Abstract
Objective
To evaluate the attention paid to larger sizes of graphic health warnings (GHWs) embedded within cigarette advertisements so as to assess their impacts on rural smokers.
Methods
Daily smokers (N = 298) were randomly assigned to view a cigarette advertisement with 3 conditions: 2 intervention conditions with GHW comprising 20% or 33% of the ad area, or a text-only control. Eye-tracking software measured attention in milliseconds. Binary outcome mediation was conducted.
Results
Intervention participants spent 24% of their time viewing the GHWs, compared to 10% for control (p < .01). The odds of GHW recall in the combined (20% and 33%) intervention group were 3.3 times higher than controls. Total dwell time mediated 33% of the effect of the graphic condition on any recall.
Conclusions
GHWs in 20% of cigarette advertisement space attracted significantly more attention than text-only warnings; larger GHWs did not increase attention. Attention was significantly associated with warning recall; total time viewing mediated warning recall. Tobacco ads should include GHWs to attract the attention of smokers.
Keywords: smoking, graphic warning, advertising, eye tracking, health communication
Cigarette advertising and promotion expenditures in the United States exceed $8.3 billion annually with approximately $23 million spent on magazine advertising.1 This size of this amount demonstrates that advertising is a robust communication channel between tobacco companies and consumers. Exposure to cigarette advertisements predicts brand loyalty and brand switching behavior among smokers, and has been associated with increased cigarette consumption and continuation of smoking.2 Tobacco product advertisements are inherently at odds with the overall intent of health warning labels, which aim to communicate information about the risks of tobacco use and promote cessation. Graphic warning labels convey the known dangers of tobacco products and are required on tobacco product packaging in over 60 countries globally.3 Most of these countries also have implemented comprehensive bans on tobacco advertising, rendering graphic warnings within advertisements unnecessary.4 In 2009, the United States Congress passed the Family Smoking Prevention and Tobacco Control Act, requiring graphic warnings to be placed on cigarette packaging and on the top 20% of advertisements for cigarettes. Although the Food and Drug Administration (FDA) issued a rule implementing this requirement, the FDA’s rule was legally challenged in 2012,5 and a new graphic warning labels are under development.6
Although the processing of information in health-warning messages is complex, to be effective, a critical first step is to draw attention so that consumers can understand, recall, and use the information for health decision making.7,8 Thus, attention paid to health warning labels is hypothesized to be necessary for informing consumers regarding smoking risks, and it may influence behavioral intent to quit smoking.9 We can improve our understanding of consumer reactions to GHWs (and the effectiveness of GHW characteristics) through research using eye-tracking equipment, which allows for detailed capture of precise eye movements when an individual is exposed to visual stimuli.10 A limited number of eye-tracking studies have focused on GHWs and demonstrated that graphic images draw greater attention than non-graphic warnings.11,12 Other studies, however, have found that smokers avoid warnings placed on product packaging.13-15
Regionally, rural residents have a higher prevalence of smoking and are more likely to be exposed to secondhand smoke, creating a disproportionate increase in risk for tobacco-related illness.16 In addition, tobacco control campaigns tend to focus on urban media markets,17 which further reduce the reach of public health messages among rural residents. Compared to the rest of the nation, Ohio is a region with higher rates of smoking, smokeless and dual use of tobacco products,18 with the highest rates observed within the rural, Appalachian counties of the state.19,20 The nature of tobacco use in this largely rural region is known to be complex, with environmental, psychological, and social influences that portray tobacco products as traditional and normative.21-23 Due to lower penetration of formal and informal tobacco control policies in this area,16 it is reasonable to assume residents have a greater exposure to tobacco use as well as greater exposure to tobacco marketing which contribute to the vulnerability to tobacco use for rural Appalachian residents.24
Outside of the US, countries have adopted GHWs, mostly on product packaging,4 that exceed the recommendations of the Framework Convention on Tobacco Control (FCTC), suggesting movement toward larger warning messages. As non-eye-tracking survey data have supported that warning size increases warning effectiveness,25 the purpose of this study was to evaluate the attention paid to larger sizes of health warning labels embedded within cigarette advertisements to assess their impacts on the vulnerable population of Ohio Appalachian smokers. Our primary hypothesis was that smokers exposed to larger GHWs would demonstrate increased attention, as measured by eye-tracking equipment dwell time (in seconds), when compared to those exposed to smaller GHWs or text-only warnings. Additionally, we hypothesized that increased attention (as measured by dwell time) would mediate an expected association between larger versus smaller or no GHWs and greater recall.
METHODS
Participants
Data were gathered as a part of the Ohio Health Warning Label (OHWL) study on tobacco users within a rural, underserved region (Ohio Appalachia) between April and October 2013. A convenience sample was recruited using flyers and brochures. Participants were invited to provide perceptions of advertising for consumer products, with recruitment materials distributed to businesses and advertisements placed in local newspapers; being a current smoker was not identified as a requirement for participation. A phone screening determined if participants met study eligibility criteria: current daily cigarette smoker; lifetime history of smoking at least 100 cigarettes; aged 21 or older; and living in one of the 32 counties designated as a part of Ohio Appalachia. Participants were excluded if they intended to quit within 30 days or if they had a history of certain eye conditions, such as macular degeneration, glaucoma, or cataracts, which are known to interfere with eye tracking equipment. Participants who completed the experiment received a $50 gift card; those unable to be calibrated on eye-tracking equipment received a $10 gift card.
Procedures
All research sessions were conducted in private areas within an office environment. Trained interviewers explained the study and obtained signed informed consent. Participants were seated comfortably in a chair within a typical viewing distance (24 to 32 inches) from a monitor equipped with the eye-tracking system and underwent calibration procedures 3 times to assure data quality before the initiation of the experiment.
Participants were instructed to imagine they were flipping through a magazine while they moved at their own pace through the experiment, answering an on-screen question after each advertisement in order to standardize a participant’s gaze between advertisements. Each participant viewed a total of 6 advertisements; one cigarette ad (always shown fourth) and 5 others for common consumer products: alcohol; a USB drive; macaroni and cheese dinner; orange juice; and an energy drink. Table 1 shows the chosen brands, corresponding survey items, and response categories for each on-screen survey question.
Table 1. Product Advertisements and Post-advertisement Survey Items from the Ohio Health Warning Label (OHWL) Study.
| Product | Brand | Post-advertisement Survey Item | Response Categories |
|---|---|---|---|
| USB Drive | iFlashDrive | I feel confident using technology. | 1-10 scale from strongly agree to strongly disagree |
| Orange Juice | Tropiciana | There is at least one full serving of fruit in 100% juice. |
1-10 scale from strongly agree to strongly disagree |
|
Macaroni and
Cheese |
Kraft | This product is a healthy choice for my family. | 1-10 scale from strongly agree to strongly disagree |
| Cigarettes | American Spirit | I am craving a cigarette right now. | 1-10 scale from strongly agree to strongly disagree |
| Energy Drink | 5 hour energy | This product is a safe way to boost my energy. | 1-10 scale from strongly agree to strongly disagree |
| Alcohol | Jose Cuervo | This advertisement is meant for people who are…? | <18, 18-20, 21+ years old |
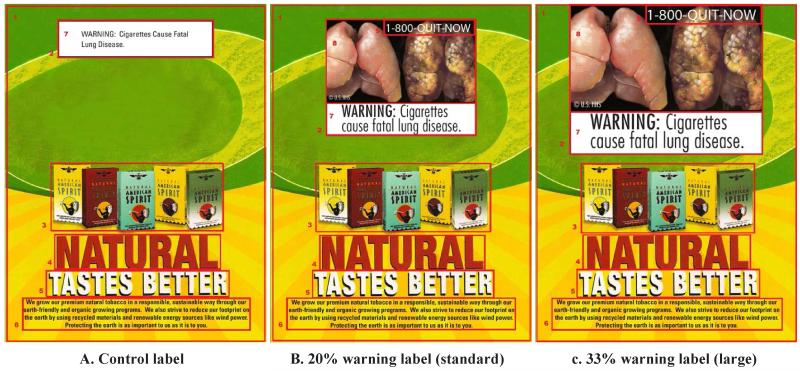
The cigarette brand selected for this experiment was based on it not being popular among smokers in Appalachian Ohio (Wewers ME et al, unpublished data, January 2012) and its use of simple graphic and text imagery. An unpopular brand was selected to minimize differential attention and recall, as smokers are highly brand loyal, and form beliefs and perceptions of the product from marketing.12,26,27 All participants viewed the selected advertisement, and were randomly assigned to one of 3 warning label study conditions: a control condition with text only, or a graphic warning label that covered either 20% or 33% of the ad area (intervention conditions, referred to as large and standard graphic, respectively). Intervention conditions differed only on the amount of space that was occupied within the ad. The amount of space allocated to the non-warning-label portion of the advertisement was fixed across all conditions; blank space (consistent with the overall aesthetics of the ad) varied by condition and was largest in the text-only condition and smallest in the 33% condition. Nine versions of the FDA-proposed warning labels28 were used, yielding 27 unique tobacco advertisements across these 3 study conditions; the control condition matched the 9 text-only messages of the warning messages. At the end of the experiment, a survey was administered by a trained interviewer. The entire protocol took approximately 45 minutes to complete.
Measures
Eye tracking measures
BeGaze software (SensoMotoric Instruments, 60 Hz RED System) was used to display the experimental stimuli (advertisements) and capture the eye-tracking data. For this analysis, the term “warning label” refers to any warning message that uses text-only or text and graphic imagery, “warning text” refers only to the textual message of the portion of a warning label, and “graphic image” refers only to the visual imagery of a warning label. The primary outcome measure was dwell time (in seconds) as a measure of attention on specific areas of interest; these areas were defined a priori for all advertisements viewed. In particular, areas of interest (AOIs) were constructed for both the warning label and the advertisement itself (the non-warning label space). Figure 1 displays the 3 study conditions. These AOIs included the (1) whole advertisement, (2) warning label, (3) cigarette packages, 2 large blocks of text with the words (4) “Natural,” (5) “Tastes better,” (6) a block of the advertisement small text, (7) graphic warning text (eg, “cigarettes are addictive”), (8) total graphic warning label, and (9) Quitline (1-800-QUIT-NOW telephone number). For each AOI listed above, the following things were measured: (1) the duration of dwell time in it in seconds; (2) the proportion of viewing time in it (calculated based on its duration of dwell time divided by total dwell time on the advertisement): (3) the first AOI to be viewed, referred to as the first fixation; and (4) revisits, measured as the sum of any repeat views to the AOI after a participant’s initial viewing. Any sections of the advertisement that were not viewed were counted as zero revisits.29
Figure 1. Cigarette Warning Advertisements with Study Conditions: Control, Standard Warning Label, Large Warning Label.
Note.
Areas of interest are labeled as (1) whole advertisement, (2) warning label, (3) packages, (4) “Natural,” (5) “Tastes better,” (6) advertisement small text, (7) graphic warning text, (8) total graphic warning, (9) Quit line.
Survey measures
Participant recall of the health warning label was determined by a series of questions that followed the conclusion of the experiment (eg, “What do you remember about the cigarette advertisement? You can describe any pictures you remember and all of the words you can recall.”) No visual aids were given to participants as a recall aid, and field staff recorded participant responses verbatim. Two trained coders (EGK, SEK) reviewed the responses independently, and assigned codes dichotomously (yes/no) for several elements: any recall of the GHW; recall of any elements of the warning text; recall of the graphic image; and recall of the Quitline (1-800-QUIT NOW). For all 4 recall elements, the kappa coefficient for inter-rater reliability was high, ranging from 98% to 100% (95% confidence interval of 95%-100%); consensus meetings were held to resolve coding disagreements.
Survey data were captured by self-report during the screening process, the experiment, and post-experiment. Items included demographic factors of age, race/ethnicity, annual household income, marital status, and sex. Behavioral factors included age of smoking initiation (in years), score (0 to 6) on the Heaviness of Smoking index,30 and a history of quitting smoking for at least 24 hours (yes/no).
Analysis
Eye-tracking measures were compared among all 3 conditions. Differences in continuous outcome measures by group were assessed via ANOVA F-tests. No gross violations of the equal variance assumption of ANOVA were found in any of the continuous variables assessed. Differences in binary outcomes (including any recall) were assessed via Wald chi-square tests. If the primary comparison (among all groups) was statistically significant, pairwise comparisons were done using Tukey (ANOVA) and Bonferroni (chi-square) post hoc tests.31
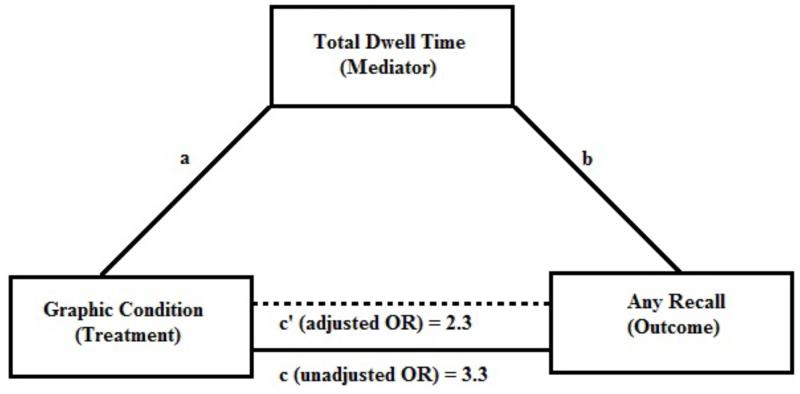
Binary outcome mediation32 analysis by logistic regression was used to explore the possibility that dwell time on the warning label mediated the effect of study condition on recall of the warning label. For these analyses only, the 2 graphic conditions were collapsed into one group so that the comparison was graphic versus text warning. Briefly, mediation analysis decomposes the total effect (c) into the mediated (indirect) effect (ab) and the direct effect (c’). If all of the effect of the treatment (graphic condition) could be explained by the mediator (dwell time), the remaining direct effect (after adjustment for the mediator) would be null.
Statistical significance was set at .05, and no adjustments were made for multiple comparisons. Due to the highly correlated outcomes, a Bonferroni correction (using alpha = .0029) is likely conservative. The sample size was estimated based on modest differences published in previous research.11 Data were analyzed using SAS 9.3 (SAS Institute, Inc.; Cary, North Carolina) and STATA 13 (StataCorp; College Station, Texas).
RESULTS
Overall, 300 participants completed the eye-tracking experiment and post-experiment survey; 12 participants were unable to be calibrated to the eye tracking equipment and 2 participants were excluded from the final analyses due to problems with incomplete eye-tracking data. The final sample (N = 298) was mostly female (66%), with an average age of 40 years; most participants (~65%) had lower educational attainment (high school or less) and lower income (below $25,000 annual household income) as the data in Table 2 show. The mean age of becoming a regular smoker was 17.5 years and participants reported an average of 21 years smoking. Participants smoked an average of 18 cigarettes per day and the mean heaviness of smoking was 2.98, approximately equal to the cutoff of 3.0 that defines high nicotine dependence.33 The majority smoked Marlboro (61%) and none reported current use of the experimental brand (data not shown.) Participants were balanced between study conditions, and no statistically significant differences existed in demographic characteristics among study conditions.
Table 2. Descriptive Characteristics of Appalachian Smokers from the Ohio Health Warning Label (OHWL) Study.
| OHWL study (N = 298) Participant Characteristics |
Total (N = 298) |
Text Only (N = 103) |
Standard Graphic (N = 97) |
Large Graphic (N = 98) |
|---|---|---|---|---|
| Demographics | ||||
|
| ||||
| Male | 33.2% | 32.0% | 34.0% | 33.7% |
|
| ||||
| Mean age (SD) (in years) | 40.5 (11.7) | 39.7 (11.8) | 40.5 (10.5) | 41.5 (12.8) |
|
| ||||
| Mean (SD) household size | 3.0 (1.5) | 3.1 (1.6) | 3.2 (1.6) | 2.8 (1.4) |
|
| ||||
| % Household income | ||||
| <$15,000 | 37.9% | 43.7% | 33.0% | 36.7% |
| $15-$24,999 | 27.2% | 25.2% | 25.8% | 30.6% |
| $25-$34,999 | 15.8% | 14.6% | 20.6% | 12.2% |
| $35-$49,999 | 10.4% | 9.7% | 10.3% | 11.2% |
| ≥$50,000 | 8.7% | 6.8% | 10.3% | 9.2% |
|
| ||||
| % Education | ||||
| <High school | 22.5% | 25.2% | 18.6% | 23.5% |
| High school | 45.0% | 43.7% | 45.4% | 45.9% |
| >High school | 32.5% | 31.1% | 36.0% | 30.6% |
|
| ||||
| Has health insurance | 68.8% | 67.0% | 73.2% | 66.3% |
| Smoking Behaviors | ||||
|
| ||||
| Mean age (SD) of initiation | 17.5 (5.6) | 17.6 (5.8) | 17.6 (6.2) | 17.3 (4.9) |
|
| ||||
| % (n) Ever made serious quit attempt | 80.5% (239) | 83.5% (86) | 81.4% (79) | 76.3% (74) |
|
| ||||
| Mean (SD) years smoking | 21.9 (11.9) | 20.7 (11.8) | 22.5 (10.6) | 22.7 (13.2) |
|
| ||||
| Mean (SD) cigarettes per day | 18.1 (8.7) | 18.1 (8.7) | 17.3 (8.4) | 18.8 (9.1) |
|
| ||||
| Mean (SD) heaviness of smoking index | 2.98 (1.52) | 2.96 (1.47) | 2.93 (1.58) | 3.04 (1.5) |
Viewing of the Advertisement
Table 3 summarizes the viewing patterns by study condition. The entire cigarette advertisement including the warning label was viewed, on average, for 12 seconds by participants in all 3 conditions (F=0.58, df=2, p = .56); total dwell time for the comparison advertisements did not differ significantly by condition but was shorter than the cigarette advertisement (result for the alcohol ad shown in the first row of Table 3; other data not shown are available by request). There were statistically significant differences in the mean dwell time on the cigarette packages by study condition (F=3.67, df=2, p = .03). There were no other statistically significant differences in the dwell time paid to other (non-warning) cigarette advertisement elements by study condition.
Table 3. Means (SD) and Proportions (N) for Dwell Times and Fixations by Condition of Warning Label and Non-warning Label of a Cigarette Advertisement from the Ohio Health Warning Label (OHWL) Study.
| OHWL study (N = 298) | Text only (N = 103) |
Standard graphic (N = 97) |
Large graphic (N = 98) |
p-value |
|---|---|---|---|---|
| Seconds of Dwell Time (SD) | ||||
| Comparison alcohol ad | 7.66 (4.11) | 6.87 (3.87) | 7.32 (4.50) | .42 |
| Cigarette ad (including warning label) | 12.74 (9.36) | 11.60 (7.52) | 12.81 (9.46) | .56 |
| “Natural” | 0.87 (1.15) | 0.72 (0.64) | 0.60 (0.50) | .06 |
| “Tastes better” | 1.40 (1.79) | 1.11 (1.15) | 0.97 (1.20) | .09 |
| Cigarette packages | 1.66 (1.39) | 1.31 (1.40) | 1.25 (1.14) | .03 b |
| Ad small text | 3.63 (5.19) | 2.25 (3.59) | 2.59 (3.82) | .06 |
| Warning label | 0.99 (1.20) | 2.36 (1.94) | 2.53 (1.83) | < .01 a,b |
| Percentage of Total Time | ||||
| % on warning label | 9.6 (11.7) | 24.1 (17.4) | 24.7 (16.8) | < .01a,b |
| % on warning text only | 9.6 (11.7) | 10.4 (9.9) | 11.2 (10.4) | .50 |
| % on graphic image only* | -- | 13.7 (12.5) | 13.7 (11.2) | .99 |
| % on Quitline* | -- | 1.3 (2.3) | 1.4 (2.3) | .59 |
| Fixation and Revisits | ||||
| First fixation on warning label | 21.4% (n=22) | 40.2% (n=39) | 41.8% (n=41) | < .01 a,b |
| Fixated on warning label | 85.4% (n=88) | 92.8% (n=90) | 91.8% (n=90) | .17 |
| # of revisits to warning label** | 0.6 (0.9) | 1.3 (1.4) | 1.3 (1.2) | < .01 a,b |
These comparisons include only individuals in the 2 intervention conditions had the opportunity to view the graphic warning elements
Among those who fixated on the warning label at least once
Note.
Shaded boxes indicate p < .05 when the 3 conditions were compared
SD = standard deviation
Pairwise comparison of text vs standard graphic significant
Pairwise comparison of text vs large graphic significant
Pairwise comparison of standard graphic vs large graphic significant
Total dwell time on the warning label was higher in both graphic conditions compared to the text-only condition (F=25.7, df=2, p < .01). Participants in both intervention conditions spent approximately 24% of their dwell time viewing the warning label, compared to less than 10% of the dwell time for control participants (F=31.2, df=2, p < .01). Almost twice as many participants in the intervention conditions viewed the warning label first, compared to those in the control condition (42%, 40%, and 21% in the large graphic, standard graphic, and text-only conditions, respectively; χ2=11.6, df=2, p = .03). Participants in both intervention conditions averaged 1.3 revisits to the area of the warning label, compared to 0.6 revisits for those in the control condition (F=9.5, df=2, p < .01).
Recall of the Warning Label and Mediation by Dwell Time
Because there were no statistically significant differences in dwell time between the 2 intervention conditions, these graphic conditions were collapsed for the mediation analysis. Recall of any portion of the warning label was higher in both graphic conditions (56% and 63%, respectively, in the large and standard graphic groups) compared to the text-only control condition (31%; χ2=22.7, p < .01; Table 4). Participants in the 2 graphic conditions were similar with respect to recall of the graphic image itself, the text of the warning label, and the Quitline.
Table 4. Percentage of Any Unaided Recall of Graphic Warning Label Elements by Condition from the Ohio Health Warning Label (OHWL) Study.
| OHWL study (N = 298) | Control (N = 103) |
20% (N = 97) |
33% (N = 98) |
p-value |
|---|---|---|---|---|
| Any warning label recall (N) | 31% (32) | 63% (61) | 56% (55) | < .01 |
| Any text element recall (N) | 16% (16) | 27% (26) | 20% (20) | 0.15 |
| Any graphic image recall (N) a | -- | 40% (39) | 39% (38) | 0.84 |
| Quit line recall (N) a | -- | 6% (6) | 4% (4) | 0.51 |
Note.
Shaded boxes indicate p < .05
These comparisons include only individuals in the intervention conditions who viewed the graphic warning elements
Mediation Analysis
In the unadjusted analysis, the odds of any recall of the warning label in the combined graphic warning label group were 3.3 times the odds of any recall in the control group. After adjusting for total dwell time on the warning label, this odds ratio decreased to 2.3 (Figure 2); total dwell time mediated 33% of the effect of graphic condition on any recall. This effect was statistically significant as the bootstrapped 95% CI excluded 0 (95% CI: 15% to 67%).
Figure 2. Diagram of Mediation Analysis.
DISCUSSION
Our study is the first to evaluate the impact of warning label size within cigarette advertisements on consumer attention. It provides empirical support for the proposition that graphic warning labels occupying at least 20% of advertising space attract significantly greater attention than smaller text only warnings. Not only did they attract more attention, but this attention mediated the effects of graphicness on memory for the warnings. In other words, having a graphic warning compared to a text-only warning caused greater attention to be drawn to it which, in turn, led to greater recall of the warning. Research on graphic warning labels implemented outside the US has indicated that warning label size is related to warning effectiveness, measured by reading and noticing GHWs, cognitive responses of thoughts of harm or quitting, or behavioral intentions to change smoking behavior.9,34 Although we did not find that increasing the size of graphic warnings from 20% to 33% of an advertisement’s space significantly increased smokers’ attention or attracted repeat views, our findings support that smokers are attending to and recalling health warning messages. Attention and noticing GHWs stimulate reactions from smokers that predict quit attempts.9
Our findings regarding the proportion of time spent on the advertisement relative to the warning label demonstrated that graphic warnings not only attract attention, but distract smokers from viewing other visual portions of the advertisement. We believe these findings highlight the importance of the GHWs themselves, as well as the context in which they are viewed by consumers. Two smaller eye-tracking studies where participants avoided pack-based warning messages, instead focused on cigarette brand information.13,35 Both studies used non-preferred brands of cigarette packs rather than product advertisements; thus, GHWs on cigarette packs may produce different responses from the same GHWs in advertisements. Alternatively, it may be that consumers’ attention threshold was reached at 20% of advertisements space; additional studies are warranted to explore means to attract the attention of smokers to GHWs in advertisements, as it is critically important to make the warnings less “invisible” to the consumer’s eye. Advertising studies have investigated the issue of congruency, or fit between the advertiser and the editorial content, and found improved consumer recognition of advertisements and incongruency improved attention and recall.36-38 Regardless of placement on tobacco products or advertisements, future research also is needed to improve understanding of warning-label factors that can be optimized to avoid message fatigue and sustain positive effects on consumers, especially considering that tobacco advertisements are likely to change in response to GHWs being added to advertising space.25
Regardless of the presence on packs or within ads, our results contribute to a robust research base demonstrating that GHWs are more effective at influencing consumer attention and/or desirable tobacco reduction behaviors than text-only messages.12,25,35,39-50 As the research on GHWs continues to grow, additional research is needed to understand the optimum characteristics of warning labels within advertisements for all tobacco products, including those products that will be newly subject to FDA regulation following the FDA “deeming” process announced in April 2014.51
The present research has some important limitations. Participants were excluded if they intended to quit smoking in the next month, so the current findings may not be applicable to smokers with immediate quit intentions. Although brain imaging studies have demonstrated variations in the response to individual GHW imagery,39 the present study was not powered to evaluate differences among the 9 individual GHWs viewed, as roughly 30 participants viewed each of the warnings. Each participant viewed a single advertisement of a non-dominant cigarette brand that used textual and graphic components. Such an ad may produce different results from advertisements that use other types of images, including people and preferred brands. Further, we cannot evaluate whether the use of an implicit health claim (the emphasis on the word “Natural” within the ad) impacted smokers’ attention or recall. The selected study design also did not include a text-only condition at 20% of the advertisement space, so we cannot determine whether greater attention could be attracted with a larger text-only warning label. Nonetheless, our results suggest a consistency in the total viewing time regardless of the portion of space allocated to the health warning, and a reduction in time spent on one ad component that appeared due to the presence versus absence of a graphic image rather than being based on the portion of allocated space to the health warning. The unaided recall was coded as any mention of warning label elements, and did not differentiate between recall of text message or warning imagery. The present study focused on rural smokers, but future studies should consider rural residences along with other vulnerable tobacco users including youth, young adults, and others considered high-risk tobacco users.
Graphic warning labels are used around the globe; they are recommended as an effective tobacco control tool on both products and advertisements. The guidelines for the Framework Convention on Tobacco Control (FCTC) note that each country “whose constitution or constitutional principles impose constraints on undertaking a comprehensive ban should, under Article 13 of the Convention, apply restrictions that are as comprehensive as possible in the light of those constraints.”52 Given that the First Amendment likely precludes a comprehensive ban on tobacco advertising in the US, the FDA instead should move forward with requiring graphic health warnings on tobacco advertisements.
IMPLICATIONS FOR TOBACCO REGULATION
Understanding the optimum characteristics of health warning labels is critically important for policymakers to consider as they seek to reduce the prevalence of smoking. Although the images used in the present study will be redesigned by the FDA, our findings support the value of placing warning labels on at least 20% of the area of tobacco advertisements, as required by the Family Smoking Prevention and Tobacco Control Act. Our results provide empirical support for the use of graphic warnings within cigarette advertising as a means to attract attention of smokers.
Acknowledgments
Research reported in this publication was supported by grant number R01CA129771-03S1 from the National Cancer Institute and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
Human Subjects Approval Statement
The study protocol was approved by The Ohio State University Institutional Review Board.
Conflict of Interest Disclosure Statement
All authors of this article declare they have no conflicts of interest.
Contributor Information
Elizabeth G. Klein, Division of Health Behavior & Health Promotion, The Ohio State University College of Public Health, Columbus, OH.
Abigail B. Shoben, Division of Biostatistics, The Ohio State University College of Public Health, Columbus, OH.
Sarah Krygowski, Division of Health Behavior & Health Promotion, The Ohio State University College of Public Health, Columbus, OH.
Amy Ferketich, Division of Epidemiology, The Ohio State University College of Public Health, Columbus, OH.
Micah Berman, Division of Health Services, Management and Policy, The Ohio State University College of Public Health, Columbus, OH.
Ellen Peters, Department of Psychology, The Ohio State University College of Arts & Sciences, Columbus, OH.
Unnava Rao, Department of Marketing and Logistics, The Ohio State University Fisher College of Business, Columbus, OH.
Mary Ellen Wewers, Division of Health Behavior & Health Promotion, The Ohio State University College of Public Health, Columbus, OH.
References
- 1.Federal Trade Commission [Accessed March 15, 2015];Cigarette report for 2011. 2013 Available at: http://www.ftc.gov/sites/default/files/documents/reports/federal-trade-commission-cigarette-report-2011/130521cigarettereport.pdf/
- 2.Capella M, Webster C, Kinard BR. A review of the effect of cigarette advertising. Int J Res Mark. 2011;28(3):269–279. [Google Scholar]
- 3.Canadian Cancer Society [Accessed March 13, 2015];Cigarette package health warnings: international status report. 2014 Available at: http://global.tobaccofreekids.org/files/pdfs/en/WL_status_report_en.pdf/
- 4.World Health Organization [Accessed March 15, 2015];Global progress report on implementation of the WHO Framework Convention. 2012 Available at: http://apps.who.int/iris/handle/10665/79170.
- 5.Almasy S. [Accessed March 12, 2015];FDA changes course on graphic warning labels for cigarettes. 2013 Available at: http://www.cnn.com/2013/03/19/health/fda-graphic-tobacco-warnings/
- 6.Kraemer JD, Baig SA. Analysis of legal and scientific issues in court challenges to graphic tobacco warnings. Am J Prev Med. 2013;45(3):334–342. doi: 10.1016/j.amepre.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Kees J, Burton S, Andrews JC, Kozup J. Understanding how graphic pictorial warnings work on cigarette packaging. Journal of Public Policy & Marketing. 2010;29(2):265–276. [Google Scholar]
- 8.Peters E. Anticipating barriers to the communication of critical information. In: Schulkin J, Anderson B, editors. Numerical Reasoning in Judgments and Decision Making about Health. Cambridge University Press; Cambridge, UK: 2014. [Google Scholar]
- 9.Borland R, Yong HH, Wilson N, et al. How reactions to cigarette packet health warnings influence quitting: findings from the ITC Four-Country survey. Addiction. 2009;104(4):669–675. doi: 10.1111/j.1360-0443.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchwoski A. Eye Tracking Methodology - Theory and Practice. 2nd ed. Springer-Verlag; London, UK: 2007. [Google Scholar]
- 11.Peterson E, Thomsen S, Lindsay G, John K. Adolescents’ attention to traditional and graphic tobacco warning labels: an eye-tracking approach. J Drug Educ. 2010;40(3):227–244. doi: 10.2190/DE.40.3.b. [DOI] [PubMed] [Google Scholar]
- 12.Strasser AA, Tang KZ, Romer D, et al. Graphic warning labels in cigarette advertisements: recall and viewing patterns. Am J Prev Med. 2012;43(1):41–47. doi: 10.1016/j.amepre.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maynard OM, Attwood A, O’Brien L, et al. Avoidance of cigarette pack health warnings among regular cigarette smokers. Drug Alcohol Depend. 2014;136:170–174. doi: 10.1016/j.drugalcdep.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maynard OM, Munafo MR, Leonards U. Visual attention to health warnings on plain tobacco packaging in adolescent smokers and non-smokers. Addiction. 2013;108(2):413–419. doi: 10.1111/j.1360-0443.2012.04028.x. [DOI] [PubMed] [Google Scholar]
- 15.Munafo MR, Roberts N, Bauld L, Leonards U. Plain packaging increases visual attention to health warnings on cigarette packs in non-smokers and weekly smokers but not daily smokers. Addiction. 2011;106(8):1505–1510. doi: 10.1111/j.1360-0443.2011.03430.x. [DOI] [PubMed] [Google Scholar]
- 16.Vander Weg MW, Cunningham CL, Howren MB, Cai X. Tobacco use and exposure in rural areas: findings from the Behavioral Risk Factor Surveillance System. Addict Behav. 2011;36(3):231–236. doi: 10.1016/j.addbeh.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Duke JC, Vallone DM, Allen JA, et al. Increasing youths’ exposure to a tobacco prevention media campaign in rural and low-population-density communities. Am J Public Health. 2009;99(12):2210–2216. doi: 10.2105/AJPH.2008.155127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention State-specific prevalence of cigarette smoking and smokeless tobacco use – United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(43):1400–1406. [PubMed] [Google Scholar]
- 19.Ohio Medicaid Assessment Survey . The Ohio Colleges of Medicine Government Resource Center; Columbus, Ohio: [Accessed March 25, 2015]. 2012. Available at: http://grc.osu.edu/omas/datadownloads/2012omaspublicdata/ [Google Scholar]
- 20.Wewers ME, Katz M, Fickle D, Paskett ED. Risky behaviors among Ohio Appalachian adults. Prev Chronic Dis. 2006;3(4):A127. [PMC free article] [PubMed] [Google Scholar]
- 21.Ahijevych K, Kuun P, Christman S, et al. Beliefs about tobacco among Appalachian current and former users. Appl Nurs Res. 2003;16(2):93–102. doi: 10.1016/s0897-1897(03)00009-0. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Nemeth JM, Klein EG, et al. Adolescent and adult perceptions of traditional and novel smokeless tobacco products and packaging in rural Ohio. Tob Control. 2014;23(3):209–214. doi: 10.1136/tobaccocontrol-2012-050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemeth J, Liu ST, Klein EG, et al. Factors influencing smokeless tobacco use in rural Ohio Appalachia. J Commun Health. 2012;37(6):1208–1217. doi: 10.1007/s10900-012-9556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein E, Ferketich AK, Abdel-Rasoul M, et al. Smokeless tobacco marketing and sales practices in Appalachian Ohio following federal regulations. Nicotine Tob Res. 2012;14(7):880–884. doi: 10.1093/ntr/ntr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borland R, Wilson N, Fong GT, et al. Impact of graphic and text warnings on cigarette packs: findings from four countries over five years. Tob Control. 2009;18(5):358–364. doi: 10.1136/tc.2008.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollay RW, Dewhirst T. The dark side of marketing seemingly “Light” cigarettes: successful images and failed fact. Tob Control. 2002;11(Suppl 1):i18–i31. doi: 10.1136/tc.11.suppl_1.i18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiffman S, Pillitteri JL, Burton SL, et al. Smokers’ beliefs about “Light” and “Ultra Light” cigarettes. Tob Control. 2001;10((Suppl)1):i17–i23. doi: 10.1136/tc.10.suppl_1.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Food & Drug Administration [Accessed June 3, 2014];Cigarette health warnings. 2013 Available at: http://www.fda.gov/tobaccoproducts/labeling/labeling/cigarettewarninglabels/default.htm/
- 29.Smith K, Moriarty S, Kenney K, Barbatsis G. Handbook of Visual Communication: Theory, Methods and Media. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- 30.Borland R, Yong HH, O’Connor RJ, et al. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010;12(Suppl):S45–S50. doi: 10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosner B. Fundamentals of Biostatistics. 7th ed. Brooks/Cole Cengage Learning; Boston, MA: 2011. [Google Scholar]
- 32.Mackinnon D, Dwyer JH. Estimating mediated effects in prevention studies. Evaluation Rev. 1993;17(2):144–158. [Google Scholar]
- 33.Schnoll RA, Goren A, Annunziata K, Suaya JA. The prevalence, predictors and associated health outcomes of high nicotine dependence using three measures among US smokers. Addiction. 2013;108(11):1989–2000. doi: 10.1111/add.12285. [DOI] [PubMed] [Google Scholar]
- 34.Azagba S, Sharaf MF. The effect of graphic cigarette warning labels on smoking behavior: evidence from the Canadian experience. Nicotine Tob Res. 2013;15(3):708–717. doi: 10.1093/ntr/nts194. [DOI] [PubMed] [Google Scholar]
- 35.Süssenbach P, Niemeier S, Glock S. Effects of and attention to graphic warning labels on cigarette packages. Psychol Health. 2013;28(10):1192–1206. doi: 10.1080/08870446.2013.799161. [DOI] [PubMed] [Google Scholar]
- 36.Burke M, Hornof A, Nilsen E, Gorman N. High-cost banner blindness: ads increase perceived workload, hinder visual search, and are forgotten. ACM Transactions on Computer-Human Interaction (TOCHI) 2005;12(4):423–445. [Google Scholar]
- 37.Hervet G, Guérard K, Tremblay S, Chtourou MS. Is banner blindness genuine? Eye tracking internet text advertising. Appl Cognit Psychol. 2011;25(5):708–716. [Google Scholar]
- 38.Simola J, Kivikangas M, Kuisma J, Krause CM. Attention and memory for newspaper advertisements: effects of adeditorial congruency and location. Appl Cognit Psychol. 2013;27(4):429–442. [Google Scholar]
- 39.Wang AL, Lowen SB, Romer D, et al. Emotional reaction facilitates the brain and behavioural impact of graphic cigarette warning labels in smokers. Tob Control. 2015 Jan 6; doi: 10.1136/tobaccocontrol-2014-051993. pii: tobaccocontrol-2014-051993. doi: 10.1136/tobaccocontrol-2014-051993. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nonnemaker JM, Choiniere CJ, Farrelly MC, et al. Reactions to graphic health warnings in the United States. Health Educ Res. 2015;30(1):46–56. doi: 10.1093/her/cyu036. [DOI] [PubMed] [Google Scholar]
- 41.McQueen A, Kreuter MW, Boyum S, et al. Reactions to FDA-proposed graphic warning labels affixed to US smokers’ cigarette packs. Nicotine Tob Res. 2015 Jan 14; doi: 10.1093/ntr/ntu339. pii: ntu339. Doi: 10.1093/ntr/ntu339 [Epub ahead of print] DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron LD, Pepper JK, Brewer NT. Responses of young adults to graphic warning labels for cigarette packages. Tob Control. 2015;24(e1):e14–e22. doi: 10.1136/tobaccocontrol-2012-050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mays D, Niaura RS, Evans WD, et al. Cigarette packaging and health warnings: the impact of plain packaging and message framing on young smokers. Tob Control. 2015;24(e1):e-87–e92. doi: 10.1136/tobaccocontrol-2013-051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thrasher JF, Rousu MC, Hammond D, et al. Estimating the impact of pictorial health warnings and “plain” cigarette packaging: evidence from experimental auctions among adult smokers in the United States. Health Policy. 2011;102(1):41–48. doi: 10.1016/j.healthpol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Bansal-Travers M, Hammond D, Smith P, Cummings KM. The impact of cigarette pack design, descriptors, and warning labels on risk perception in the U.S. Am J Prev Med. 2011;40(6):674–682. doi: 10.1016/j.amepre.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vardavas CI, Connolly G, Karamanolis K, Kafatos A. Adolescents perceived effectiveness of the proposed European graphic tobacco warning labels. Eur J Public Health. 2009;19(2):212–217. doi: 10.1093/eurpub/ckp015. [DOI] [PubMed] [Google Scholar]
- 47.Thrasher JF, Rousu MC, Anaya-Ocampo R, et al. Estimating the impact of different cigarette package warning label policies: the auction method. Addict Behav. 2007;32(12):2916–2925. doi: 10.1016/j.addbeh.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Thrasher JF, Hammond D, Fong GT, Arillo-Santillan E. Smokers’ reactions to cigarette package warnings with graphic imagery and with only text: a comparison between Mexico and Canada. Salud Publica Mex. 2007;49(Suppl 2):S233–S240. doi: 10.1590/s0036-36342007000800013. [DOI] [PubMed] [Google Scholar]
- 49.Hammond D, Fong GT, Borland R, et al. Text and graphic warnings on cigarette packages: findings from the international tobacco control four country study. Am J Prev Med. 2007;32(3):202–209. doi: 10.1016/j.amepre.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kees J, Burton S, Andrews JC, Kozup J. Tests of graphic visuals and cigarette package warning combinations: implications for the Framework Convention on Tobacco Control. Journal of Public Policy & Marketing. 2006;25:212–223. [Google Scholar]
- 51.Proposed rule: Federal Register. 2014. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products; pp. 23141–23207. [PubMed] [Google Scholar]
- 52.World Health Organization [Accessed April 3, 2015];WHO Framework Convention on Tobacco Control. 2003 Available at: http://www.who.int/fctc/text_download/en/