Abstract
Background & Aims
Colorectal cancer (CRC) screening decisions for elderly individuals are often made based primarily on age—other factors that affect the effectiveness and cost effectiveness of screening are often not considered. We investigated the relative importance of factors that could be used to identify those elderly individuals most likely to benefit from CRC screening and determined the maximum ages at which screening remains cost effective based on these factors.
Methods
We used a microsimulation model (Microsimulation Screening Analysis-Colon) that was calibrated to the incidence of CRC in the US and the prevalence of adenomas reported in autopsy studies to determine the appropriate age to stop colonoscopy screening in 19,200 cohorts of individuals defined by sex, race, screening history, background risk for CRC, and comorbidity status. We applied a willingness-to-pay threshold of $100,000 per quality-adjusted life-year (QALY) gained.
Results
When considering the factors included in the model, a less intensive screening history, a higher background risk for CRC, and fewer comorbidities were associated with cost-effective screening at older ages. Sex and race had only a small effect on the appropriate age to stop screening. For some individuals likely to be screened in current practice, screening resulted in a loss of QALYs, rather than a gain. For some individuals unlikely to be screened in current practice, screening was highly cost effective. Although screening some previously screened, low-risk individuals was not cost effective even when they were 66 y old, screening some healthy, high-risk individuals remained cost effective until they reached an age of 88 y.
Conclusion
The current approach to CRC screening in elderly individuals, in which decisions are often based primarily on age, is inefficient, resulting in underuse of screening for some and overuse of screening for others. CRC screening could be more effective and cost effective if individual factors for each patient are considered.
Keywords: Colon cancer screening, individualized care, MISCAN, tumor
Introduction
Screening for colorectal cancer (CRC) has been shown to be effective and cost effective in reducing CRC mortality and is therefore widely recommended.1-4 The U.S. Preventive Services Task Force (USPSTF), for example, calls for routine screening for average risk individuals starting at age 50 years and continuing up to age 75 years.1 Although clinicians are generally aware that factors other than age affect the effectiveness and cost effectiveness of CRC screening,5 many make their decisions on screening for elderly individuals primarily based on age. This practice is in concordance with existing age-based guidelines and performance measures.6 Moreover, a substantial minority of clinicians still offer CRC screening to elderly individuals with a life-expectancy less than 5 years.5, 7-9 Hence, screening is not always targeted at those elderly individuals most likely to benefit.
The effectiveness and cost effectiveness of screening for a particular elderly individual depend on two key variables: CRC risk and life-expectancy. Both of these variables are affected by age. With increasing age, the average risk for CRC increases, but simultaneously the average life-expectancy declines. This results in a deterioration of the effectiveness and cost effectiveness of screening with age. An individual's risk for CRC, however, is also affected by the individual's sex, race, screening history, and level of exposure to other risk factors for CRC, such as a family history of CRC and smoking (i.e., the individual's “background risk for CRC”). Similarly, an individual's life-expectancy is affected by the individual's sex, race, and comorbidity status. Therefore, ideally all these factors should be considered when making decisions about offering CRC screening.
The objective of this study was to determine the appropriate age to stop colonoscopy screening (i.e., the maximum age at which screening is cost effective) given an individual's sex, race, screening history, background risk for CRC, and comorbidity status. We focused on colonoscopy screening, because it is the most commonly used screening modality in the United States today.10 Through this work, we hope to facilitate a more personalized approach to CRC screening for elderly individuals, which would ultimately result in more efficient screening. As the population ages, and clinicians are faced with increasing numbers of both healthy and unhealthy elderly individuals, such an approach will become increasingly relevant to clinical practice.
Methods
To quantify the effectiveness and cost effectiveness of screening we used Microsimulation Screening Analysis-Colon (MISCAN-Colon).
MISCAN-Colon
MISCAN-Colon is a well-established microsimulation model for CRC developed at the Department of Public Health of the Erasmus University Medical Center (Rotterdam, the Netherlands). The model's structure, underlying assumptions, and calibration are described in Appendix 1. In brief, MISCAN-Colon simulates the life histories of a large population of persons from birth to death. As each simulated person ages, one or more adenomas may develop. These adenomas can progress from small (≤5mm in diameter), to medium (6-9mm), to large size (≥10mm). Some adenomas can develop into preclinical cancer, which may progress through stages I to IV. However, during each stage, CRC may also be diagnosed because of symptoms. Survival after clinical diagnosis is determined by the stage at diagnosis, the localization of the cancer, and the person's age.
Screening will alter some of the simulated life histories. Some cancers will be prevented by the detection and removal of adenomas; other cancers will be detected in an earlier stage with a more favorable survival. However, screening can also result in serious complications and overdiagnosis and overtreatment of CRC. By comparing all life histories with screening with the corresponding life histories without screening, MISCAN-Colon quantifies the effectiveness of screening as well as the associated costs.
For our current study, we calibrated four distinct versions of MISCAN-Colon: a version for white men, white women, black men, and black women (Appendix 1). To do so, we used sex- and race-specific data on the age-, stage-, and localization-specific incidence of CRC as observed in the U.S. before the introduction of mass endoscopic screening (i.e. between 1990 and 1994) and data on the age-specific prevalence and multiplicity distribution of adenomas as observed in autopsy studies.11-21 Moreover, we used U.S. sex- and race-specific CRC survival data.22 We assumed that the average preclinical duration of CRC and adenoma dwell-time were independent of sex and race. These durations were calibrated to the rates of interval and surveillance-detected cancers observed in randomized controlled trials evaluating screening using guaiac fecal occult blood tests and a once-only sigmoidoscopy.23-27
Model Inputs
Populations Simulated
We simulated a cohort of 10 million individuals for each combination of:
Age (66/ 67/ (…)/ 90 years);
Sex (men/ women);
Race (black/ white);
Screening history (negative finding from a screening colonoscopy 10 years prior/15 years prior/ 20 years prior/ no prior screening);
Background risk for CRC (white men: 17 levels, white women: 14 levels, black men: 18 levels, black women: 15 levels (see below)); and
Comorbidity status (no/ moderate/ severe comorbidities (see below)).
This amounted to a total of 19,200 cohorts. Our analysis does not address individuals previously diagnosed with an adenoma or CRC.
Background Risk for CRC
To determine plausible sex- and race-specific ranges for the background risk for CRC, we used the National Cancer Institute's Colorectal Cancer Risk Assessment Tool (Appendix 2).28, 29 This tool allowed us to determine an individual's background risk for CRC based on the following risk factors: the number of first-degree relatives with CRC, current leisure-time vigorous activity, aspirin/ NSAID use, vegetable intake, body mass index, current and past smoking (men only), and estrogen status within the last two years (women only). Based on these risk factors, the background risk for CRC in white females ranged from 0.5 times up to 3.5 times the average background risk in white women. The corresponding ranges in white men, black women, and black men were 0.5-4.9, 0.4-3.5, and 0.5-5.3, respectively. For each combination of sex and race, we modeled cohorts with the minimum risk, the maximum risk, all risks between the minimum risk and average risk using an increment of 0.1, all risks between the average risk and twice the average risk using an increment of 0.2, and all risks between twice the average risk and the maximum risk using an increment of 0.5. We modeled different risk levels by multiplying the age-specific onset rates of adenomas. We did not exclude individuals with a family history of CRC from our analyses.
Comorbidity Status
To simulate individuals with no, moderate, and severe comorbidities, we used U.S. sex-, race-, and comorbidity status specific life-tables.30 Individuals are classified as having moderate comorbidities if diagnosed with an ulcer, rheumatologic disease, peripheral vascular disease, diabetes, paralysis, or cerebrovascular disease and in case of a history of acute myocardial infarction; as having severe comorbidities if diagnosed with chronic obstructive pulmonary disease, congestive heart failure, moderate or severe liver disease, chronic renal failure, dementia, cirrhosis and chronic hepatitis, or AIDS; and as having no comorbidities if none of these conditions is present.
Screening Strategy
Within each cohort, we simulated a screening colonoscopy for all individuals simulated. Individuals in whom adenomas were removed were assumed to undergo colonoscopy surveillance according to the current guidelines.31 We assumed that surveillance continued until the diagnosis of CRC or death. Adherence to surveillance was assumed to be 100%.
Test Characteristics of Colonoscopy
The sensitivity of colonoscopy for the detection of adenomas and CRC was obtained from a systematic review on miss rates observed in tandem colonoscopy studies and was 75% for small adenomas (≤5mm in diameter), 85% for medium-sized adenomas (6-9mm), and 95% for large adenomas (≥10mm) and CRC.32 We assumed that 95% of all colonoscopies reached the cecum; for the remaining 5% the reach of the procedure was assumed to be distributed uniformly over colon and rectum. Age-specific risks for non-lethal complications of colonoscopy were derived from a study by Warren and colleagues.33, 34 _ENREF_31 We assumed that one of every 30,000 colonoscopies involving a polypectomy resulted in death.34, 35
Utility Losses Associated with Colonoscopy Screening
We assumed a utility loss (i.e., a loss of quality of life) equivalent to two full days of life per colonoscopy (0.0055 quality-adjusted life-years [QALYs]) and two weeks of life per complication (0.0384 QALYs). Utility losses for life-years (LYs) with CRC care were derived from a study by Ness and colleagues (Table 1).36
Table 1.
Model Inputs: Utility Losses and Costs Associated with Colonoscopy Screening.
| UTILITY LOSS (QALYs)1 | ||||
|---|---|---|---|---|
| Per colonoscopy | ||||
| without polypectomy/ biopsy | 0.0055 | |||
| with polypectomy/ biopsy | 0.0055 | |||
| Per complication of colonoscopy | 0.0384 | |||
| Per LY with CRC care2, 3 | Initial care | Continuing care | Terminal care Death CRC | Terminal care Death other cause |
| Stage I CRC | 0.12 | 0.05 | 0.70 | 0.05 |
| Stage II CRC | 0.18 | 0.05 | 0.70 | 0.05 |
| Stage III CRC | 0.24 | 0.24 | 0.70 | 0.24 |
| Stage IV CRC | 0.70 | 0.70 | 0.70 | 0.70 |
|
| ||||
| COSTS (2013 US$)4 | ||||
|
| ||||
| Per colonoscopy | ||||
| without polypectomy/ biopsy | 887 | |||
| with polypectomy/ biopsy | 1,096 | |||
| Per complication of colonoscopy | 6,045 | |||
| Per life-year with CRC care2 | Initial care | Continuing care | Terminal care Death CRC | Terminal care Death other cause |
| Stage I CRC | 36,683 | 3,050 | 63,809 | 19,176 |
| Stage II CRC | 49,234 | 2,870 | 63,555 | 17,279 |
| Stage III CRC | 59,759 | 4,021 | 67,041 | 21,457 |
| Stage IV CRC | 77,790 | 12,178 | 88,368 | 49,866 |
QALY = quality-adjusted life-year; LY = life-year; CRC = colorectal cancer
The loss of quality of life associated with a particular event.
Care for CRC was divided in three clinically relevant phases: the initial, continuing, and terminal care phase. The initial care phase was defined as the first 12 months after diagnosis; the terminal care phase was defined as the final 12 months of life; the continuing care phase was defined as all months in between. In the terminal care phase, we distinguished between CRC patients dying from CRC and CRC patients dying from another cause. For patients surviving less than 24 months, the final 12 months were allocated to the terminal care phase and the remaining months were allocated to the initial care phase.
Utility losses for LYs with initial care were derived from a study by Ness and colleagues.36 For LYs with continuing care for stage I and II CRC, we assumed a utility loss of 0.05 QALYs; for LYs with continuing care for stage III and IV CRC, we assumed the corresponding utility losses for LYs with initial care. For LYs with terminal care for CRC, we assumed the utility loss for LYs with initial care for stage IV CRC. For LYs with terminal care for another cause, we assumed the corresponding utility losses for LYs with continuing care.
Costs include copayments and patient time costs (i.e. the opportunity costs of spending time on screening or being treated for a complication or CRC), but do not include travel costs, costs of lost productivity, and unrelated health care and non-health care costs in added years of life. We assumed that the value of patient time was equal to the median wage rate in 2012: $16.71 per hour.39 We assumed that colonoscopies and complications used up 8 and 16 hours of patient time, respectively. Patient time costs were already included in the estimates for the costs of LYs with CRC care obtained from a study by Yabroff and colleagues.40
Costs Associated with Colonoscopy Screening
The cost effectiveness analyses were conducted from a societal perspective. The costs of colonoscopies were based on 2007 Medicare payment rates and copayments (Table 1).37 The costs of complications were obtained from a cost analysis of cases of unexpected hospital use after endoscopy in 2007.38 We added patient time costs to both.39 The costs of LYs with CRC care were obtained from an analysis of Surveillance, Epidemiology, and End Results-Medicare linked data and included patient deductibles, copayments, and patient time costs.40 We adjusted all costs to reflect the 2013 level using the U.S. Consumer Price Index.41
Outcomes
For each cohort, we quantified the effectiveness of screening (i.e., the number of CRC cases prevented, CRC deaths prevented, LYs gained, and QALYs gained by screening) as well as the associated costs, applying the conventional 3% annual discount rate to both.42 We expressed the cost effectiveness of screening in terms of the costs per QALY gained.
Analyses
For all demographic groups, we first quantified the effect of age on the effectiveness and cost effectiveness of screening. To demonstrate the (relative) importance of also considering factors other than age, we subsequently quantified the effect of screening history, background risk for CRC, and comorbidity status on the effectiveness and cost effectiveness of screening. Finally, we determined the appropriate age to stop screening given an individual's sex and race, screening history, background risk for CRC, and comorbidity status, applying the currently recommended willingness-to-pay threshold of $100,000 per QALY gained.43
Sensitivity Analyses
We repeated our analyses assuming 1) 50% higher and 50% lower utility losses for colonoscopies and complications; 2) 25% higher and 25% lower costs for colonoscopies; 3) 25% higher and 25% lower costs for CRC care; and 4) a cost effectiveness threshold of $50,000 per QALY gained. Moreover, we performed a multivariate probabilistic sensitivity analysis on the above-mentioned utility losses and costs for one representative case (details on this analysis are given in Appendix 6).
Results
The Effect of Age on the Effectiveness and Cost Effectiveness of Screening
The effectiveness of colonoscopy screening declined with increasing age. While screening healthy, average risk, white women with a negative screening colonoscopy 10 years prior resulted in 27.8 QALYs gained per 1,000 women aged 66 years, it resulted in a loss of QALYs, rather than a gain in women aged 85 years and older (Figure 1A). On the other hand, the costs of screening increased with age: from $602,000 per 1,000 women aged 66 years to $1,061,000 per 1,000 women aged 90 years (Figure 1B). As a result, the cost effectiveness of screening deteriorated with age. While screening was associated with a cost of $22,000 per QALY gained for women aged 66 years, it was associated with a cost of nearly $4M per QALY gained for women aged 84 years (Figure 1C). Sex and race had relatively little effect on the effectiveness and cost effectiveness of screening (Figure 1).
Figure 1.
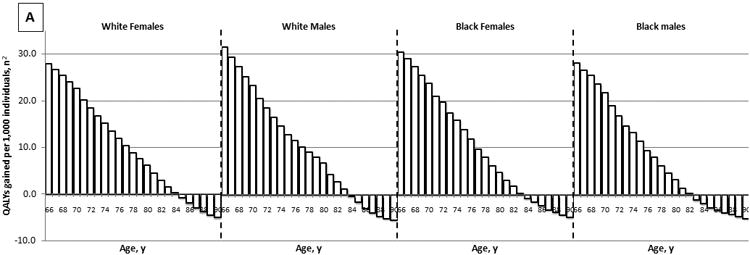
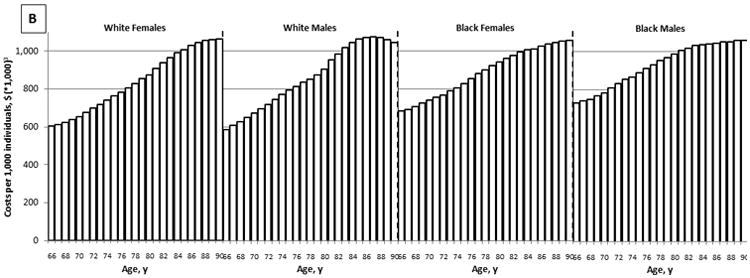
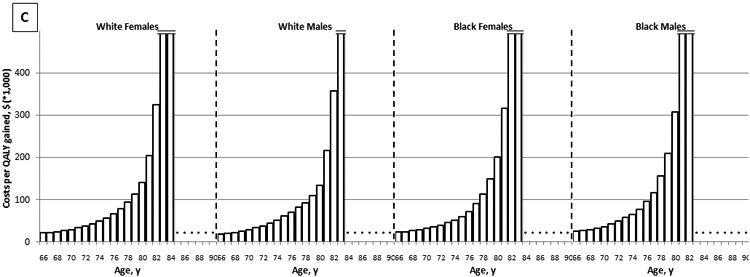
The Effect of Age on the Effectiveness (A), Costs (B), and Cost Effectiveness (C) of Colonoscopy Screening: Results for Average Risk Individuals with a Negative Screening Colonoscopy 10 Years Prior and No Comorbidities (QALYs gained and costs per 1,000 individuals; 3% discounted).1
QALY = quality-adjusted life-year
1Detailed results on the effectiveness and costs of screening can be found in Appendix 3.
2The effect of screening on quantity and quality of life incorporated in one measure (i.e. the net health benefit of screening). A negative value indicates that screening is associated with a net harm, rather than a net health benefit.
3The costs of screening and surveillance colonoscopies, complications of colonoscopy, and overtreatment of CRC minus the savings associated with preventing CRC treatment.
The Effect of Factors Other than Age on the Effectiveness and Cost Effectiveness of Screening
Screening history and comorbidity status had a large effect on the effectiveness of colonoscopy screening. While screening healthy, average risk, 75-year-old, white women with a negative screening colonoscopy 10 years prior (our “reference cohort” for this comparison) resulted in 13.4 QALYs gained per 1,000 women, screening women with identical characteristics, but without prior screening, resulted in 55.2 QALYs gained per 1,000 women (factor difference = 55.2 QALYs gained/ 13.4 QALYs gained = 4.1) (Figure 2A). Similarly, screening women with severe, rather than no comorbidities resulted in 4.3 QALYs gained per 1,000 women (factor difference = 3.1). These relative differences in effectiveness were comparable to the difference in effectiveness between screening 70-year-old and 80-year-old women. Nevertheless, the most important factor influencing the effectiveness of screening was the individual's background risk for CRC. While screening women with the lowest possible background risk resulted in 0.8 QALYs gained per 1,000 women, screening women with the highest possible background risk resulted in 106.5 QALYs gained per 1,000 women (factor difference = 137.7). The relative effect of all factors on the cost effectiveness of screening was slightly larger than the corresponding effect on the effectiveness of screening (Figure 2B). The relative importance of considering factors other than age did not differ substantially by sex and race (Figure 2).
Figure 2.
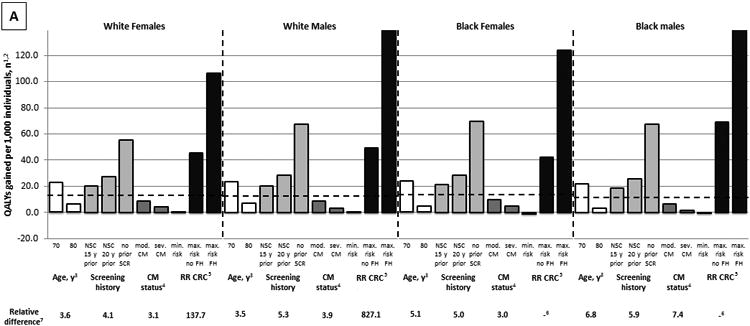
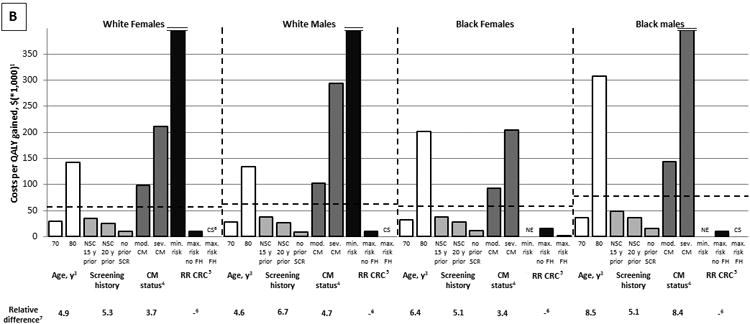
The Relative Effect of Age, Screening History, Comorbidity Status, and Background Risk for CRC on the Effectiveness (A) and Cost Effectiveness (B) of Colonoscopy Screening (QALYs gained per 1,000 individuals; 3% discounted).
CRC = colorectal cancer; QALY = quality-adjusted life-year; NSC = negative screening colonoscopy; SCR = screening; RR CRC = background risk for CRC; CM = comorbidities; FH = family history; CS = cost saving; NE = negative effect
1The dashed lines indicate results for healthy, average risk, 75-year-old individuals with a negative screening colonoscopy 10 years prior.
2The effect of screening on quantity and quality of life incorporated in one measure (i.e. the net health benefit of screening). A negative value indicates that screening is associated with a net harm, rather than a net health benefit.
3See also Figure 1.
4Individuals are classified as having moderate comorbidities if diagnosed with an ulcer, rheumatologic disease, peripheral vascular disease, diabetes, paralysis, or cerebrovascular disease and in case of a history of acute myocardial infarction; as having severe comorbidities if diagnosed with chronic obstructive pulmonary disease, congestive heart failure, moderate or severe liver disease, chronic renal failure, dementia, cirrhosis and chronic hepatitis, or AIDS; and as having no comorbidities if none of these conditions is pre‘Moderate comorbidities’ corresponds with ‘low/medium comorbidity’ and ‘severe comorbidities’ corresponds with high comorbidity’ as used in the study by Cho and colleagues.30
5The range of the background risk for CRC is based on the National Cancer Institute's Colorectal Cancer Risk Assessment Tool. This tool incorporates the following risk factors: the number of first-degree relatives with CRC, current leisure-time vigorous activity, aspirin/ NSAID use, vegetable intake, body mass index, current and past smoking (men only), and estrogen status within the last two years (women only). In white women, the minimum background risk for CRC is 0.5, the maximum background risk in the absence of a family history of CRC is 2.0, and the maximum background risk in the presence of a family history of CRC is 3.5. In white men, black women, and black men, the corresponding risks are 0.5, 2.0, and 4.9; 0.4, 1.8, and 3.5; and 0.5, 2.5, and 5.3, respectively.
6Cannot be calculated.
The Appropriate Ages to Stop Screening
As expected, screening was cost effective up to a more advanced age for individuals without prior screening compared with individuals with prior screening; for individuals without comorbidities compared with individuals with comorbidities; and for individuals with a high background risk for CRC compared with individuals with a low background risk for CRC (Table 2).
Table 2.
The Costs per QALY Gained (*$1,000) of Colonoscopy Screening for White Females by Screening History, Comorbidity Status, Background Risk for CRC, and Age (3% discounted).1
| NEGATIVE SCREENING COLONOSCOPY 10 YEARS PRIOR | NO PRIOR SCREENING COLONOSCOPY | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||||||||||
| NO COMORBIDITY2 | NO COMORBIDITY2 | ||||||||||||||||||||||||||||
| Background risk for CRC3 | Background risk for CRC3 | ||||||||||||||||||||||||||||
| Age | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | 1.2 | 1.4 | 1.6 | 1.8 | 2.0 | 2.54 | 3.04 | 3.54 | Age | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | 1.2 | 1.4 | 1.6 | 1.8 | 2.0 | 2.54 | 3.04 | 3.54 |
| 66 | 124 | 73 | 51 | 37 | 28 | 22 | 14 | 9 | 5 | 3 | 1 | CS | CS | CS | 66 | 16 | 11 | 9 | 6 | 5 | 3 | 1 | CS | CS | CS | CS | CS | CS | CS |
| 68 | 152 | 86 | 59 | 42 | 31 | 24 | 16 | 10 | 6 | 4 | 2 | CS | CS | CS | 68 | 17 | 12 | 9 | 7 | 5 | 4 | 2 | 0 | CS | CS | CS | CS | CS | CS |
| 70 | 202 | 108 | 70 | 50 | 38 | 29 | 19 | 12 | 8 | 5 | 3 | CS | CS | CS | 70 | 19 | 14 | 10 | 8 | 6 | 5 | 2 | 1 | CS | CS | CS | CS | CS | CS |
| 72 | 324 | 153 | 93 | 67 | 48 | 38 | 24 | 17 | 12 | 8 | 5 | 1 | CS | CS | 72 | 24 | 17 | 13 | 10 | 8 | 7 | 4 | 2 | 1 | CS | CS | CS | CS | CS |
| 74 | >500 | 232 | 130 | 88 | 64 | 49 | 32 | 22 | 16 | 12 | 8 | 3 | CS | CS | 74 | 29 | 21 | 17 | 13 | 11 | 9 | 6 | 4 | 2 | 1 | 0 | CS | CS | CS |
| 76 | >500 | 417 | 193 | 123 | 86 | 66 | 43 | 30 | 22 | 17 | 13 | 6 | 2 | CS | 76 | 36 | 27 | 22 | 18 | 15 | 12 | 9 | 7 | 5 | 4 | 2 | 0 | CS | CS |
| 78 | NE | >500 | 343 | 186 | 126 | 94 | 59 | 41 | 31 | 24 | 19 | 10 | 6 | 3 | 78 | 49 | 36 | 30 | 24 | 21 | 18 | 14 | 11 | 9 | 7 | 6 | 3 | 1 | CS |
| 80 | NE | NE | >500 | 323 | 201 | 141 | 86 | 59 | 45 | 36 | 28 | 17 | 11 | 7 | 80 | 67 | 51 | 41 | 35 | 30 | 26 | 21 | 17 | 15 | 13 | 11 | 8 | 5 | 3 |
| 82 | NE | NE | NE | >500 | >500 | 325 | 168 | 107 | 80 | 64 | 51 | 34 | 24 | 18 | 82 | 115 | 85 | 69 | 58 | 50 | 45 | 37 | 31 | 27 | 24 | 21 | 17 | 14 | 11 |
| 84 | NE | NE | NE | NE | NE | >500 | 468 | 237 | 164 | 125 | 98 | 65 | 48 | 36 | 84 | 235 | 161 | 126 | 105 | 90 | 79 | 65 | 56 | 49 | 45 | 40 | 33 | 28 | 25 |
| 86 | NE | NE | NE | NE | NE | NE | NE | >500 | >500 | 349 | 245 | 142 | 99 | 79 | 86 | >500 | 441 | 299 | 229 | 189 | 163 | 129 | 110 | 98 | 87 | 79 | 66 | 57 | 51 |
| 88 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | >500 | 322 | 234 | 88 | NE | NE | NE | >500 | >500 | >500 | 405 | 312 | 263 | 222 | 199 | 160 | 134 | 120 |
| 90 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | 90 | NE | NE | NE | NE | NE | NE | NE | NE | NE | >500 | >500 | >500 | >500 | 453 |
|
| |||||||||||||||||||||||||||||
| MODERATE COMORBIDITY2 | MODERATE COMORBIDITY2 | ||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Background risk for CRC3 | Background risk for CRC3 | ||||||||||||||||||||||||||||
| Age | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | 1.2 | 1.4 | 1.6 | 1.8 | 2.0 | 2.54 | 3.04 | 3.54 | Age | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | 1.2 | 1.4 | 1.6 | 1.8 | 2.0 | 2.54 | 3.04 | 3.54 |
| 66 | 180 | 100 | 68 | 50 | 38 | 30 | 19 | 13 | 9 | 6 | 4 | 0 | CS | CS | 66 | 22 | 17 | 13 | 10 | 8 | 7 | 4 | 2 | 1 | CS | CS | CS | CS | CS |
| 68 | 238 | 123 | 82 | 58 | 44 | 34 | 23 | 16 | 11 | 8 | 5 | 1 | CS | CS | 68 | 25 | 18 | 14 | 11 | 9 | 8 | 5 | 3 | 2 | 1 | CS | CS | CS | CS |
| 70 | 367 | 168 | 103 | 73 | 55 | 42 | 28 | 20 | 14 | 10 | 7 | 3 | CS | CS | 70 | 29 | 21 | 17 | 14 | 11 | 9 | 7 | 5 | 3 | 2 | 1 | CS | CS | CS |
| 72 | >500 | 260 | 144 | 100 | 71 | 55 | 37 | 26 | 19 | 14 | 11 | 5 | 2 | CS | 72 | 35 | 26 | 21 | 17 | 14 | 12 | 9 | 7 | 5 | 4 | 2 | 0 | CS | CS |
| 74 | NE | >500 | 244 | 154 | 105 | 79 | 52 | 37 | 28 | 22 | 17 | 10 | 5 | 2 | 74 | 47 | 36 | 29 | 24 | 20 | 18 | 14 | 11 | 9 | 7 | 6 | 3 | 1 | CS |
| 76 | NE | NE | >500 | 277 | 172 | 127 | 79 | 55 | 42 | 34 | 27 | 17 | 11 | 7 | 76 | 67 | 51 | 41 | 35 | 30 | 26 | 21 | 17 | 15 | 13 | 11 | 8 | 5 | 3 |
| 78 | NE | NE | >500 | >500 | 300 | 200 | 116 | 79 | 61 | 48 | 39 | 25 | 18 | 13 | 78 | 93 | 70 | 57 | 47 | 41 | 36 | 30 | 25 | 22 | 19 | 17 | 13 | 10 | 8 |
| 80 | NE | NE | NE | >500 | >500 | 378 | 191 | 120 | 92 | 73 | 58 | 38 | 28 | 22 | 80 | 139 | 103 | 82 | 69 | 60 | 53 | 44 | 38 | 33 | 30 | 27 | 22 | 18 | 15 |
| 82 | NE | NE | NE | NE | NE | >500 | >500 | 259 | 180 | 138 | 107 | 70 | 53 | 42 | 82 | 289 | 193 | 149 | 122 | 104 | 92 | 76 | 66 | 57 | 52 | 47 | 39 | 34 | 30 |
| 84 | NE | NE | NE | NE | NE | NE | NE | >500 | >500 | 383 | 259 | 150 | 106 | 81 | 84 | >500 | >500 | 373 | 284 | 225 | 192 | 151 | 128 | 111 | 101 | 91 | 76 | 66 | 58 |
| 86 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | >500 | 389 | 232 | 177 | 86 | NE | NE | >500 | >500 | >500 | >500 | 343 | 268 | 227 | 197 | 176 | 142 | 122 | 108 |
| 88 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | >500 | >500 | 88 | NE | NE | NE | NE | NE | NE | NE | >500 | >500 | >500 | >500 | >500 | 391 | 328 |
| 90 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | 90 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE |
|
| |||||||||||||||||||||||||||||
| SEVERE COMORBIDITY2 | SEVERE COMORBIDITY2 | ||||||||||||||||||||||||||||
|
| |||||||||||||||||||||||||||||
| Background risk for CRC3 | Background risk for CRC3 | ||||||||||||||||||||||||||||
| Age | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | 1.2 | 1.4 | 1.6 | 1.8 | 2.0 | 2.54 | 3.04 | 3.54 | Age | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | 1.2 | 1.4 | 1.6 | 1.8 | 2.0 | 2.54 | 3.04 | 3.54 |
| 66 | 335 | 160 | 104 | 74 | 56 | 45 | 30 | 21 | 16 | 12 | 9 | 4 | 1 | CS | 66 | 34 | 26 | 21 | 17 | 14 | 12 | 9 | 7 | 5 | 3 | 2 | CS | CS | CS |
| 68 | >500 | 210 | 129 | 89 | 66 | 52 | 35 | 25 | 18 | 14 | 11 | 5 | 2 | CS | 68 | 38 | 29 | 23 | 19 | 16 | 14 | 10 | 8 | 6 | 4 | 3 | 1 | CS | CS |
| 70 | >500 | 373 | 187 | 125 | 92 | 70 | 47 | 34 | 25 | 20 | 15 | 9 | 5 | 2 | 70 | 47 | 36 | 29 | 24 | 21 | 18 | 14 | 11 | 9 | 7 | 6 | 3 | 1 | CS |
| 72 | NE | >500 | 348 | 208 | 139 | 104 | 67 | 49 | 36 | 29 | 23 | 14 | 9 | 6 | 72 | 65 | 48 | 39 | 33 | 29 | 25 | 20 | 17 | 14 | 12 | 10 | 7 | 4 | 3 |
| 74 | NE | NE | >500 | 407 | 229 | 160 | 98 | 69 | 53 | 42 | 34 | 22 | 15 | 11 | 74 | 88 | 66 | 54 | 46 | 39 | 35 | 28 | 24 | 20 | 18 | 16 | 11 | 9 | 7 |
| 76 | NE | NE | NE | >500 | 427 | 272 | 148 | 100 | 75 | 60 | 49 | 32 | 23 | 18 | 76 | 124 | 91 | 73 | 61 | 53 | 47 | 38 | 33 | 28 | 25 | 23 | 18 | 14 | 12 |
| 78 | NE | NE | NE | NE | >500 | >500 | 298 | 177 | 128 | 99 | 79 | 53 | 39 | 31 | 78 | 216 | 149 | 118 | 96 | 83 | 73 | 61 | 52 | 45 | 41 | 37 | 31 | 26 | 22 |
| 80 | NE | NE | NE | NE | NE | NE | >500 | 334 | 220 | 164 | 127 | 81 | 60 | 48 | 80 | 425 | 256 | 190 | 154 | 131 | 113 | 93 | 80 | 70 | 63 | 58 | 48 | 41 | 36 |
| 82 | NE | NE | NE | NE | NE | NE | NE | >500 | >500 | 461 | 311 | 170 | 122 | 96 | 82 | NE | >500 | >500 | 388 | 296 | 244 | 190 | 157 | 133 | 120 | 109 | 91 | 78 | 70 |
| 84 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | >500 | 472 | 267 | 196 | 84 | NE | NE | NE | >500 | >500 | >500 | 491 | 363 | 295 | 253 | 223 | 177 | 149 | 131 |
| 86 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | >500 | >500 | 86 | NE | NE | NE | NE | NE | NE | NE | NE | >500 | >500 | >500 | >500 | 437 | 364 |
| 88 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | 88 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | >500 |
| 90 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | 90 | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE | NE |
QALY = quality-adjusted life-year; CRC = colorectal cancer; CS = cost saving; NE = negative effect
The dashed lines indicate a willingness-to-pay threshold of $100,000 per QALY gained. Green cells indicate that screening is cost-effective given this threshold. Red cells indicate that screening is not cost-effective given this threshold. Similar tables for white males, black females, and black males are given in Appendix 4.
Detailed information on the assessment of comorbidity status is given in Figure 2, footnote 4.
Detailed information on the assessment of background risk for CRC is given in Figure 2, footnote 5.
Background risk for CRC only possible in case of a family history of CRC.
Table 3 shows the appropriate ages to stop screening for all individuals. Although screening some previously screened, low risk individuals was not even cost effective at age 66 years, screening some healthy, high risk individuals was cost effective up to age 88 years. Results were comparable across the demographic groups.
Table 3.
The Appropriate Ages to Stop Colonoscopy Screening: Results by Screening History, Comorbidity Status, and Background Risk for CRC.1
| WHITE FEMALES | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| SCREENING HISTORY | |||||||||||||||
| Negative screening colonoscopy 10 years prior |
Negative screening colonoscopy 15 years prior |
Negative screening colonoscopy 20 years prior |
No prior screening | ||||||||||||
|
| |||||||||||||||
| COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | ||||||||||||
| RR CRC3 | No comorbidity |
Moderate comorbidity |
Severe comorbidity |
RR CRC3 | No comorbidity |
Moderate comorbidity |
Severe comorbidity |
RR CRC3 | No comorbidity |
Moderate comorbidity |
Severe comorbidity |
RR CRC3 | No comorbidity |
Moderate comorbidity |
Severe comorbidity |
| 0.5 | <665 | <665 | <665 | 0.5 | 70 | 67 | <665 | 0.5 | 73 | 70 | 67 | 0.5 | 81 | 78 | 74 |
| 0.6 | 69 | 66 | <665 | 0.6 | 73 | 70 | 67 | 0.6 | 76 | 73 | 70 | 0.6 | 82 | 79 | 76 |
| 0.7 | 72 | 69 | <665 | 0.7 | 76 | 72 | 70 | 0.7 | 78 | 75 | 72 | 0.7 | 83 | 80 | 77 |
| 0.8 | 74 | 71 | 68 | 0.8 | 78 | 74 | 71 | 0.8 | 80 | 76 | 73 | 0.8 | 83 | 81 | 78 |
| 0.9 | 76 | 73 | 70 | 0.9 | 79 | 76 | 73 | 0.9 | 80 | 78 | 74 | 0.9 | 84 | 81 | 78 |
| 1.0 | 78 | 75 | 71 | 1.0 | 80 | 77 | 74 | 1.0 | 81 | 79 | 76 | 1.0 | 84 | 82 | 79 |
| 1.2 | 80 | 77 | 74 | 1.2 | 81 | 79 | 76 | 1.2 | 82 | 80 | 77 | 1.2 | 85 | 82 | 80 |
| 1.4 | 81 | 79 | 75 | 1.4 | 83 | 80 | 77 | 1.4 | 83 | 81 | 78 | 1.4 | 85 | 83 | 80 |
| 1.6 | 82 | 80 | 77 | 1.6 | 83 | 81 | 78 | 1.6 | 84 | 82 | 79 | 1.6 | 86 | 83 | 81 |
| 1.8 | 83 | 81 | 78 | 1.8 | 84 | 82 | 79 | 1.8 | 85 | 82 | 80 | 1.8 | 86 | 83 | 81 |
| 2.0 | 84 | 81 | 78 | 2.0 | 85 | 82 | 80 | 2.0 | 85 | 83 | 80 | 2.0 | 86 | 84 | 81 |
|
| |||||||||||||||
| 2.54 | 85 | 82 | 80 | 2.54 | 85 | 83 | 81 | 2.54 | 86 | 84 | 81 | 2.54 | 87 | 84 | 82 |
| 3.04 | 86 | 83 | 81 | 3.04 | 86 | 84 | 82 | 3.04 | 86 | 84 | 82 | 3.04 | 87 | 85 | 82 |
| 3.54 | 86 | 84 | 82 | 3.54 | 87 | 85 | 82 | 3.54 | 87 | 85 | 82 | 3.54 | 87 | 85 | 83 |
|
| |||||||||||||||
| WHITE FEMALES | |||||||||||||||
|
| |||||||||||||||
| SCREENING HISTORY | |||||||||||||||
| Negative screening colonoscopy 10 years prior | Negative screening colonoscopy 15 years prior | Negative screening colonoscopy 20 years prior | No prior screening | ||||||||||||
|
| |||||||||||||||
| COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | ||||||||||||
| RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity | RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity | RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity | RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity |
|
| |||||||||||||||
| 0.5 | <665 | <665 | <665 | 0.5 | 70 | 68 | <665 | 0.5 | 73 | 71 | 67 | 0.5 | 81 | 79 | 74 |
| 0.6 | 69 | 66 | <665 | 0.6 | 73 | 70 | 67 | 0.6 | 76 | 73 | 69 | 0.6 | 82 | 80 | 76 |
| 0.7 | 71 | 69 | <665 | 0.7 | 75 | 73 | 69 | 0.7 | 78 | 75 | 71 | 0.7 | 83 | 80 | 77 |
| 0.8 | 74 | 71 | 67 | 0.8 | 78 | 74 | 70 | 0.8 | 80 | 77 | 72 | 0.8 | 83 | 81 | 77 |
| 0.9 | 76 | 73 | 69 | 0.9 | 79 | 76 | 72 | 0.9 | 80 | 77 | 73 | 0.9 | 83 | 81 | 78 |
| 1.0 | 78 | 74 | 70 | 1.0 | 80 | 77 | 73 | 1.0 | 81 | 78 | 75 | 1.0 | 84 | 81 | 78 |
| 1.2 | 80 | 77 | 73 | 1.2 | 81 | 79 | 75 | 1.2 | 82 | 80 | 76 | 1.2 | 84 | 82 | 79 |
| 1.4 | 81 | 79 | 75 | 1.4 | 82 | 80 | 76 | 1.4 | 83 | 80 | 77 | 1.4 | 85 | 82 | 79 |
| 1.6 | 82 | 80 | 76 | 1.6 | 83 | 81 | 77 | 1.6 | 83 | 81 | 78 | 1.6 | 85 | 83 | 80 |
| 1.8 | 83 | 80 | 77 | 1.8 | 83 | 81 | 78 | 1.8 | 84 | 82 | 79 | 1.8 | 85 | 83 | 80 |
| 2.0 | 83 | 81 | 78 | 2.0 | 84 | 82 | 79 | 2.0 | 84 | 82 | 79 | 2.0 | 85 | 83 | 80 |
|
| |||||||||||||||
| 2.54 | 84 | 82 | 80 | 2.54 | 85 | 83 | 80 | 2.54 | 85 | 83 | 80 | 2.54 | 86 | 83 | 81 |
| 3.04 | 85 | 83 | 80 | 3.04 | 85 | 83 | 81 | 3.04 | 86 | 83 | 81 | 3.04 | 86 | 84 | 81 |
| 3.54 | 85 | 83 | 81 | 3.54 | 86 | 84 | 81 | 3.54 | 86 | 84 | 81 | 3.54 | 86 | 84 | 81 |
| 4.04 | 86 | 84 | 81 | 4.04 | 86 | 84 | 81 | 4.04 | 86 | 84 | 81 | 4.04 | 86 | 84 | 82 |
| 4.54 | 86 | 84 | 82 | 4.54 | 86 | 84 | 82 | 4.54 | 87 | 84 | 82 | 4.54 | 87 | 84 | 82 |
| 4.94 | 86 | 84 | 82 | 4.94 | 87 | 84 | 82 | 4.94 | 87 | 84 | 82 | 4.94 | 87 | 84 | 82 |
|
| |||||||||||||||
| BLACK FEMALES | |||||||||||||||
|
| |||||||||||||||
| SCREENING HISTORY | |||||||||||||||
| Negative screening colonoscopy 10 years prior | Negative screening colonoscopy 15 years prior | Negative screening colonoscopy 20 years prior | No prior screening | ||||||||||||
|
| |||||||||||||||
| COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | ||||||||||||
| RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity | RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity | RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity | RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity |
|
| |||||||||||||||
| 0.4 | <665 | <665 | <665 | 0.4 | <665 | <665 | <665 | 0.4 | 68 | <665 | <665 | 0.4 | 80 | 78 | 73 |
| 0.5 | <665 | <665 | <665 | 0.5 | 69 | 67 | <665 | 0.5 | 72 | 70 | <665 | 0.5 | 81 | 79 | 75 |
| 0.6 | 68 | <665 | <665 | 0.6 | 73 | 70 | <665 | 0.6 | 75 | 73 | 69 | 0.6 | 82 | 80 | 77 |
| 0.7 | 72 | 70 | <665 | 0.7 | 75 | 73 | 68 | 0.7 | 77 | 75 | 71 | 0.7 | 83 | 81 | 77 |
| 0.8 | 74 | 72 | 67 | 0.8 | 77 | 75 | 70 | 0.8 | 79 | 77 | 72 | 0.8 | 84 | 81 | 78 |
| 0.9 | 76 | 74 | 69 | 0.9 | 78 | 76 | 72 | 0.9 | 80 | 78 | 74 | 0.9 | 84 | 82 | 79 |
| 1.0 | 77 | 75 | 71 | 1.0 | 79 | 78 | 73 | 1.0 | 81 | 79 | 75 | 1.0 | 85 | 82 | 80 |
| 1.2 | 79 | 77 | 73 | 1.2 | 81 | 79 | 76 | 1.2 | 82 | 80 | 77 | 1.2 | 85 | 83 | 80 |
| 1.4 | 80 | 79 | 75 | 1.4 | 82 | 80 | 77 | 1.4 | 83 | 81 | 78 | 1.4 | 85 | 83 | 81 |
| 1.6 | 81 | 80 | 77 | 1.6 | 83 | 81 | 78 | 1.6 | 84 | 82 | 79 | 1.6 | 86 | 84 | 81 |
| 1.8 | 82 | 80 | 77 | 1.8 | 83 | 81 | 79 | 1.8 | 84 | 82 | 80 | 1.8 | 86 | 84 | 82 |
|
| |||||||||||||||
| 2.04 | 83 | 81 | 78 | 2.04 | 84 | 82 | 80 | 2.04 | 85 | 83 | 80 | 2.04 | 86 | 84 | 82 |
| 2.54 | 84 | 82 | 80 | 2.54 | 85 | 83 | 81 | 2.54 | 86 | 84 | 82 | 2.54 | 87 | 85 | 82 |
| 3.04 | 85 | 83 | 81 | 3.04 | 86 | 84 | 82 | 3.04 | 86 | 84 | 82 | 3.04 | 87 | 86 | 83 |
| 3.54 | 86 | 84 | 82 | 3.54 | 87 | 85 | 83 | 3.54 | 87 | 86 | 83 | 3.54 | 88 | 86 | 83 |
|
| |||||||||||||||
| BLACK FEMALES | |||||||||||||||
|
| |||||||||||||||
| SCREENING HISTORY | |||||||||||||||
| Negative screening colonoscopy 10 years prior | Negative screening colonoscopy 15 years prior | Negative screening colonoscopy 20 years prior | No prior screening | ||||||||||||
|
| |||||||||||||||
| COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | COMORBIDITY STATUS2 | ||||||||||||
| RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity | RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity | RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity | RR CRC3 | No comorbidity | Moderate comorbidity | Severe comorbidity |
|
| |||||||||||||||
| 0.5 | <665 | <665 | <665 | 0.5 | 68 | <665 | <665 | 0.5 | 71 | 69 | <665 | 0.5 | 80 | 78 | 72 |
| 0.6 | 66 | <665 | <665 | 0.6 | 71 | 69 | <665 | 0.6 | 74 | 71 | 67 | 0.6 | 81 | 79 | 73 |
| 0.7 | 70 | 68 | <665 | 0.7 | 74 | 71 | 67 | 0.7 | 76 | 73 | 68 | 0.7 | 82 | 80 | 75 |
| 0.8 | 72 | 70 | <665 | 0.8 | 76 | 72 | 67 | 0.8 | 77 | 74 | 70 | 0.8 | 82 | 80 | 75 |
| 0.9 | 74 | 71 | 67 | 0.9 | 77 | 74 | 70 | 0.9 | 79 | 76 | 71 | 0.9 | 83 | 81 | 76 |
| 1.0 | 76 | 73 | 68 | 1.0 | 78 | 76 | 70 | 1.0 | 80 | 78 | 72 | 1.0 | 84 | 81 | 76 |
| 1.2 | 78 | 75 | 70 | 1.2 | 80 | 78 | 72 | 1.2 | 81 | 79 | 73 | 1.2 | 84 | 81 | 76 |
| 1.4 | 79 | 78 | 72 | 1.4 | 81 | 79 | 73 | 1.4 | 82 | 80 | 75 | 1.4 | 84 | 81 | 77 |
| 1.6 | 80 | 78 | 73 | 1.6 | 82 | 80 | 75 | 1.6 | 82 | 81 | 76 | 1.6 | 85 | 82 | 79 |
| 1.8 | 81 | 79 | 75 | 1.8 | 82 | 81 | 76 | 1.8 | 83 | 81 | 76 | 1.8 | 85 | 82 | 79 |
| 2.0 | 82 | 80 | 75 | 2.0 | 83 | 81 | 76 | 2.0 | 84 | 81 | 77 | 2.0 | 86 | 82 | 80 |
| 2.5 | 83 | 81 | 77 | 2.5 | 84 | 82 | 79 | 2.5 | 85 | 82 | 79 | 2.5 | 86 | 83 | 80 |
|
| |||||||||||||||
| 3.04 | 84 | 82 | 79 | 3.04 | 85 | 82 | 80 | 3.04 | 86 | 83 | 80 | 3.04 | 87 | 83 | 80 |
| 3.54 | 85 | 82 | 80 | 3.54 | 86 | 83 | 80 | 3.54 | 86 | 83 | 80 | 3.54 | 87 | 84 | 80 |
| 4.04 | 86 | 83 | 80 | 4.04 | 86 | 84 | 80 | 4.04 | 87 | 84 | 80 | 4.04 | 87 | 84 | 80 |
| 4.54 | 87 | 83 | 80 | 4.54 | 87 | 84 | 81 | 4.54 | 87 | 84 | 81 | 4.54 | 88 | 84 | 81 |
| 5.04 | 87 | 84 | 81 | 5.04 | 87 | 84 | 82 | 5.04 | 87 | 84 | 82 | 5.04 | 88 | 84 | 82 |
| 5.34 | 87 | 84 | 81 | 5.34 | 88 | 84 | 82 | 5.34 | 88 | 84 | 82 | 5.34 | 88 | 84 | 82 |
CRC = colorectal cancer; RR CRC = background risk for CRC
Given a willingness-to-pay threshold of $100,000 per QALY gained.
Detailed information on the assessment of comorbidity status is given in Figure 2, footnote 4.
Detailed information on the assessment of background risk for CRC is given in Figure 2, footnote 5.
Background risk for CRC only possible in case of a family history of CRC.
In these cohorts screening was not cost-effective at age 66 years. We did not perform analyses for individuals aged 65 years or younger.
Sensitivity Analyses
Results were robust to varying the utility losses associated with colonoscopies and complications and to varying the costs of colonoscopies and CRC care (Appendix 5 [univariate deterministic sensitivity analyses] and Appendix 6 [multivariate probabilistic sensitivity analysis]). Applying a willingness-to-pay threshold of $50,000 instead of $100,000 per QALY gained reduced the maximum age at which screening was cost effective by an average of 3 years.
Discussion
In current practice, decisions on CRC screening for elderly individuals are often based primarily on age.6 Our study shows that this approach is inefficient, resulting in underuse of screening for some and overuse of screening for others. An 81-year-old black man with no comorbidities, an average background risk for CRC, and no prior screening, for example, might currently be denied screening, while our study shows that screening these individuals is highly cost effective (costs per QALY gained: $50,000). A 74-year-old white woman with moderate comorbidities (e.g. diabetes), half the average background risk for CRC, and a negative screening colonoscopy 10 years prior, on the other hand, might currently be offered screening, while our study shows that screening these individuals is harmful. Although screening some previously screened, low risk individuals is not even cost effective at age 66 years, screening healthy, high risk individuals can remain cost effective up to age 88 years. To facilitate the use of our results in clinical practice, we developed a web tool that can be used to quantify the cost effectiveness of colonoscopy screening for individual elderly patients. This tool can be accessed at: http://www.frankly.yetes.nl/.
Although our study shows that the appropriate age to stop screening varies widely among individuals, our results are in line with the USPSTF recommendation to discontinue routine screening for CRC in individuals aged over 75 years.1 Based on our analyses, the appropriate age to stop screening for average risk, white women with a negative screening colonoscopy 10 years prior is 78 years for those with no comorbidities, 75 years for those with moderate comorbidities, and 71 years for those with severe comorbidities. Results for the other demographic groups were comparable. Our results are also in agreement with the results of an earlier study on the cost effectiveness of screening in elderly individuals without prior screening.44 In that study, in which we did not consider previously screened individuals, nor stratify results by sex and race or background risk for CRC, we found colonoscopy screening to be cost effective up to age 85, 82, and 79 years for average risk individuals with no, moderate, and severe comorbidities, respectively. For white women, the corresponding ages found in our current study were 84, 82, and 79 years. Finally, our results are in line with the results of an earlier study on FIT screening in elderly individuals with an adequate screening history.45 In that study, in which we did not consider previously unscreened individuals, nor stratify results by sex and race or background risk for CRC, we found FIT screening to have a favorable balance between benefits and harms up to age 76, 74, and 71 years for average risk individuals with no, moderate, and severe comorbidities, respectively. For white women, the corresponding ages to stop colonoscopy screening found in our current study were 78, 75, and 71 years.
The idea of personalizing screening decisions for elderly patients is not new. Walter and Covinsky described a theoretical framework for personalization in elderly individuals in 2001.46 This framework, which focused primarily on the effect of life-expectancy on the effectiveness of screening, was followed by many studies that examined the univariate relationships between sex and race, screening history, comorbidity status, and cancer risk on the one hand, and the effectiveness and cost effectiveness of CRC screening on the other.47-49 However, none of these studies considered all relevant factors simultaneously, using a multivariate approach. This complicates the use of the results of these studies in clinical practice. For example, how should one approach an individual with a high risk for CRC, but a poor life-expectancy, or vice versa? We believe that our study is more applicable to the complex situations commonly encountered in clinical practice. Additionally, our work may prove useful to policy makers aiming at improving the efficiency of cancer prevention.
Our study has several important limitations. First, in our analyses we did not consider individuals with a negative screening colonoscopy less than 10 years prior. Because some screening guidelines recommend a screening interval of 5 years for individuals with a family history of CRC, we decided to provide results for high risk individuals with a negative screening colonoscopy 5 years prior in an appendix (Appendix 7). Second, we only considered screening by colonoscopy. Since the costs of a screening sigmoidoscopy and, particularly, a fecal occult blood test are considerably lower than those of a colonoscopy, these screening modalities might remain cost effective up to a more advanced age. However, in an earlier study, age differences between tests were found to be small.44 Third, we did not consider individuals with multiple prior negative screening colonoscopies. However, since the interval since the last negative screening colonoscopy is likely to be the most important screening-related predictor of CRC risk, having had multiple prior negative screening colonoscopies is unlikely to substantially lower the appropriate age to stop screening. Finally, the National Cancer Institute's CRC Risk Assessment Tool only provides risk estimates for average risk adenoma patients. Since recommendations for surveillance in adenoma patients should be tailored according to the characteristics of adenomas removed during colonoscopy, we could not use our current approach to provide guidance for elderly individuals with adenomas removed during a prior colonoscopy. Given that the majority of colonoscopies in elderly patients are performed for post-polypectomy surveillance,50 this is an important area for future research.
Although we believe that effectiveness and cost effectiveness should be important criteria when making decisions about offering medical interventions, we recognize that decisions on CRC screening should also be based on patient preferences. This requires reliable, personalized information on the benefits, burden, and harms of screening. Hence, additional studies focusing on those outcomes most meaningful to patients are required. Another important future research direction is the development of new prediction models for both CRC risk and life-expectancy. Research in this area should not only focus on developing more sophisticated and accurate models, but also on developing simpler models that are less time consuming to use than the currently available models. Along these lines, it is important to realize that implementing personalized screening in clinical practice will be challenging: many patients are reluctant to discontinue screening even if the expected benefit is low,51 physicians might find personalization cumbersome, and system-level incentives, which currently encourage “one size fits all” age-based screening, need to be aligned with benefit.
In conclusion: The current approach to CRC screening in elderly individuals, in which the decision to offer screening is often based primarily on age, is inefficient, resulting in underuse of screening for some and overuse of screening for others. A more personalized approach to screening has great potential to increase the efficiency of screening. As the population ages, this will become increasingly important.
Supplementary Material
Acknowledgments
Grant Support: This study was made possible by funding from the University of Michigan Medical School (contract number: 3001705234) and Veterans Affairs Health Services Research and Development (contract numbers: IIR 12-411 and CDA 09-213-2). This study was partially made possible by the National Cancer Institute as part of the Cancer Intervention and Surveillance Modeling Network (CISNET), which supported the development of MISCAN-Colon (grant number: U01-CA152959). The sponsors had no role in the designs and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- CRC
colorectal cancer
- LY
life-year
- MISCAN-Colon
Microsimulation Screening Analysis-Colon
- QALY
quality-adjusted life-year
- USPSTF
U.S. Preventive Services Task Force
Footnotes
Disclosures: For none of the authors there is a conflict of interest to disclose.
Author Contributions: FvH and SDS were responsible for drafting the manuscript. All authors contributed to the conception and design of the study; the generation, collection, assembly, analysis and/or interpretation of data; and the revision of the manuscript. All authors approved of the final version of the manuscript.
References
- 1.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Qaseem A, Denberg TD, Hopkins RH, Jr, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378–86. doi: 10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 5.Kahi CJ, van Ryn M, Juliar B, et al. Provider recommendations for colorectal cancer screening in elderly veterans. J Gen Intern Med. 2009;24:1263–8. doi: 10.1007/s11606-009-1110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saini SD, Vijan S, Schoenfeld P, et al. Role of quality measurement in inappropriate use of screening for colorectal cancer: retrospective cohort study. BMJ. 2014;348:g1247. doi: 10.1136/bmj.g1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin JS, Singh A, Reddy N, et al. Overuse of screening colonoscopy in the Medicare population. Arch Intern Med. 2011;171:1335–43. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheffield KM, Han Y, Kuo YF, et al. Potentially inappropriate screening colonoscopy in Medicare patients: variation by physician and geographic region. JAMA Intern Med. 2013;173:542–50. doi: 10.1001/jamainternmed.2013.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150:465–73. doi: 10.7326/0003-4819-150-7-200904070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro JA, Klabunde CN, Thompson TD, et al. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:895–904. doi: 10.1158/1055-9965.EPI-12-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arminski TC, McLean DW. Incidence and Distribution of Adenomatous Polyps of the Colon and Rectum Based on 1,000 Autopsy Examinations. Dis Colon Rectum. 1964;7:249–61. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 12.Bombi JA. Polyps of the colon in Barcelona, Spain. An autopsy study Cancer. 1988;61:1472–6. doi: 10.1002/1097-0142(19880401)61:7<1472::aid-cncr2820610734>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157:223–6. doi: 10.1097/00000658-196302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark JC, Collan Y, Eide TJ, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36:179–86. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 15.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand Gut. 1992;33:1508–14. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989;24:799–806. doi: 10.3109/00365528909089217. [DOI] [PubMed] [Google Scholar]
- 17.Rickert RR, Auerbach O, Garfinkel L, et al. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43:1847–57. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49:819–25. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23:835–42. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blatt LJ. Polyps of the colon and rectum: incidence and distribution. Dis Colon Rectum. 1961;4:277–282. [Google Scholar]
- 21.Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence-SEER 9 Regs Limited-Use, Nov 2002 Sub (1973-2000) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2003, based on the November 2002 submission. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 22.Rutter CM, Johnson EA, Feuer EJ, et al. Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst. 2013;105:1806–13. doi: 10.1093/jnci/djt299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 24.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 25.Jorgensen OD, Kronborg O, Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002;50:29–32. doi: 10.1136/gut.50.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, et al. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer. 2009;115:2410–9. doi: 10.1002/cncr.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandel JS, Church TR, Ederer F, et al. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 28.Freedman AN, Slattery ML, Ballard-Barbash R, et al. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol. 2009;27:686–93. doi: 10.1200/JCO.2008.17.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park Y, Freedman AN, Gail MH, et al. Validation of a colorectal cancer risk prediction model among white patients age 50 years and older. J Clin Oncol. 2009;27:694–8. doi: 10.1200/JCO.2008.17.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H, Klabunde CN, Yabroff KR, et al. Comorbidity-adjusted life expectancy: a new tool to inform recommendations for optimal screening strategies. Ann Intern Med. 2013;159:667–76. doi: 10.7326/0003-4819-159-10-201311190-00005. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 32.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 33.van Hees F, Zauber AG, Klabunde CN, et al. The appropriateness of more intensive colonoscopy screening than recommended in Medicare beneficiaries: a modeling study. JAMA Intern Med. 2014;174:1568–76. doi: 10.1001/jamainternmed.2014.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849–57. doi: 10.7326/0003-4819-150-12-200906160-00008. W152. [DOI] [PubMed] [Google Scholar]
- 35.Gatto NM, Frucht H, Sundararajan V, et al. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–6. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 36.Ness RM, Holmes AM, Klein R, et al. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94:1650–7. doi: 10.1111/j.1572-0241.1999.01157.x. [DOI] [PubMed] [Google Scholar]
- 37.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, Ballegooijen Mv, Kuntz KM. Rockville, MD: Agency for Healthcare Research and Quality; 2007. [Accessed May 10, 2015]. Cost-effectiveness of DNA stool testing to screen for colorectal cancer, 2007. http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id52TA.pdf. [PubMed] [Google Scholar]
- 38.Leffler DA, Kheraj R, Garud S, et al. The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. Arch Intern Med. 2010;170:1752–7. doi: 10.1001/archinternmed.2010.373. [DOI] [PubMed] [Google Scholar]
- 39.Bureau of Labor Statistics, US Department of Labor. [Accessed May 10, 2015];Occupational Employment Statistics: May 2012 National Occupational Employment and Wage Estimates-United States. http://www.bls.gov/oes/2012/may/oes_nat.htm.
- 40.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–41. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 41.Bureau of Labor Statistics, US Department of Labor. CPI Detailed Report: Data for January 2013. [Accessed May 10, 2015]; http://www.bls.gov/cpi/cpid1301.pdf.
- 42.Siegel JE, Torrance GW, Russell LB, et al. Guidelines for pharmacoeconomic studies. Recommendations from the panel on cost effectiveness in health and medicine. Panel on cost Effectiveness in Health and Medicine. Pharmacoeconomics. 1997;11:159–68. doi: 10.2165/00019053-199711020-00005. [DOI] [PubMed] [Google Scholar]
- 43.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 44.van Hees F, Habbema JD, Meester RG, et al. Should colorectal cancer screening be considered in elderly persons without previous screening? A cost-effectiveness analysis. Ann Intern Med. 2014;160:750–9. doi: 10.7326/M13-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lansdorp-Vogelaar I, Gulati R, Mariotto AB, et al. Personalizing age of cancer screening cessation based on comorbid conditions: model estimates of harms and benefits. Ann Intern Med. 2014;161:104–12. doi: 10.7326/M13-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285:2750–6. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 47.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009;70:96–108. 108 e1–24. doi: 10.1016/j.gie.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon LF, Jr, Hayward R, Saint S, et al. Univariate solutions in a multivariate world: can we afford to practice as in the ‘good old days’? Am J Manag Care. 2005;11:473–6. [PubMed] [Google Scholar]
- 49.Wilschut JA, Steyerberg EW, van Leerdam ME, et al. How much colonoscopy screening should be recommended to individuals with various degrees of family history of colorectal cancer? Cancer. 2011;117:4166–74. doi: 10.1002/cncr.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lieberman DA, Williams JL, Holub JL, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. 2014;80:133–43. doi: 10.1016/j.gie.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Torke AM, Schwartz PH, Holtz LR, et al. Older adults and forgoing cancer screening: ‘I think it would be strange’. JAMA Intern Med. 2013;173:526–31. doi: 10.1001/jamainternmed.2013.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


