Abstract
Rationale
Reports of cognitive decline, particularly in the domains of executive functions (EFs), are common among menopausal women.
Objective
This study aims to determine the impact of the psychostimulant lisdexamfetamine (LDX) on subjective and objective cognitive function among menopausal women who report new-onset EF complaints.
Methods
Thirty-two healthy perimenopausal and early postmenopausal women experiencing mid-life-onset executive function difficulties as measured using the Brown Attention Deficit Disorder Scale (BADDS) were administered LDX 40–60 mg/day for 4 weeks in this double-blind, placebo-controlled, cross-over study. Diagnosis of lifetime ADHD was exclusionary. BADDS total and subscale scores and performance on verbal memory and working memory tasks were outcomes of interest.
Results
Analyses revealed a significant effect of LDX treatment over placebo for total BADDS scores (p=0.0001) and for four out of the five BADDS subscales (all p<0.004). LDX treatment also resulted in significant improvement in delayed paragraph recall (p=0.018), but there was no significant effect of treatment on other cognitive measures. Systolic blood pressure (p=0.017) and heart rate increased significantly (p= 0.006) when women were on LDX but remained, on average, within the normal range.
Conclusions
LDX 40–60 mg/day was well tolerated and improved the subjective measures of executive function as well as objective measures of delayed verbal recall in this sample of healthy menopausal women.
Keywords: Menopause, Cognition, Executive function, ADHD, Psychostimulant, Lisdexamfetamine, Brown Attention Deficit Disorder Scale, Verbal memory, Paragraph recall
Introduction
Cognitive complaints are common among menopausal women, but until recently it has been unclear to what degree the menopause or aging itself contributes to these symptoms (Luetters et al. 2007; Greendale et al. 2011; Epperson et al. 2013; Maki et al. 2010; Weber et al. 2014). We previously reported findings from a large community cohort confirming an age-independent decline in immediate and delayed verbal recall despite controlling for mood, education, race, and body mass index (Epperson et al. 2013). Loss of estradiol is suspected to contribute, albeit in part, to this phenomenon as estradiol has potent modulatory effects on multiple neurotransmitter systems and brain regions implicated in learning and memory (Shanmugan and Epperson 2014). However, the cognitive benefits of postmenopausal estradiol treatment (ET) are debatable and depend upon the age of menopause, the type of estrogen used, the duration of hypogonadism, and the overall health of the central nervous system at the time of ET initiation (Weber et al. 2014). Moreover, systemic ET is contraindicated in women who have had or are at risk for gynecologic cancer.
We previously reported that cognitively intact perimenopausal and early postmenopausal women reported new-onset executive function (EF) difficulties in the domains of organization, working memory, focus, and attention (Epperson et al. 2011). The severity of executive dysfunction in a subset of women was consistent with that observed in adults diagnosed with attention deficit hyperactivity disorder (ADHD). Although the true prevalence and severity of mid-life onset of EF difficulties among women experiencing a natural menopause are not known, complaints are likely to be more severe in a minority of women and depend upon factors such as baseline intelligence, education, and age of menopause (Rapp et al. 2013; Ryan et al. 2014; Weber et al. 2014; Wegesin and Stern 2007). The risk for cognitive difficulties with menopause is accentuated in those women who experience an early and/or abrupt transition to hypogonadism (Ryan et al. 2014; Sherwin 2005). Women who undergo a premature menopause as defined by being 40 or younger at the final menstrual period (FMP) are at heightened risk of poor cognition later in life (Ryan et al. 2014). Interestingly, estradiol treatment (ET) protected some, but not all, aspects of cognition in these prematurely postmenopausal women.
With 52 as the average age of natural menopause, roughly 45 million postmenopausal women live in the USA alone, and the vast majority of these women are living at least a third of their lives in the postmenopausal state. Hence, promoting healthy cognitive aging among menopausal women should be a major public health goal. At present, our ability to predict which women will have the gravest difficulties with hypogonadism is minimal, and few interventions other than ET have been tested.
To this end, we sought to determine whether treatment with the psychostimulant lisdexamfetamine (LDX; Shire®) would improve EFs among healthy perimenopausal and early postmenopausal women reporting subjective cognitive difficulties. Psychostimulants are primarily marketed for the treatment of ADHD but have been utilized with variable success to treat radiation- or chemotherapy-induced fatigue or cognitive difficulties in cancer patients (Gehring et al. 2012) including postmenopausal women (Johnson et al. 2010). The primary mechanism of action of psychostimulants in the treatment of EF difficulties is through enhancement of prefrontal cortex (PFC) dopaminergic tone, which is impaired in ADHD and other disorders characterized by EF difficulties (Arnsten 2009). Similarly, estradiol has potent modulatory effects on PFC dopaminergic function (Jacobs and D’Esposito 2011). Whether altered dopaminergic function is responsible for unmasking EF difficulties or is a target for therapy in some menopausal women is not known, but of considerable interest. We therefore sought to determine the impact of LDX on subjective complaints of executive dysfunction among late perimenopausal and early postmenopausal women who report impairment in these domains. While the literature suggests the value of normed, self-report, ecologically based scales in assessing EF impairments (Barkley and Murphy 2010), objective measures of cognitive function have been included as secondary outcomes.
Methods
Participants
Women between the ages of 45 and 60 who reported onset of EF difficulties during the menopause transition and were within 5 years of their FMP were recruited to the Penn Center for Women’s Behavioral Wellness. Perimenopausal women were required to have had irregular menstrual cycles for at least the past 12 months, with no period for at least 3 months, and a serum follicle stimulating hormone (FSH) level of ≥20 IU/L. LMP of ≥12 months and serum FSH levels ≥35 UI/L indicated postmenopausal status. The Brown Attention Deficit Disorder Scale (BADDS) was used to assess the severity of the subjective symptoms of EF difficulties. A BADDS score of 20 or greater with onset of symptoms reported to coincide with the initiation of menstrual cycle irregularity was required. The participants were English-speaking and gave written informed consent for participation in this study, which was approved by the Perelman School of Medicine at the University of Pennsylvania Institutional Review Board.
Structured Clinical Interview for Diagnosis—DSM-IV (SCID)-Non-patient Version (First et al. 1995) was administered, and women with a lifetime history of a psychotic disorder, substance abuse disorder within the previous year, lifetime diagnosis of psychostimulant abuse, or present Axis I psychiatric disorder were excluded. The study psychiatrist confirmed via clinical interview that the participants did not meet the criteria for ADHD. Likewise, regular use of psychotropic medications, use of ET within the previous 6 months, positive urine pregnancy test (perimenopausal women), Minimental Status Examination score of <26, full-scale IQ of ≤90 based upon the Wide Range Achievement Test, history seizures, cardiac disease, active hypertension, or an abnormal electrocardiogram at screening were all exclusionary.
Assessment of subjective executive function
The BADDS is a validated subjective measure of EFs (Sandra Kooij et al. 2008) and has been used successfully to assess ADHD-related EF impairments in adults and in clinical trials of ADHD medications (Brown and Perrin 2007; Brown and Landgraf 2010; Brown et al. 2010; Brown et al. 2011). The BADDS is a clinician administered 40-item questionnaire that assesses the frequency and severity of five clusters of symptoms reflective of executive dysfunction reported by individuals with ADHD. These subscales focus on difficulties in (1) organization and activating for work, (2) sustaining attention and concentration, (3) sustaining alertness, effort, and processing speed, (4) managing affective interference, and (5) using working memory and accessing recall. Respondents are asked to rate the frequency and severity of a symptom on a scale from 0 to 3, with 0 meaning that the problem described does not relate to them and 3 indicating that the problem is very true for them and occurs almost daily. The range of severity for the total BADDS score is 0 to 120, with scores of 55 and above being consistent with full-syndrome ADHD (Brown et al. 2009). The total BADDS score served as the primary outcome variable, while subscale scores were examined to more fully determine the impact of LDX treatment on various domains of EF and to determine whether any sub-categories of executive difficulty were more responsive to LDX treatment (Brown and Perrin 2007; Brown et al. 2010; Epperson et al. 2011). The cutoff of 20 on the BADDS was chosen for this study as this severity of symptoms is consistent with cognitive complaints among middle-aged women who do not carry the diagnosis of ADHD but report symptom interference in their daily life (Epperson et al. 2011).
Neurocognitive testing
Penn Continuous Performance Test—number and letter version (sPCPT-nl (Kurtz et al. 2001))
The sPCPT-nl is a measure of visual attention and vigilance. In this task, a series of red vertical and horizontal lines flash in a digital numeric frame (resembling a digital clock). The participant must press the spacebar whenever these lines form complete numbers or complete letters. The sPCPT-nl is scored based on the number of true/false positives and true negative responses and their respective median response times.
Letter N-back task (LNB (Ragland et al. 2002))
The LNB3 is a measure of attention and working memory. In this task, participants are asked to pay attention to flashing letters on the computer screen, one at a time, and to press the spacebar according to four different principles or rules: the 0-back, the 1-back, the 2-back, and the 3-back. The total number and median response time of true positives were outcomes of interest, assessed separately at each task level as cognitive load is likely to impact on detection of treatment effects on performance (Barrouillet et al. 2007).
NYU Paragraph Recall Task (Kluger et al. 1999)
Subjects hear two brief narratives, each containing 19–21 informational bits, and are asked to recall as many details as possible immediately after hearing each paragraph (A and B) and again following a 30-min delay. Subjects receive credit for each informational bit recalled verbatim. Different paragraphs were read at each assessment.
Behavioral assessments
At screening, and prior to randomization (baseline), participants were administered the Hamilton Depression (HAM-D (Hamilton 1967)) and Anxiety (HAM-A (Hamilton 1959)) Scales. Participants completed the Beck Depression and Anxiety Inventories (BDI (BECK et al. 1961), BAI (Beck et al. 1988)) and Pittsburgh Sleep Quality Index (PSQI (Buysse et al. 1989)) at baseline and after each treatment arm to account for depression and anxiety symptoms and sleep quality as possible confounding factors affecting cognitive performance. The PSQI is a ten-item self-rated questionnaire focusing on perceived sleep quality within the previous month. At baseline and at the end of each treatment trial, individuals were queried regarding their sleep over the previous month or 2 weeks, respectively. Scores greater than 5 reflect relatively poor sleep quality.
Safety measures
Weight and resting blood pressure and heart rate were measured at study visits. Likewise, participants were queried regarding side effects, including but not limited to headache, jitteriness, dry mouth, appetite change, insomnia, racing heart or palpitations, and upset stomach.
Randomization and medication treatment
Randomization and medication dispensing was conducted by the Penn Investigational Drug Service. Study investigators, clinical research coordinators, and participants were blind to group assignment. Participants began each treatment trial by taking one pill of the study medication (LDX 20 or look-alike placebo) for the first week and two pills (40 mg total) each morning for the second week. Dosage was increased to three pills (60 mg/day) for the final 2 weeks of each trial if tolerated. Upon completion of the first 4-week trial, participants underwent a 2-week washout and were then crossed over to the other treatment condition.
Statistical analysis
Statistical power and sample size were determined assuming the cross-over design, i.e., two replicate measures per woman, active baseline and placebo baseline, and a two-sided type I error rate of 5 %. Assuming perfect correlation among the measures, this study has 80 % power to detect an effect size of 1/2 SD. Because the primary and secondary outcome measures of BADDS scores and cognitive test results utilize ordinal (as opposed to interval) measures and are not normally distributed, we employed nonparametric mixed effects models to compare the ranks of change-from-baseline scores measured at the end of the active drug trial vs. those measured at the end of the placebo phase for each participant, which accounts for within-woman correlation among repeated measures; p-values were adjusted for analysis of variance-type statistics (ATS) (Brunner et al. 2002). All results reported are from models containing the baseline outcome measure as a covariate, as well as a binary indicator of treatment sequence. The latter is included to ensure that the “washout” period between trials was sufficient to prevent any carryover effects from an initial active drug trial into a subsequent placebo trial. Except as noted, this treatment sequence indicator was not statistically significant.
In order to test whether baseline levels of EF difficulties had a moderating effect on treatment, we utilized a median split for the BADDS total score and each of the five subscales in models with interaction terms. For consistency, we used the same type of nonparametric mixed effects models comparing ranks of change-from-baseline levels to investigate the drug treatment effect on participants’ vital signs (systolic and diastolic blood pressure, heart rate, and weight), as well as in those models testing for interactions with mood or sleep problem indicators.
In addition to the above analysis, we determined the relative impact of LDX versus placebo treatment on responder status, with response being defined as having at least 50 % reduction in total BADDS score from baseline to the end of a treatment arm. We used this binary outcome in a repeated-measures logistic regression, again controlling for treatment sequence.
We tested the primary outcomes of BADDS total score and subscales using both an intent-to-treat (ITT) analysis (N=35) and a completers-only analysis (N=32), with completers defined as those women for whom we have BADDS results at baseline and at the end of both trials. Since the results of these two analyses were nearly identical, we conducted the remaining analyses using only the 32 completers (refer to Supplemental Materials for other ITT analyses). Study data were stored and managed using REDCap electronic data capture tools (Harris et al. 2009) hosted at the University of Pennsylvania. All statistical analyses were conducted using SAS version 9.3, with two-sided p-values <0.05 considered as statistically significant.
Results
Participants
Of the 55 subjects who were consented for screening, 20 (36.4 %) were excluded for the following reasons: BADDS score <20 (n=3), abnormal electrocardiogram (n=1), exclusionary psychiatric or substance use disorder (n=4), FSH<20 pg/ml (n=6), decided not to participate (n=4), medical contraindication to psychostimulant treatment (n=1), and other (n=1). Of the remaining 35 subjects, 32 completed both active and placebo treatment trials. Two participants (both on LDX) dropped out, one due to reported jitteriness and the other secondary to a personal issue, while one participant (on placebo) dropped out citing time constraints (CONSORT figure; Online Resource 1). Baseline characteristics for the 32 women who completed both active LDX and placebo conditions are depicted in Table 1. In summary, mean (standard deviation, SD) age and number of months since the FMP were 53.1 (2.8) years and 24.8 (19.7) months, respectively, at study enrollment. Respectively, mean (SD) FSH and estradiol levels at baseline were 80.0 (29.7) mIU/ml (range 27–152) and 25.7 (14.2) pg/ml, consistent with peri- or postmenopausal status. Only three women reported having ever used hormone therapy in the peri- or postmenopause. The majority (68.8 %) were Caucasian, presently married (62.5 %), and highly educated, with 34.4 % having a graduate or professional degree. Final LDX and placebo dose was 60 mg or three pills per day for all participants excepting two, one who remained at 20 mg/day and another who remained at 40 mg/day of LDX due to side effects of feeling jittery/activated.
Table 1.
Participant characteristics
| Mean (SD) or number (%) | |
|---|---|
| Age (years) | 53.1 (2.8) |
| Time since last menstrual period (months) | 24.8 (19.7) |
| Intelligence quotient | 113.3 (10.9) |
| Mini-mental state examination | 28.9 (1.2) |
| Marital status | |
| Single | 6 (18.8) |
| Married | 20 (62.5) |
| Divorced/separated | 4 (12.5) |
| Widowed | 1 (3.1) |
| Did not disclose | 1 (3.1) |
| Menopause status | |
| Perimenopause | 9 (28.1) |
| Postmenopause | 23 (91.9) |
| Race | |
| Caucasian | 22 (68.8) |
| African American | 7 (21.9) |
| American Indian/Alaska native | 1 (3.1) |
| Others | 2 (6.3) |
| Ethnicity | |
| Hispanic | 2 (6.2) |
| Non-Hispanic | 30 (93.8) |
| Education | |
| High school diploma | 2 (6.2) |
| Some college/vocational | 5 (15.6) |
| College graduate/some graduate school | 14 (43.8) |
| Graduate/professional degree | 11 (34.4) |
| Household income | |
| Unknown or preferred not to disclose | 7 (21.9) |
| < $50,000 | 4 (12.5) |
| $50,000 to $100,000 | 6 (18.8) |
| $100,000 to $200,000 | 12 (37.5) |
| > $200,0000 | 3 (9.3) |
Executive function
Mean baseline total BADDS score was 35.7 (16.8) with 37.5 % scoring above 40 and 5.5 % scoring above 55. There was a significant effect of LDX treatment on total BADDS scores, with participants having a mean total score of 21.2 (16.8) while on the active drug versus a mean of 29.8 (17.7) while on placebo (Table 2). Change-from-baseline BADDS scores with active drug were significantly lower than change-from-baseline with placebo (numerator degrees of freedom [num df]=1, ATS=15.42, p<0.001) (Fig. 1). Similarly, four out of the five BADDS subscales also showed significant treatment effects (all p<0.004) (Fig. 2). In addition, women were significantly more likely to respond to the active treatment than to the placebo, with responders defined as those who had at least 50 % reduction from baseline in their total BADDS score (O.R.=7.36 (2.46–22.07), p=0.0004.)
Table 2.
Assessments at baseline and after each treatment arm
| Baseline mean (SD) | Activea mean (SD) | Placebo mean (SD) | |
|---|---|---|---|
| Brown Attention Deficit Disorder Scale (total) | 35.7 (16.8) | 21.2 (16.8)a | 29.8 (17.7) |
| Subscales | |||
| Organization and motivation for work | 8.4 (5.3) | 5.2 (5.4)a | 6.8 (5.6) |
| Attention and concentration | 10.8 (5.5) | 5.6 (4.6)a | 8.6 (5.5) |
| Alertness, effort and processing speed | 5.5 (4.3) | 3.3 (3.9)a | 4.8 (4.3) |
| Managing affective interference | 3.9 (2.9) | 2.8 (2.7) | 3.6 (3.6) |
| Working memory and accessing recall | 7.1 (3.7) | 4.3 (3.5)a | 6.1 (3.5) |
| Neurocognitive measures | |||
| Immediate paragraph recall A | 7.09 (2.56) | 7.48 (2.63) | 7.00 (2.70) |
| Immediate paragraph recall B | 6.38 (2.12) | 7.45 (3.20) | 6.75 (2.53) |
| Delayed paragraph recall A | 4.68 (2.65) | 5.90 (3.38)a | 4.94 (3.02) |
| Delayed paragraph recall B | 7.81 (3.58) | 9.77 (3.53)a | 8.47 (2.97) |
| Continuous performance task (CPT; # correct) | 53.29 (3.78) | 56.37 (3.59) | 56.21 (3.27) |
| CPT (reaction time correct responses; ms) | 503.23 (50.21) | 495.02 (48.71) | 497.75 (50.15) |
| N-back: 0-back # correct | 14.07 (2.91) | 14.68 (0.79) | 14.45 (1.18) |
| N-back: 0-back reaction time (ms) | 540.34 (97.32) | 523.40 (81.93) | 524.13 (95.89) |
| N-back: 2-back # correct | 9.79 (3.17) | 9.94 (3.39) | 10.35 (3.60) |
| N-back: 2-back reaction time (ms) | 734.29 (169.88) | 694.29 (141.30) | 672.87 (136.14) |
| N-back: 3-back # correct | 9.21 (2.53) | 9.81 (3.09) | 9.19 (3.33) |
| N-back: 3-back reaction time (ms) | 688.31 (148.07) | 703.84 (170.78) | 702.23 (150.83) |
| Behavioral measures | |||
| Pittsburgh sleep quality inventory | 6.9 (3.5) | 5.4 (2.5) | 6.0 (3.4) |
| Beck depression inventory | 6.8 (5.1) | 5.1 (4.4) | 5.9 (6.7) |
| Beck anxiety inventory | 7.7 (5.5) | 7.8 (6.1) | 6.9 (6.0) |
Significant active treatment effect (all p<0.05)
Fig. 1.
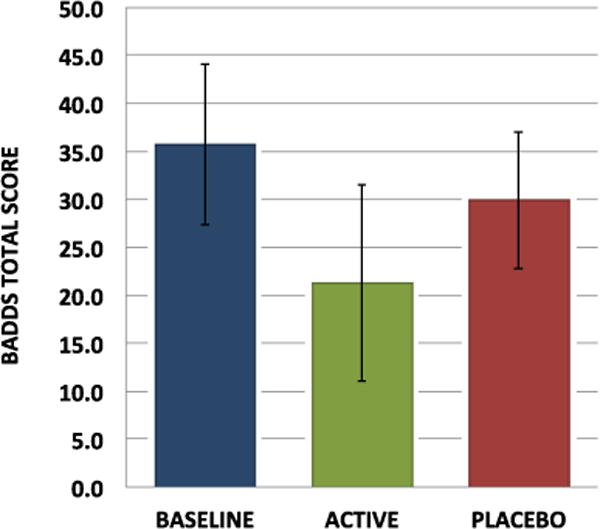
BADDS scores across the study. Non-parametric mixed models to compare ranks of change-from-baseline scores indicate a significant effect of active treatment on total BADDS scores (numerator degrees of freedom (num df=1, ATS= 15.42, p<0.001). Bars represent standard deviations
Fig. 2.
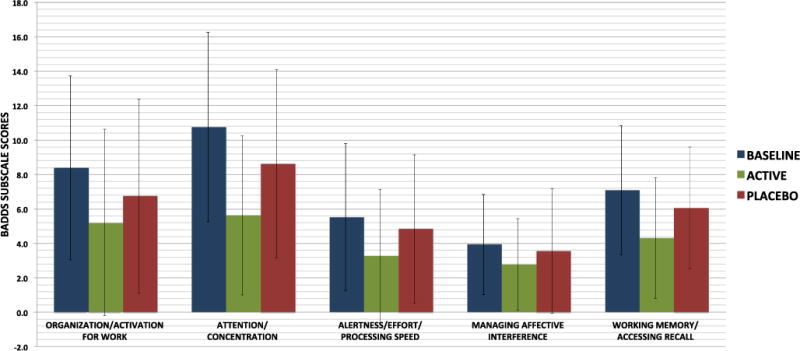
Lisdexamfetamine treatment effects on five domains of executive function as assessed by the BADDS subscale scores. Change from baseline was greater with LDX versus placebo for all BADDS subscales except for managing affective interference (p=0.11). For organization and activation, num df=1, ATS=8.69, p=0.003; for attention and concentration, num df=1, ATS=11.46, p<0.001; for alertness/effort/processing speed, num df=1, ATS=10.89, p=0.001; for working memory/accessing recall, num df=1, ATS=10.89, p=0.001. Bars represent standard deviations
Neither the total BADDS score nor any of the five subscales showed a statistically significant effect of the treatment sequence; this implies that the washout period between trials was sufficient to prevent any carryover effects. In all cases except for the organization/activation for work subscale, the main effect of baseline BADDS score was significant when included in the model, and in all cases the beta-estimate for this baseline parameter was negative, meaning that, on average, those with more severe symptoms at baseline showed more improvement from baseline during the trial periods. When we tested for an interaction of baseline severity of BADDS symptoms with the LDX treatment effect, the interaction term was only significant in the attention/concentration subscale model. That is, the LDX treatment was significantly associated with improvement in this domain only for those women whose baseline scores were above the median of 10, indicating more problems with attention and concentration at baseline (interaction num df=1, ATS=7.04, p=0.008). There were no significant interactions between participant characteristics such as age, education, race, ever use of oral contraceptives or hormone therapy, and treatment effect on BADDS scores.
Cognitive performance
The impact of LDX treatment on performance on the delayed aspect of the NYU Paragraph Recall Task (Fig. 3) was significant for both paragraph A (num df=1, ATS=4.07, p=0.044) and paragraph B (num df=1, ATS=5.59, p=0.018), but there was no impact of treatment on the immediate recall tasks. Delayed recall of paragraphs was the only neuropsychological test administered in this study, which showed any statistically significant differences in performance with LDX treatment vs. placebo.
Fig. 3.
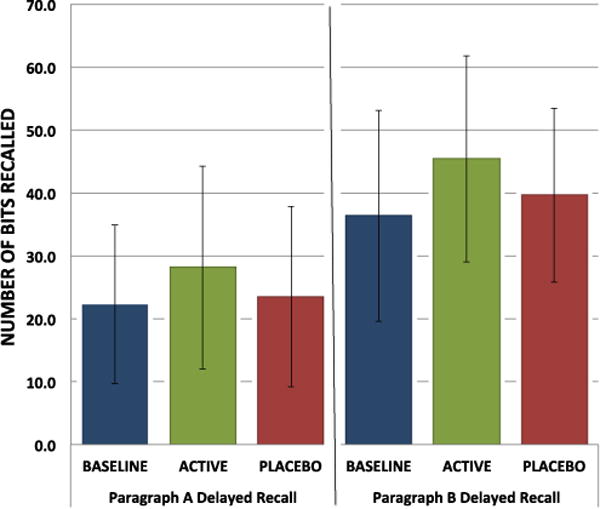
Delayed verbal recall at baseline and at the end of each treatment arm. Means and standard deviations for the NYU Paragraph Recall Task (delayed). Non-parametric mixed models to compare ranks of change-from-baseline scores indicate a significant effect of active treatment on performance on paragraph A (num df=1, ATS=4.07, p=0.44) and paragraph B (num df=1, ATS=5.59, p=0.018). Bars represent standard deviations. Bars represent standard deviations
Depression and anxiety
Consistent with enrollment criteria, at screening, depression and anxiety levels were within the asymptomatic range and remained so on average across the study, as measured by BAI and BDI following each 4-week treatment phase (Table 2).
Sleep quality
Baseline scores on the PSQI ranged from 2 to 13, with a mean of 6.9 (3.5) and numerically, but not statistically significant, lower scores during LDX 5.4 (2.5) than placebo 6.0 (3.4) trials (p=0.183) (Table 2). However, when we tested a model including the interaction of treatment and baseline score, the interaction term was significant (num df=1, ATS=12.62, p<0.001). Specifically, those women who had baseline PSQI scores above the median level of 6 showed significantly more improvement in sleep quality with the LDX treatment than with placebo (Supplemental Materials).
Safety measures
There was a significant treatment effect of LDX vs. placebo on systolic blood pressure (SBP) (num df=1, ATS=5.66, p=0.017) and heart rate (HR) (num df=1, ATS=7.60, p=0.006), with both increasing from baseline to the end of LDX treatment. The mean increase in SBP was 2.9 (14.7) for patients during the LDX phase versus a decrease of 2.2 (15.7) while on placebo. For heart rate, the mean increase was 4.2 (12.0) for patients during the LDX phase versus a decrease of 1.5 (10.0) while on placebo. The sequence of treatment had no clinically significant effect on blood pressure or heart rate.
Women lost an average of 3.2 (4.7) pounds during the active phase vs. a decrease of 1.0 (4.5) pounds during the placebo phase (num df=1, ATS=10.51, p=0.001). Here, we did observe a significant effect of treatment sequence (num df=1, ATS=6.96, p=0.008), with women who were randomized to the active drug first losing an average of 7.9 (8.1) pounds across both trials vs. only 0.8 (7.1) pounds across both trials for women who received the placebo treatment in the first trial (data not shown).
Discussion
To our knowledge, this is the first study to examine the impact of a psychostimulant on EF difficulties reported to have emerged de novo during the natural transition to menopause. Women experienced significantly greater improvement during the LDX versus placebo arms of the study with respect to overall EF difficulties as assessed by the BADDS and with respect to organization/activation, attention/concentration, alertness/effort/processing speed, and working memory/recall in specific. The treatment effect failed to reach statistical significance for the subscale focusing on affective interference, a domain that is receiving considerable attention in adolescent and adult ADHD research as a predictor of functional impairment and psychopathology (Brown 2014). While a common problem among individuals with ADHD, our study cohort scored the cluster of symptoms related to managing affective interference as the lowest in severity of all the symptom clusters. Whether relatively intact management of affective interference in the context of other EF difficulties is a phenomenological distinction between women with mid-life-onset EF difficulties and adults with ADHD or is secondary to our exclusion of women with frank mood disorders is not known. Improvement in attention/concentration was significant only in those women who experienced the more severe symptoms in this domain at baseline, suggesting that LDX treatment may be useful in women with complaints of attention and concentration difficulties in particular. Interestingly, the domain that is most consistent with our participants’ original complaints during screening is the subscale focusing on working memory and accessing recall. This subscale is comprised of the fewest questions and practically tied for mean score per question with the subscale focusing on attention/concentration (1.18 compared to 1.19), suggesting that this instrument has ecological validity in capturing the subjective cognitive experience of this population.
With respect to performance on neurocognitive measures, the benefit of LDX treatment was limited to the verbal memory domain, specifically the more challenging delayed recall task. Interestingly, our group (Epperson et al. 2013) recently reported that the natural menopause transition exerts an age-independent, detrimental effect on both immediate and delayed verbal memory with significance noted in delayed recall relatively early in the menopause transition when women are experiencing initial changes in their menstrual cycle length. Verbal memory declines have been documented with both natural (Epperson et al. 2013) and surgical menopause (Sherwin 2005) and may be particularly amenable to improvement if addressed early in the menopause transition. Similarly, a previous study (Epperson et al. 2011) demonstrated that atomoxetine, a non-stimulant medication FDA-approved for the treatment of ADHD (Asherson et al. 2014) and studied with variable success in other disorders (Beglinger et al. 2009; Marsh et al. 2009; Stahl 2003), significantly improved subjective EFs and performance on the verbal paired associates memory task in a similar sample of menopausal women.
While these findings are compelling, there are several aspects of the study design that limit our ability to generalize these data to all women with cognitive complaints during the menopause transition. Probably the most obvious concern is that women may have been able to identify whether they were being treated with LDX or placebo, contributing to the overall efficacy of LDX over placebo. We believe that the relative tolerability of the LDX even at 60 mg/day aided us in maintaining the blind. Likewise, there was no order effect for our primary behavioral outcomes, suggesting that women did not adjust their report of EF symptoms based upon knowing when they were on active medication. For our participants, the menopause occurred naturally around the average age of menopause of 52. Whether LDX would be beneficial in women who are undergoing a premature and/or abrupt menopause is not known but of interest as these women are at even greater risk of cognitive decline, particularly if ET is not used (Ryan et al. 2014).
Another limitation is that we could not fully confirm that women enrolled in this study were without EF difficulties prior to moving into the menopause transition. However, the fact that all participants were seeking treatment for their cognitive difficulties for the first time and they had functioned well in their professions for their entire adult life diminishes the likelihood that significant EF difficulties were present prior to menopause. Our careful clinical interview regarding earlier history of inattentiveness, hyperactivity, or academic difficulties would have identified women with possible childhood ADHD. Whether menopausal women who present with new-onset EF difficulties are phenotypically distinct or have a distinct response to LDX compared to age-matched individuals seeking treatment for the first time for ADHD cannot be determined without the ADHD comparison group. To our knowledge, there are no studies indicating the severity of EF difficulties among non-treatment-seeking menopausal women. We chose a cutoff of 20 on the BADDS for study enrollment based upon our previous experience with menopausal women who participated in our RCT of atomoxetine in the treatment of new-onset EF difficulties. This degree of severity is considerably lower than that reported by adults with ADHD (Brown et al. 2009) but appears to be consistent with subjective distress among women for whom these types of symptoms represent a change from their lifetime norm.
Finally, mean immediate paragraph recall scores at baseline were similar (7.1 bits of information) with the mean scores (7.6 bits of information) from a normative sample of older individuals with an above average IQ (Mathews et al. 2013). This later observation diminishes the possibility that participants in the present study were experiencing early signs of pathological cognitive decline. The robust response to LDX observed in this group of menopausal women is further reflective of their overall cognitive health as psychostimulants have not been shown to significantly improve cognition among individuals with dementia (Dolder et al. 2010).
Although anxiety and depression are common among menopausal women, those with a current affective disorder were excluded from participation. One of the most common reasons women did not meet the study criteria was the use of psychotropic medications, particularly antidepressants. Hence, the impact of depression, anxiety, or antidepressant use on outcomes of interest could not be determined but is worthy of consideration in future research as psychostimulants have been shown to impact anxiety and depression. Finally, the educational status of the participants in this study was high and therefore not representative of the general population. Not surprisingly, the participants performed well on all cognitive measures at baseline, making it difficult to detect an objective benefit of LDX on cognitive tasks performance. Based upon their professional status, women presenting for this study are typically functioning at the highest cognitive levels. As cognitive load increases, so do demands on the prefrontal cortex, perhaps making these women particularly vulnerable to EF difficulties or at least more likely to notice even relatively small changes in EFs (Rypma et al. 2002). Mid-life-onset cognitive difficulties experienced by some women during and after menopause are not insignificant and contribute to needless concerns regarding early-onset dementia (Brown 2014). Such concerns can be a source of considerable stress and may interfere with multiple aspects of occupational and social role functioning.
In summary, these data provide the first evidence that a psychostimulant is well tolerated and improves EFs in healthy menopausal women who report an unprecedented subjective decline in EFs. Further research is needed to ascertain whether psychostimulants other than LDX are helpful and well tolerated in this population and whether such treatments may be beneficial for women who experience EF difficulties after surgery- or chemotherapy-induced menopause. While psychostimulants enhance dopaminergic and noradrenergic function in the PFC, the mechanism by which LDX was beneficial in this sample of mid-life women is not known but may be investigated, albeit indirectly, using neuroimaging techniques. Additional research could also be helpful to determine whether particular domains of EF such as attention/concentration or other phenotypic characteristics are predictive of overall LDX treatment response among menopausal women and distinguish them from adults with ADHD. Although the tolerability and safety profile of short-term LDX treatment was observed in this study, long-term studies of menopausal women receiving LDX would be required to confirm a positive risk–benefit profile like that observed for the drug among adults with ADHD (Maneeton et al. 2014). Moreover, it is important for clinicians to confirm that a woman’s complaints of worsening memory are temporally related to the menopause transition and are not the harbinger of some other pathological cognitive impairment before prescribing a trial of LDX.
Supplementary Material
Acknowledgments
Funding and disclosures This project was funded in part by Shire Pharmaceuticals through an Investigator-Initiated Grant, the National Institute of Mental Health (P50 MH099910; CNE and DRK), the National Institute on Aging (R01 AG048839; CNE), and the National Institute on Drug Abuse (R01 DA030301; CNE). Dr. Epperson discloses research grant support from Shire and Novartis and stock holdings in Pfizer, Johnson and Johnson, Abbott, Abbvie and Merck. Dr. Brown discloses research grant support from Lilly and Shire and publication royalties from PsychCorp/Pearson, Yale University Press, Routledge, and Wiley. Dr. Sammel and Dr. Kim and Ms. Iannelli, Appleby, Bradley, Czarkowski and Shanmugan report no competing interests. As the lead author, Dr. Epperson can attest that the authors have had full control of all primary data and the journal Psychopharmacology may have access to the data if necessary.
Footnotes
Author’s contributions As the corresponding author, CNE confirms that all authors have contributed to conceptualizing the study design, conducting some or part of the study, analyzing and interpreting findings, and preparing the manuscript.
Electronic supplementary material The online version of this article (doi:10.1007/s00213-015-3953-7) contains supplementary material, which is available to authorized users.
References
- Arnsten AF. The emerging neurobiology of attention deficit hyperactivity disorder: the key role of the prefrontal association cortex. J Pediatr. 2009;154:I–S43. doi: 10.1016/j.jpeds.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherson P, Bushe C, Saylor K, Tanaka Y, Deberdt W, Upadhyaya H. Efficacy of atomoxetine in adults with attention deficit hyperactivity disorder: an integrated analysis of the complete database of multicenter placebo-controlled trials. J Psychopharmacol. 2014;28:837–846. doi: 10.1177/0269881114542453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR. Impairment in occupational functioning and adult ADHD: the predictive utility of executive function (EF) ratings versus EF tests. Arch Clin Neuropsychol. 2010;25:157–173. doi: 10.1093/arclin/acq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrouillet P, Bernardin S, Portrat S, Vergauwe E, Camos V. Time and cognitive load in working memory. J Exp Psychol Learn Mem Cogn. 2007;33:570–585. doi: 10.1037/0278-7393.33.3.570. [DOI] [PubMed] [Google Scholar]
- Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beglinger LJ, Adams WH, Paulson H, Fiedorowicz JG, Langbehn DR, Duff K, Leserman A, Paulsen JS. Randomized controlled trial of atomoxetine for cognitive dysfunction in early Huntington disease. J Clin Psychopharmacol. 2009;29:484–487. doi: 10.1097/JCP.0b013e3181b2ac0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE. Smart but stuck: emotions in teens and adults with ADHD. Jossey-Bass; San Francisco: 2014. [Google Scholar]
- Brown TE, Landgraf JM. Improvements in executive function correlate with enhanced performance and functioning and health-related quality of life: evidence from 2 large, double-blind, randomized, placebo-controlled trials in ADHD. Postgrad Med. 2010;122:42–51. doi: 10.3810/pgm.2010.09.2200. [DOI] [PubMed] [Google Scholar]
- Brown RT, Perrin JM. Measuring outcomes in attention-deficit/hyperactivity disorder. J Pediatr Psychol. 2007;32:627–630. doi: 10.1093/jpepsy/jsm001. [DOI] [PubMed] [Google Scholar]
- Brown TE, Reichel PC, Quinlan DM. Executive function impairments in high IQ adults with ADHD. J Atten Disord. 2009;13:161–167. doi: 10.1177/1087054708326113. [DOI] [PubMed] [Google Scholar]
- Brown TE, Brams M, Gao J, Gasior M, Childress A. Open-label administration of lisdexamfetamine dimesylate improves executive function impairments and symptoms of attention-deficit/hyperactivity disorder in adults. Postgrad Med. 2010;122:7–17. doi: 10.3810/pgm.2010.09.2196. [DOI] [PubMed] [Google Scholar]
- Brown TE, Brams M, Gasior M, Adeyi B, Babcock T, Dirks B, Scheckner B, Wigal T. Clinical utility of ADHD symptom thresholds to assess normalization of executive function with lisdexamfetamine dimesylate treatment in adults. Curr Med Res Opin. 2011;27(Suppl 2):23–33. doi: 10.1185/03007995.2011.605441. [DOI] [PubMed] [Google Scholar]
- Brunner E, Domhof S, Langer F. Nonparametric analysis of longitudinal data in factorial experiments. Wiley; New York: 2002. [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Dolder CR, Davis LN, McKinsey J. Differential regulation of psychostimulant-induced gene expression of brain derived neurotrophic factor and the immediate-early gene Arc in the juvenile and adult brain. Ann Pharmacother. 2010;44(10):1624–32. [Google Scholar]
- Epperson CN, Pittman B, Czarkowski KA, Bradley J, Quinlan DM, Brown TE. Impact of atomoxetine on subjective attention and memory difficulties in perimenopausal and postmenopausal women. Menopause. 2011;18:542–548. doi: 10.1097/gme.0b013e3181fcafd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab. 2013;98:3829–3838. doi: 10.1210/jc.2013-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorders—patient edition SCID-I/P 1995 [Google Scholar]
- Gehring K, Patwardhan SY, Collins R, Groves MD, Etzel CJ, Meyers CA, Wefel JS. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J Neurooncol. 2012;107:165–174. doi: 10.1007/s11060-011-0723-1. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Derby CA, Maki PM. Differential regulation of psychostimulant-induced gene expression of brain derived neurotrophic factor and the immediate-early gene Arc in the juvenile and adult brain. Obstet Gynecol Clin North Am. 2011;38(3):519–35. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Block I, Gold MA, Markwell S, Zupancic M. Effect of methylphenidate on fatigue in women with recurrent gynecologic cancer. Psychooncology. 2010;19:955–958. doi: 10.1002/pon.1646. [DOI] [PubMed] [Google Scholar]
- Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol. 1999;12:168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Ragland JD, Bilker W, Gur RC, Gur RE. Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res. 2001;48:307–316. doi: 10.1016/s0920-9964(00)00060-8. [DOI] [PubMed] [Google Scholar]
- Luetters C, Huang MH, Seeman T, Buckwalter G, Meyer PM, Avis NE, Sternfeld B, Johnston JM, Greendale GA. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the Study of Women’s Health Across the Nation (SWAN) J Womens Health (Larchmt) 2007;16(3):331–44. doi: 10.1089/jwh.2006.0057. [DOI] [PubMed] [Google Scholar]
- Maki PM, Freeman EW, Greendale GA, Henderson VW, Newhouse PA, Schmidt PJ, Scott NF, Shively CA, Soares CN. Summary of the national institute on aging-sponsored conference on depressive symptoms and cognitive complaints in the menopausal transition. Menopause. 2010;17:815–822. doi: 10.1097/gme.0b013e3181d763d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneeton N, Maneeton B, Suttajit S, Reungyos J, Srisurapanont M, Martin SD. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685–93. doi: 10.2147/DDDT.S68393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L, Biglan K, Gerstenhaber M, Williams JR. Atomoxetine for the treatment of executive dysfunction in Parkinson’s disease: a pilot open-label study. Mov Disord. 2009;24:277–282. doi: 10.1002/mds.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M, Abner E, Caban-Holt A, Dennis BC, Kryscio R, Schmitt F. Characteristic neurocognitive profile associated with adult attention-deficit/hyperactivity disorder. Curr Alzheimer Res. 2013;10(7):776–83. doi: 10.2174/15672050113109990140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Manson JE, Resnick SM, Bryan NR, Smoller S, Coker LH, Phillips LS, Stefanick ML, Sarto GE, Women’s Health Initiative Memory Study Educational attainment, MRI changes, and cognitive function in older postmenopausal women from the Women’s Health Initiative Memory Study. Int J Psychiatry Med. 2013;46:121–143. doi: 10.2190/PM.46.2.a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, Scali J, Carriere I, Amieva H, Rouaud O, Berr C, Ritchie K, Ancelin ML. Impact of a premature menopause on cognitive function in later life. BJOG. 2014 doi: 10.1111/1471-0528.12828. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Sandra Kooij JJ, Marije Boonstra A, Swinkels SH, Bekker EM, de Noord I, Buitelaar JK. Reliability, validity, and utility of instruments for self-report and informant report concerning symptoms of ADHD in adult patients. J Atten Disord. 2008;11:445–458. doi: 10.1177/1087054707299367. [DOI] [PubMed] [Google Scholar]
- Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35:847–865. doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann N YAcad Sci. 2005;1052:3–10. doi: 10.1196/annals.1347.001. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Neurotransmission of cognition, part 2. Selective NRIs are smart drugs: exploiting regionally selective actions on both dopamine and norepinephrine to enhance cognition. J Clin Psychiatry. 2003;64:110–111. doi: 10.4088/jcp.v64n0201. [DOI] [PubMed] [Google Scholar]
- Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: a systematic review and meta-analysis. J Steroid Biochem Mol Biol. 2014;142:90–98. doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegesin DJ, Stern Y. Effects of hormone replacement therapy and aging on cognition: evidence for executive dysfunction. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:301–328. doi: 10.1080/13825580600802893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


