Abstract
BACKGROUND
It is unclear whether using fetal electrocardiographic (ECG) ST-segment analysis as an adjunct to conventional intrapartum electronic fetal heart-rate monitoring modifies intrapartum and neonatal outcomes.
METHODS
We performed a multicenter trial in which women with a singleton fetus who were attempting vaginal delivery at more than 36 weeks of gestation and who had cervical dilation of 2 to 7 cm were randomly assigned to “open” or “masked” monitoring with fetal ST-segment analysis. The masked system functioned as a normal fetal heart-rate monitor. The open system displayed additional information for use when uncertain fetal heart-rate patterns were detected. The primary outcome was a composite of intrapartum fetal death, neonatal death, an Apgar score of 3 or less at 5 minutes, neonatal seizure, an umbilical-artery blood pH of 7.05 or less with a base deficit of 12 mmol per liter or more, intubation for ventilation at delivery, or neonatal encephalopathy.
RESULTS
A total of 11,108 patients underwent randomization; 5532 were assigned to the open group, and 5576 to the masked group. The primary outcome occurred in 52 fetuses or neonates of women in the open group (0.9%) and 40 fetuses or neonates of women in the masked group (0.7%) (relative risk, 1.31; 95% confidence interval, 0.87 to 1.98; P = 0.20). Among the individual components of the primary outcome, only the frequency of a 5-minute Apgar score of 3 or less differed significantly between neonates of women in the open group and those in the masked group (0.3% vs. 0.1%, P = 0.02). There were no significant between-group differences in the rate of cesarean delivery (16.9% and 16.2%, respectively; P = 0.30) or any operative delivery (22.8% and 22.0%, respectively; P = 0.31). Adverse events were rare and occurred with similar frequency in the two groups.
CONCLUSIONS
Fetal ECG ST-segment analysis used as an adjunct to conventional intrapartum electronic fetal heart-rate monitoring did not improve perinatal outcomes or decrease operative-delivery rates. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and Neoventa Medical; ClinicalTrials.gov number, NCT01131260.)
Continuous intrapartum fetal heart-rate monitoring has caused considerable controversy in obstetrics. Despite decades of use and an associated rise in cesarean-delivery rates based at least in part on nonreassuring fetal heart-rate patterns, evidence that such monitoring has reduced the rate of hypoxia-induced neonatal encephalopathy is lacking. In 2005, the Food and Drug Administration (FDA) granted conditional approval of the STAN S31 device (Neoventa Medical) for use as an adjunct to conventional electronic fetal heart-rate monitoring.1 This technology was designed to provide fetal electrocardiographic (ECG) information reflective of myocardial metabolism and acid–base balance. The rationale is that fetal acidemia is associated with fetal ECG ST-segment elevation and increased T-wave amplitude.2-6 The monitor for fetal ECG ST-segment analysis uses proprietary software to detect these ECG changes and then issues a visual alert (“ST event”) when these changes occur.
Initial FDA approval was based primarily on European studies7-9 that suggested that fetal ST-segment analysis technology reduced the rates of neonatal encephalopathy, acidemia, and operative delivery. However, the relevance of these results to usual obstetrical practice is unclear, given inconsistent findings among published trials and questions about eligibility criteria, choice and definition of primary outcomes, and intrapartum management, as discussed by Øian and Blix10 and Steer and Hvidman.11 Moreover, there are substantive differences between U.S. and European practices. We designed a large, multi-institutional, randomized trial to assess the effects of using fetal ECG ST-segment analysis on perinatal outcomes.
METHODS
STUDY DESIGN AND OVERSIGHT
The study, which consisted of a pilot phase and the randomized trial, was conducted at 16 university-based clinical centers — each comprising 1 to 5 delivery hospitals (26 total) — in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal–Fetal Medicine Units (MFMU) Network. The protocol was approved by the institutional review board at each hospital and is available with the full text of this article at NEJM.org. Written informed consent was obtained from all participants before participation. A small group of investigators (protocol subcommittee) provided oversight for the trial. The study was supported by the NICHD and by funding from the manufacturer (Neoventa Medical). Neoventa did not participate in the monitoring of the study; data collection, management, or analysis; or manuscript preparation. The first, second, third, and fifth authors take responsibility for the fidelity of the report to the study protocol and for the accuracy and completeness of the reported data.
CERTIFICATION, TRAINING, AND PILOT PHASE
All participating care providers and research personnel were trained and certified in the correct use of the fetal ECG ST-segment analysis system to a level exceeding FDA requirements. Each hospital participated in a pilot phase that consisted of enrollment and care of at least 50 patients monitored with fetal ECG ST-segment analysis, with central review of labor-management decisions and retraining as needed before approval of the hospital to start the trial. Details of the certification and monitoring process are provided in the Supplementary Appendix, available at NEJM.org; guidelines for the management of labor on the basis of fetal ECG analysis are summarized in Table 1, and Figure S1 in the Supplementary Appendix.
Table 1.
Fetal Heart Rate (FHR) Classification System for Electrocardiographic ST-Segment Analysis and Management Guidelines.*
| FHR Pattern | FHR Characteristics | Management Guidelines | |||
|---|---|---|---|---|---|
| Baseline Heart Rate | Variability | Decelerations | No ST-Segment Event | ST-Segment Event | |
| Green zone | 110–160 bpm | Moderate variability (defined as 6–25 bpm); accelera- tions present |
Early decelerations; variable de-
celerations with a duration of <60 sec and depth of <60 beats |
Expectant management; contin- ued observation |
Expectant management; contin- ued observation |
| Yellow zone | Bradycardia <110 bpm; tachycardia >160 bpm; >150 bpm with minimal variability |
Minimal variability (≤5 bpm) for >40 min; marked variability (>25 bpm) for >40 min |
Variable decelerations with a du- ration of ≥60 sec or depth of ≥60 beats; recurrent late de- celerations; prolonged decel- eration for >2 min regardless of variability or reactivity |
Expectant management, closer observation; if >60 min (or earlier if FHR shows rapid de- terioration of fetal condition), direct physician assessment of fetal state |
Direct physician assessment; intrauterine resuscitation as appropriate; if no improve- ment in fetal condition, expe- ditious operative delivery; in second stage of labor with ac- tive pushing, expeditious op- erative delivery |
| Red zone | Absent variability regardless of other FHR patterns; sinusoidal pattern | Expeditious operative delivery regardless of any ST-segment changes |
Expeditious operative delivery regardless of any ST-segment changes |
||
After the fetal heart rate is classified according to the color zone, management is predicated on the zone and the presence or absence of an ST-segment event. An ST-segment event is defined by an episodic increase in the ratio of the T-wave height to the amplitude of the QRS complex (T:QRS ratio), a baseline increase in the T:QRS ratio, or two occurrences of a bi-phasic ST segment. In the first stage of labor, operative delivery is recommended within 60 minutes after an ST-segment event with a yellow-zone pattern and the failure of intrauterine resuscitation efforts to effect a return to a green-zone pattern. In the second stage of labor, operative delivery is recommended within 30 minutes after an ST-segment event with a yellow-zone pattern. Expectant management is defined by continued observation without any specified intervention. Italics indicate differences from the corresponding categories (I, II, and III) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).12 Absent variability indicates that the amplitude range was undetectable.
Once a hospital was approved to participate in the randomized trial, the independent data coordinating center sent a software card containing the encrypted randomization module to the designated local biomedical technician to be installed on the S31 monitors at that hospital. No other personnel had access to the software or were able to adjust the monitors. A separate randomization sequence was created for each monitor.
TRIAL PROTOCOL
Women with a singleton fetus at more than 36 weeks of gestation who were attempting vaginal delivery and had cervical dilation of 2 to 7 cm were invited to participate. The main exclusion criteria were noncephalic presentation, planned cesarean delivery, a need for immediate delivery, absent fetal heart-rate variability (amplitude range undetectable) or a sinusoidal pattern, minimal fetal heart-rate variability in the 20 minutes before randomization, or other fetal or maternal conditions that would preclude a trial of labor or the placement of a scalp electrode. The full eligibility criteria are provided in the Supplementary Appendix.
After spontaneous or artificial membrane rupture, a Goldtrace fetal scalp electrode (Neoventa Medical) was placed in each woman who consented to participate in the trial. If it was not possible to obtain or maintain an adequate fetal ECG signal after three attempts at electrode placement or if an ST-segment event occurred during the attempts to obtain an adequate signal, the woman was excluded from randomization. Immediately after successful electrode placement resulting in an adequate ST-segment analysis signal, a researcher activated the randomization module, which then automatically set the S31 monitor into “masked” or “open” mode according to the internal preassigned randomization scheme.
The masked S31 monitors functioned as conventional electronic fetal heart-rate monitors. The care of patients in the masked group was managed at the discretion of the attending physician or midwife. S31 monitors in the open mode displayed ECG ST-segment information intended for use when uncertain fetal heart-rate patterns were detected. Management of the labor and delivery for women in this group was dictated by the ST-segment analysis guidelines (Table 1, and Fig. S1 in the Supplementary Appendix). To monitor adherence to the guidelines, all tracings from fetuses of patients who subsequently had cesarean or operative vaginal (forceps or vacuum-assisted) deliveries and from fetuses who had ST-segment events or primary-outcome events, as well as tracings from fetuses of a sample of patients who subsequently had vaginal deliveries, were centrally reviewed by the protocol subcommittee, whose members were unaware of the outcome of the neonate. If the group determined that the guidelines for fetal ECG ST-segment analysis had not been followed, the provider received additional training.
Paired arterial and venous umbilical-cord blood gas measurements were obtained from all neonates. The base deficit was calculated with the use of measurements from the extracellular fluid and the modified Siggaard-Andersen curve.13 To be valid, the arterial pH had to be lower than the venous pH, and the partial pressure of arterial carbon dioxide had to be greater than the partial pressure of venous carbon dioxide. All enrolled women and their infants were followed until hospital discharge. Trained research staff collected maternal and perinatal data, including the interpretation by the clinical providers of the fetal heart-rate pattern (NICHD category12 or fetal ECG ST-segment analysis category).
PRIMARY OUTCOME
The primary outcome was a composite of intrapartum fetal death, neonatal death, an Apgar score of 3 or less at 5 minutes, neonatal seizure, an umbilical-artery blood pH of 7.05 or less with a base deficit of 12 mmol per liter or more, intubation for ventilation at delivery, or neonatal encephalopathy.14 This composite outcome represents a cluster of neonatal outcomes that manifest early, that indicate that the fetus may have been compromised during labor, that are associated with a risk of long-term neurologic adverse outcomes, and that potentially could be avoided by more prompt delivery.15 The protocol subcommittee, whose members were unaware of the study-group assignments, conducted chart reviews of all pregnancies that were designated as having met the primary outcome criteria to confirm that they had met these criteria and also reviewed charts of a sample of neonates who had longer hospital stays or who required more-than-routine resuscitation at delivery.
Maternal secondary outcomes were cesarean delivery (with indication), forceps or vacuum-assisted delivery, chorioamnionitis, maternal blood transfusion, duration of labor after randomization, shoulder dystocia, postpartum endometritis, and length of hospital stay. Neonatal secondary outcomes were individual components of the primary outcome, Apgar score at 5 minutes, umbilical-artery blood gas results, and admission to the intermediate care nursery or neonatal intensive care unit.
STATISTICAL ANALYSIS
We calculated that a sample size of 11,000 patients would give the study more than 85% power to detect a 40% reduction in the primary outcome in the open group, assuming a rate of 1.75% in the masked group (derived from a previous MFMU Network randomized trial involving women in labor at term), at a two-sided type I error rate of 5%.16 We estimated that with the same power and type I error rate, our sample size would also be sufficient to detect a 10% reduction in the cesarean-delivery rate in the open group. Given a cesarean-delivery rate for nonreassuring fetal status as low as 5%, there was adequate power (88%) to detect a 25% reduction in cesarean delivery for this specific indication.
We performed an intention-to-treat analysis, in which patients were included in the group to which they had been randomly assigned, regardless of the completeness of ST-segment monitoring or provider adherence to the protocol. Categorical variables were compared with the use of the chi-square test or Fisher’s exact test, as appropriate. Relative risks and 95% confidence intervals were calculated. Interaction tests in prespecified subgroups (category I vs. category II fetal heart-rate tracing at baseline, multiparous vs. nulliparous status, center, enrollment before vs. after the midpoint of recruitment for each site, and race or ethnic group) and in post hoc subgroups (induced vs. spontaneous labor and baseline cervical dilation of 2 to 5 cm vs. 6 to 7 cm) were conducted with the use of the Breslow–Day method for the following end points: primary outcome, cesarean delivery, and any operative delivery (cesarean and operative vaginal delivery combined). Continuous variables were compared with the use of the Wilcoxon rank-sum test. An independent data and safety monitoring committee monitored the trial for safety and trial performance. The committee did not review interim data on the primary outcome; therefore, no adjustment to the type I error rate was necessary. For all secondary outcomes, a nominal P value of less than 0.05, without adjustment for multiple comparisons, was considered to indicate statistical significance.
RESULTS
RECRUITMENT AND CHARACTERISTICS OF THE PARTICIPANTS
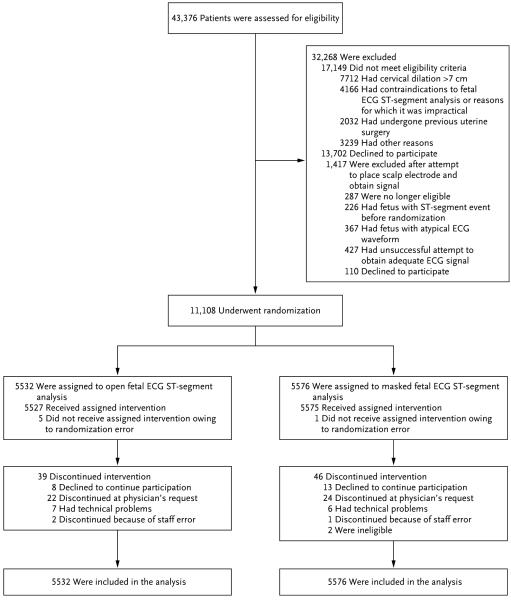
Recruitment to the pilot study started in April 2010; by April 2013, a total of 26 hospitals were authorized to start the randomized trial. Recruitment to the trial began in November 2010 and ended in March 2014. A total of 43,376 women were screened for inclusion, of whom 18,456 did not meet eligibility criteria, 13,812 declined participation, and 11,108 were randomly assigned to the open group (5532 women) or the masked group (5576 women) (Fig. 1). A total of 85 women discontinued open or masked ST-segment monitoring before delivery; however, outcome data were obtained for all women and their neonates.
Figure 1. Enrollment and Outcomes.
ECG denotes electrocardiographic.
There were no significant differences in baseline characteristics between the two groups (Table 2). Assessment of fetal heart rate immediately before randomization showed that 73% of fetal heart-rate tracings were NICHD category I (reassuring) and 27% were category II (indeterminate)12 (Fig. S2 in the Supplementary Appendix). Of the category II tracings, 43% had either recurrent late or recurrent variable decelerations.
Table 2.
Characteristics of the Patients Enrolled in the Study.*
| Characteristic | Open Group (N = 5532) |
Masked Group (N = 5576) |
|---|---|---|
| Age — yr | 27.4±5.9 | 27.2±5.8 |
| Week of pregnancy at randomization | 39.4±1.2 | 39.4±1.2 |
| Race — no. (%)† | ||
| Black | 1326 (24.0) | 1350 (24.2) |
| White | 3297 (59.6) | 3281 (58.8) |
| Other | 909 (16.4) | 945 (16.9) |
| Body-mass index before pregnancy‡ | 27.4±7.2 | 27.4±7.0 |
| Educational level — yr | 12.8±2.6 | 12.8±2.7 |
| Cervical dilation at randomization — cm |
||
| Median | 5 | 5 |
| Interquartile range | 4–6 | 4–6 |
| Type of labor — no. (%) | ||
| Spontaneous | 2259 (40.8) | 2311 (41.4) |
| Induced | 3273 (59.2) | 3265 (58.6) |
| Nulliparous — no. (%) | 2354 (42.6) | 2373 (42.6) |
Plus–minus values are means ±SD. There were no significant differences in baseline characteristics between the two groups.
Race was self-reported.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
OUTCOMES
Valid umbilical-cord blood gas measurements were obtained for 96.5% of all neonates. The primary outcome occurred in 52 neonates (0.9%) of patients in the open group and in 40 neonates (0.7%) of patients in the masked group (relative risk, 1.31; 95% confidence interval, 0.87 to 1.98; P = 0.20) (Table 3). The groups did not differ significantly with respect to the incidences of individual components of the primary outcome, with one exception: the frequency of an Apgar score of 3 or less at 5 minutes was higher among neonates of patients in the open group than among neonates of patients in the masked group (0.3% vs. 0.1%; P = 0.02). However, most components of the primary outcome occurred in no more than 0.1% of neonates in either group.
Table 3.
Primary Outcome and Components.*
| Outcome | Open Group (N = 5532) |
Masked Group (N = 5576) |
Relative Risk (95% CI) |
P Value |
|---|---|---|---|---|
| no. (%) | ||||
| Primary composite outcome† | 52 (0.94) | 40 (0.72) | 1.31 (0.87–1.98) | 0.20 |
| Intrapartum fetal death | 0 | 0 | ||
| Neonatal death | 3 (0.05) | 1 (0.02) | 3.02 (0.31–29.1) | 0.37 |
| Apgar score ≤3 at 5 min | 17 (0.31) | 6 (0.11) | 2.86 (1.13–7.24) | 0.02 |
| Neonatal seizure | 3 (0.05) | 4 (0.07) | 0.76 (0.17–3.38) | 1.0 |
| Umbilical-artery blood pH ≤7.05 and base deficit in extracellular fluid ≥12 mmol/liter‡ |
3 (0.06) | 8 (0.15) | 0.37 (0.10–1.41) | 0.13 |
| Intubation for ventilation at delivery | 42 (0.76) | 27 (0.48) | 1.57 (0.97–2.54) | 0.07 |
| Neonatal encephalopathy | 4 (0.07) | 5 (0.09) | 0.81 (0.22–3.00) | 1.0 |
CI denotes confidence interval.
The primary outcome was a composite of intrapartum fetal death, neonatal death, an Apgar score of 3 or less at 5 minutes, neonatal seizure, an umbilical-artery blood pH of 7.05 or less with a base deficit of 12 mmol per liter or more, intubation for ventilation at delivery, or neonatal encephalopathy.
Data were available for 5362 patients in the open group and 5359 in the masked group. An umbilical-artery blood pH of 7.05 or less and a base deficit in blood (not extracellular fluid) of 12 mmol per liter or more occurred in 34 deliveries (0.6%) in the open group and 39 deliveries (0.7%) in the masked group (relative risk, 0.87; 95% CI, 0.55 to 1.38; P = 0.56).
There were no significant differences between the two groups in the overall rates of cesarean delivery (P = 0.30) or any operative delivery (P = 0.31). Overall, cesarean delivery because of fetal indications occurred in 287 women (5.2%) in the open group and 298 women (5.3%) in the masked group (P = 0.48). There were no significant differences between groups in any secondary outcomes (Table 4). Adverse events related to the ST-segment monitoring were rare, and the rates of individual adverse events were similar in the two groups (Table S1 in the Supplementary Appendix). There were no significant differences between any of the subgroups we assessed with respect to the effect of monitoring with ST-segment analysis on the primary outcome, cesarean delivery, and any operative delivery (P>0.05 for interaction for all comparisons) (Fig. S3, S4, and S5 in the Supplementary Appendix).
Table 4.
Maternal and Infant Outcomes.*
| Outcome | Open Group (N = 5532) |
Masked Group (N = 5576) |
P Value |
|---|---|---|---|
| Maternal | |||
| Delivery method — no. (%) | 0.17 | ||
| Spontaneous | 4269 (77.2) | 4348 (78.0) | |
| Forceps | 128 (2.3) | 103 (1.8) | |
| Vacuum-assisted | 201 (3.6) | 224 (4.0) | |
| Cesarean | 934 (16.9) | 901 (16.2) | |
| Indication for cesarean delivery — no./total no. (%) | 0.45† | ||
| Fetal indication‡ | 287/934 (30.7) | 298/901 (33.1) | |
| Dystocia | 621/934 (66.5) | 583/901 (64.7) | |
| Other | 26/934 (2.8) | 20/901 (2.2) | |
| Indication for forceps or vacuum-assisted delivery — no./total no. (%) |
0.94† | ||
| Fetal indication‡ | 225/329 (68.4) | 218/327 (66.7) | |
| Dystocia | 95/329 (28.9) | 101/327 (30.9) | |
| Other | 9/329 (2.7) | 8/327 (2.4) | |
| Duration of labor after randomization — hr | |||
| Median | 3.8 | 3.9 | 0.32 |
| Interquartile range | 2.1–6.4 | 2.2–6.7 | |
| Shoulder dystocia — no. (%) | 141 (2.5) | 158 (2.8) | 0.35 |
| Chorioamnionitis — no. (%) | 286 (5.2) | 269 (4.8) | 0.40 |
| Blood transfusion — no. (%) | 80 (1.4) | 74 (1.3) | 0.59 |
| Postpartum endometritis — no. (%) | 71 (1.3) | 88 (1.6) | 0.19 |
| Hospital stay — days | |||
| Median | 2 | 2 | 0.77 |
| Interquartile range | 2–2 | 2–2 | |
| Neonatal | |||
| Apgar score at 5 min | |||
| Median | 9 | 9 | 0.54 |
| Interquartile range | 9–9 | 9–9 | |
| Nursery admission — no. (%) | 0.28 | ||
| Well-baby nursery | 5034 (91.0) | 5106 (91.6) | |
| Intermediate care nursery or NICU | 498 (9.0) | 470 (8.4) | |
| Meconium aspiration syndrome — no. (%) | 20 (0.4) | 20 (0.4) | 0.98 |
| Major congenital malformation — no. (%) | 38 (0.7) | 23 (0.4) | 0.05 |
Plus–minus values are means ±SD. NICU denotes neonatal intensive care unit.
The P value was calculated on the basis of the entire cohort.
Fetal indication was determined according to fetal electrocardiographic ST-segment analysis guidelines in the open group and according to NICHD guidelines12 for fetal heart-rate monitoring in the masked group.
PROTOCOL ADHERENCE
The protocol subcommittee reviewed 43.9% of the tracings in the open group (2427 of 5532 tracings): 27.3% of the tracings of fetuses later delivered by spontaneous vaginal deliveries (1164 of 4269 tracings), 100% of the tracings of fetuses later delivered by operative vaginal deliveries (329 of 329 tracings), and 100% of the tracings of fetuses later delivered by cesarean deliveries (934 of 934 tracings) (Table S2 in the Supplementary Appendix). Among the 2427 women assigned to the open group whose fetus’ tracings were reviewed, 163 (6.7%) were determined not to have received care according to ST-segment analysis guidelines. In 95 cases (3.9%), expeditious delivery did not occur when recommended, and in 68 cases (2.8%), delivery was expedited when the guidelines indicated that continued observation was warranted. The primary outcome occurred in 5 of the 95 cases in which delivery did not occur as expeditiously as recommended and in 0 of the 68 in which delivery was expedited when not recommended. Even if the 5 cases were recoded as not associated with a primary outcome, there would be no significant difference between the two groups (P = 0.43). Of the 5 patients, 2 had a cesarean delivery and 3 a spontaneous vaginal delivery. An as-treated analysis in which patients for whom ST-segment analysis guidelines were not followed were included with the masked group did not reveal any significant differences between the groups (Table S3 in the Supplementary Appendix).
DISCUSSION
This large, randomized trial showed that in a U.S. population of pregnant women in whom labor and delivery were managed according to U.S. practices, ST-segment analysis as an adjunct to continuous electronic fetal monitoring neither improved neonatal outcomes nor reduced the rates of cesarean delivery or operative vaginal delivery. Although our findings differ from those of two randomized trials,8,9 they are in agreement with those of other randomized trials.17-19 Our findings of no improvement in neonatal outcomes or reduction in cesarean-delivery rates are also consistent with the results of a meta-analysis of individual-patient data from ST-segment analysis trials,20 which showed that electronic fetal monitoring with adjunctive ST-segment analysis, as compared with conventional electronic fetal monitoring alone, did not reduce the rates of neonatal metabolic acidosis, need for intubation, hypoxic–ischemic encephalopathy, a composite neonatal outcome, or cesarean delivery.20 However, that meta-analysis showed a reduction in the frequency of fetal blood sampling (which is not routine in the United States) and operative vaginal delivery among women who underwent electronic fetal monitoring and adjunctive ST-segment analysis, as compared with those who underwent electronic fetal monitoring alone.20
One possible explanation for our negative findings is that clinicians did not follow the guidelines correctly in all cases. In the open group, 5.9% of cesarean deliveries occurred when ST-segment analysis guidelines indicated that labor should continue (Table S2 in the Supplementary Appendix). Even if these 55 cases had resulted in a vaginal delivery, there would have been no significant difference in the cesarean-delivery rate. A similar result is true for operative vaginal delivery. Moreover, if we assumed that all the cases in which operative delivery was indicated had actually occurred in accordance with ST-segment analysis guidelines and that 5 cases of the primary outcome had been avoided, the incidence of the primary outcome would have remained similar in the two groups.
There could have been a Hawthorne effect (the tendency for some people to perform better when they believe that they are being watched) resulting in improved interpretation of fetal heart-rate patterns, consistent with the suggestion that a systematic approach to fetal monitoring, rather than ST-segment analysis itself, may be what is effective.11 It has also been suggested that there is a learning curve with ST-segment analysis. A reanalysis of the meta-analysis by Schuit et al. showed that labor management with the use of ST-segment analysis reduced metabolic acidosis and adverse neonatal outcomes in the second half of the trials.21,22 We incorporated a pilot phase in our study; moreover, our preplanned analysis of the first 50% of randomly assigned women according to center as compared with the second 50% showed no significant differences.
The frequency of operative vaginal delivery in our trial was lower than the 13 to 14% frequency in the meta-analysis by Schuit et al.20 Operative vaginal delivery is much more common in Europe than in the United States23 where the current rate is approximately 3%.24 In addition, two aspects of fetal heart-rate monitoring practice in Europe differ from the practice in the United States; in Europe, there is a slower horizontal scaling (1 cm per minute) on monitors (resulting in a compressed view that potentially affects the interpretation of heart-rate variability), and fetal blood sampling (for confirming or ruling out fetal acidosis) is more common. These differences could have influenced intrapartum care and may have contributed to differences between our results and those of European trials.
Our study had a lower-than-anticipated incidence of the primary outcome and, therefore, reduced power to show between-group differences. However, the lower boundary of the 95% confidence interval for the relative risk of the primary outcome was 0.87, implying at best a reduction of 13% in the open group. This is far lower than the hypothesized effect and may be of questionable clinical significance. Even with lower rates of cesarean delivery and operative vaginal delivery than expected (16.5% and 5.9%, respectively), the trial still had 80% power to detect relative differences of 11% and 15%, respectively, in these outcomes. To be eligible for ST-segment analysis, patients must have cervical dilatation of at least 2 cm and have ruptured membranes. These women are likely to have a lower cesarean-delivery rate than the overall rate in all women attempting vaginal delivery, some of whom are undergoing labor induction or in spontaneous labor at less than 2 cm. However, insofar as the clinical use of ST-segment analysis outside the trial would require the same eligibility criteria, we consider these results to be generalizable to clinical practice in the United States.
In conclusion, this large and closely monitored randomized, controlled trial showed no significant benefit of the adjunctive use of ST-segment analysis in reducing a composite of neonatal outcomes or in reducing cesarean or operative vaginal deliveries in a U.S. population undergoing conventional intrapartum continuous electronic fetal heart-rate monitoring.
Supplementary Material
Acknowledgments
Supported by grants (HD34208, HD53097, HD40545, HD40560, HD27869, HD40485, HD40512, HD27915, HD40544, HD40500, HD68282, HD68268, HD27917, HD21410, and HD36801) from the NICHD and by funding from Neoventa Medical.
We thank Kim Hill, R.N., B.S.N., and Ashley Salazar, R.N., M.S.N., W.H.N.P., for assistance with protocol development and coordination between clinical research centers; Steven J. Weiner, M.S., for protocol development, data management, and statistical analysis; and Michael W. Varner, M.D., and Catherine Y. Spong, M.D., for protocol development, oversight, and outcome review.
Footnotes
Additional members of this network are listed in the Supplementary Appendix, available at NEJM.org.
The comments and views expressed in this article are those of the authors and do not necessarily represent the views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Presented in part at the 35th annual meeting of the Society for Maternal–Fetal Medicine, San Diego, CA, February 2–7, 2015.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Food and Drug Administration Approval letter — STAN S31 fetal heart monitor. 2005 Nov 1; http://www.accessdata.fda.gov/cdrh_docs/pdf2/p020001a.pdf. [Google Scholar]
- 2.Rosén KG, Isaksson O. Alterations in fetal heart rate and ECG correlated to glycogen, creatine phosphate and ATP levels during graded hypoxia. Biol Neonate. 1976;30:17–24. [Google Scholar]
- 3.Hökegård KH, Eriksson BO, Kjellmer I, Magno R, Rosén KG. Myocardial metabolism in relation to electrocardiographic changes and cardiac function during graded hypoxia in the fetal lamb. Acta Physiol Scand. 1981;113:1–7. doi: 10.1111/j.1748-1716.1981.tb06853.x. [DOI] [PubMed] [Google Scholar]
- 4.Gelli MG, Bergström J, Hultman E, Thalme B. Heart muscle and plasma electrolytes in normal and glucose-loaded rabbit foetuses under anoxia. Acta Obstet Gynecol Scand. 1969;48:34–55. doi: 10.3109/00016346909156625. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe T, Okamura K, Tanigawara S, et al. Change in electrocardiogram T-wave amplitude during umbilical cord compression is predictive of fetal condition in sheep. Am J Obstet Gynecol. 1992;166:246–55. doi: 10.1016/0002-9378(92)91867-a. [DOI] [PubMed] [Google Scholar]
- 6.Westgate JA, Bennet L, Brabyn C, Williams CE, Gunn AJ. ST waveform changes during repeated umbilical cord occlusions in near-term fetal sheep. Am J Obstet Gynecol. 2001;184:743–51. doi: 10.1067/mob.2001.111932. [DOI] [PubMed] [Google Scholar]
- 7.Neilson JP. Fetal electrocardiogram (ECG) for fetal monitoring during labour. Cochrane Database Syst Rev. 2003;2:CD000116. doi: 10.1002/14651858.CD000116. [DOI] [PubMed] [Google Scholar]
- 8.Westgate J, Harris M, Curnow JS, Greene KR. Plymouth randomized trial of cardiotocogram only versus ST waveform plus cardiotocogram for intrapartum monitoring in 2400 cases. Am J Obstet Gynecol. 1993;169:1151–60. doi: 10.1016/0002-9378(93)90273-l. [DOI] [PubMed] [Google Scholar]
- 9.Amer-Wåhlin I, Hellsten C, Norén H, et al. Cardiotocography only versus cardiotocography plus ST analysis of fetal electrocardiogram for intrapartum fetal monitoring: a Swedish randomised controlled trial. Lancet. 2001;358:534–8. doi: 10.1016/s0140-6736(01)05703-8. [DOI] [PubMed] [Google Scholar]
- 10.Øian P, Blix E. Scarce scientific evidence for the use of cardiotocography plus fetal ECG ST interval analysis (STAN) Acta Obstet Gynecol Scand. 2014;93:570. doi: 10.1111/aogs.12414. [DOI] [PubMed] [Google Scholar]
- 11.Steer PJ, Hvidman LE. Scientific and clinical evidence for the use of fetal ECG ST segment analysis (STAN) Acta Obstet Gynecol Scand. 2014;93:533–8. doi: 10.1111/aogs.12369. [DOI] [PubMed] [Google Scholar]
- 12.American College of Obstetricians and Gynecologists ACOG practice bulletin no. 106: intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114:192–202. doi: 10.1097/AOG.0b013e3181aef106. [DOI] [PubMed] [Google Scholar]
- 13.Siggaard-Andersen O. An acid-base chart for arterial blood with normal and pathophysiological reference areas. Scand J Clin Lab Invest. 1971;27:239–45. doi: 10.3109/00365517109080214. [DOI] [PubMed] [Google Scholar]
- 14.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 15.Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901. doi: 10.1097/01.AOG.0000445580.65983.d2. Executive summary: neonatal encephalopathy and neurologic outcome, second edition. [DOI] [PubMed] [Google Scholar]
- 16.Bloom SL, Spong CY, Thom EA, et al. Fetal pulse oximetry and cesarean delivery. N Engl J Med. 2006;355:2195–202. doi: 10.1056/NEJMoa061170. [DOI] [PubMed] [Google Scholar]
- 17.Ojala K, Vääräsmäki M, Mäkikallio K, Valkama M, Tekay A. A comparison of intrapartum automated fetal electrocardiography and conventional cardiotocography — a randomised controlled study. BJOG. 2006;113:419–23. doi: 10.1111/j.1471-0528.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 18.Vayssière C, David E, Meyer N, et al. A French randomized controlled trial of ST-segment analysis in a population with abnormal cardiotocograms during labor. Am J Obstet Gynecol. 2007;197(3):299.e1–299.e6. doi: 10.1016/j.ajog.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Westerhuis ME, van Horen E, Kwee A, van der Tweel I, Visser GH, Moons KG. Inter- and intra-observer agreement of intrapartum ST analysis of the fetal electrocardiogram in women monitored by STAN. BJOG. 2009;116:545–51. doi: 10.1111/j.1471-0528.2008.02092.x. [DOI] [PubMed] [Google Scholar]
- 20.Schuit E, Amer-Wahlin I, Ojala K, et al. Effectiveness of electronic fetal monitoring with additional ST analysis in vertex singleton pregnancies at >36 weeks of gestation: an individual participant data metaanalysis. Am J Obstet Gynecol. 2013;208(3):187.e1–187.e13. doi: 10.1016/j.ajog.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Rosén KG. ST analysis reviewed. Am J Obstet Gynecol. 2013;209:394. doi: 10.1016/j.ajog.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Schuit E. Reply: to PMID 23333546. Am J Obstet Gynecol. 2013;209:394–5. doi: 10.1016/j.ajog.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Begley C, Devane D, Clarke M, et al. Comparison of midwife-led and consultant-led care of healthy women at low risk of childbirth complications in the Republic of Ireland: a randomised trial. BMC Pregnancy Childbirth. 2011;11:85. doi: 10.1186/1471-2393-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–65. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.