Introduction
The National Birth Defects Prevention Network (NBDPN) published the first Congenital Malformations Surveillance Report in 1997 and has annually released a report since 2000 that contains state-specific population-based data on major birth defects and a directory describing data collection information for population-based birth defects surveillance programs in the United States. The birth defects in these reports have included conditions affecting major organs of the central nervous, eye, ear, cardiovascular, orofacial, gastrointestinal, genitourinary, and musculoskeletal systems, as well as other disorders, including trisomies and amniotic band sequence.
In 2014, the NBDPN released an updated list of major birth defects as part of its national standards development for birth defects surveillance. The criteria used to guide deliberations for inclusion on the reportable list were: (1) public health importance; (2) accuracy of diagnosis; (3) amenable to prevention/intervention; (4) state of knowledge; (5) structural malformations, diagnosed within the first year of life; and (6) ability to separate into syndromic/nonsyndromic. For example, the NBDPN list now includes all 12 critical congenital heart defects (CCHDs) that are primary and secondary targets of pulse oximetry screening as a result of the addition of CCHD to the U.S. Recommended Universal Screening Panel for newborns (Mahle et al., 2012). Other noncardiac conditions that were added include clubfoot, cloacal exstrophy, craniosynostosis, deletion 22q11.2, holoprosencephaly, small intestinal atresia/stenosis, and Turner syndrome. These additions were balanced with the removal of several conditions, including: amniotic bands, aniridia, congenital hip dislocation, epispadias, fetus or newborn affected by maternal alcohol use, Hirschsprung disease (congenital megacolon), hydrocephalus, microcephalus, patent ductus arterious, and pyloric stenosis. Additional modifications to the list resulted in the regrouping of some conditions. Upper and lower limb deficiencies were collapsed into all limb deficiencies, while cleft lip with or without cleft palate was separated into cleft lip alone and cleft lip with cleft palate. Finally, obstructive genitourinary defect was limited to just the reporting of congenital posterior urethral valves. Table 1 presents the new reported list of birth defects and their diagnostic codes (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM]; and Centers for Disease Control and Prevention/British Pediatric Association Classification of Diseases [CDC/BPA]).
TABLE 1.
Disease Classification Codes for Major Birth Defects Included in the 2014 NBDPN Annual Report
| Birth defects | ICD-9-CM codes | CDC/BPA codes |
|---|---|---|
| Central nervous system | ||
| Anencephaly | 740.0 – 740.1 | 740.00 – 740.10 |
| Spina bifida without anencephaly | 741.0, 741.9 w/o 740.0 – 740.1 | 741.00 – 741.99 w/o 740.00 – 740.10 |
| Encephalocele | 742.0 | 742.00 – 742.09 |
| Holoprosencephaly | 742.2 | 742.26 |
| Eye | ||
| Anophthalmia/microphthalmia | 743.0, 743.1 | 743.00 – 743.10 |
| Congenital cataract | 743.30 – 743.34 | 743.32 |
| Ear | ||
| Anotia/microtia | 744.01, 744.23 | 744.01, 744.21 |
| Cardiovascular | ||
| Common truncus (truncus arteriosus) | 745.0 | 745.00 (excluding 745.01) |
| Transposition of the great arteries (TGA) | 745.10, .12, .19 | 745.10 – 745.12, 745.18 – 745.19 |
| dextro-Transposition of great arteries (d-TGA) – for CCHD screeninga | 745.10 | 745.10, 745.11,745.19 |
| Tetralogy of Fallot | 745.2 | 745.20 – 745.21, 747.31 |
| Ventricular septal defect | 745.4 | 745.40 – 745.49 (excluding 745.487, 745.498) |
| Atrial septal defect | 745.5 | 745.51 – 745.59 |
| Atrioventricular septal defect (endocardial cushion defect) | 745.60, .61, .69 | 745.60 – 745.69, 745.487 |
| Pulmonary valve atresia and stenosis | 746.01, 746.02 | 746.00, 746.01 |
| Pulmonary valve atresia – for CCHD screeninga | 746.01 | 746.00 |
| Tricuspid valve atresia and stenosis | 746.1 | 746.100, 746.106 (excluding 746.105) |
| Tricuspid valve atresia– for CCHD screeninga | 746.1 | 746.100 |
| Ebstein anomaly | 746.2 | 746.20 |
| Aortic valve stenosis | 746.3 | 746.30 |
| Hypoplastic left heart syndrome | 746.7 | 746.70 |
| Coarctation of aorta | 747.10 | 747.10 – 747.19 |
| Total anomalous pulmonary venous connection | 747.41 | 747.42 |
| Single ventricle | 745.3 | 745.3 |
| Interrupted aortic arch | 747.11 | 747.215 – 747.217 |
| Double outlet right ventricle | 745.11 | 745.13 – 745.15 |
| Orofacial | ||
| Cleft palate alone (without cleft lip) | 749.0 | 749.00 – 749.09 |
| Cleft lip alone (without cleft palate) | 749.1 | 749.10 – 749.19 |
| Cleft lip with cleft palate | 749.20–749.25 | 749.20 – 749.29 |
| Choanal atresia | 748.0 | 748.00 |
| Gastrointestinal | ||
| Esophageal atresia/tracheoesophageal fistula | 750.3 | 750.30 – 750.35 |
| Rectal and large intestinal atresia/stenosis | 751.2 | 751.20 – 751.24 |
| Biliary atresia | 751.61 | 751.65 |
| Small intestinal atresia/stenosis | 751.1 | 751.10 – 751.19 |
| Genitourinary | ||
| Renal agenesis/hypoplasia | 753.0 | 753.00 – 753.01 |
| Bladder exstrophy | 753.5 | 753.50 |
| Hypospadias | 752.61 | 752.60 – 752.62(excluding 752.61 and 752.621) |
| Congenital posterior urethral valves | 753.6 | 753.60 |
| Cloacal exstrophy | 751.5 | 751.555 |
| Musculoskeletal | ||
| Gastroschisis | 756.73 (as of 10/1/09) | 756.71 |
| Omphalocele | 756.72 (as of 10/1/09) | 756.70 |
| Diaphragmatic hernia | 756.6 | 756.610 – 756.617 |
| Limb deficiencies (reduction defects) | 755.2 – 755.4 | 755.20 – 755.49 |
| Craniosynostosis | No specific code | 756.00 – 756.03 |
| Clubfoot | 754.51, 754.70 | 754.50, 754.73(excluding 754.735) |
| Chromosomal | ||
| Trisomy 13 | 758.1 | 758.10 – 758.19 |
| Trisomy 21 (Down syndrome) | 758.0 | 758.00 – 758.09 |
| Trisomy 18 | 758.2 | 758.20 – 758.29 |
| Turner syndrome | 758.6 | 758.60 – 758.69 |
| Deletion 22q11.2 | 758.32 | 758.37 |
ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; CDC/BPA, Centers for Disease Control and Prevention/British Pediatric Association Classification of Diseases; NBDPN, National Birth Defects Prevention Network; w/o, without; CCHD, critical congenital heart defect.
The primary targets for CCHD screening include 7 conditions: hypoplastic left heart syndrome, pulmonary atresia with intact septum, tetralogy of Fallot, total anomalous pulmonary venous connection, dextro-transposition of great arteries (d-TGA), tricuspid atresia, and truncus arteriosus. The NBDPN traditionally monitors all TGA, and both atresia and stenosis for pulmonary and tricuspid valve conditions; however, for CCHD screening reporting purpose, these conditions are also reported as d-TGA, pulmonary valve atresia, and tricuspid valve atresia.
The current report includes state-specific data from 39 population-based birth defects surveillance programs for the updated list of 47 major birth defects, and an accompanying directory describes program data collection status and contacts for state birth defects surveillance activities. In addition, the report highlights orofacial clefts (OFCs) from 29 state programs.
State-specific Data Collection and Presentation of 47 major birth defects
DATA COLLECTION
The NBDPN Data Committee, in collaboration with CDC, issued a call for data to population-based birth defects surveillance programs in April 2014. State programs were provided with a data dictionary and data table creation tools in Excel and SAS. CDC performed data quality checks, and state programs validated their data and approved final data table presentation.
Participating birth defects surveillance programs submitted case counts of the reportable birth defects shown in Table 1 and live births occurring from January 1, 2007 through December 31, 2011. These cases were stratified by U.S. Census maternal racial/ethnic groups: non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian/Pacific Islander, non-Hispanic American Indian/Alaska Native, and other/unknown. Additionally, trisomy conditions (trisomy 21 [Down syndrome], trisomy 13, and trisomy 18) were stratified by six maternal age categories: less than 20 years, 20 to 24 years, 25 to 29 years, 30 to 34 years, 35 to 39 years, and 40+ years.
DATA PRESENTATION
State-specific data from 39 population-based birth defects surveillance programs for 2007 to 2011 are included in the supplemental materials. The data are presented in two tables for each state. The first table shows defect counts and prevalence per 10,000 live births by maternal racial/ethnic categories, and the second table presents counts and prevalence for trisomies by two maternal age categories (less than 35 years, 35+ years). The prevalence is calculated by dividing the number of birth defect cases for any pregnancy outcome by the total number of live births for the reported years and then multiplying by 10,000 (Mason et al., 2005). The denominator used to calculate the prevalence for all birth defects is total live births except for hypospadias, which is calculated using total male live births.
Although the NBDPN provided a data dictionary and attempted to obtain the data in a uniform manner, variability can be expected in the reported birth defects data by state programs, given differences in coding systems used for case inclusion, case-finding methodology, and available data sources. State-specific notes and clarification about the data, such as methodological changes and probable/possible diagnoses, are included in the data tables. Additional information about each state program data collection methodology is available in the accompanying program directory.
Highlighting Orofacial Clefts
In addition to submitting data for the 47 NBDPN reportable birth defects, 29 state programs submitted supplemental data for this feature on orofacial clefts (OFCs). OFC are a phenotypically and etiologically diverse group of malformations that include cleft lip alone, cleft palate alone, and cleft lip with cleft palate, as well as several atypical cleft variations (Watkins et al., 2014). Orofacial clefts are among the most common major structural birth defects. In the United States, approximately 1 in 940 infants are born with cleft lip with or without cleft palate, and approximately 1 in 1574 infants are born with cleft palate (Parker et al., 2010).
Cleft lip alone and cleft lip with cleft palate both involve a bilateral, unilateral, or central defect of the upper lip that is visible in the newborn and often can be detected by prenatal ultrasound. In cleft lip alone, the defect can extend to the nasal floor, while in cleft lip with cleft palate, there also is a malformation of the upper gums (maxillary alveoli) or roof of the mouth (palate) that is often continuous with the separation of the lip. Cleft palate alone involves a hole or separation in the hard palate, soft palate, or the uvula (dangling structure at the rear of the soft palate), without a cleft lip.
Like other types of birth defects, OFCs are often classified by the presence or absence of other major malformations. Nonisolated clefts, which occur more commonly when the palate is involved (Genisca et al., 2009), are defined by the presence of at least one unrelated defect of another organ system or body part that also has surgical, medical, or serious cosmetic consequences (Rasmussen et al., 2003). Without another major birth defect, OFCs are classified as isolated; a third classification category, syndromic, is used in birth defects studies when a single gene or chromosomal etiology has been identified for the cleft. However, this terminology has been applied inconsistently in the literature. Some researchers use the term nonsyndromic when referring to isolated clefts and syndromic to refer to nonisolated clefts, the latter sometimes being subdivided into syndromes of known cause, such as when a single gene disorder or chromosomal anomaly has been diagnosed, and syndromes of unknown cause (or idiopathic syndromic) when the specific etiology is undetermined (Watkins et al., 2014). It is important to note that accurate classification of birth defects often requires review by a clinical geneticist, and few birth defects surveillance programs routinely conduct such reviews on all birth defects, including OFCs. The data presented in this report include both isolated and nonisolated cases combined; therefore, caution should be used when comparing these data with other published reports that may be restricted to only isolated cases.
Children with OFCs typically require extensive multidisciplinary team care, especially during infancy and early childhood, and this care may continue throughout life (ACPA, 2009). Their care includes feeding assistance, counseling, plastic/reconstructive surgery, orthodontics and dental care, otolaryngology, speech and audiology, psychosocial, and developmental follow-up. Depending on the cleft type, children may need different services, and the recommended timing of these services may differ (ACPA, 2009).
Due to the high prevalence of OFCs and health care use and costs associated with their treatment, improving the health of these children is an important public health goal. Disparities in prevalence, risk factors, health service use and access to care among children with OFCs recently were identified as public health research priorities by several convened expert groups sponsored by CDC (Yazdy et al., 2007). Evidence suggests that the three cleft phenotypes differ in etiology (especially for preventable risk factors), recurrence risk, treatment and management, and health service use (Harville et al., 2005; Cassell et al., 2008; ACPA, 2009; Boulet et al., 2009; Weiss et al., 2009).
DATA PRESENTATION OF OROFACIAL CLEFTS
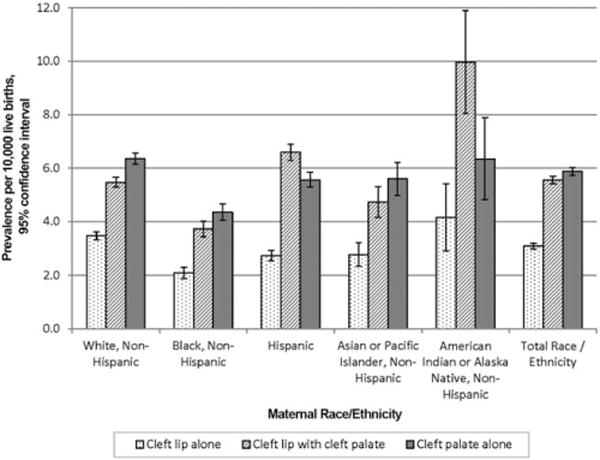
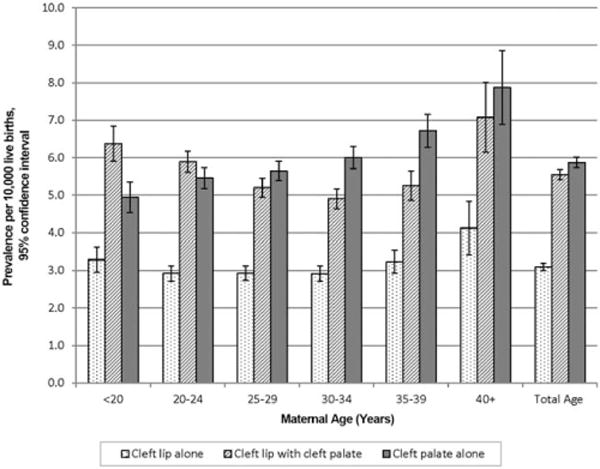
Table 2 presents the counts and prevalence for OFCs from 2007 to 2011 by case-finding methodology and pregnancy outcome from 29 population-based birth defects surveillance programs in the United States. Data are also presented in Table 2 for each phenotype and combined total (cleft lip alone, cleft lip with cleft palate, and cleft palate alone) by maternal race/ethnicity, maternal age, and infant sex. A graphic display of the prevalence of OFCs by maternal race/ethnicity is shown in Figure 1 and by maternal age (years) in Figure 2. Table 3 further stratifies the prevalence of OFCs by presenting a cross-tabulation of each OFC phenotype and combined total by maternal race/ethnicity and maternal age (years).
TABLE 2.
Orofacial Cleft Counts, Prevalence and 95% Confidence Interval for 29 U.S. States, 2007 to 2011 (Prevalence per 10,000 Live Births)a
| Cleft lip alone | Cleft lip with cleft palate | Cleft palate alone | Total | |||||
|---|---|---|---|---|---|---|---|---|
| n | Prevalence (95% CI) | n | Prevalence (95% CI) | n | Prevalence (95% CI) | n | Prevalence (95% CI) | |
| Total Cases | 3,509 | 3.1 (3.0, 3.2) | 6,286 | 5.6 (5.4, 5.7) | 6,651 | 5.9 (5.7, 6.0) | 16,446 | 14.5 (14.3, 14.8) |
| Case-finding methodologyb | ||||||||
| Active case finding | 1,354 | 3.3 (3.1, 3.5) | 2,659 | 6.5 (6.3, 6.7) | 2,495 | 6.1 (5.9, 6.3) | 6,508 | 15.9 (15.5, 16.3) |
| Passive case finding | 2,155 | 3.0 (2.9, 3.1) | 3,627 | 5.0 (4.9, 5.2) | 4,156 | 5.7 (5.6, 5.9) | 9,938 | 13.7 (13.5, 14.0) |
| Pregnancy outcome inclusionc | ||||||||
| Live births only | 916 | 2.7 (2.6, 2.9) | 1,574 | 4.7 (4.5, 4.9) | 1,823 | 5.4 (5.2, 5.7) | 4,313 | 12.9 (12.5, 13.3) |
| Live births and stillbirths | 1,423 | 3.1 (2.9, 3.3) | 2,427 | 5.3 (5.1, 5.5) | 2,587 | 5.6 (5.4, 5.9) | 6,437 | 14.0 (13.7, 14.4) |
| All pregnancy outcomes | 1,170 | 3.5 (3.3, 3.7) | 2,285 | 6.8 (6.5, 7.0) | 2,241 | 6.6 (6.4, 6.9) | 5,696 | 16.8 (16.4, 17.3) |
| Maternal Race/ethnicityd | ||||||||
| White, Non-Hispanic | 2,091 | 3.5 (3.3, 3.6) | 3,292 | 5.5 (5.3, 5.7) | 3,822 | 6.4 (6.2, 6.6) | 9,205 | 15.4 (15.0, 15.7) |
| Black, Non-Hispanic | 381 | 2.1 (1.9, 2.3) | 678 | 3.7 (3.5, 4.0) | 789 | 4.4 (4.1, 4.7) | 1,848 | 10.2 (9.7, 10.7) |
| Hispanic | 746 | 2.8 (2.6, 3.0) | 1,793 | 6.6 (6.3, 6.9) | 1,510 | 5.6 (5.3, 5.9) | 4,049 | 14.9 (14.5, 15.4) |
| Asian or Pacific Islander, | 156 | 2.8 (2.3, 3.2) | 266 | 4.7 (4.2, 5.3) | 315 | 5.6 (5.0, 6.2) | 737 | 13.2 (12.2, 14.1) |
| Non-Hispanic | ||||||||
| American Indian or Alaska Native, Non-Hispanic | 43 | 4.2 (2.9, 5.4) | 104 | 10.1 (8.1, 12.0) | 66 | 6.4 (4.8, 7.9) | 213 | 20.5 (17.7, 23.2) |
| Maternal Age (years)d | ||||||||
| <20 | 371 | 3.3 (3.0, 3.6) | 718 | 6.4 (5.9, 6.9) | 557 | 5.0 (4.5, 5.4) | 1,646 | 14.6 (13.9, 15.3) |
| 20–24 | 807 | 2.9 (2.7, 3.1) | 1,627 | 5.9 (5.6, 6.2) | 1,506 | 5.5 (5.2, 5.7) | 3,940 | 14.3 (13.8, 14.7) |
| 25–29 | 927 | 2.9 (2.7, 3.1) | 1,649 | 5.2 (5.0, 5.5) | 1,790 | 5.7 (5.4, 5.9) | 4,366 | 13.8 (13.4, 14.2) |
| 30–34 | 772 | 2.9 (2.7, 3.1) | 1,297 | 4.9 (4.6, 5.2) | 1,588 | 6.0 (5.7, 6.3) | 3,657 | 13.8 (13.4, 14.3) |
| 35–39 | 426 | 3.2 (2.9, 3.5) | 692 | 5.3 (4.9, 5.7) | 884 | 6.7 (6.3, 7.2) | 2,002 | 15.2 (14.5, 15.9) |
| 40+ | 130 | 4.1 (3.4, 4.8) | 223 | 7.1 (6.2, 8.0) | 250 | 7.9 (7.0, 8.9) | 603 | 19.1 (17.6, 20.7) |
| Infant Sexd | ||||||||
| Male | 2,107 | 3.6 (3.5, 3.8) | 3,810 | 6.6 (6.4, 6.8) | 3,017 | 5.2 (5.0, 5.4) | 8,934 | 15.4 (15.1, 15.7) |
| Female | 1,390 | 2.5 (2.4, 2.6) | 2,453 | 4.4 (4.3, 4.6) | 3,622 | 6.5 (6.3, 6.8) | 7,465 | 13.5 (13.2, 13.8) |
CI: Confidence interval
Contributing states included: AR, AZ, CO, FL, GA, IA, IL, KS, KY, MA, ME, MI, MN, MS, NC, NE, NJ, NV, NY, OK, PR, RI, TN, VA, VT, and WI; LA provided data for 2007–2010 only, OH for 2008 only, and TX for 2007–2009 only.
Program primary case-finding methodology: Active case-finding: AR, AZ, GA, IA, LA, MA, MN, NC, OK, PR, TX; Passive case-finding: CO, FL, IL, KS, KY, ME, MI, MS, NE, NJ, NV, NY, OH, RI, TN, VA, VT, WI
Program case inclusion: Live births only: FL, LA, MN, NJ, NV, NY, VT; Live births and stillbirths: AZ, IL, KS, KY, MA, ME, MI, MS, NE, OH, TN, VA, WI; All pregnancy outcomes: AR, CO, GA, IA, NC, OK, PR, RI, TX
Counts of unknown and/or other are not reported for Maternal Race/ethnicity, Maternal Age, and Infant sex
FIGURE 1.
Prevalence of orofacial clefts by maternal race/ethnicity, 29 U.S. states, 2007 to 2011.
FIGURE 2.
Prevalence of orofacial clefts by maternal age (years), 29 U.S. states, 2007 to 2011.
TABLE 3.
Orofacial Cleft Counts and Prevalence by Maternal Race/Ethnicity and Age, 29 U.S. States, 2007 to 2011 (Prevalence per 10,000 Live Births)a
| Maternal Race/Ethnicity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal Age (years) | White, Non-Hispanic | Black, Non-Hispanic | Hispanic | Asian or Pacific Islander, Non-Hispanic | American Indian or Alaska Native, Non-Hispanic | Total race/ethnicityb | ||||||
| n | Prevalence | n | Prevalence | n | Prevalence | n | Prevalence | n | Prevalence | n | Prevalence | |
| Cleft lip alone | ||||||||||||
| <35 | 1737 | 3.5 | 336 | 2.1 | 621 | 2.6 | 106 | 2.4 | 38 | 4.0 | 2877 | 3.0 |
| 35+ | 344 | 3.6 | 41 | 2.1 | 100 | 3.2 | 49 | 4.1 | 5 | 6.3 | 556 | 3.4 |
| Total Ageb | 2091 | 3.5 | 381 | 2.1 | 746 | 2.8 | 156 | 2.8 | 43 | 4.2 | 3509 | 3.1 |
| Cleft lip with cleft palate | ||||||||||||
| <35 | 2788 | 5.5 | 594 | 3.7 | 1542 | 6.4 | 188 | 4.3 | NR | NR | 5291 | 5.5 |
| 35+ | 495 | 5.2 | 82 | 4.2 | 242 | 7.7 | 77 | 6.4 | NR | NR | 915 | 5.6 |
| Total Ageb | 3292 | 5.5 | 678 | 3.7 | 1793 | 6.6 | 266 | 4.7 | 104 | 10.3 | 6286 | 5.6 |
| Cleft palate alone | ||||||||||||
| <35 | 3139 | 6.2 | 678 | 4.2 | 1260 | 5.3 | 234 | 5.3 | 59 | 6.1 | 5441 | 5.6 |
| 35+ | 676 | 7.1 | 108 | 5.5 | 238 | 7.5 | 80.0 | 6.6 | 7.0 | 8.8 | 1134 | 7.0 |
| Total Ageb | 3822 | 6.4 | 789 | 4.4 | 1510 | 5.6 | 315 | 5.6 | 66 | 6.5 | 6651 | 5.9 |
NR: Not reported due to small counts for selected category.
Contributing states included: AR, AZ, CO, FL, GA, IA, IL, KS, KY, MA, ME, MI, MN, MS, NC, NE, NJ, NV, NY, OK, PR, RI, TN, VA, VT, and WI; LA provided data for 2007–2010 only, OH for 2008 only, and TX for 2007–2009 only.
Total included unknown and/or other.
Infant sex-specific prevalence by maternal race/ethnicity and maternal age for each OFC phenotype is shown in Table 4. The 14 contributing states for Table 4 are a subset of the 29 states included in Tables 2 and 3.
TABLE 4.
Sex-specific Counts and Prevalence of Orofacial Clefts by Maternal Race/Ethnicity and Age, 14 U.S. States, 2007 to 2011 (Prevalence per 10,000 Live Births)a
| Infant sex | ||||||
|---|---|---|---|---|---|---|
| Male | Female | Total infant sex | ||||
| n | Prevalence | n | Prevalence | n | Prevalence | |
| Maternal Race/Ethnicity | ||||||
| Cleft lip alone | ||||||
| White, Non-Hispanic | 730 | 4.2 | 388 | 2.3 | 1119 | 3.3 |
| Black, Non-Hispanic | 91 | 1.7 | 104 | 2 | 195 | 1.8 |
| Hispanic | 166 | 2.6 | 123 | 2 | 289 | 2.3 |
| Asian or Pacific Islander, Non-Hispanic | 53 | 2.6 | 47 | 2.5 | 100 | 2.6 |
| American Indian or Alaska Native, Non-Hispanic | 3 | 4.0 | 1 | 1.4 | 4 | 2.7 |
| Total Race/Ethnicityb | 1069 | 3.3 | 679 | 2.2 | 1749 | 2.8 |
| Cleft lip with cleft palate | ||||||
| White, Non-Hispanic | 1159 | 6.7 | 662 | 4 | 1826 | 5.4 |
| Black, Non-Hispanic | 189 | 3.5 | 184 | 3.5 | 373 | 3.5 |
| Hispanic | 467 | 7.2 | 322 | 5.2 | 791 | 6.2 |
| Asian or Pacific Islander, Non-Hispanic | 98 | 4.9 | 79 | 4.2 | 177 | 4.5 |
| American Indian or Alaska Native, Non-Hispanic | 6 | 7.9 | 8 | 10.8 | 14 | 9.4 |
| Total Race/Ethnicityb | 1949 | 6.1 | 1283 | 4.2 | 3239 | 5.2 |
| Cleft palate alone | ||||||
| White, Non-Hispanic | 1022 | 5.9 | 1174 | 7.1 | 2196 | 6.5 |
| Black, Non-Hispanic | 216 | 4.0 | 257 | 4.9 | 473 | 4.4 |
| Hispanic | 305 | 4.7 | 391 | 6.3 | 696 | 5.5 |
| Asian or Pacific Islander, Non-Hispanic | 94 | 4.7 | 119 | 6.3 | 213 | 5.5 |
| American Indian or Alaska Native, Non-Hispanic | NR | NR | NR | |||
| Total Race/Ethnicityb | 1672 | 5.2 | 1978 | 6.5 | 3651 | 5.9 |
| Maternal Age (in years) | ||||||
| Cleft lip alone | ||||||
| <35 | 862 | 3.2 | 551 | 2.2 | 1414 | 2.7 |
| 35+ | 196 | 3.8 | 121 | 2.4 | 317 | 3.1 |
| Total Ageb | 1069 | 3.3 | 679 | 2.2 | 1749 | 2.8 |
| Cleft lip with cleft palate | ||||||
| <35 | 1655 | 6.2 | 1048 | 4.1 | 2710 | 5.2 |
| 35+ | 291 | 5.6 | 229 | 4.6 | 520 | 5.1 |
| Total Ageb | 1949 | 6.1 | 1283 | 4.2 | 3239 | 5.2 |
| Cleft palate alone | ||||||
| <35 | 1351 | 5.1 | 1600 | 6.3 | 2951 | 5.6 |
| 35+ | 312 | 6.0 | 372 | 7.5 | 685 | 6.8 |
| Total Ageb | 1672 | 5.2 | 1978 | 6.5 | 3651 | 5.9 |
NR: Not reported due to small cell counts.
The contributing state programs for Table 4 are a subset of the 29 state programs used for Tables (2–3). These 14 states include: AR, CO, FL, IL, KS, LA (2007–2010 only), MA, ME, MN, NJ, NY, OH (2008 only), TN, and VA.
Total included unknown and/or other.
Orofacial Cleft Discussion
OBSERVED PREVALENCE
The prevalence for cleft lip alone is 3.1 per 10,000 live births, 5.6 per 10,000 live births for cleft lip with cleft palate, and 5.9 per 10,000 live births for cleft palate alone. The overall unadjusted prevalence of all OFCs is 14.5, or approximately 1 in 690 births. Separating cleft lip with or without cleft palate into two categories results in approximately one-third of the cases as cleft lip alone and two-thirds as cleft lip with cleft palate. The prevalence of cleft lip with or without cleft palate is similar when compared with the data collected for the 2013 NBDPN annual report (results not shown).
Worldwide, the prevalence of OFCs varies considerably. However, it is not clear to what extent differences in case ascertainment, case definition, and other surveillance methods versus true differences in population prevalence contribute to the geographic variability (IPDTOC, 2011; Mossey and Little, 2002). For example, the birth prevalence of cleft lip with or without cleft palate in Japan is 20.0 per 10,000 births—approximately twice the prevalence reported in the United States, Canada, and Australia (IPDTOC, 2011). Internationally, the birth prevalence of cleft palate shows even more striking geographic variation, with a 10- to 20-fold difference being reported, although it is likely that much of this variation is due to the difficulty in diagnosing some forms of cleft palate during the newborn period (Mossey and Modell, 2012).
RISK FACTORS
Orofacial clefts have a multifactorial etiology, involving a combination of both genetic and environmental risk factors, and complex gene-environment interaction, which are poorly understood. Several putative risk factors have been identified that tend to vary according to cleft phenotype. Many of these risk factors are preventable, notably maternal smoking (Little et al., 2004; Honein et al., 2007; US DHHS, 2014), alcohol consumption (Lorente et al., 2000; Romitti et al., 2007), diabetes and obesity (Cedergren and Kallen, 2005; Correa et al., 2008; Villamor et al., 2008), maternal diet (Munger, 2002), and certain medications (Hernandez-Diaz et al., 2000; Holmes et al., 2004; Werler et al., 2011; Margulis et al., 2012). In this report, we examine prevalence of OFCs by maternal race/ethnicity, maternal age, and infant sex.
MATERNAL RACE/ETHNICITY
The overall estimated prevalence for OFCs for non-Hispanic whites was 15.4 per 10,000 live births (Table 2). Compared with non-Hispanic whites, the prevalence was relatively similar for Hispanics (14.9 per 10,000 live births) and lower for other racial/ethnic groups except for non-Hispanic American Indians/Alaska Natives (20.5 per 10,000 live births). However, results should be interpreted with caution for the prevalence of OFCs for non-Hispanic American Indians/Alaska Natives due to small numbers.
The variation differed when examining the prevalence by OFC phenotypes. Compared with non-Hispanic whites, the estimated prevalence of each OFC phenotype for non-Hispanic blacks remained significantly lower while for non-Hispanic Asians/Pacific Islanders, the prevalence was slightly lower or not statistically significant. The prevalence of cleft lip with cleft palate was significantly higher for both Hispanics and non-Hispanic American Indians/Alaska Natives compared with non-Hispanic whites while the observed prevalence for cleft lip alone and cleft palate alone among Hispanics was significantly lower but the increased prevalence among non-Hispanic American Indians/Alaska Natives was nonsignificant (Table 2 and Fig. 1).
Published studies showing OFCs by maternal race/ethnicity varied in several methodological aspects, including: (1) study population (for example: live births, live births and fetuses, inpatient admissions); (2) time periods; (3) geography; (4) case classification (for example: overall cleft cases, cleft lip with and without cleft palate, cleft lip alone, cleft palate alone, isolated cases); and (5) inclusion or exclusion of Hispanic ethnicity and the source of ethnicity information. Despite these differences, statistically significant observations for various case classifications consistently noted lower occurrence in non-Hispanic blacks compared with non-Hispanic whites and Hispanics (Kirby et al., 2000; Genisca et al., 2009; Lebby et al., 2010). Several studies that have examined a broader range of maternal racial/ethnic groups reported similar findings, but also showed non-Hispanic American Indians/Alaska Natives with the highest occurrence of OFCs (Croen et al., 1998; Hashmi et al., 2005; Canfield et al., 2014). Consistent findings were seen with a lower prevalence of cleft palate alone among Hispanics compared with non-Hispanic whites; however, the prevalence for cleft lip alone varied depending on case classification. The studies reporting combined cleft lip with or without cleft palate showed no difference or a slight increase in the prevalence of OFCs among Hispanics compared with non-Hispanic whites. Genisca et al. (2009) presented estimated prevalences for the three OFC phenotypes by three maternal race/ethnicity categories (non-Hispanic white, non-Hispanic black, and Hispanic) and found a decreased prevalence among Hispanics for cleft lip alone and a nonsignificant but slightly higher prevalence for cleft lip with cleft palate. We had similar findings except the prevalence for cleft lip with cleft palate was significantly higher among Hispanics compared with non-Hispanic whites. A strength of our study was the ability to examine the three OFC phenotypes by the five maternal U.S. Census racial/ethnic groups.
MATERNAL AGE
We found that mothers who were greater than or equal to 35 years old had a higher prevalence of OFCs compared with those less than 35 years old. The prevalence for cleft lip alone and cleft lip with cleft palate was relatively stable across all maternal ages except that the prevalence was higher in mothers 40+ years old. For cleft palate alone, the prevalence increased with advanced maternal age, and the prevalence for mothers who were 40+ years old was approximately two-thirds higher than that of mothers less than 20 years old (Table 2 and Fig. 2). This may be due, in part, to the higher rate of certain chromosomal birth defects among older women, such as trisomy 18 and trisomy 13, which are often associated with cleft palate.
Published studies showed inconsistent findings between maternal age and OFCs. Some reported an increase in prevalence with advanced maternal age, while others reported no evidence of an association (Vieira et al., 2002; Bille et al., 2005). One study using data from a surveillance program found a statistically significant increase of isolated cleft lip with or without cleft palate among infants of mothers less than 20 years old but this was not observed for nonisolated cleft lip (DeRoo et al., 2003).
In general, our data showed the observed crude prevalence of OFCs was higher among mothers age 35 years and older within each racial/ethnic category with some exceptions (Table 3). For cleft lip with cleft palate among non-Hispanic whites and for cleft lip alone among non-Hispanic whites and non-Hispanic blacks, the prevalence was relatively similar between the maternal age categories.
INFANT SEX
The data in Table 2 indicated a higher prevalence of cleft lip alone, cleft lip with cleft palate, and overall for OFCs among males compared with females, but the prevalence was lower for cleft palate alone. Previous literature supports our results (Shaw et al., 1991; Forrester and Merz, 2004; Genisca et al., 2009; Messer et al., 2010;).
Table 4 presents the sex-specific prevalence of OFCs by maternal race/ethnicity and maternal age for 14 states, a subset of the 29 contributing states for this report. These findings are consistent with the previous literature that prevalence differs among the cleft phenotypes by infant sex and maternal race/ethnicity (Shaw et al., 1991; Croen et al., 1998; Kirby et al., 2000; Forrester and Merz, 2004; Hashmi et al., 2005; Genisca et al., 2009; Lebby et al., 2010; Messer et al., 2010; Canfield et al., 2014).
Conclusion
The 2014 NBDPN Congenital Malformations Surveillance Report, which includes data from 39 population-based surveillance programs, continues to provide unique and important information to aid in the understanding of the occurrence and public health importance of birth defects in the United States. The focus on OFCs in the present report, using pooled surveillance data from 29 states, is intended to provide more detailed information on the occurrence of these serious birth defects. We hope the current population-based prevalence estimates of cleft lip alone, cleft lip with cleft palate, and cleft palate alone by maternal race/ethnicity, maternal age, and infant sex in the United States will provide those using this report with the in-depth data they seek. This information can also guide clinicians, scientists, and public health officials concerned with treatment, management, and service planning for children with orofacial clefts.
Supplementary Material
Acknowledgments
We thank the state birth defects surveillance programs that submitted data for this report: Arkansas Reproductive Health Monitoring System; Arizona Birth Defects Monitoring Program; California Birth Defects Monitoring Program; Colorado Responds To Children With Special Needs; Delaware Birth Defects Surveillance Project; U.S. Department of Defense Birth and Infant Health Registry; Florida Birth Defects Registry; Metropolitan Atlanta Congenital Defects Program; Iowa Registry for Congenital and Inherited Disorders; Illinois Adverse Pregnancy Outcomes Reporting System; Kansas Birth Defects Information System; Kentucky Birth Surveillance Registry; Louisiana Birth Defects Monitoring Network; Massachusetts Center for Birth Defects Research and Prevention; Maryland Birth Defects Reporting and Information System; Maine Birth Defects Program; Michigan Birth Defects Registry; Minnesota Birth Defects Information System; Mississippi Birth Defects Registry; North Carolina Birth Defects Monitoring Program; North Dakota Birth Defects Monitoring System; Nebraska Birth Defects Registry; New Hampshire Birth Conditions Program; New Jersey Special Child Health Services Registry; Nevada Birth Outcomes Monitoring System; New York State Congenital Malformations Registry; Ohio Connections for Children with Special Needs; Oklahoma Birth Defects Registry; Puerto Rico Birth Defects Surveillance and Prevention System; Rhode Island Birth Defects Program; South Carolina Birth Defects Program; Tennessee Birth Defects Registry; Texas Birth Defects Epidemiology and Surveillance Branch; Utah Birth Defect Network; Virginia Congenital Anomalies Reporting and Education System; Vermont Birth Information Network; Wisconsin Birth Defects Registry; and West Virginia Congenital Abnormalities Registry, Education and Surveillance System.
Footnotes
Additional Supporting Information may be found in the online version of this article.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- American Cleft Palate-Craniofacial Association (ACPA) Parameters for evaluation and treatment of patients with cleft lip/palate or other craniofacial anomalies. 2009 Available at: http://www.acpa-cpf.org/uploads/site/parameters_rev_2009.pdf Accessed July 7, 2014.
- Bille C, Skytthe A, Vach W, et al. Parent’s age and the risk of oral clefts. Epidemiology. 2005;16:311–316. doi: 10.1097/01.ede.0000158745.84019.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet SL, Grosse SD, Honein MA, Correa-Villasenor A. Children with orofacial clefts: health-care use and costs among a privately insured population. Public Health Rep. 2009;124:447–453. doi: 10.1177/003335490912400315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield MA, Mai CT, Wang Y, et al. The association between race/ethnicity and major birth defects in the United States, 1999–2007. Am J Public Health. 2014;104:e14–e23. doi: 10.2105/AJPH.2014.302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell CH, Meyer R, Daniels J. Health care expenditures among Medicaid enrolled children with and without orofacial clefts in North Carolina, 1995–2002. Birth Defects Res A. 2008;82:785–794. doi: 10.1002/bdra.20522. [DOI] [PubMed] [Google Scholar]
- Cedergren M, Kallen B. Maternal obesity and the risk for orofacial clefts in the offspring. Cleft Palate Craniofac J. 2005;42:367–371. doi: 10.1597/04-012.1. [DOI] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237.e1–237.e9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, Wasserman CR, Tolarova MM. Racial and ethnic variations in the prevalence of orofacial clefts in California, 1983–1992. Am J Med Genet. 1998;79:42–47. doi: 10.1002/(sici)1096-8628(19980827)79:1<42::aid-ajmg11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- DeRoo LA, Gaudino JA, Edmonds LD. Orofacial cleft malformations: associations with maternal and infant characteristics in Washington state. Birth Defects Res A. 2003;67:637–642. doi: 10.1002/bdra.10114. [DOI] [PubMed] [Google Scholar]
- Forrester MB, Merz RD. Descriptive epidemiology of oral clefts in a multiethnic population, Hawaii, 1986–2000. Cleft Palate Craniofac J. 2004;41:622–628. doi: 10.1597/03-089.1. [DOI] [PubMed] [Google Scholar]
- Genisca AE, Frías JL, Broussard CS, et al. Orofacial clefts in the National Birth Defects Prevention Study, 1997–2004. Am J Med Genet A. 2009;149A:1149–1158. doi: 10.1002/ajmg.a.32854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harville EW, Wilcox AJ, Lie RT, et al. Cleft lip and palate versus cleft lip only: are they distinct defects? Am J Epidemiol. 2005;162:448–453. doi: 10.1093/aje/kwi214. [DOI] [PubMed] [Google Scholar]
- Hashmi SS, Waller DK, Langlois P, et al. Prevalence of nonsyndromic oral clefts in Texas: 1995–1999. Am J Med Genet A. 2005;134:368–372. doi: 10.1002/ajmg.a.30618. [DOI] [PubMed] [Google Scholar]
- Hernandez-Diaz S, Werler MM, Walker AH, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343:1608–1614. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Wyszynski DF, Lieberman E. The AED (antiepileptic drug) pregnancy registry: a 6-year experience. Arch Neurol. 2004;61:673–678. doi: 10.1001/archneur.61.5.673. [DOI] [PubMed] [Google Scholar]
- Honein MA, Rasmussen SA, Reefhuis J, et al. Maternal smoking and environmental tobacco smoke exposure and the risk of orofacial clefts. Epidemiology. 2007;18:226–233. doi: 10.1097/01.ede.0000254430.61294.c0. [DOI] [PubMed] [Google Scholar]
- International Perinatal Database of Typical Orofacial Clefts (IPD-TOC) Working Group. Prevalence at birth of cleft lip with or without cleft palate: data from the International Perinatal Database of Typical Oral Clefts. Cleft Palate Craniofac J. 2011;48:66–81. doi: 10.1597/09-217. [DOI] [PubMed] [Google Scholar]
- Kirby R, Petrini J, Alter C. Collecting and interpreting birth defects surveillance data by Hispanic ethnicity: a comparative study. Teratology. 2000;61:21–27. doi: 10.1002/(SICI)1096-9926(200001/02)61:1/2<21::AID-TERA5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lebby KD, Tan F, Brown CP. Maternal factors and disparities associated with oral clefts. Ethn Dis. 2010;20:S1–146-9. [PMC free article] [PubMed] [Google Scholar]
- Little J, Cardy A, Munger RG. Tobacco smoking and oral clefts: a meta-analysis. Bull World Health Org. 2004;82:213–218. [PMC free article] [PubMed] [Google Scholar]
- Lorente C, Cordier S, Goujard J, et al. Tobacco and alcohol use during pregnancy and risk of oral clefts. Occupational Exposure and Congenital Malformation Working Group. Am J Public Health. 2000;90:415–419. doi: 10.2105/ajph.90.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahle WT, Martin GR, Beekman RH, III, et al. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129:190–192. doi: 10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- Margulis AV, Mitchell AA, Gilboa SM, et al. Use of topiramate in pregnancy and risk of oral clefts. Am J Obstet Gynecol. 2012;207:405.e1–e7. doi: 10.1016/j.ajog.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CA, Kirby RS, Sever LE, Langlois PH. Prevalence is the preferred measure of frequency of birth defects. Birth Defects Res A. 2005;73:690–692. doi: 10.1002/bdra.20211. [DOI] [PubMed] [Google Scholar]
- Messer LC, Luben TJ, Mendola P, et al. Urban-rural residence and the occurrence of cleft lip and cleft palate in Texas, 1999–2003. Ann Epidemiol. 2010;20:32–39. doi: 10.1016/j.annepidem.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Mossey PA, Little J. Epidemiology of oral clefts: an international perspective. In: Wyszynski DF, editor. Cleft lip and palate: from origins to treatment. New York: Oxford University Press; 2002. pp. 127–158. [Google Scholar]
- Mossey PA, Modell B. Epidemiology of oral clefts: an international perspective. Front Oral Biol. 2012;16:1–18. doi: 10.1159/000337464. [DOI] [PubMed] [Google Scholar]
- Munger R. Maternal nutrition and oral clefts. In: Wyszynski DF, editor. Cleft lip and palate: from origins to treatment. New York: Oxford University Press; 2002. pp. 170–192. [Google Scholar]
- Parker SE, Mai CT, Canfield MA, et al. Updated national prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Olney RS, Holmes LB, et al. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A. 2003;67:193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- Romitti PA, Sun L, Honein MA, et al. Maternal periconceptional alcohol consumption and risk of orofacial clefts. Am J Epidemiol. 2007;166:775–785. doi: 10.1093/aje/kwm146. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Croen LA, Curry CJ. Isolated oral cleft malformations: associations with maternal and infant characteristics in a California population. Teratology. 1991;43:225–228. doi: 10.1002/tera.1420430306. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (U.SDHHS) The health consequences of smoking—50 years of progress A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- Vieira AR, Orioli IM, Murray JC. Maternal age and oral clefts: a reappraisal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:530–535. doi: 10.1067/moe.2002.128875. [DOI] [PubMed] [Google Scholar]
- Villamor E, Sparen P, Cnattingius S. Risk of oral clefts in relation to prepregnancy weight change and interpregnancy interval. Am J Epidemiol. 2008;167:1305–1311. doi: 10.1093/aje/kwn065. [DOI] [PubMed] [Google Scholar]
- Watkins SE, Meyer RE, Strauss RP, Aylsworth AS. Classification, epidemiology, and genetics of orofacial clefts. Clin Plast Surg. 2014;41:149–163. doi: 10.1016/j.cps.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Weiss J, Kotelchuck M, Grosse SD, et al. Hospital use and associated costs of children aged zero-to-two years with craniofacial malformations in Massachusetts. Birth Defects Res A. 2009;85:925–934. doi: 10.1002/bdra.20635. [DOI] [PubMed] [Google Scholar]
- Werler MM, Ahrens KA, Bosco JL, et al. Use of antiepileptic medications in pregnancy in relation to risks of birth defects. Ann Epidemiol. 2011;21:842–850. doi: 10.1016/j.annepidem.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdy MM, Honein MA, Rasmussen SA, Frias JL. Priorities for future public health research in orofacial clefts. Cleft Palate Craniofac J. 2007;44:351–357. doi: 10.1597/06-233.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


