooberry LK, Nair M, Paranjape S, McConathy WJ, Lacko AG. Receptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrier. Journal of Drug Targeting. 2010;18:53–58. doi: 10.3109/10611860903156419. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. The Journal of Clinical Investigation. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira AP, Cavassani KA, Ismailoglu UB, Hullinger R, Dunleavy MP, Knight DA, et al. The protective role of TLR6 in a mouse model of asthma is mediated by IL-23 and IL-17A. The Journal of Clinical Investigation. 2011;121:4420–4432. doi: 10.1172/JCI44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Munoz-Garcia B, Martin-Ventura JL, Madrigal-Matute J, Orbe J, Paramo JA, et al. The CD163-expressing macrophages recognize and internalize TWEAK: Potential consequences in atherosclerosis. Atherosclerosis. 2009;207:103–110. doi: 10.1016/j.atherosclerosis.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Moser B, Janik S, Schiefer AI, Mullauer L, Bekos C, Scharrer A, et al. Expression of RAGE and HMGB1 in thymic epithelial tumors, thymic hyperplasia and regular thymic morphology. PLoS One. 2014;9:e94118. doi: 10.1371/journal.pone.0094118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nature Reviews Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: Agents of antigen cross-presentation. Expert Review of Vaccines. 2008;7:1019–1030. doi: 10.1586/14760584.7.7.1019. [DOI] [PubMed] [Google Scholar]
- Murshid A, Gong J, Calderwood SK. Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I. Journal of Immunology. 2010;185:2903–2917. doi: 10.4049/jimmunol.0903635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KY, Kim HS, Jung WW, Sung JY, Kalil RK, Kim YW, et al. CXCL16 and CXCR6 in Ewing sarcoma family tumor. Human Pathology. 2014;45:753–760. doi: 10.1016/j.humpath.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Nabeshima A, Matsumoto Y, Fukushi J, Iura K, Matsunobu T, Endo M, et al. Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3K/Akt pathways. British Journal of Cancer. 2015;112:547–555. doi: 10.1038/bjc.2014.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju GP, Dontula R, El-Rayes BF, Lakka SS. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis. 2014;35:967–973. doi: 10.1093/carcin/bgu072. [DOI] [PubMed] [Google Scholar]
- Naito Y, Takagi T, Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Archives of Biochemistry and Biophysics. 2014;564:83–88. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Nakagawa-Toyama Y, Hirano K, Tsujii K, Nishida M, Miyagawa J, Sakai N, et al. Human scavenger receptor class B type I is expressed with cell-specific fashion in both initial and terminal site of reverse cholesterol transport. Atherosclerosis. 2005;183:75–83. doi: 10.1016/j.atherosclerosis.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Nasser MW, Wani N, Ahirwar DK, Powell CA, Ravi J, Elbaz MM, et al. RAGE mediates S100A7-induced breast cancer growth and metastasis by modulating the tumor microenvironment. Cancer Research. 2015;75:974–985. doi: 10.1158/0008-5472.CAN-14-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. Apo A-1 mimetic peptides as atheroprotective agents in murine models. Current Drug Targets. 2008;9:204–209. doi: 10.2174/138945008783755584. [DOI] [PubMed] [Google Scholar]
- Neuhaus J, Schiffer E, von Wilcke P, Bauer HW, Leung H, Siwy J, et al. Seminal plasma as a source of prostate cancer peptide biomarker candidates for detection of indolent and advanced disease. PLoS One. 2013;8:e67514. doi: 10.1371/journal.pone.0067514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyen C, Pluddemann A, Mukhopadhyay S, Maniati E, Bossard M, Gordon S, et al. Macrophage scavenger receptor a promotes tumor progression in murine models of ovarian and pancreatic cancer. Journal of Immunology. 2013;190:3798–3805. doi: 10.4049/jimmunol.1203194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KK, Lovell JF, Zheng G. Lipoprotein-inspired nanoparticles for cancer theranostics. Accounts of Chemical Research. 2011;44:1105–1113. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Schwartz EJ, West RB, Warnke RA, Arber DA, Natkunam Y. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. The American Journal of Surgical Pathology. 2005;29:617–624. doi: 10.1097/01.pas.0000157940.80538.ec. [DOI] [PubMed] [Google Scholar]
- Nickel T, Schmauss D, Hanssen H, Sicic Z, Krebs B, Jankl S, et al. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis. 2009;205:442–450. doi: 10.1016/j.atherosclerosis.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Nieva C, Marro M, Santana-Codina N, Rao S, Petrov D, Sierra A. The lipid phenotype of breast cancer cells characterized by Raman microspectroscopy: Towards a stratification of malignancy. PLoS One. 2012;7:e46456. doi: 10.1371/journal.pone.0046456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- No JH, Moon JM, Kim K, Kim YB. Prognostic significance of serum soluble CD163 level in patients with epithelial ovarian cancer. Gynecologic and Obstetric Investigation. 2013;75:263–267. doi: 10.1159/000349892. [DOI] [PubMed] [Google Scholar]
- Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, et al. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. Journal of Thoracic Oncology. 2010;5:1507–1515. doi: 10.1097/JTO.0b013e3181eba692. [DOI] [PubMed] [Google Scholar]
- Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo R, De Marchis F, Pusterla T, Conti A, Alessio M, Bianchi ME. Src family kinases are necessary for cell migration induced by extracellular HMGB1. Journal of Leukocyte Biology. 2009;86:617–623. doi: 10.1189/jlb.0908581. [DOI] [PubMed] [Google Scholar]
- Pan H, Niu W, He L, Wang B, Cao J, Zhao F, et al. Contributory role of five common polymorphisms of RAGE and APE1 genes in lung cancer among Han Chinese. PLoS One. 2013;8:e69018. doi: 10.1371/journal.pone.0069018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlato S, Romagnoli G, Spadaro F, Canini I, Sirabella P, Borghi P, et al. LOX-1 as a natural IFN-alpha-mediated signal for apoptotic cell uptake and antigen presentation in dendritic cells. Blood. 2010;115:1554–1563. doi: 10.1182/blood-2009-07-234468. [DOI] [PubMed] [Google Scholar]
- Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- Platt N, Suzuki H, Kodama T, Gordon S. Apoptotic thymocyte clearance in scavenger receptor class A-deficient mice is apparently normal. Journal of Immunology. 2000;164:4861–4867. doi: 10.4049/jimmunol.164.9.4861. [DOI] [PubMed] [Google Scholar]
- Pluddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, et al. Stabilin-1 and −2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. The Biochemical Journal. 2002;362:155–164. doi: 10.1042/0264-6021:3620155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhudas M, Bowdish D, Drickamer K, Febbraio M, Herz J, Kobzik L, et al. Standardizing scavenger receptor nomenclature. Journal of Immunology. 2014;192:1997–2006. doi: 10.4049/jimmunol.1490003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupovac A, Foster CM, Sluyter R. Human P2X7 receptor activation induces the rapid shedding of CXCL16. Biochemical and Biophysical Research Communications. 2013;432:626–631. doi: 10.1016/j.bbrc.2013.01.134. [DOI] [PubMed] [Google Scholar]
- Qian H, Johansson S, McCourt P, Smedsrod B, Ekblom M, Johansson S. Stabilins are expressed in bone marrow sinusoidal endothelial cells and mediate scavenging and cell adhesive functions. Biochemical and Biophysical Research Communications. 2009;390:883–886. doi: 10.1016/j.bbrc.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Sun BL, Zhang WY, Ke J, Zhu J. Gly82Ser polymorphism of the receptor for advanced glycation end-product (RAGE) potential high risk in patients with colorectal cancer. Tumour Biology. 2014;35:3171–3175. doi: 10.1007/s13277-013-1414-7. [DOI] [PubMed] [Google Scholar]
- Qian J, Yi H, Guo C, Yu X, Zuo D, Chen X, et al. CD204 suppresses large heat shock protein-facilitated priming of tumor antigen gp100-specific T cells and chaperone vaccine activity against mouse melanoma. Journal of Immunology. 2011;187:2905–2914. doi: 10.4049/jimmunol.1100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachidi SM, Qin T, Sun S, Zheng WJ, Li Z. Molecular profiling of multiple human cancers defines an inflammatory cancer-associated molecular pattern and uncovers KPNA2 as a uniform poor prognostic cancer marker. PLoS One. 2013;8:e57911. doi: 10.1371/journal.pone.0057911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ortiz ZG, Pendergraft WF, 3rd, Prasad A, Byrne MH, Iram T, Blanchette CJ, et al. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nature Immunology. 2013;14:917–926. doi: 10.1038/ni.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramprasad MP, Fischer W, Witztum JL, Sambrano GR, Quehenberger O, Steinberg D. The 94- to 97-kDa mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9580–9584. doi: 10.1073/pnas.92.21.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB Journal. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- Raycroft MT, Harvey BP, Bruck MJ, Mamula MJ. Inhibition of antigen trafficking through scavenger receptor A. The Journal of Biological Chemistry. 2012;287:5310–5316. doi: 10.1074/jbc.M111.316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MA, Li SL, Sahar S, Kim YS, Xu ZG, Lanting L, et al. Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. The Journal of Biological Chemistry. 2006;281:13685–13693. doi: 10.1074/jbc.M511425200. [DOI] [PubMed] [Google Scholar]
- Reinartz S, Schumann T, Finkernagel F, Wortmann A, Jansen JM, Meissner W, et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. International Journal of Cancer. 2014;134:32–42. doi: 10.1002/ijc.28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. The Journal of Experimental Medicine. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert H, Zeigler-Johnson C, Mittal RD, Tan YC, Sadowl CM, Edwards J, et al. Analysis of the RNASEL/HPC1, and macrophage scavenger receptor 1 in Asian-Indian advanced prostate cancer. Urology. 2008;72:456–460. doi: 10.1016/j.urology.2007.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riuzzi F, Sorci G, Sagheddu R, Donato R. HMGB1-RAGE regulates muscle satellite cell homeostasis through p38-MAPK- and myogenin-dependent repression of Pax7 transcription. Journal of Cell Science. 2012;125:1440–1454. doi: 10.1242/jcs.092163. [DOI] [PubMed] [Google Scholar]
- Rohrer L, Freeman M, Kodama T, Penman M, Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990;343:570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- Rost MS, Sumanas S. Hyaluronic acid receptor Stabilin-2 regulates Erk phosphorylation and arterial–venous differentiation in zebrafish. PLoS One. 2014;9:e88614. doi: 10.1371/journal.pone.0088614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Komohara Y, Niino D, Horlad H, Ohnishi K, Takeya H, et al. Role of CD204-positive tumor-associated macrophages in adult T-cell leukemia/lymphoma. Journal of Clinical and Experimental Hematopathology. 2014;54:59–65. doi: 10.3960/jslrt.54.59. [DOI] [PubMed] [Google Scholar]
- Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- Schramme A, Abdel-Bakky MS, Kampfer-Kolb N, Pfeilschifter J, Gutwein P. The role of CXCL16 and its processing metalloproteinases ADAM10 and ADAM17 in the proliferation and migration of human mesangial cells. Biochemical and Biophysical Research Communications. 2008;370:311–316. doi: 10.1016/j.bbrc.2008.03.088. [DOI] [PubMed] [Google Scholar]
- Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala EH, Ikonen T, Autio V, Rokman A, Mononen N, Matikainen MP, et al. Germ-line alterations in MSR1 gene and prostate cancer risk. Clinical Cancer Research. 2003;9:5252–5256. [PubMed] [Google Scholar]
- Shabo I, Olsson H, Elkarim R, Sun XF, Svanvik J. Macrophage infiltration in tumor stroma is related to tumor cell expression of CD163 in colorectal cancer. Cancer Microenvironment. 2014;7:61–69. doi: 10.1007/s12307-014-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabo I, Olsson H, Sun XF, Svanvik J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. International Journal of Cancer. 2009;125:1826–1831. doi: 10.1002/ijc.24506. [DOI] [PubMed] [Google Scholar]
- Shabo I, Stal O, Olsson H, Dore S, Svanvik J. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. International Journal of Cancer. 2008;123:780–786. doi: 10.1002/ijc.23527. [DOI] [PubMed] [Google Scholar]
- Shabo I, Svanvik J. Expression of macrophage antigens by tumor cells. Advances in Experimental Medicine and Biology. 2011;714:141–150. doi: 10.1007/978-94-007-0782-5_7. [DOI] [PubMed] [Google Scholar]
- Shahzad MM, Mangala LS, Han HD, Lu C, Bottsford-Miller J, Nishimura M, et al. Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia. 2011;13:309–319. doi: 10.1593/neo.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar J, Messenberg A, Chan J, Underhill TM, Foster LJ, Nabi IR. Pseudopodial actin dynamics control epithelial-mesenchymal transition in metastatic cancer cells. Cancer Research. 2010;70:3780–3790. doi: 10.1158/0008-5472.CAN-09-4439. [DOI] [PubMed] [Google Scholar]
- Shetty S, Weston CJ, Oo YH, Westerlund N, Stamataki Z, Youster J, et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. Journal of Immunology. 2011;186:4147–4155. doi: 10.4049/jimmunol.1002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka M, Urakawa N, Nakamura T, Nishio M, Watajima T, Kuroda D, et al. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Science. 2013;104:1112–1119. doi: 10.1111/cas.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka T, Kume N, Minami M, Hayashida K, Kataoka H, Kita T, et al. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. The Journal of Biological Chemistry. 2000;275:40663–40666. doi: 10.1074/jbc.C000761200. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Kume N, Minami M, Hayashida K, Sawamura T, Kita T, et al. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. Journal of Immunology. 2001;166:5108–5114. doi: 10.4049/jimmunol.166.8.5108. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Nakayama T, Kume N, Takahashi S, Yamaguchi J, Minami M, et al. Cutting edge: SR-PSOX/CXC chemokine ligand 16 mediates bacterial phagocytosis by APCs through its chemokine domain. Journal of Immunology. 2003;171:1647–1651. doi: 10.4049/jimmunol.171.4.1647. [DOI] [PubMed] [Google Scholar]
- Shin JY, Yang Y, Heo P, Lee JC, Kong B, Cho JY, et al. pH-responsive high-density lipoprotein-like nanoparticles to release paclitaxel at acidic pH in cancer chemotherapy. International Journal of Nanomedicine. 2012;7:2805–2816. doi: 10.2147/IJN.S29817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, et al. Macrophage polarization in tumour progression. Seminars in Cancer Biology. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Sierra-Filardi E, Vega MA, Sanchez-Mateos P, Corbi AL, Puig-Kroger A. Heme oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology. 2010;215:788–795. doi: 10.1016/j.imbio.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Simantov R, Febbraio M, Silverstein RL. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biology. 2005;24:27–34. doi: 10.1016/j.matbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, Kosma VM. Hyaluronan in human malignancies. Experimental Cell Research. 2011;317:383–391. doi: 10.1016/j.yexcr.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Son H, Moon A. Epithelial-mesenchymal transition and cell invasion. Toxicological Research. 2010;26:245–252. doi: 10.5487/TR.2010.26.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Lee C, Schindler C. Deletion of the murine scavenger receptor CD68. Journal of Lipid Research. 2011;52:1542–1550. doi: 10.1194/jlr.M015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: Chaperoning of the innate and adaptive immune responses. Annual Review of Immunology. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nature Immunology. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Chien M, Lin C, Chen M, Yang S. RAGE gene polymorphism and environmental factor in the risk of oral cancer. Journal of Dental Research. 2015;94:403–411. doi: 10.1177/0022034514566215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya M, Miyagaki T, Ohmatsu H, Suga H, Kai H, Kamata M, et al. Association of the numbers of CD163(+) cells in lesional skin and serum levels of soluble CD163 with disease progression of cutaneous T cell lymphoma. Journal of Dermatological Science. 2012;68:45–51. doi: 10.1016/j.jdermsci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Mitsunaga S, Yoshikawa K, Kato Y, Gotohda N, Takahashi S, et al. Prognostic impact of M2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas. European Journal of Cancer. 2014;50:1900–1908. doi: 10.1016/j.ejca.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Sun M, Finnemann SC, Febbraio M, Shan L, Annangudi SP, Podrez EA, et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: A potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. The Journal of Biological Chemistry. 2006;281:4222–4230. doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Turner A, Xu J, Gronberg H, Isaacs W. Genetic variability in inflammation pathways and prostate cancer risk. Urologic Oncology. 2007;25:250–259. doi: 10.1016/j.urolonc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- Tateno H, Ogawa T, Muramoto K, Kamiya H, Saneyoshi M. Rhamnose-binding lectins from steelhead trout (Oncorhynchus mykiss) eggs recognize bacterial lipopolysaccharides and lipoteichoic acid. Bioscience, Biotechnology, and Biochemistry. 2002;66:604–612. doi: 10.1271/bbb.66.604. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nature Immunology. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur SA, Hamilton RF, Jr, Holian A. Role of scavenger receptor a family in lung inflammation from exposure to environmental particles. Journal of Immunotoxicology. 2008;5:151–157. doi: 10.1080/15476910802085863. [DOI] [PubMed] [Google Scholar]
- Theriault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. Journal of Immunology. 2006;177:8604–8611. doi: 10.4049/jimmunol.177.12.8604. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Nordestgaard BG, Kobzik L, Dahl M. Genetic variation in the scavenger receptor MARCO and its association with chronic obstructive pulmonary disease and lung infection in 10,604 individuals. Respiration; International Review of Thoracic Diseases. 2013;85:144–153. doi: 10.1159/000342354. [DOI] [PubMed] [Google Scholar]
- Tiainen S, Tumelius R, Rilla K, Hamalainen K, Tammi M, Tammi R, et al. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology. 2014;66:873–883. doi: 10.1111/his.12607. [DOI] [PubMed] [Google Scholar]
- Todt JC, Hu B, Curtis JL. The scavenger receptor SR-A I/II (CD204) signals via the receptor tyrosine kinase Mertk during apoptotic cell uptake by murine macrophages. Journal of Leukocyte Biology. 2008;84:510–518. doi: 10.1189/jlb.0307135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure F, Zahm JM, Garnotel R, Lambert E, Bonnet N, Schmidt AM, et al. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. The Biochemical Journal. 2008;416:255–261. doi: 10.1042/BJ20080054. [DOI] [PubMed] [Google Scholar]
- Tripathy S, Vinokour E, McMahon KM, Volpert OV, Thaxton CS. High density lipoprotein nanoparticles deliver RNAi to endothelial cells to inhibit angiogenesis. Particle and Particle Systems Characterization. 2014;31:1141–1150. doi: 10.1002/ppsc.201400036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiddy AL, Cox ME, Wasan KM. Knockdown of scavenger receptor class B type I reduces prostate specific antigen secretion and viability of prostate cancer cells. Prostate. 2012;72:955–965. doi: 10.1002/pros.21499. [DOI] [PubMed] [Google Scholar]
- Uray IP, Liang Y, Hyder SM. Estradiol down-regulates CD36 expression in human breast cancer cells. Cancer Letters. 2004;207:101–107. doi: 10.1016/j.canlet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- van der Laan LJ, Dopp EA, Haworth R, Pikkarainen T, Kangas M, Elomaa O, et al. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. Journal of Immunology. 1999;162:939–947. [PubMed] [Google Scholar]
- van Dongen M, Savage ND, Jordanova ES, Briaire-de Bruijn IH, Walburg KV, Ottenhoff TH, et al. Anti-inflammatory M2 type macrophages characterize metastasized and tyrosine kinase inhibitor-treated gastrointestinal stromal tumors. International Journal of Cancer. 2010;127:899–909. doi: 10.1002/ijc.25113. [DOI] [PubMed] [Google Scholar]
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature Cell Biology. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez JA, Martinez-Ruiz A, Sancho-Rodriguez N, Martinez-Hernandez P, Noguera-Velasco JA. The real role of prediagnostic high-density lipoprotein cholesterol and the cancer risk: A concise review. European Journal of Clinical Investigation. 2014;44:103–114. doi: 10.1111/eci.12185. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nature Medicine. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- Wadsack C, Hirschmugl B, Hammer A, Levak-Frank S, Kozarsky KF, Sattler W, et al. Scavenger receptor class B, type I on non-malignant and malignant human epithelial cells mediates cholesteryl ester-uptake from high density lipoproteins. The International Journal of Biochemistry & Cell Biology. 2003;35:441–454. doi: 10.1016/s1357-2725(02)00272-8. [DOI] [PubMed] [Google Scholar]
- Wadsack C, Hrzenjak A, Hammer A, Hirschmugl B, Levak-Frank S, Desoye G, et al. Trophoblast-like human choriocarcinoma cells serve as a suitable in vitro model for selective cholesteryl ester uptake from high density lipoproteins. European Journal of Biochemistry/FEBS. 2003;270:451–462. doi: 10.1046/j.1432-1033.2003.03394.x. [DOI] [PubMed] [Google Scholar]
- Wang JL, Bi Z, Zou JW, Gu XM. Combination therapy with lentinan improves outcomes in patients with esophageal carcinoma. Molecular Medicine Reports. 2012;5:745–748. doi: 10.3892/mmr.2011.718. [DOI] [PubMed] [Google Scholar]
- Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. Journal of Immunology. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- Wang XY, Facciponte J, Chen X, Subjeck JR, Repasky EA. Scavenger receptor-A negatively regulates antitumor immunity. Cancer Research. 2007;67:4996–5002. doi: 10.1158/0008-5472.CAN-06-3138. [DOI] [PubMed] [Google Scholar]
- Wang XY, Facciponte JG, Subjeck JR. Molecular chaperones and cancer immunotherapy. Handbook of Experimental Pharmacology. 2006;172:305–329. doi: 10.1007/3-540-29717-0_13. [DOI] [PubMed] [Google Scholar]
- Wang J, Lu Y, Koch AE, Zhang J, Taichman RS. CXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamycin signaling pathway. Cancer Research. 2008;68:10367–10376. doi: 10.1158/0008-5472.CAN-08-2780. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang L, McDonnell SK, Cunningham JM, Hebbring S, Jacobsen SJ, Cerhan JR, et al. No association of germline alteration of MSR1 with prostate cancer risk. Nature Genetics. 2003;35:128–129. doi: 10.1038/ng1239. [DOI] [PubMed] [Google Scholar]
- Wang XY, Subjeck JR. High molecular weight stress proteins: Identification, cloning and utilisation in cancer immunotherapy. International Journal of Hyperthermia. 2013;29:364–375. doi: 10.3109/02656736.2013.803607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Sun X, Chen X, Facciponte J, Repasky EA, Kane J, et al. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. Journal of Immunology. 2010;184:6309–6319. doi: 10.4049/jimmunol.0903891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sun M, Gu C, Wang X, Chen D, Zhao E, et al. Expression of CD163, interleukin-10, and interferon-gamma in oral squamous cell carcinoma: Mutual relationships and prognostic implications. European Journal of Oral Sciences. 2014;122:202–209. doi: 10.1111/eos.12131. [DOI] [PubMed] [Google Scholar]
- Weis N, Weigert A, von Knethen A, Brune B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Molecular Biology of the Cell. 2009;20:1280–1288. doi: 10.1091/mbc.E08-10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente MN, Gaida MM, Mayer C, Michalski CW, Haag N, Giese T, et al. Expression and potential function of the CXC chemokine CXCL16 in pancreatic ductal adenocarcinoma. International Journal of Oncology. 2008;33:297–308. [PubMed] [Google Scholar]
- Wermeling F, Chen Y, Pikkarainen T, Scheynius A, Winqvist O, Izui S, et al. Class A scavenger receptors regulate tolerance against apoptotic cells, and auto-antibodies against these receptors are predictive of systemic lupus. The Journal of Experimental Medicine. 2007;204:2259–2265. doi: 10.1084/jem.20070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan FJ, Meehan CJ, Golding GB, McConkey BJ, Bowdish DM. The evolution of the class A scavenger receptors. BMC Evolutionary Biology. 2012;12:227. doi: 10.1186/1471-2148-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K, El Khoury J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. International Journal of Alzheimer’s Disease. 2012;2012:489456. doi: 10.1155/2012/489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman G, Sage EH. Identification of a sequence in the matricellular protein SPARC that interacts with the scavenger receptor stabilin-1. Journal of Cellular Biochemistry. 2011;112:1003–1008. doi: 10.1002/jcb.23015. [DOI] [PubMed] [Google Scholar]
- Xia W, Xu Y, Mao Q, Dong G, Shi R, Wang J, et al. Association of RAGE polymorphisms and cancer risk: A meta-analysis of 27 studies. Medical Oncology. 2015;32:442. doi: 10.1007/s12032-014-0442-5. [DOI] [PubMed] [Google Scholar]
- Xie J, Zhu H, Guo L, Ruan Y, Wang L, Sun L, et al. Lectin-like oxidized low-density lipoprotein receptor-1 delivers heat shock protein 60-fused antigen into the MHC class I presentation pathway. Journal of Immunology. 2010;185:2306–2313. doi: 10.4049/jimmunol.0903214. [DOI] [PubMed] [Google Scholar]
- Xing YN, Xu XY, Nie XC, Yang X, Yu M, Xu HM, et al. Role and clinicopathologic significance of CXC chemokine ligand 16 and chemokine (C-X-C motif ) receptor 6 expression in gastric carcinomas. Human Pathology. 2012;43:2299–2307. doi: 10.1016/j.humpath.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Xu J, Zheng SL, Komiya A, Mychaleckyj JC, Isaacs SD, Hu JJ, et al. Germline mutations and sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Nature Genetics. 2002;32:321–325. doi: 10.1038/ng994. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhu H, Wang T, Sun Y, Ni P, Liu Y, et al. Exogenous high-mobility group box 1 inhibits apoptosis and promotes the proliferation of lewis cells via RAGE/ TLR4-dependent signal pathways. Scandinavian Journal of Immunology. 2014;79:386–394. doi: 10.1111/sji.12174. [DOI] [PubMed] [Google Scholar]
- Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S, Matsui T, Fukami K. Role of receptor for advanced glycation end products (RAGE) and its ligands in cancer risk. Rejuvenation Research. 2015;18:48–56. doi: 10.1089/rej.2014.1625. [DOI] [PubMed] [Google Scholar]
- Yan N, Zhang S, Yang Y, Cheng L, Li C, Dai L, et al. Therapeutic upregulation of Class A scavenger receptor member 5 inhibits tumor growth and metastasis. Cancer Science. 2012;103:1631–1639. doi: 10.1111/j.1349-7006.2012.02350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung OW, Lo C, Ling C, Qi X, Geng W, Li C, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. Journal of Hepatology. 2014;62:607–616. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Yi H, Guo C, Yu X, Gao P, Qian J, Zuo D, et al. Targeting the immunoregulator SRA/CD204 potentiates specific dendritic cell vaccine-induced T-cell response and antitumor immunity. Cancer Research. 2011a;71:6611–6620. doi: 10.1158/0008-5472.CAN-11-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Yu X, Gao P, Wang Y, Baek SH, Chen X, et al. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113:5819–5828. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Zuo D, Yu X, Hu F, Manjili MH, Chen Z, et al. Suppression of antigen-specific CD4+ T cell activation by SRA/CD204 through reducing the immunostimulatory capability of antigen-presenting cell. Journal of Molecular Medicine (Berlin, Germany) 2012;90:413–426. doi: 10.1007/s00109-011-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Liu YN, Tillman H, Barrett B, Hewitt S, Ylaya K, et al. AR-regulated TWEAK-FN14 pathway promotes prostate cancer bone metastasis. Cancer Research. 2014;74:4306–4317. doi: 10.1158/0008-5472.CAN-13-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Luistro L, Zhong H, Smith M, Nevins T, Schostack K, et al. RG7212 anti-TWEAK mAb inhibits tumor growth through inhibition of tumor cell proliferation and survival signaling and by enhancing the host antitumor immune response. Clinical Cancer Research. 2013;19:5686–5698. doi: 10.1158/1078-0432.CCR-13-0405. [DOI] [PubMed] [Google Scholar]
- Yoon TJ, Koppula S, Lee KH. The effects of beta-glucans on cancer metastasis. Anti-Cancer Agents in Medicinal Chemistry. 2013;13:699–708. doi: 10.2174/1871520611313050004. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Mitsunaga S, Kinoshita T, Konishi M, Takahashi S, Gotohda N, et al. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Science. 2012;103:2012–2020. doi: 10.1111/j.1349-7006.2012.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Tseng GC, Yu YP, Gavel T, Nelson J, Wells A, et al. CSR1 suppresses tumor growth and metastasis of prostate cancer. The American Journal of Pathology. 2006;168:597–607. doi: 10.2353/ajpath.2006.050620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang XY. Antagonizing the innate pattern recognition receptor CD204 to improve dendritic cell-targeted cancer immunotherapy. Oncoimmunology. 2012;1:770–772. doi: 10.4161/onci.19728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Yi H, Guo C, Zuo D, Wang Y, Kim HL, et al. Pattern precognition scavenger receptor CD204 attenuates toll-like receptor 4-induced NF-{kappa}B activation by directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. The Journal of Biological Chemistry. 2011;286:18795–18806. doi: 10.1074/jbc.M111.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zuo D, Subjeck JR, Wang X-Y. Scavenger receptor A (SRA/ CD204): A multifaceted regulator of inflammatory response and immunity. In: Manjili Masoud H., editor. Cytokines: Mechanisms, functions and abnormalities. New York: NOVA Science; 2012. pp. 173–196. [Google Scholar]
- Zhang S, Hou X, Zi S, Wang Y, Chen L, Kong B. Polymorphisms of receptor for advanced glycation end products and risk of epithelial ovarian cancer in Chinese patients. Cellular Physiology and Biochemistry. 2013;31:525–531. doi: 10.1159/000350073. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Wu LQ, Zhang T, Han YF, Lin X. Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncology Reports. 2015;33:1630–1638. doi: 10.3892/or.2015.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CB, Bao JM, Lu YJ, Zhao T, Zhou XH, Zheng DY, et al. Co-expression of RAGE and HMGB1 is associated with cancer progression and poor patient outcome of prostate cancer. American Journal of Cancer Research. 2014;4:369–377. [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Liu Y, Jin H, Pan S, Qian Y, Huang C, et al. Scavenger receptor B1 is a potential biomarker of human nasopharyngeal carcinoma and its growth is inhibited by HDL-mimetic nanoparticles. Theranostics. 2013;3:477–486. doi: 10.7150/thno.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou DN, Deng YF, Li RH, Yin P, Ye CS. Concurrent alterations of RAGE, RECK, and MMP9 protein expression are relevant to Epstein-Barr virus infection, metastasis, and survival in nasopharyngeal carcinoma. International Journal of Clinical and Experimental Pathology. 2014;7:3245–3254. [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. Journal of Immunology. 2012;189:3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo D, Yu X, Guo C, Wang H, Qian J, Yi H, et al. Scavenger receptor a restrains T-cell activation and protects against concanavalin A-induced hepatic injury. Hepatology. 2013;57:228–238. doi: 10.1002/hep.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]