Abstract
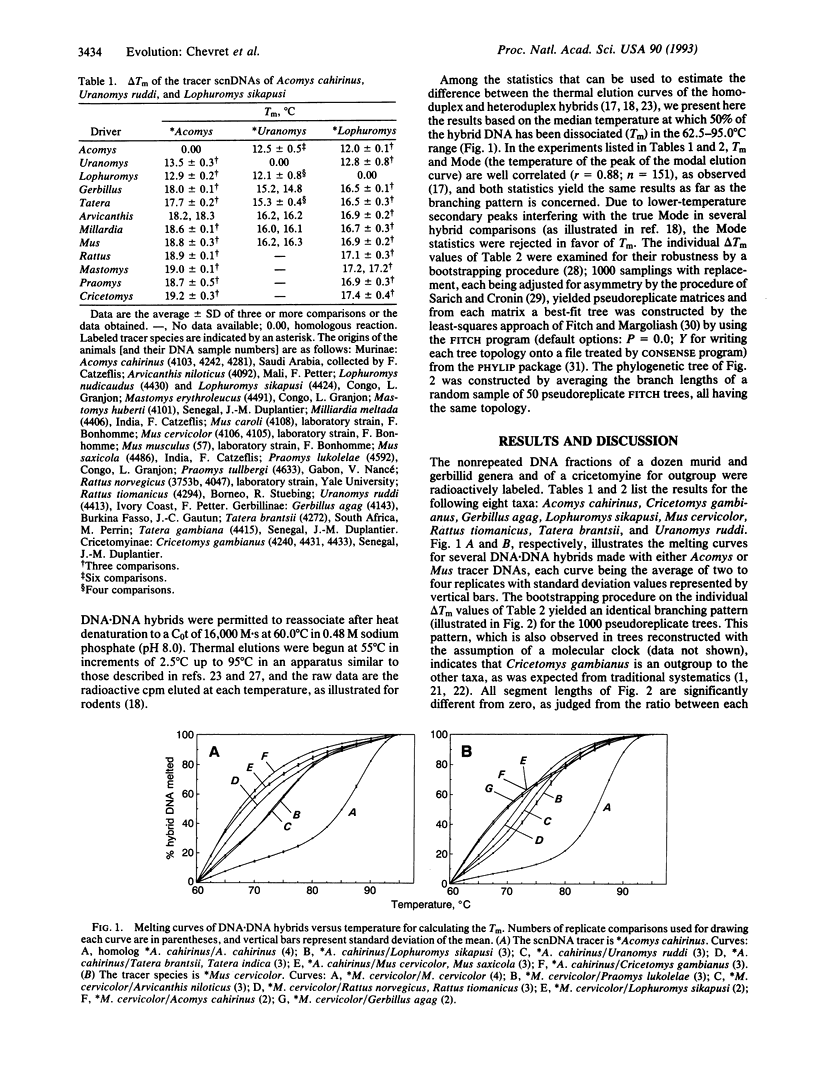
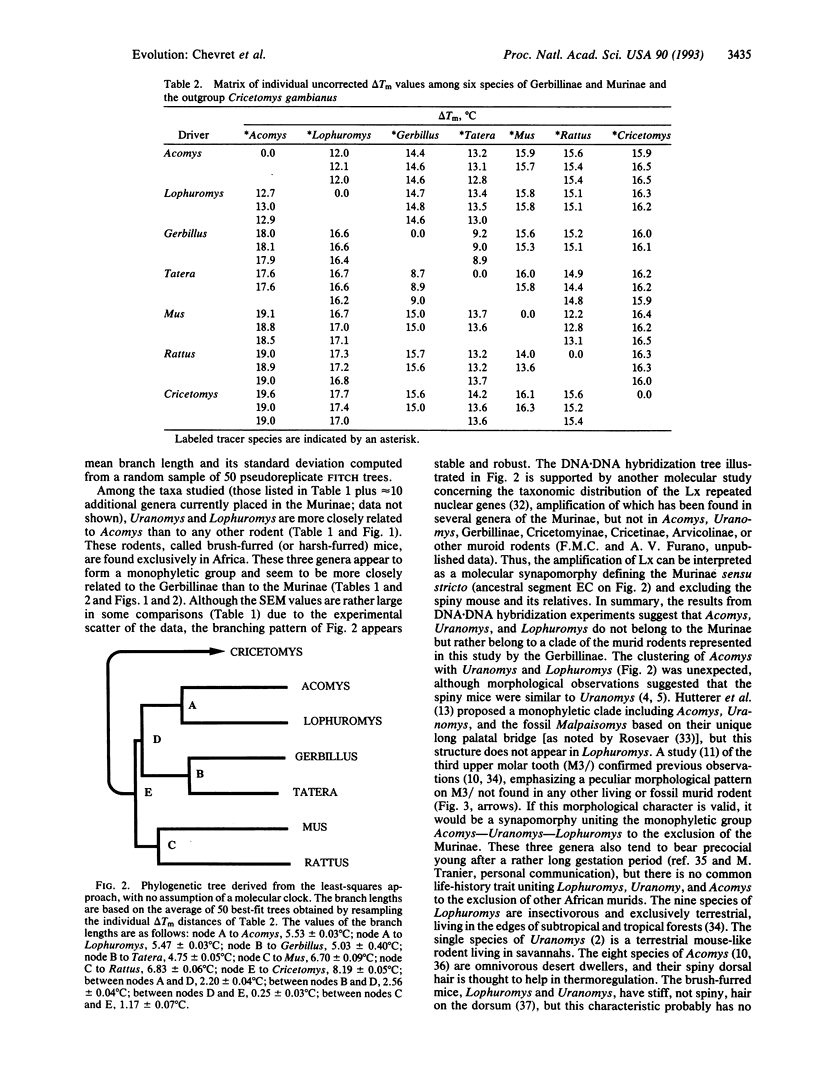
Spiny mice of the genus Acomys traditionally have been classified as members of the Murinae, a subfamily of rodents that also includes rats and mice with which spiny mice share a complex set of morphological characters, including a unique molar pattern. The origin and evolution of this molar pattern, documented by many fossils from Southern Asia, support the hypothesis of the monophyly of Acomys and all other Murinae. This view has been challenged by immunological studies that have suggested that Acomys is as distantly related to mice (Mus) as are other subfamilies (e.g., hamsters: Cricetinae) of the muroid rodents. We present molecular evidence derived from DNA.DNA hybridization data that indicate that the spiny mouse Acomys and two African genera of Murinae, Uranomys and Lophuromys, constitute a monophyletic clade, a view that was recently suggested on the basis of dental characters. However, our DNA.DNA hybridization data also indicate that the spiny mice (Acomys) are more closely related to gerbils (Gerbillinae) than to the true mice and rats (Murinae) with which they have been classified. Because Acomys and the brush-furred mice Uranomys and Lophuromys share no derived morphological characters with the Gerbillinae, their murine morphology must have evolved by convergence, including the molar pattern previously considered to support the monophyly of the Murinae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catzeflis F. M., Sheldon F. H., Ahlquist J. E., Sibley C. G. DNA-DNA hybridization evidence of the rapid rate of muroid rodent DNA evolution. Mol Biol Evol. 1987 May;4(3):242–253. doi: 10.1093/oxfordjournals.molbev.a040444. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Hunt J. A., Hall T. J., Britten R. J. Evolutionary distances in Hawaiian Drosophila measured by DNA reassociation. J Mol Evol. 1981;17(6):361–367. doi: 10.1007/BF01734358. [DOI] [PubMed] [Google Scholar]
- Kirsch J. A., Ganje R. J., Olesen K. G., Hoffman D. W., Bledsoe A. H. TED, an improved thermal elution device for the simultaneous hydroxyapatite chromatography of solution DNA/DNA hybrids. Biotechniques. 1990 May;8(5):504–506. [PubMed] [Google Scholar]
- Pascale E., Valle E., Furano A. V. Amplification of an ancestral mammalian L1 family of long interspersed repeated DNA occurred just before the murine radiation. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9481–9485. doi: 10.1073/pnas.87.23.9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon F. H., Bledsoe A. H. Indexes to the reassociation and stability of solution DNA hybrids. J Mol Evol. 1989 Oct;29(4):328–343. doi: 10.1007/BF02103620. [DOI] [PubMed] [Google Scholar]
- Springer M., Krajewski C. DNA hybridization in animal taxonomy: a critique from first principles. Q Rev Biol. 1989 Sep;64(3):291–318. doi: 10.1086/416360. [DOI] [PubMed] [Google Scholar]



