Summary
Urban transmission of arthropod-vectored disease has increased in recent decades. Understanding and managing transmission potential in urban landscapes requires integration of sociological and ecological processes that regulate vector population dynamics, feeding behavior, and vector-pathogen interactions in these unique ecosystems. Vectorial capacity is a key metric for generating predictive understanding about transmission potential in systems with obligate vector transmission. This review evaluates how urban conditions, specifically habitat suitability and local temperature regimes, and the heterogeneity of urban landscapes can influence the biologically-relevant parameters that define vectorial capacity: vector density, survivorship, biting rate, extrinsic incubation period, and vector competence.
Urban landscapes represent unique mosaics of habitat. Incidence of vector-borne disease in urban host populations is rarely, if ever, evenly distributed across an urban area. The persistence and quality of vector habitat can vary significantly across socio-economic boundaries to influence vector species composition and abundance, often generating socio-economically distinct gradients of transmission potential across neighborhoods.
Urban regions often experience unique temperature regimes, broadly termed urban heat islands (UHI). Arthropod vectors are ectothermic organisms and their growth, survival, and behavior are highly sensitive to environmental temperatures. Vector response to UHI conditions is dependent on regional temperature profiles relative to the vector’s thermal performance range. In temperate climates UHI can facilitate increased vector development rates while having countervailing influence on survival and feeding behavior. Understanding how urban heat island (UHI) conditions alter thermal and moisture constraints across the vector life cycle to influence transmission processes is an important direction for both empirical and modeling research.
There remain persistent gaps in understanding of vital rates and drivers in mosquito-vectored disease systems, and vast holes in understanding for other arthropod vectored diseases. Empirical studies are needed to better understand the physiological constraints and socio-ecological processes that generate heterogeneity in critical transmission parameters, including vector survival and fitness. Likewise, laboratory experiments and transmission models must evaluate vector response to realistic field conditions, including variability in sociological and environmental conditions.
Keywords: arthropod, climate, disease, mosquito, pathogen, socio-ecology, tick, triatomine, urban heat island, vector, vectorial capacity
Graphical Abstract

Pathogens transmitted by the bite of arthropod vectors pose a significant human health burden worldwide, and mosquito-vectored diseases alone cause more than 1300 deaths each day (WHO 2013). The landscape of vector-borne disease risk has changed dramatically in recent decades, due in part to global exchanges of goods and people. This is evident in the spread of tick-borne disease across the eastern United States and in the emergence of mosquito-borne chikungunya virus in temperate Italy. Urban regions are often a focus for arrivals and pathogens transmitted by biting arthropods have been increasingly reported in urban areas across the temperate zone.
This paper reviews how urban climate and habitat conditions influence population density, biting behaviour, and vector-pathogen interactions across a range of arthropod-pathogen systems. Predictive capacity needed to manage transmission risk is often limited to broad spatial and temporal scales, yet disease incidence in urban regions is fundamentally patchy. Urban landscapes represent unique mosaics of habitat. The persistence and quality of vector habitat can vary significantly across socio-economic boundaries to influence vector species composition and abundance. The changes in the environmental temperature and moisture regimes observed in urban systems can dramatically influence predictions for vector population growth and transmission potential. Synthesis of research highlights the heterogeneity in transmission potential across urban landscapes, created in part by socio-economic conditions that can influence both vector species composition and fitness to generate distinct gradients of risk across neighborhoods.
There remain persistent gaps in understanding of vital rates and drivers in even the most studied mosquito-vectored disease systems, and vast holes in understanding for other arthropod vectored diseases. Empirical studies are needed to better understand the physiological constraints and socio-ecological processes that generate landscape heterogeneity in transmission. Likewise, laboratory experiments and transmission models must evaluate vector response to realistic field conditions, including variability in sociological and environmental conditions.
Introduction
Human activities have facilitated the emergence and resurgence of many vector-borne diseases (Gratz 1999; Lounibos 2002; Wilcox & Gubler 2005; Kilpatrick & Randolph 2012; Weaver 2013). Although once considered rural diseases, local transmission of suburban and urban cases of malaria (Keiser et al. 2004), Chagas disease (Guzman-Tapia, Ramirez-Sierra & Dumonteil 2007; Medrano-Mercado et al. 2008; Delgado et al. 2011), Leishmaniasis (Jeronimo et al. 1994; Harhay et al. 2011), have increased in recent decades, often challenging public health responses and management strategies (Geissbuhler et al. 2007; Levy et al. 2010). Likewise, increases in cases of locally-transmitted arboviruses in suburban and urban populations have increasingly raised public health concern even in temperate regions (Rezza et al. 2007; Kyle & Harris 2008; Rey et al. 2010; Leisnham & Juliano 2012; Weaver 2013). The processes that facilitate pathogen emergence and rises in urban transmission are complex but changes in the abiotic and biotic quality of habitat supporting vector populations and host exposure in urban landscapes are critical (Leisnham & Slaney 2009; Kilpatrick & Randolph 2012; Weaver 2013).
Here we review current understanding of the ecological properties of urban landscapes that regulate local transmission of vector-borne pathogens. We define a vector-borne disease as a pathological condition in humans, domestic animals, or wildlife that is caused by an etiological agent (i.e, pathogen) that is transmitted by another organism, the vector. This review is focused on arthropod-vectored diseases because a vast majority of important disease-vectors around the world are hematophagous (blood-feeding) arthropods. Arthropod vectors are capable of transmitting viral (e.g., dengue, West Nile virus (WNV)), rickettsial (e.g., typhus, ehrlichiosis), bacterial (e.g., plague, Lyme), protozoan (e.g., malaria, trypanosomiasis) and nematode (i.e., filariasis) pathogens between vertebrate hosts. Likewise, a majority of the studies we discuss are focused on understanding vector transmission to humans. This reflects the distribution of published literature that can inform a mechanistic understanding of transmission parameters in urban systems, although both domestic animals and wildlife can also experience changes in prevalence (Bradley & Altizer 2007; Kellner et al. 2012; Jennett, Smith & Wall 2013; Giraudeau et al. 2014; Paras, O’Brien & Reiskind 2014) and increased susceptibility to some pathogens in urban habitat (Bradley & Altizer 2007; LaDeau et al. 2011; Giraudeau et al. 2014).
The most unpredictable influence on vectorial capacity in urban ecosystems is humans. Socio-economic variability, cultural practices, and human behavior all help shape the biotic and abiotic components of urban ecosystems and the species interactions underlying ecological disease systems (Leisnham & Slaney 2009; Levy et al. 2014a). Urban infrastructure can alter habitat availability and quality, affecting fundamental rates of vector and host life cycles and interactions (Joshi et al. 2006; Reisen et al. 2009; Leisnham, LaDeau & Juliano 2014; Levy et al. 2014b). For example, poverty and degraded urban infrastructure are associated with high mosquito production in two temperate U.S. cities (LaDeau et al. 2013), but human behavior can also effectively decouple mosquito population growth from environmental regulation when residents water urban gardens during dry periods (Becker, Leisnham & LaDeau 2014).
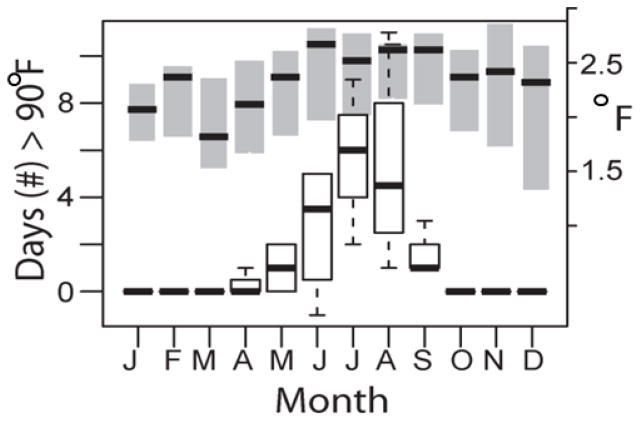
Urban areas can have temperature (Oke 1982; Arnfield 2003) and precipitation regimes (Lacke, Mote & Shepherd 2009; Shepherd et al. 2010; Niyogi et al. 2011) that are distinct from the surrounding region (Pierce and Negri 2002), with important consequences for all organisms that use city habitats. The replacement of natural soil and vegetation with built surfaces in cities can elevate temperatures several degrees above the surrounding region (Oke 1982). In the continental United States researchers found that urban areas were on average 2.9°C warmer than surrounding areas, except in arid biomes where differences could be reversed (Imhoff et al. 2010). This is evident in Baltimore, Maryland (Figure 1) where both monthly temperature and numbers of days with maximum temperatures over 32.2°C were higher in urban versus nearby suburban sites. Urban heat island (UHI) effects can also lead to reduced daily temperature ranges (DTR) due to higher night temperatures in cities relative to forested surroundings (Kalnay & Cai 2003). Arthropod vectors are small ectothermic organisms and their growth, survival, and behavior are highly sensitive to environmental conditions (Thomas & Blanford 2003; Paaijmans et al. 2013). However, the specific responses of arthropod vectors to UHI conditions are dependent on regional temperatures relative to the vector’s thermal performance range (Huey et al. 2012a).
Figure 1. An urban heat island.
Differences in climate metrics for 2011–2013 between urban and suburban sites in Baltimore, Maryland. Boxplots show the additional number of days with maximum daily temperature ≥ 90°F (left axis) at the urban versus suburban site. Right axis shows increase in mean monthly temperature at the urban site (shaded bars). Standard boxplot designations used; dark band denotes median values. Data from CDC.NOAA.gov, collected from NOAA stations located at Baltimore-Washington International Airport and Maryland Science Center.
The predominance of research on mosquito-vectored systems in this paper reflects a dominance of mosquito studies in the published literature. Mosquito-vectored diseases pose a significant human health burden worldwide (Lounibos 2002) and cause more than 1300 deaths each day (WHO 2013). However, there are other vector-borne systems that represent considerable and growing health threats in and around urban environments, including Chagas disease (Trypanosoma cruzi), transmitted by dozens of triatomine insect species (Reduviidae) (Levy et al. 2006; Guzman-Tapia, Ramirez-Sierra & Dumonteil 2007; Medrano-Mercado et al. 2008) and a growing number of pathogens with tick (Ixodidae) vectors (Maetzel, Maier & Kampen 2005; Hamer et al. 2012; Queirogas et al. 2012; Uspensky 2014). An incomplete understanding of the ecological processes that regulate mosquito and non-mosquito vector population growth and pathogen transmission continues to limit predictive capacity needed to effectively manage vector-borne diseases, especially in urban systems.
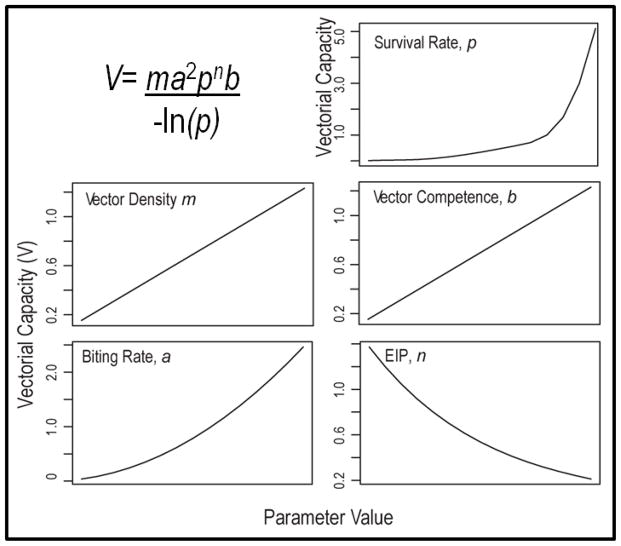
We organize the remainder of this review around the mechanistic components of vectorial capacity (Garrett-Jones 1964), V, a key metric for generating predictive understanding about transmission potential in systems with obligate vector transmission (Figure 2). Unlike the widely used basic reproductive number, R0 (MacDonald 1957), V is derived from entomological parameters and is not dependent on knowledge of host incubation and/or recovery rates. V can also be estimated and evaluated for locations and times with or without pathogen presence (Anderson & May 1992) to evaluate spatio-temporal heterogeneity in transmission potential. Although constrained by important and often unrealistic assumptions such as homogenous transmission and constant mortality and biting rates, see (Wonham et al. 2006; Bellan 2010; Smith et al. 2014), V still provides a useful framework for evaluating the constituent processes and environmental variables that define transmission potential. While the vectorial capacity equation (Figure 2) was formulated specifically to characterize transmission of malaria causing Plasmodium by Anopheles mosquitoes to humans, we use it here as a conceptual roadmap for investigating how various ecological processes associated with urbanization can affect transmission potential of diverse pathogens with obligate arthropod vectors. For recent and thoughtful reviews of the derivation of these epidemiological metrics and application to mosquito control (see Smith et al. 2012 and Reiner et al. 2013). For each of the parameters below we evaluate current understanding of how arthropod vectors respond to urbanization, and specifically to urban heat island and habitat conditions. In Figure 3 we provide a conceptual framework to evaluate current understanding of vectorial capacity in urban landscapes.
Figure 2. A metric for understanding vector-borne disease transmission and risk.
Vectorial capacity (V) describes transmission potential by integrating the potential for a competent vector to bite an infected host and then survive long enough to become infectious. The trend lines in each panel (above) show how increasing each of the five biologically-relevant vector parameters influences transmission potential (V). The value of each of the vector parameters may be influenced by local conditions within the urban environment. EIP is the extrinsic incubation period of a pathogen inside the vector.
Figure 3. A conceptual depiction of urban influence on vectorial capacity.
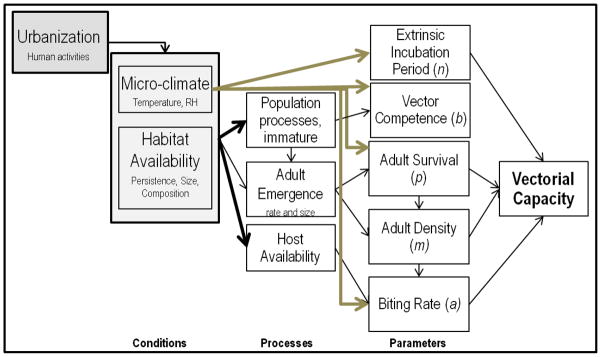
Urbanization involves replacing natural land cover with built surfaces and infrastructure, which directly alters micro-climate and habitat availability for vector and host species. These environmental characteristics influence vector population processes, with consequences for each of the parameters defining vectorial capacity (Fig. 2). Lighter arrows highlight parameters of V that are directly influenced by changes in urban temperatures. Thicker arrows denote pathways with >2 published studies (in mosquito vectors) showing consistent directional effect.
Vector Density (m)
Urban conditions can effectively limit vector populations and pathogen transmission in some systems. Drainage of wetlands and stream burial during urbanization can reduce habitat for Anopheles mosquitoes and may reduce malaria incidence (Hay et al. 2005; Tatem et al. 2013), although urban malaria can persist when agricultural ecosystems are present (De Silva & Marshall 2012). Similarly, deforestation can result in changes to vector access to wildlife hosts, reducing development rates of Ixodid ticks (Horobik, Keesing & Ostfeld 2006). However, there are increasing reports of established tick and pathogen populations in even small remnant forests within urban environments (Magnarelli et al. 1995; Jobe et al. 2007; Jennett, Smith & Wall 2013). Quantifying urban influences on vector density often requires a full evaluation of impacts at different life-stages, which may differ radically in habitat requirements. For example, adult mosquitoes transmit pathogens between hosts, but food web processes and abiotic conditions at the egg and aquatic juvenile stages are important for regulating adult emergence and subsequent density (Juliano 2007; Beck-Johnson et al. 2013).
Urban microclimates and UHIs have a large impact on spatio-temporal patterns of arthropod population growth. In regions where vector growth rates and fitness are already at the peak thermal performance, warmer urban conditions should limit vector populations (Huey et al. 2012b; Paaijmans et al. 2012). However, in temperate regions especially, warmer urban environments could facilitate overwinter survival and speed vector development (Alto & Juliano 2001; Ogden et al. 2004) to alter population growth rates. Even small shifts in microclimate near impervious surfaces can lead to substantial changes in vector development rates and population growth (Beck-Johnson et al. 2013). For example, models using malaria vector and climate data from Africa found that a 0.5 °C increase in mean temperature could lead to a 30–100% increase in mosquito abundance (Pascual et al. 2006). Model’s parameterized with data from different sites often show differences in relative importance of environmental conditions (Johansson, Dominici & Glass 2009), demonstrating that the influence of the abiotic environment on vector density is a function of the temperature or moisture regime currently limiting vector fitness. There is growing recognition of the need to investigate vector performance under field conditions, where thermal conditions can fluctuate more widely and rapidly than is easily reproduced in laboratory settings and when vector responses are conditionally dependent on regional climate (Cator et al. 2013; Mordecai et al. 2013; Paaijmans et al. 2013).
The vector species that feed on humans are often the species that are best able to adapt to environmental changes associated with urbanization. Urban habitats, both terrestrial and aquatic, can offer high resources (e.g., food, resting sites) with sparse populations of competitor and predator species that regulate vector population growth in more natural systems (Chase & Shulman 2009; Freed & Leisnham 2014). The viral pathogens that cause dengue, yellow fever and the emergent chikungunya virus are transmitted by Aedes spp. mosquito vectors that are well-adapted to human-dominated landscapes (Gratz 2004; Hammond et al. 2007). Immature development occurs in artificial water-filled containers (e.g., trash receptacles, garden pots) or ephemeral pools (e.g., drainage ditches, bioswales) associated with human activities (Bartlett-Healy et al. 2012; Quintero et al. 2014; Townroe & Callaghan 2014). Likewise, subterranean urban storm water drains can support population growth for a range of mosquito species and may account for as much as 78% of the resident urban mosquito population in the dry season in Australian cities (Kay et al. 2000). Habitat permanence, size and nutrient status are important characteristics of larval mosquito habitat and can influence species composition and vector abundance (Silver 2008). Unmanaged container habitat supporting immature mosquito development is often more abundant around vacant lots in lower socio-economic neighborhoods (LaDeau et al. 2013), while more permanent habitat such as garden water features and ponds are more likely to be found in higher income neighborhoods (Bartlett-Healy et al. 2012; Dowling et al. 2013). The aggressive human-biting Asian tiger mosquito (Aedes albopictus) is especially capable of exploiting unmanaged container habitat and is a superior competitor to the predominant WNV vector, Culex pipiens, in small to medium sized containers (Carrieri et al. 2003; Costanzo et al. 2011). However, the heterogeneous availability and quality of aquatic habitat across socio-economically diverse neighborhoods in the mid-Atlantic region of the United States appears to support local-scale coexistence of Ae. albopictus and Cx. pipiens (LaDeau et al. 2013). The mosaic of land use and socio-economic status at both local and regional scales is important. Vector management actions can effectively limit population density, although socio-economic and political boundaries influence which regions and neighborhoods can institute effective control strategies (Tedesco, Ruiz & McLafferty 2010). The intersection of agricultural and urban landscapes can create geographic overlap between vector species and human populations (Ljumba & Lindsay 2001; Afrane et al. 2004; Mackenzie, Gubler & Petersen 2004; Matthys et al. 2006) and the process of urbanization can facilitate pathogen emergence when urban areas expand into natural areas of existing high vector densities (Bradley & Altizer 2007; Leisnham & Slaney 2009; Brearley et al. 2013). The greatest number of human cases of Lyme disease in the northeastern and north-central regions of the United States occurs most often in the suburban and exurban communities that intersect with forest ecosystems containing hosts, vectors and pathogen (Steere, Coburn & Glickstein 2004; Bacon, Kugeler & Mead 2008; Tran & Waller 2013).
Strategies for prevention and control of vector-borne disease generally focus on reducing the density of vector populations. Positive associations between the abundance of arthropod vectors and disease incidence has been demonstrated across numerous systems (Gratz 1999; Andreadis et al. 2004; Walk et al. 2009; Levy et al. 2011), although this association does not hold in all locations even for a specific pathogen (Scott et al. 2000). Habitat availability is certainly a prerequisite for vector population growth, but the timing and quality of available habitat have complex impacts on vector populations. For example, reduced availability of larval habitat during droughts can cause female mosquitoes to retain eggs, which has been associated with reduced fitness and propensity to take a second blood meal in some Aedes and Anopholes species (Dieter, Huestis & Lehmann 2012; Charlwood et al. 2013) but has relatively low fitness cost in Cx. pipiens (Johnson & Fonseca 2014). Persistent gaps in mechanistic understanding of what regulates vector population growth rates in built environments continue to limit predictive capacity for managing transmission risk.
Vector Survival (p)
Vector survival is necessary to achieve high vector density. However, it is important to note that the duration of vector survival (longevity) is a critical determinant of V in itself. Even highly abundant and competent vectors are unlikely to pose a transmission risk if their lifespan is shorter than the time required for extrinsic incubation (n) and subsequent host feeding. Urban conditions can have complex impacts on vector survival that transverse life-stages. While temperatures can directly influence survival of adult mosquitoes (Alto & Bettinardi 2013), environmental conditions at the larval life-stage can also affect adult longevity (Delatte et al. 2009; Alto 2011; Araujo, Gil & e-Silva 2012). Larval mosquitoes reared at warm mean temperatures (26°C) had slower development, decreased survival and reduced adult reproduction when daily temperature fluctuations were large (Carrington et al. 2013c, but see Ciota et al. 2014). Juliano and colleagues used both laboratory experiments and field collected female Ae. aegypti to demonstrate that intraspecific competition in larval habitat produced smaller females that had reduced survival and were less likely to be infected with dengue (Juliano et al. 2014). Thus, heterogeneity in habitat persistence, size and quality across urban landscapes is likely to generate important variability in vector survivorship and transmission potential.
There are few studies of survivorship in non-mosquito vectors that are relevant to urban landscape comparisons, yet local urban transmission is evident in sand fly (Harhay et al. 2011), triatomine (Guzman-Tapia, Ramirez-Sierra & Dumonteil 2007; Medrano-Mercado et al. 2008) and tick-borne systems (Schwartz et al. 1991; Buczek et al. 2014; Uspensky 2014). In general, tick survival is negatively related to temperature (Bertrand & Wilson 1996) but most sensitive to low or variable humidity conditions (Nieto, Holmes & Foley 2010; Buczek et al. 2014). Similarly, a principal vector of Chagas disease, Triatoma infestans, is sensitive to shifts in both temperature and relative humidity in laboratory studies (Lorenzo & Lazzari 1999) and is typically found in human residences where building materials dampen variation in these factors (Vazquez-Prokopec et al. 2002). The malaria vector An. stephensi was also found to prefer indoor resting habitat where daily temperature ranges were moderated, even though these were on average warmer than ambient (outdoor) temperatures (Cator et al. 2013).
The physiological constraints on arthropod survival through temperature or humidity extremes are clear, but further research is needed to better understand how variability in survival and its interaction with the extrinsic incubation period (EIP) across urban landscapes can contribute to heterogeneous transmission risk. Despite the importance of mosquito survivorship, there are relatively few studies that have evaluated mosquito age in the field (Harrington et al. 2008) and many models assume constant population mortality rates (see references in Bellan 2010). Advances in use of transcriptional profiles to estimate adult mosquito age (Cook et al. 2006, Cook and Sinkins 2010, Hugo et al. 2010) provide an opportunity for estimating adult mosquito age from field-collected samples and make this an exciting and critically important direction for future work. Likewise, empirical data informing understanding of overwinter or inter-seasonal vector survival are generally sparse, although those studies available suggest egg and adult survival between seasons may be important determinants of seasonal population dynamics and pathogen persistence (Andreadis, Armstrong & Bajwa 2010; Lounibos et al. 2010; Fischer et al. 2011; Andreadis, Dimotsiou & Savopoulou-Soultani 2014). The classical assumption of constant mortality rates is not supported by empirical studies, meaning that common transmission models overestimate the efficacy of control strategies aimed at reducing survival rates (Bellan 2010).
Host Feeding or Biting Rate (a)
Most human vector-borne pathogens and all arboviruses originated as zoonoses and many still function as multi-host systems that require vectors to consume bloodmeals from more than one vertebrate species. Borrelia burgdorferi (Lyme) and WNV require non-human (zoonotic) hosts for pathogen amplification and persistence, and any human infection indicates spill-over from zoonotic cycles. The pathogens causing malaria and dengue virus in humans originated through similar zoonotic cycles (Wolfe, Dunavan & Diamond 2007), although most forms of these pathogens are now effectively adapted to mosquito transmission exclusively between humans.
In the vectorial capacity (V) equation the parameter a is the number of human bites per mosquito per day. To encompass our focus on diverse arthropod vectors, we interpret the biting rate more broadly to evaluate feeding activity across available host species. Thus, the biting rate for a given host species is a function of both the time needed to find (i.e., composition and density of available host species) and process the blood meal (e.g., gonotrophic cycle in mosquitoes). In V, the parameter a is squared to reflect the fact that a vector must feed twice to transmit a pathogen: first to acquire it and then a second time to pass it on to a new host. These sequential host feeding probabilities may be equal (i.e., a2), although systems in which transmission to humans relies on occurrence of a previous zoonotic bloodmeal (e.g., WNV, Lyme) could be represented by separate feeding parameters. In some cases, infection with a pathogen may also influence vector host-seeking behavior (as in human malaria (Cator et al. 2014)). Likewise, there are many studies demonstrating that mosquito biting rates vary across individuals in host populations (Ansell et al. 2002; Harrington et al. 2014), although models and derived control strategies generally assume constant biting rates.
As with other parameters, urban habitat modification and temperature regimes potentially have strong impacts on host-seeking and biting behavior across arthropod vectors. Laboratory studies have shown that Anopheles mosquitoes reared at lower temperatures take more time between emergence and initial blood feeding (Paaijmans, Cator & Thomas 2013) and that temperature-dependent feeding rates vary among important WNV vector Culex species (Ciota et al. 2014). Similarly, thermal preferences are evident in the spatial distribution, feeding activity and dispersal of Triatoma species (Guarneri et al. 2003; Minoli & Lazzari 2003), and host-seeking activity in many tick species is negatively associated with temperature and sensitive to humidity (Ogden et al. 2004; Berger et al. 2014).
The composition of potential host species is often a critical determinant of pathogen transmission. The suite of host animals required to support persistent (endemic) pathogen transmission can be complex community networks (LoGiudice et al. 2003; Keesing, Holt & Ostfeld 2006; Kilpatrick et al. 2006; Garcia et al. 2007; Hamer et al. 2011). Human infection is often the event that motivates research and management in disease systems, but humans are not always an active player in transmission (i.e., pathogens with a non-human reservoir host). The human malaria parasite Plasmodium is effectively transmitted to and from humans through sequential mosquito feeding (Service & Townson 2002). Thus, the density of susceptible human hosts and their availability to biting mosquitoes is an important determinant of mosquito feeding rates and human transmission potential for malaria-causing Plasmodium. Control strategies such as the use of bednets to prohibit biting can effectively limit transmission and ultimately, local pathogen persistence (Lindblade et al. 2004; Muller et al. 2006). Mosquito feeding behavior varies substantially across sites, due both to differences in host availability and to geographic differences in host preference (Apperson et al. 2004; Kilpatrick et al. 2006; Molaei et al. 2006; Hamer et al. 2009; Faraji et al. 2014b). Aedes albopictus host-use includes ten to twenty percent avian species in some regions (Richards et al. 2006; Sawabe et al. 2010), although most published studies report few to no bird bloodmeals (Munoz et al. 2011; Faraji et al. 2014a). High densities of non-human animals may divert some vector species from biting humans (Franco et al. 2014). The presence of outdoor pets (dogs and cats) in an urban New Jersey neighborhood was associated with a decrease in Ae. albopictus human feeding rates relative to nearby suburban sites where pets were generally kept indoors (Faraji et al. 2014a). Heterogeneity in feeding preferences across populations is, however, also evident. For example, Ae. albopictus preference for human hosts despite availability of other mammalian host species is reported from studies in the southern U.S. (Richards et al. 2006), Cameroon (Kamgang et al. 2012), and Spain (Munoz et al. 2011).
By comparison, transmission and persistence of the etiological agents of Lyme borreliosis rely on a complex sequence of ticks feeding on multiple animal reservoir species (LoGiudice et al. 2003). Although humans may be infected by a tick bite, they are not infectious to subsequent biting ticks. The relative density of zoonotic host species is an important determinant of Borrelia burgdorferi infection in ticks and human disease risk. Small forest fragments, including those that occur in urban landscapes, may pose elevated risk of exposure to tick-borne Lyme disease due to high densities of a key tick host and pathogen reservoir, the white-footed mouse (Peromyscus leucopus), and low densities of predators and competitors (Rosenblatt et al. 1999; Nupp & Swihart 2000; Logiudice et al. 2008). As a result, tick density and infection prevalence with the Lyme pathogen have been shown to negatively correlate with forest fragment area or vertebrate host species richness in both empirical and theoretical studies (Allan, Keesing & Ostfeld 2003; Brownstein et al. 2005; Logiudice et al. 2008). Recent studies suggest that invasion by exotic shrubs, which are widespread in urban ecosystems, may also increase tick-borne disease risk via changes in reservoir host abundance (Williams et al. 2009; Allan et al. 2010). Trypanosoma cruzi (the etiological agent of Chagas disease) falls somewhere in between—humans can transmit the parasite, but other animal hosts are usually implicated in areas of high incidence and host species composition near human residences is an important component determining human exposure (Gurtler et al. 2014).
Creation of vector habitat close to humans and domestic animals can dramatically influence host feeding success and disease transmission, although human behavior can be the largest determinant of feeding success in these situations (Guillet et al. 2001; Gurtler et al. 2014). Triatomine bugs are particularly adept at finding resting places in the cracks and crevices that occur between bricks and stones (Levy et al. 2006) close to the humans and domestic animals that support persistent local T. cruzi transmission during and after the urbanization process in Peru (Levy et al. 2006; Foley et al. 2013; Gurtler et al. 2014; Levy et al. 2014b). In a study of tick-borne ehrlichiosis in the metropolitan area of St. Louis, MO, where tick-borne disease risk increases with distance from the urban core, prevention measures taken by humans were greatest in exurban habitats (Bayles, Evans & Allan 2013). Thus while vector density and corresponding disease risk may change along human land-use gradients, human knowledge and prevention of disease may change as well, necessitating an interdisciplinary approach to the study of vector-borne disease dynamics in human-dominated ecosystems.
Vector Competence (b)
A vector ingests a pathogen while feeding on an infected host, and, in many cases, the pathogen must pass through the gut and enter the salivary glands before the vector is infectious (exceptions include T. cruzi and Typhus systems in which transmission occurs via the vector’s feces). A pathogen’s interaction with the vector’s gut epithelium is often specific to vector physiology and many species can ingest pathogens but fail to become infectious. In triatomine vectors it may be the chemical make-up of the feces that define variation in T. cruzi competence across species (Antunes et al. 2013). Species competence across a taxonomic group (or across populations within a species) can vary from not competent to fully competent for pathogen transmission according to vector physiology, environmental conditions, and interactions between vector and pathogen genetics (Weaver et al. 2004).
Arboviral competence can vary among mosquito populations within a species (Kilpatrick et al. 2010; Charan et al. 2013; Fansiri et al. 2013; Tabachnick 2013) and are often temperature-sensitive (Dohm, O’Guinn & Turell 2002; Reisen, Fang & Martinez 2006; Kilpatrick et al. 2008; Carrington et al. 2013b). Dengue virus infection and transmission rates increase at higher temperatures (Halstead 2008), although a growing body of models and laboratory experiments suggest that fluctuations in daily temperature modify this relationship (Lambrechts et al. 2011; Carrington et al. 2013a). Adult mosquitoes held at 26°C mean temperature were less likely to have midgut infections when DTR was large (Carrington et al. 2013b), while infection and transmission probability were increased when DTR was large at lower mean temperature (20°C) (Carrington et al. 2013a). Vector immunity (Murdock et al. 2012) and interactions between vector and viral genetics (Zouache et al. 2014) can be important determinants of vector competence in mosquitoes. Immune function and genetic interactions may explain some of the mechanism for temperature regulation of vector competence, as both are influenced by environmental temperatures (Kilpatrick et al. 2008; Murdock, Moller-Jacobs & Thomas 2013; Zouache et al. 2014). Empirical studies that evaluate the role of vector immunity and the role of vector-pathogen genetics in vector competence across urban landscapes are an important research priority. Relatively few studies examine vector competence across species of ticks feeding on the same hosts (Henning et al. 2014). The influence of ambient temperature on vector competence in tick species was not existent for Venezuelan equine encephalomyelitis (Dohm & Linthicum 1993) and temperatures above 27°C were not conducive to vector competence for B. burgdorferi (Shih, Telford & Spielman 1995).
For vectors with complex life-cycles such as mosquitoes, environmental conditions experienced in the larval life-stage can also influence the vector competence of adults. For example, larval competition between Ae. albopictus and Ae. aegypti increases the susceptibility of Ae. albopictus to infection by dengue-2 virus as adults (Alto et al. 2008). Similarly, larval Ae. aegypti raised at higher temperatures in the presence of the pesticide malathion experienced increased virus dissemination as adults (Muturi & Alto 2011), indicating potential for complex interactions between UHI and environmental pollutants to influence vector competence in urban landscapes.
Extrinsic Incubation Period (n)
The EIP describes the number of days between the time a vector acquires an infectious pathogen and when it is capable of transmitting the pathogen to a subsequent host. Pathogen transmission can only occur if the vector bites a host after sufficient time for parasite development (e.g., malaria) or replication (infection by viruses, T. cruzi, etc.). Urban heat island characteristics, including both high temperatures and reduced daily temperature variation, may directly influence the rate of pathogen development inside vectors.
Nymphal ticks reared at warmer temperatures (33–37°C) were less likely to be infected with B. burgdorferi (Shih, Telford & Spielman 1995). Likewise, the development of T. cruzi within the triatomine bugs is highly temperature dependent (Neves 1971); the presence of infectious parasites in the vector Rhodnius prolixus was observed in 2 versus 7 days when the temperature was increased from 20°C to 30°C (Phillips 1960; Brener 1973).
Much of our current understanding about how environment influences EIP is once again derived from mosquito models, primarily in malaria and dengue systems. In regions where temperature means are generally below the thermal peak for EIP performance, urban heat island conditions will generally shorten EIP, although dampening of daily temperature fluctuations may have contrasting effects (Blanford et al. 2013; Carrington et al. 2013a). The extrinsic incubation of the malaria parasite for example, was faster when daily temperature range (DTR) was large at cooler mean temperatures but large DTR slowed EIP when mean temperatures were already high (Blanford et al. 2013). More rapid viral replication (and shortened EIP) in response to increased temperature has been demonstrated for many (Dohm, O’Guinn & Turell 2002; Reisen, Fang & Martinez 2006; Richards et al. 2012), although not all arboviruses (Kramer, Hardy & Presser 1983) and some evidence suggests that cooler temperatures may increase vector susceptibility to infection (Adelman et al. 2013). Likewise, cooler larval habitat has been shown to increase vector susceptibility to dengue (Alto & Bettinardi 2013).
Discussion
The ability to predict and effectively manage arthropod-vectored disease outbreaks depends critically on how well we understand the mechanistic components of vectorial capacity. The emergence and re-emergence of vector-borne diseases in urban areas across the globe is occurring in parallel with a surge in urban populations and public health challenges will continue to increase (Patz et al. 2004; Leisnham & Slaney 2009; Myers & Patz 2009). Our review demonstrates that while there is much we do know, there remain persistent gaps in understanding of critical rates and drivers in mosquito vectored systems, and vast holes in knowledge for other arthropod vectored diseases.
A majority of arboviruses lack effective vaccines, while clinical diagnosis and treatments of other vector-borne diseases (including those caused by parasites and bacteria) are limited. Thus, managing the entomological exposure risk is critical. The most effective management of vector-borne disease in humans has often focused on limiting the distribution and abundance of vector populations and thus, processes regulating vector density are the most studied component of vectorial capacity. However, predicting and managing transmission of even well-studied, mosquito-vectored pathogens is often difficult due to limited understanding of the processes controlling vector-pathogen interactions and vector-host contacts in real environmental conditions.
Vectorial capacity captures many important aspects of vector-borne disease transmission but the simple formulation lies atop of a number of assumptions, such as homogenous mixing of vectors and hosts and the absence of migration. These assumptions were never meant to be realistic, even for idealized rural environments (Smith et al. 2012). For cities they can be especially misleading. Urban areas typically serve as regional transport hubs and are often the point of entry for exotic organisms (Lockwood, Cassey & Blackburn 2009). Indeed Aedes mosquito species have spread to urban areas worldwide (Lounibos 2002; Tatem, Hay & Rogers 2006). The establishment of these species in cities has been a determining factor in the spread of chikungunya and dengue viruses (Delatte et al. 2008; Lambrechts, Scott & Gubler 2010). Likewise, neither vector nor host populations within cities are likely to be ‘well-mixed’. Vector density can vary significantly across neighborhoods within a city (Foley et al. 2013; LaDeau et al. 2013) but current understanding of urban dispersal barriers and heterogeneity in vector fitness at this scale is limited. City streets can serve as a barrier to insect dispersal, as some species may be unwilling or unable to cross a swath of unprotected pavement or asphalt (Hemme et al. 2010; Barbu et al. 2013). Variation in temperature and humidity across impervious surfaces and residential infrastructure can also influence dispersal activity (Vazquez-Prokopec et al. 2004). The divisions that urban infrastructure may create in vector populations could lead to more complex metapopulation transmission dynamics, such as source-sink metapopulations (Hanski & Hanski 1999) in which vector fitness is high in some blocks/patches but not others. Vector genetics are important determinants of life history traits and vector competence among individual populations within a species (Zouache et al. 2014). Population structure is likely influenced by land use and socio-economic heterogeneity in urban environments, but data are needed to identify relevant spatial scales and quantify their importance in urban landscapes.
The motivation behind development of the vectorial capacity model was to guide control strategies (Garrett-Jones 1964; Smith et al. 2012). The equation for V predicts high sensitivity to adult survival and many studies demonstrate how this conclusion is highly dependent on a suite of assumptions that may be unrealistic in real field settings where mortality rates and population mixing are not constant, vector-host interactions are environmentally dependent and vector-pathogen interactions vary with genetic structure (Bellan 2010; Smith et al. 2014). Perhaps less well-recognized is the importance of the socio-economic environment of modern cities that can affect entomological parameters and efficacy of vector control (Bartlett-Healy et al. 2011; Fonseca et al. 2013; Barbu et al. 2014; Buttenheim et al. 2014). Social factors interact with the biophysical environment to create a complex and often hard to predict, socio-ecological context within which vector-borne diseases circulate.
The predictive capacity needed to manage transmission is often limited to broad spatial and temporal scales, yet human disease incidence within a region is often patchy (Reiter et al. 2003; Liu et al. 2009; Sugumaran, Larson & DeGroote 2009; Foley et al. 2013). New data and models are needed to better understand how UHI and habitat suitability regulate both vector-pathogen and vector-host interactions. Infrastructure, transportation grids, and human behavior create an urban habitat mosaic that is fundamentally different from many of the model systems or laboratory experiments used to generate inference on transmission risk. Data are required to better understand how physiological constraints associated with urban temperature and regimes influence the individual parameters that define transmission potential (Chown & Duffy 2015). Modeling disease metrics for even the most well-studied mosquito vectored system, human malaria, still relies on too few data points from a limited suite of species, including some that are not relevant to transmission of Plasmodium parasites (Mordecai et al. 2013). Empirical work is needed to better understand the range of conditions experienced by vectors in the field and evaluate the integrative impacts of urban habitat and microclimate conditions on vectorial capacity. Finally, while the many studies reviewed here provide valuable insights into the processes that define vectorial capacity, few address the complexity of interacting socio-ecological factors that ultimately determine transmission risk in urban habitats. In addition to a call for more data to parameterize models to reflect realistic field conditions, we also highlight the need for models that can accommodate the influence of socio-economic drivers to better predict transmission risk at spatio-temporal scales relevant to management strategies.
Acknowledgments
The authors wish to thank the subject editors, Drs. Amy Hahs and Karl Evans and two anonymous reviewers. Time for SLL and PTL to work on this review was provided by USDA-NIFA and the Northeastern Integrated Pest Management Center (MD-2011-00540) and the NSF-Couple Natural Human Systems Program (DEB-1211797). MZL was supported by NIH-NIAID 5R01AI101229 and NICHD R01HD075869.
Footnotes
Data Accessibility
This paper is a review of existing research and does not use new data.
References
- Adelman ZN, Anderson MAE, Wiley MR, Murreddu MG, Samuel GH, Morazzani EM, Myles KM. Cooler Temperatures Destabilize RNA Interference and Increase Susceptibility of Disease Vector Mosquitoes to Viral Infection. Plos Neglected Tropical Diseases. 2013:7. doi: 10.1371/journal.pntd.0002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrane YA, Klinkenberg E, Drechsel P, Owusu-Daaku K, Garms R, Kruppa T. Does irrigated urban agriculture influence the transmission of malaria in the city of Kumasi, Ghana? Acta Tropica. 2004;89:125–134. doi: 10.1016/j.actatropica.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Allan BF, Dutra HP, Goessling LS, Barnett K, Chase JM, Marquis RJ, Pang G, Storch GA, Thach RE, Orrock JL. Invasive honeysuckle eradication reduces tick-borne disease risk by altering host dynamics. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18523–18527. doi: 10.1073/pnas.1008362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan BF, Keesing F, Ostfeld RS. Effect of forest fragmentation on Lyme disease risk. Conservation Biology. 2003;17:267–272. [Google Scholar]
- Alto BW. Interspecific Larval Competition Between Invasive Aedes japonicus and Native Aedes triseriatus (Diptera: Culicidae) and Adult Longevity. Journal of Medical Entomology. 2011;48:232–242. doi: 10.1603/me09252. [DOI] [PubMed] [Google Scholar]
- Alto BW, Bettinardi D. Temperature and Dengue Virus Infection in Mosquitoes: Independent Effects on the Immature and Adult Stages. American Journal of Tropical Medicine and Hygiene. 2013;88:497–505. doi: 10.4269/ajtmh.12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Juliano SA. Precipitation and temperature effects on populations of Aedes albopictus (Diptera : Culicidae): Implications for range expansion. Journal of Medical Entomology. 2001;38:646–656. doi: 10.1603/0022-2585-38.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proceedings of the Royal Society B-Biological Sciences. 2008;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford University Press; Oxford, UK: 1992. [Google Scholar]
- Andreadis SS, Dimotsiou OC, Savopoulou-Soultani M. Variation in adult longevity of Culex pipiens f. pipiens, vector of the West Nile Virus. Parasitology Research. 2014;113:4315–4319. doi: 10.1007/s00436-014-4152-x. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: A five-year analysis of mosquito data 1999–2003. Vector-Borne and Zoonotic Diseases. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Armstrong PM, Bajwa WI. Studies On Hibernating Populations Of Culex Pipiens From A West Nile Virus Endemic Focus In New York City: Parity Rates And Isolation Of West Nile Virus. Journal of the American Mosquito Control Association. 2010;26:257–264. doi: 10.2987/10-6004.1. [DOI] [PubMed] [Google Scholar]
- Ansell J, Hamilton KA, Pinder M, Walraven GEL, Lindsay SW. Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:113–116. doi: 10.1016/s0035-9203(02)90271-3. [DOI] [PubMed] [Google Scholar]
- Antunes LCM, Han J, Pan JX, Moreira CJC, Azambuja P, Borchers CH, Carels N. Metabolic Signatures of Triatomine Vectors of Trypanosoma cruzi Unveiled by Metabolomics. Plos One. 2013:8. doi: 10.1371/journal.pone.0077283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, Farajollahi A, Crans W, Daniels TJ, Falco RC, Benedict M, Anderson M, McMillen L, Unnasch TR. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector-Borne and Zoonotic Diseases. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo MD, Gil LHS, e-Silva AD. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malaria Journal. 2012:11. doi: 10.1186/1475-2875-11-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnfield AJ. Two decades of urban climate research: a review of turbulence, exchanges of energy and water, and the urban heat island. International Journal of Climatology. 2003;23:1–26. [Google Scholar]
- Bacon RM, Kugeler KJ, Mead PSSfLd US 1992–2006; C.f.D.C.a.P. Department of Health & Human Services. Surveillance for Lyme disease--United States, 1992–2006. 2008. [PubMed] [Google Scholar]
- Barbu CM, Buttenheim AM, Pumahuanca MLH, Calderon JEQ, Salazar R, Carrion M, Rospigliossi AC, Chavez FSM, Alvarez KO, del Carpio JC, Naquira C, Levy MZ. Residual Infestation and Recolonization during Urban Triatoma infestans Bug Control Campaign, Peru. Emerging Infectious Diseases. 2014;20:2055–2063. doi: 10.3201/eid2012.131820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbu CM, Hong A, Manne JM, Small DS, Calderon JEQ, Sethuraman K, Quispe-Machaca V, Ancca-Juarez J, del Carpio JGC, Chavez FSM, Naquira C, Levy MZ. The Effects of City Streets on an Urban Disease Vector. Plos Computational Biology. 2013:9. doi: 10.1371/journal.pcbi.1002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett-Healy K, Hamilton G, Healy S, Crepeau T, Unlu I, Farajollahi A, Fonseca D, Gaugler R, Clark GG, Strickman D. Source Reduction Behavior as an Independent Measurement of the Impact of a Public Health Education Campaign in an Integrated Vector Management Program for the Asian Tiger Mosquito. International Journal of Environmental Research and Public Health. 2011;8:1358–1367. doi: 10.3390/ijerph8051358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett-Healy K, Unlu I, Obenauer P, Hughes T, Healy S, Crepeau T, Farajollahi A, Kesavaraju B, Fonseca D, Schoeler G, Gaugler R, Strickman D. Larval Mosquito Habitat Utilization and Community Dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae) Journal of Medical Entomology. 2012;49:813–824. doi: 10.1603/me11031. [DOI] [PubMed] [Google Scholar]
- Bayles BR, Evans G, Allan BF. Knowledge and prevention of tick-borne diseases vary across an urban-to-rural human land-use gradient. Ticks and Tick-Borne Diseases. 2013;4:352–358. doi: 10.1016/j.ttbdis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjornstad ON. The Effect of Temperature on Anopheles Mosquito Population Dynamics and the Potential for Malaria Transmission. Plos One. 2013:8. doi: 10.1371/journal.pone.0079276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Leisnham PT, LaDeau SL. A Tale of Two City Blocks: Differences in Immature and Adult Mosquito Abundances between Socioeconomically Different Urban Blocks in Baltimore (Maryland, USA) International Journal of Environmental Research and Public Health. 2014;11:3256–3270. doi: 10.3390/ijerph110303256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellan SE. The Importance of Age Dependent Mortality and the Extrinsic Incubation Period in Models of Mosquito-Borne Disease Transmission and Control. Plos One. 2010;5:e10165. doi: 10.1371/journal.pone.0010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KA, Ginsberg HS, Gonzalez L, Mather TN. Relative Humidity and Activity Patterns of Ixodes scapularis (Acari: Ixodidae) Journal of Medical Entomology. 2014;51:769–776. doi: 10.1603/me13186. [DOI] [PubMed] [Google Scholar]
- Bertrand MR, Wilson ML. Microclimate-dependent survival of unfed adult Ixodes scapularis (Acari: Ixodidae) in nature: Life cycle and study design implications. Journal of Medical Entomology. 1996;33:619–627. doi: 10.1093/jmedent/33.4.619. [DOI] [PubMed] [Google Scholar]
- Blanford JI, Blanford S, Crane RG, Mann ME, Paaijmans KP, Schreiber KV, Thomas MB. Implications of temperature variation for malaria parasite development across Africa. Scientific Reports. 2013:3. doi: 10.1038/srep01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends in Ecology & Evolution. 2007;22:95–102. doi: 10.1016/j.tree.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brearley G, Rhodes J, Bradley A, Baxter G, Seabrook L, Lunney D, Liu Y, McAlpine C. Wildlife disease prevalence in human-modified landscapes. Biological Reviews. 2013;88:427–442. doi: 10.1111/brv.12009. [DOI] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annual Reviews in Microbiology. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Brownstein JS, Skelly DK, Holford TR, Fish D. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia. 2005;146:469–475. doi: 10.1007/s00442-005-0251-9. [DOI] [PubMed] [Google Scholar]
- Buczek A, Ciura D, Bartosik K, Zajac Z, Kulisz J. Threat of attacks of Ixodes ricinus ticks (Ixodida: Ixodidae) and Lyme borreliosis within urban heat islands in southwestern Poland. Parasites & Vectors. 2014:7. doi: 10.1186/s13071-014-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttenheim AM, Paz-Soldan V, Barbu C, Skovira C, Calderon JQ, Riveros LMM, Cornejo JO, Small DS, Bicchieri C, Naquira C, Levy MZ. Is participation contagious? Evidence from a household vector control campaign in urban Peru. Journal of Epidemiology and Community Health. 2014;68:103–109. doi: 10.1136/jech-2013-202661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri M, Bacchi M, Bellini R, Maini S. On the competition occurring between Aedes albopictus and Culex pipiens (Diptera : Culicidae) in Italy. Environmental Entomology. 2003;32:1313–1321. [Google Scholar]
- Carrington LB, Armijos MV, Lambrechts L, Scott TW. Fluctuations at a Low Mean Temperature Accelerate Dengue Virus Transmission by Aedes aegypti. Plos Neglected Tropical Diseases. 2013a:7. doi: 10.1371/journal.pntd.0002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington LB, Seifert SN, Armijos MV, Lambrechts L, Scott TW. Reduction of Aedes aegypti Vector Competence for Dengue Virus under Large Temperature Fluctuations. American Journal of Tropical Medicine and Hygiene. 2013b;88:689–697. doi: 10.4269/ajtmh.12-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington LB, Seifert SN, Willits NH, Lambrechts L, Scott TW. Large Diurnal Temperature Fluctuations Negatively Influence Aedes aegypti (Diptera: Culicidae) Life-History Traits. Journal of Medical Entomology. 2013c;50:43–51. doi: 10.1603/me11242. [DOI] [PubMed] [Google Scholar]
- Cator L, Thomas S, Paaijmans K, Ravishankaran S, Justin J, Mathai M, Read A, Thomas M, Eapen A. Characterizing microclimate in urban malaria transmission settings: a case study from Chennai, India. Malaria Journal. 2013;12:1–10. doi: 10.1186/1475-2875-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, Lynch PA, Thomas MB, Read AF. Alterations in mosquito behaviour by malaria parasites: potential impact on force of infection. Malaria Journal. 2014:13. doi: 10.1186/1475-2875-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan SS, Pawar KD, Severson DW, Patole MS, Shouche YS. Comparative analysis of midgut bacterial communities of Aedes aegypti mosquito strains varying in vector competence to dengue virus. Parasitology Research. 2013;112:2627–2637. doi: 10.1007/s00436-013-3428-x. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Cuamba N, Tomas EVE, Briet OJT. Living on the edge: a longitudinal study of Anopheles funestus in an isolated area of Mozambique. Malaria Journal. 2013:12. doi: 10.1186/1475-2875-12-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase JM, Shulman RS. Wetland isolation facilitates larval mosquito density through the reduction of predators. Ecological Entomology. 2009;34:741–747. [Google Scholar]
- Chown SL, Duffy GA. Thermal physiology and urbanization: perspectives on exit, entry and transformation rules. Functional Ecology. 2015 this issue. [Google Scholar]
- Ciota AT, Matacchiero AC, Kilpatrick AM, Kramer LD. The Effect of Temperature on Life History Traits of Culex Mosquitoes. Journal of Medical Entomology. 2014;51:55–62. doi: 10.1603/me13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PE, Hugo LE, Iturbe-Ormaetxe I, Williams CR, Chenoweth SF, Ritchie SA, Ryan PA, Kay BH, Blows MW, O’Neill SL. The use of transcriptional profiles to predict adult mosquito age under field conditions. Proceedings of the National Academy of Sciences. 2006;103:18060–18065. doi: 10.1073/pnas.0604875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PE, Sinkins SP. Transcriptional profiling of Anopheles gambiae mosquitoes for adult age estimation. Insect Molecular Biology. 2010;19:745–751. doi: 10.1111/j.1365-2583.2010.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo KS, Muturi EJ, Lampman HL, Alto BW. The Effects of Resource Type and Ratio on Competition With Aedes albopictus and Culex pipiens (Diptera: Culicidae) Journal of Medical Entomology. 2011;48:29–38. doi: 10.1603/me10085. [DOI] [PubMed] [Google Scholar]
- De Silva PM, Marshall JM. Factors Contributing to Urban Malaria Transmission in Sub-Saharan Africa: A Systematic Review. Journal of Tropical Medicine. 2012;2012:10. doi: 10.1155/2012/819563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of Temperature on Immature Development, Survival, Longevity, Fecundity, and Gonotrophic Cycles of Aedes albopictus, Vector of Chikungunya and Dengue in the Indian Ocean. Journal of Medical Entomology. 2009;46:33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, Fontenille D. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: Biology and control. Parasite-Journal De La Societe Francaise De Parasitologie. 2008;15:3–13. doi: 10.1051/parasite/2008151003. [DOI] [PubMed] [Google Scholar]
- Delgado S, Neyra RC, Machaca VRQ, Juarez JA, Chu LC, Verastegui MR, Apaza GMM, Bocangel CD, Tustin AW, Sterling CR, Comrie AC, Naquira C, del Carpio JGC, Gilman RH, Bern C, Levy MZ. A History of Chagas Disease Transmission, Control, and Re-Emergence in Peri-Rural La Joya, Peru. Plos Neglected Tropical Diseases. 2011:5. doi: 10.1371/journal.pntd.0000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter KL, Huestis DL, Lehmann T. The effects of oviposition-site deprivation on Anopheles gambiae reproduction. Parasites & Vectors. 2012:5. doi: 10.1186/1756-3305-5-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm DJ, Linthicum KJ. Effects Of Temperature On Fecundity And Viral Replication In Amblyomma-Cajennense (Arachnida, Ixodidae) Infected With Venezuelan Equine Encephalomyelitis Virus. Journal of Medical Entomology. 1993;30:286–290. doi: 10.1093/jmedent/30.1.286. [DOI] [PubMed] [Google Scholar]
- Dohm DJ, O’Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera : Culicidae) to transmit West Nile virus. Journal of Medical Entomology. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Dowling Z, Ladeau SL, Armbruster P, Biehler D, Leisnham PT. Socioeconomic Status Affects Mosquito (Diptera: Culicidae) Larval Habitat Type Availability and Infestation Level. Journal of Medical Entomology. 2013;50:764–772. doi: 10.1603/me12250. [DOI] [PubMed] [Google Scholar]
- Fansiri T, Fontaine A, Diancourt L, Caro V, Thaisomboonsuk B, Richardson JH, Jarman RG, Ponlawat A, Lambrechts L. Genetic Basis Of Vector Competence For Field Dengue Virus Isolates In A Wild Aedes Aegypti Population. Pathogens and Global Health. 2013;107:402–402. [Google Scholar]
- Faraji A, Egizi A, Fonseca DM, Unlu I, Crepeau T, Healy SP, Gaugler R. Comparative Host Feeding Patterns of the Asian Tiger Mosquito, Aedes albopictus, in Urban and Suburban Northeastern USA and Implications for Disease Transmission. PLoS Negl Trop Dis. 2014a;8:e3037. doi: 10.1371/journal.pntd.0003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraji A, Egizi A, Fonseca DM, Unlu I, Crepeau T, Healy SP, Gaugler R. Comparative Host Feeding Patterns of the Asian Tiger Mosquito, Aedes albopictus, in Urban and Suburban Northeastern USA and Implications for Disease Transmission. Plos Neglected Tropical Diseases. 2014b:8. doi: 10.1371/journal.pntd.0003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Alem IS, De Majo MS, Campos RE, Schweigmann N. Cold season mortality and hatching behavior of Aedes aegypti L. (Diptera: Culicidae) eggs in Buenos Aires City, Argentina. Journal of Vector Ecology. 2011;36:94–99. doi: 10.1111/j.1948-7134.2011.00145.x. [DOI] [PubMed] [Google Scholar]
- Foley EA, Khatchikian CE, Hwang J, Ancca-Juarez J, Borrini-Mayori K, Quispe-Machaca VR, Levy MZ, Brisson D Chagas Dis Working Grp, A. Population structure of the Chagas disease vector, Triatoma infestans, at the urban-rural interface. Molecular Ecology. 2013;22:5162–5171. doi: 10.1111/mec.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca DM, Unlu I, Crepeau T, Farajollahi A, Healy SP, Bartlett-Healy K, Strickman D, Gaugler R, Hamilton G, Kline D, Clark GG. Area-wide management of Aedes albopictus. Part 2: Gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest Management Science. 2013;69:1351–1361. doi: 10.1002/ps.3511. [DOI] [PubMed] [Google Scholar]
- Franco AO, Gomes MGM, Rowland M, Coleman PG, Davies CR. Controlling Malaria Using Livestock-Based Interventions: A One Health Approach. Plos One. 2014:9. doi: 10.1371/journal.pone.0101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed TZ, Leisnham PT. Roles of spatial partitioning, competition, and predation in the North American invasion of an exotic mosquito. Oecologia. 2014;175:601–611. doi: 10.1007/s00442-014-2909-7. [DOI] [PubMed] [Google Scholar]
- Garcia ES, Ratcliffe NA, Whitten MMA, Gonzalez MS, Azambuja P. Exploring the role of insect host factors in the dynamics of Trypanosoma cruzi-Rhodnius prolixus interactions. Journal of Insect Physiology. 2007;53:11–21. doi: 10.1016/j.jinsphys.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bulletin of World Health Organization. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- Geissbuhler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, Mtasiwa D, Mshinda H, Fillinger U, Lindsay SW, Kannady K, de Castro MC, Tanner M, Killeen GF. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malaria Journal. 2007:6. doi: 10.1186/1475-2875-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudeau M, Mousel M, Earl S, McGraw K. Parasites in the City: Degree of Urbanization Predicts Poxvirus and Coccidian Infections in House Finches (Haemorhous mexicanus) Plos One. 2014:9. doi: 10.1371/journal.pone.0086747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz NG. Emerging and resurging vector-borne diseases. Annual Review of Entomology. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- Gratz NG. Critical review of the vector status of Aedes albopictus. Medical and Veterinary Entomology. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Guarneri AA, Lazzari C, Xavier AAP, Diotaiuti L, Lorenzo MG. The effect of temperature on the behaviour and development of Triatoma brasiliensis. Physiological Entomology. 2003;28:185–191. [Google Scholar]
- Guillet P, N’Guessan R, Darriet F, Traore-Lamizana M, Chandre F, Carnevale P. Combined pyrethroid and carbamate ‘two-in-one’ treated mosquito nets: field efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus. Medical and Veterinary Entomology. 2001;15:105–112. doi: 10.1046/j.1365-2915.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- Gurtler RE, Cecere MC, Vazquez-Prokopec GM, Ceballos LA, Gurevitz JM, Fernandez MD, Kitron U, Cohen JE. Domestic Animal Hosts Strongly Influence Human-Feeding Rates of the Chagas Disease Vector Triatoma infestans in Argentina. Plos Neglected Tropical Diseases. 2014:8. doi: 10.1371/journal.pntd.0002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Tapia Y, Ramirez-Sierra MJ, Dumonteil E. Urban infestation by Triatoma dimidiata in the city of Merida, Yucatan, Mexico. Vector-Borne and Zoonotic Diseases. 2007;7:597–606. doi: 10.1089/vbz.2007.0133. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Dengue virus - Mosquito interactions. Annual Review of Entomology. 2008:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Chaves LF, Anderson TK, Kitron UD, Brawn JD, Ruiz MO, Loss SR, Walker ED, Goldberg TL. Fine-Scale Variation in Vector Host Use and Force of Infection Drive Localized Patterns of West Nile Virus Transmission. Plos One. 2011:6. doi: 10.1371/journal.pone.0023767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, Hayes DB, Walker ED. Host Selection by Culex pipiens Mosquitoes and West Nile Virus Amplification. American Journal of Tropical Medicine and Hygiene. 2009;80:268–278. [PubMed] [Google Scholar]
- Hamer SA, Goldberg TL, Kitron UD, Brawn JD, Anderson TK, Loss SR, Walker ED, Hamer GL. Wild Birds and Urban Ecology of Ticks and Tick-borne Pathogens, Chicago, Illinois, USA, 2005–2010. Emerging Infectious Diseases. 2012;18:1589–1595. doi: 10.3201/eid1810.120511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SN, Gordon AL, Lugo EDC, Moreno G, Kuan GM, Lopez MM, Lopez JD, Delgado MA, Valle SI, Espinoza PM, Harris E. Characterization of Aedes aegypti (Diptera : Culcidae) production sites in urban Nicaragua. Journal of Medical Entomology. 2007;44:851–860. doi: 10.1603/0022-2585(2007)44[851:coaadc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hanski I, Hanski IA. Metapopulation ecology. Oxford University Press; Oxford: 1999. [Google Scholar]
- Harhay MO, Olliaro PL, Costa DL, Costa CHN. Urban parasitology: visceral leishmaniasis in Brazil. Trends in Parasitology. 2011;27:403–409. doi: 10.1016/j.pt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Harrington LC, Fleisher A, Ruiz-Moreno D, Vermeylen F, Wa CV, Poulson RL, Edman JD, Clark JM, Jones JW, Kitthawee S, Scott TW. Heterogeneous Feeding Patterns of the Dengue Vector, Aedes aegypti, on Individual Human Hosts in Rural Thailand. Plos Neglected Tropical Diseases. 2014:8. doi: 10.1371/journal.pntd.0003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LC, Vermeylen F, Jones JJ, Kitthawee S, Sithiprasasna R, Edman JD, Scott TW. Age-dependent survival of the dengue vector Aedes aegypti (Diptera : Culicidae) demonstrated by simultaneous release-recapture of different age cohorts. Journal of Medical Entomology. 2008;45:307–313. doi: 10.1603/0022-2585(2008)45[307:asotdv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nature Reviews Microbiology. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemme RR, Thomas CL, Chadee DD, Severson DW. Influence of Urban Landscapes on Population Dynamics in a Short-Distance Migrant Mosquito: Evidence for the Dengue Vector Aedes aegypti. PLoS Negl Trop Dis. 2010;4:e634. doi: 10.1371/journal.pntd.0000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning TC, Orr JM, Smith JD, Arias JR, Norris DE. Spotted Fever Group Rickettsiae in Multiple Hard Tick Species from Fairfax County, Virginia. Vector-Borne and Zoonotic Diseases. 2014;14:482–485. doi: 10.1089/vbz.2013.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horobik V, Keesing F, Ostfeld RS. Abundance and Borrelia burgdorferi-infection prevalence of nymphal Ixodes scapularis ticks along forest-field edges. Ecohealth. 2006;3:262–268. [Google Scholar]
- Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. 2012a doi: 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philosophical Transactions of the Royal Society B-Biological Sciences. 2012b;367:1665–1679. doi: 10.1098/rstb.2012.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo LE, Cook PE, Johnson PH, Rapley LP, Kay BH, Ryan PA, Ritchie S, O’Neill S. Field validation of a transcriptional assay for the prediction of age of uncaged Aedes aegypti mosquitoes in northern Australia. PLoS Neglected Tropical Diseases. 2010;4:e608. doi: 10.1371/journal.pntd.0000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff ML, Zhang P, Wolfe RE, Bounoua L. Remote sensing of the urban heat island effect across biomes in the continental USA. Remote Sensing of Environment. 2010;114:504–513. [Google Scholar]
- Jennett AL, Smith FD, Wall R. Tick infestation risk for dogs in a peri-urban park. Parasites & Vectors. 2013:6. doi: 10.1186/1756-3305-6-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo SMB, Oliveira RM, Mackay S, Costa RM, Sweet J, Nascimento ET, Luz KG, Fernandes MZ, Jernigan J, Pearson RD. An Urban Outbreak of Visceral Leishmaniasis in Natal, Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1994;88:386–388. doi: 10.1016/0035-9203(94)90393-x. [DOI] [PubMed] [Google Scholar]
- Jobe DA, Nelson JA, Adam MD, Martin SA. Lyme disease in urban areas, Chicago. Emerging Infectious Diseases. 2007;13:1799–1800. doi: 10.3201/eid1311.070801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MA, Dominici F, Glass GE. Local and Global Effects of Climate on Dengue Transmission in Puerto Rico. Plos Neglected Tropical Diseases. 2009:3. doi: 10.1371/journal.pntd.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJ, Fonseca DM. The effects of forced-egg retention on the blood-feeding behavior and reproductive potential of Culex pipiens (Diptera: Culicidae) Journal of Insect Physiology. 2014;66:53–58. doi: 10.1016/j.jinsphys.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Joshi V, Sharma RC, Sharma Y, Adha S, Sharma K, Singh H, Purohit A, Singhi M. Importance of socioeconomic status and tree holes in distribution of Aedes mosquitoes (Diptera : Culicidae) in Jodhpur, Rajasthan, India. Journal of Medical Entomology. 2006;43:330–336. doi: 10.1603/0022-2585(2006)043[0330:iossat]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Population dynamics. Journal of the American Mosquito Control Association. 2007;23:265–275. doi: 10.2987/8756-971x(2007)23[265:pd]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Ribeiro GS, Maciel-De-Freitas R, Castro MG, Codeco C, Lourenco-de-Oliveira R, Lounibos LP. She’s a femme fatale: low-density larval development produces good disease vectors. Memorias Do Instituto Oswaldo Cruz. 2014;109:1070–U1112. doi: 10.1590/0074-02760140455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnay E, Cai M. Impact of urbanization and land-use change on climate. Nature. 2003;423:528–531. doi: 10.1038/nature01675. [DOI] [PubMed] [Google Scholar]
- Kamgang B, Nchoutpouen E, Simard F, Paupy C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasites & Vectors. 2012:5. doi: 10.1186/1756-3305-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay BH, Ryan PA, Russell BM, Holt JS, Lyons SA, Foley PN. The importance of subterranean mosquito habitat to arbovirus vector control strategies in north Queensland Australia. Journal of Medical Entomology. 2000;37:846–853. doi: 10.1603/0022-2585-37.6.846. [DOI] [PubMed] [Google Scholar]
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecology Letters. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Keiser J, Utzinger J, De Castro MC, Smith TA, Tanner M, Singer BH. Urbanization in sub-Saharan Africa and implication for malaria control. American Journal of Tropical Medicine and Hygiene. 2004;71:118–127. [PubMed] [Google Scholar]
- Kellner KF, Page LK, Downey M, McCord SE. Effects of Urbanization on Prevalence of Baylisascaris procyonis in Intermediate Host Populations. Journal of Wildlife Diseases. 2012;48:1083–1087. doi: 10.7589/2011-09-267. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates West Nile virus transmission. Proceedings of the Royal Society B-Biological Sciences. 2006;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Fonseca DM, Ebel GD, Reddy MR, Kramer LD. Spatial and Temporal Variation in Vector Competence of Culex pipiens and Cx. restuans Mosquitoes for West Nile Virus. American Journal of Tropical Medicine and Hygiene. 2010;83:607–613. doi: 10.4269/ajtmh.2010.10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. Plos Pathogens. 2008:4. doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Randolph SE. Zoonoses 2 Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946–1955. doi: 10.1016/S0140-6736(12)61151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD, Hardy JL, Presser SB. Effect Of Temperature Of Extrinsic Incubation On The Vector Competence Of Culex-Tarsalis For Western Equine Encephalomyelitis Virus. American Journal of Tropical Medicine and Hygiene. 1983;32:1130–1139. doi: 10.4269/ajtmh.1983.32.1130. [DOI] [PubMed] [Google Scholar]
- Kyle JL, Harris E. Global Spread and Persistence of Dengue. Annual Review of Microbiology. 2008:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- Lacke MC, Mote TL, Shepherd JM. Aerosols and associated precipitation patterns in Atlanta. Atmospheric Environment. 2009;43:4359–4373. [Google Scholar]
- LaDeau SL, Calder CA, Doran PJ, Marra PP. West Nile virus impacts in American crow populations are associated with human land use and climate. Ecological Research. 2011;26:909–916. doi: 10.1007/s11284-010-0725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDeau SL, Leisnham PT, Biehler D, Bodner D. Higher Mosquito Production in Low-Income Neighborhoods of Baltimore and Washington, DC: Understanding Ecological Drivers and Mosquito-Borne Disease Risk in Temperate Cities. International Journal of Environmental Research and Public Health. 2013;10:1505–1526. doi: 10.3390/ijerph10041505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Scott TW, Gubler DJ. Consequences of the Expanding Global Distribution of Aedes albopictus for Dengue Virus Transmission. Plos Neglected Tropical Diseases. 2010:4. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham P, Juliano S. Impacts of Climate, Land Use, and Biological Invasion on the Ecology of Immature Aedes Mosquitoes: Implications for La Crosse Emergence. Ecohealth. 2012;9:217–228. doi: 10.1007/s10393-012-0773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham P, Slaney D. Urbanization and the increasing risk from mosquito-borne diseases: Linking human well-being with ecosystem health. In: De Smet LM, editor. Focus on Urbanization Trends. Nova Science Publishers, Inc; Hauppauge, New York: 2009. pp. 47–82. [Google Scholar]
- Leisnham PT, LaDeau SL, Juliano SA. Spatial and Temporal Habitat Segregation of Mosquitoes in Urban Florida. Plos One. 2014:9. doi: 10.1371/journal.pone.0091655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MZ, Barbu CM, Castillo-Neyra R, Quispe-Machaca VR, Ancca-Juarez J, Escalante-Mejia P, Borrini-Mayori K, Niemierko M, Mabud TS, Behrman JR, Naquira-Velarde C, Arequipa CDWG. Urbanization, land tenure security and vector-borne Chagas disease. Proceedings of the Royal Society B-Biological Sciences. 2014a:281. doi: 10.1098/rspb.2014.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MZ, Barbu CM, Castillo-Neyra R, Quispe-Machaca VR, Ancca-Juarez J, Escalante-Mejia P, Borrini-Mayori K, Niemierko M, Mabud TS, Behrman JR, Naquira-Velarde C Chagas Dis Working Grp A. Urbanization, land tenure security and vector-borne Chagas disease. Proceedings of the Royal Society B-Biological Sciences. 2014b:281. doi: 10.1098/rspb.2014.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MZ, Bowman NM, Kawai V, Waller LA, del Carpio JGC, Benzaquen EC, Gilman RH, Bern C. Periurban Trypanosoma cruzi-infected Triatoma infestans, Arequipa, Peru. Emerging Infectious Diseases. 2006;12:1345–1352. doi: 10.3201/eid1209.051662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MZ, Chavez FSM, del Carpio JGC, Vilhena DA, McKenzie FE, Plotkin JB. Rational spatio-temporal strategies for controlling a Chagas disease vector in urban environments. Journal of the Royal Society Interface. 2010;7:1061–1070. doi: 10.1098/rsif.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MZ, Small DS, Vilhena DA, Bowman NM, Kawai V, del Carpio JGC, Cordova-Benzaquen E, Gilman RH, Bern C, Plotkin JB. Retracing Micro-Epidemics of Chagas Disease Using Epicenter Regression. Plos Computational Biology. 2011:7. doi: 10.1371/journal.pcbi.1002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblade KA, Eisele TP, Gimnig JE, Alaii JA, Odhiambo F, ter Kuile FO, Hawley WA, Wannemuehler KA, Phillips-Howard PA, Rosen DH, Nahlen BL, Terlouw DJ, Adazu K, Vulule JM, Slutsker L. Sustainability of reductions in malaria transmission and infant mortality in Western Kenya with use of insecticide-treated bednets - 4 to 6 years of follow-up. Jama-Journal of the American Medical Association. 2004;291:2571–2580. doi: 10.1001/jama.291.21.2571. [DOI] [PubMed] [Google Scholar]
- Liu A, Lee V, Galusha D, Slade M, Diuk-Wasser M, Andreadis T, Scotch M, Rabinowitz P. Risk factors for human infection with West Nile virus in Connecticut: a multi-year analysis. International Journal of Health Geographics. 2009:8. doi: 10.1186/1476-072X-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljumba JN, Lindsay SW. Impact of irrigation on malaria in Africa: Paddies paradox. Journal of Medical and Veterinary Entomology. 2001;15:1–11. doi: 10.1046/j.1365-2915.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- Lockwood JL, Cassey P, Blackburn TM. The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Diversity and Distributions. 2009;15:904–910. [Google Scholar]
- Logiudice K, Duerr STK, Newhouse MJ, Schmidt KA, Killilea ME, Ostfeld RS. Impact Of Host Community Composition On Lyme Disease Risk. Ecology. 2008;89:2841–2849. doi: 10.1890/07-1047.1. [DOI] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo MG, Lazzari CR. Temperature and relative humidity affect the selection of shelters by Triatoma infestans, vector of Chagas disease. Acta Tropica. 1999;72:241–249. doi: 10.1016/s0001-706x(98)00094-1. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annual Review of Entomology. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, O’Meara GF, Juliano SA, Nishimura N, Escher RL, Reiskind MH, Cutwa M, Greene K. Differential Survivorship of Invasive Mosquito Species in South Florida Cemeteries: Do Site-Specific Microclimates Explain Patterns of Coexistence and Exclusion? Annals of the Entomological Society of America. 2010;103:757–770. doi: 10.1603/AN09142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald G. The Epidemiology and Control of Malaria. Oxford University Press; London: 1957. [Google Scholar]
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature Medicine. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- Maetzel D, Maier WA, Kampen H. Borrelia burgdorferi infection prevalences in questing Ixodes ricinus ticks (Acari : Ixodidae) in urban and suburban Bonn, western Germany. Parasitology Research. 2005;95:5–12. doi: 10.1007/s00436-004-1240-3. [DOI] [PubMed] [Google Scholar]
- Magnarelli LA, Denicola A, Stafford KC, Anderson JF. Borrelia-Burgdorferi in an Urban-Environment - White-Tailed Deer with Infected Ticks and Antibodies. Journal of Clinical Microbiology. 1995;33:541–544. doi: 10.1128/jcm.33.3.541-544.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys B, N’Goran EK, Kone M, Koudou BG, Vounatsou P, Cisse G, Tschannen AB, Tanner M, Utzinger J. Urban agricultural land use and characterization of mosquito larval habitats in a medium-sized town of Cote d’Ivoire. Journal of Vector Ecology. 2006;31:319–333. doi: 10.3376/1081-1710(2006)31[319:ualuac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Medrano-Mercado N, Ugarte-Fernandez R, Butron V, Uber-Busek S, Guerra HL, de Araujo-Jorge TC, Correa-Oliveira R. Urban transmission of Chagas disease in Cochabamba, Bolivia. Memorias Do Instituto Oswaldo Cruz. 2008;103:423–430. doi: 10.1590/s0074-02762008000500003. [DOI] [PubMed] [Google Scholar]
- Minoli SA, Lazzari CR. Chronobiological basis of thermopreference in the haematophagous bug Triatoma infestans. Journal of Insect Physiology. 2003;49:927–932. doi: 10.1016/s0022-1910(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis TA, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerging Infectious Diseases. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, Moor E, McNally A, Pawar S, Ryan SJ, Smith TC, Lafferty KD. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecology Letters. 2013;16:22–30. doi: 10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]
- Muller O, Traore C, Kouyate B, Ye Y, Frey C, Coulibaly B, Becher H. Effects of insecticide-treated bednets during early infancy in an African area of intense malaria transmission: a randomized controlled trial. Bulletin of the World Health Organization. 2006;84:120–126. doi: 10.2471/blt.05.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J, Eritja R, Alcaide M, Montalvo T, Soriguer RC, Figuerola J. Host-Feeding Patterns of Native Culex pipiens and Invasive Aedes albopictus Mosquitoes (Diptera: Culicidae) in Urban Zones From Barcelona, Spain. Journal of Medical Entomology. 2011;48:956–960. doi: 10.1603/me11016. [DOI] [PubMed] [Google Scholar]
- Murdock CC, Moller-Jacobs LL, Thomas MB. Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proceedings Biological sciences/The Royal Society. 2013;280:20132030. doi: 10.1098/rspb.2013.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock CC, Paaijmans KP, Bell AS, King JG, Hillyer JF, Read AF, Thomas MB. Complex effects of temperature on mosquito immune function. Proceedings of the Royal Society B-Biological Sciences. 2012;279:3357–3366. doi: 10.1098/rspb.2012.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muturi EJ, Alto BW. Larval Environmental Temperature and Insecticide Exposure Alter Aedes aegypti Competence for Arboviruses. Vector-Borne and Zoonotic Diseases. 2011;11:1157–1163. doi: 10.1089/vbz.2010.0209. [DOI] [PubMed] [Google Scholar]
- Myers SS, Patz JA. Emerging Threats to Human Health from Global Environmental Change. Annual Review of Environment and Resources. 2009;34:223–252. [Google Scholar]
- Neves DP. Influencia da temperatura na evoluçao do Trypanosoma cruzi em triatominos. Revista do Instituto de Medicina Tropical de São Paulo. 1971;13:155–161. [PubMed] [Google Scholar]
- Nieto NC, Holmes EA, Foley JE. Survival rates of immature Ixodes pacificus (Acari: Ixodidae) ticks estimated using field-placed enclosures. Journal of Vector Ecology. 2010;35:43–49. doi: 10.1111/j.1948-7134.2010.00026.x. [DOI] [PubMed] [Google Scholar]
- Niyogi D, Pyle P, Lei M, Arya SP, Kishtawal CM, Shepherd M, Chen F, Wolfe B. Urban Modification of Thunderstorms: An Observational Storm Climatology and Model Case Study for the Indianapolis Urban Region. Journal of Applied Meteorology and Climatology. 2011;50:1129–1144. [Google Scholar]
- Nupp TE, Swihart RK. Landscape-level correlates of small-mammal assemblages in forest fragments of farmland. Journal of Mammalogy. 2000;81:512–526. [Google Scholar]
- Ogden NH, Lindsay LR, Beauchamp G, Charron D, Maarouf A, O’Callaghan CJ, Waltner-Toews D, Barker IK. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari : Ixodidae) in the laboratory and field. Journal of Medical Entomology. 2004;41:622–633. doi: 10.1603/0022-2585-41.4.622. [DOI] [PubMed] [Google Scholar]
- Oke TR. THE ENERGETIC BASIS OF THE URBAN HEAT-ISLAND. Quarterly Journal of the Royal Meteorological Society. 1982;108:1–24. [Google Scholar]
- Paaijmans KP, Blanford S, Chan BHK, Thomas MB. Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biology Letters. 2012;8:465–468. doi: 10.1098/rsbl.2011.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Cator LJ, Thomas MB. Temperature-Dependent Pre-Bloodmeal Period and Temperature-Driven Asynchrony between Parasite Development and Mosquito Biting Rate Reduce Malaria Transmission Intensity. Plos One. 2013:8. doi: 10.1371/journal.pone.0055777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Heinig RL, Seliga RA, Blanford JI, Blanford S, Murdock CC, Thomas MB. Temperature variation makes ectotherms more sensitive to climate change. Global Change Biology. 2013;19:2373–2380. doi: 10.1111/gcb.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paras KL, O’Brien VA, Reiskind MH. Comparison of the vector potential of different mosquito species for the transmission of heartworm, Dirofilaria immitis, in rural and urban areas in and surrounding Stillwater, Oklahoma, USA. Medical and Veterinary Entomology. 2014;28:60–67. doi: 10.1111/mve.12069. [DOI] [PubMed] [Google Scholar]
- Pascual M, Ahumada JA, Chaves LF, Rodo X, Bouma M. Malaria resurgence in the East African highlands: Temperature trends revisited. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5829–5834. doi: 10.1073/pnas.0508929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, Wolfe ND, Kilpatrick AM, Foufopoulos J, Molyneux D, Bradley DJ Working Grp Land Use Change, D. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environmental Health Perspectives. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips NR. Experimental studies on the quantitative transmission of Trypanosoma cruzi: considerations regarding the standardization of materials. Annals of Tropical Medicine and Parasitology. 1960;54:60–70. doi: 10.1080/00034983.1960.11685957. [DOI] [PubMed] [Google Scholar]
- Queirogas VL, Del Claro K, Nascimento ART, Szabo MPJ. Capybaras and ticks in the urban areas of Uberlandia, Minas Gerais, Brazil: ecological aspects for the epidemiology of tick-borne diseases. Experimental and Applied Acarology. 2012;57:75–82. doi: 10.1007/s10493-012-9533-1. [DOI] [PubMed] [Google Scholar]
- Quintero J, Brochero H, Manrique-Saide P, Barrera-Perez M, Basso C, Romero S, Caprara A, Cunha JCD, Beltran-Ayala E, Mitchell-Foster K, Kroeger A, Sommerfeld J, Petzold M. Ecological, biological and social dimensions of dengue vector breeding in five urban settings of Latin America: a multi-country study. Bmc Infectious Diseases. 2014:14. doi: 10.1186/1471-2334-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner RC, Perkins TA, Barker CM, Niu TC, Chaves LF, Ellis AM, George DB, Le Menach A, Pulliam JRC, Bisanzio D, Buckee C, Chiyaka C, Cummings DAT, Garcia AJ, Gatton ML, Gething PW, Hartley DM, Johnston G, Klein EY, Michael E, Lindsay SW, Lloyd AL, Pigott DM, Reisen WK, Ruktanonchai N, Singh BK, Tatem AJ, Kitron U, Hay SI, Scott TW, Smith DL. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. Journal of the Royal Society Interface. 2013:10. doi: 10.1098/rsif.2012.0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Carroll BD, Takahashi R, Fang Y, Garcia S, Martinez VM, Quiring R. Repeated West Nile Virus Epidemic Transmission in Kern County, California, 2004–2007. Journal of Medical Entomology. 2009;46:139–157. doi: 10.1603/033.046.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera : Culicidae) Journal of Medical Entomology. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, Baber L, Amador M, Thirion J, Hayes J, Seca C, Mendez J, Ramirez B, Robinson J, Rawlings J, Vorndam V, Waterman S, Gubler D, Clark G, Hayes E. Texas lifestyle limits transmission of dengue virus. Emerging Infectious Diseases. 2003;9:86–89. doi: 10.3201/eid0901.020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey JR, Lounibos LP, Padmanabha H, Mosquera M. Emergence Of Dengue Fever In America: Patterns, Processes And Prospects. Interciencia. 2010;35:800–806. [Google Scholar]
- Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A, Grp CS. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Richards SL, Anderson SL, Lord CC, Tabachnick WJ. Effects of Virus Dose and Extrinsic Incubation Temperature on Vector Competence of Culex nigripalpus (Diptera: Culicidae) for St. Louis Encephalitis Virus. Journal of Medical Entomology. 2012;49:1502–1506. doi: 10.1603/me12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS. Host-feeding patterns of Aedes albopictus (Diptera : Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central north Carolina. Journal of Medical Entomology. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt DL, Heske EJ, Nelson SL, Barber DH, Miller MA, MacAllister B. Forest fragments in east-central Illinois: Islands or habitat patches for mammals? American Midland Naturalist. 1999;141:115–123. [Google Scholar]
- Sawabe K, Isawa H, Hoshin K, Sasaki T, Roychoudhury S, Higa Y, Kasai S, Tsuda Y, Nishiumi I, Hisai N, Hamao S, Kobayashi M. Host-Feeding Habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) Collected at the Urban and Suburban Residential Areas of Japan. Journal of Medical Entomology. 2010;47:442–450. doi: 10.1603/ME09256. [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Hofmeister E, Glass GE, Arthur RR, Childs JE, Cranfield MR. Lyme Borreliosis In An Inner-City Park In Baltimore. American Journal of Public Health. 1991;81:803–804. doi: 10.2105/ajph.81.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera : Culicidae) in Thailand and Puerto Rico: Population dynamics. Journal of Medical Entomology. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- Service MW, Townson H. Essential Malariology. Arnold; London: 2002. [Google Scholar]
- Shepherd JM, Carter M, Manyin M, Messen D, Burian S. The impact of urbanization on current and future coastal precipitation: a case study for Houston. Environment and Planning B-Planning & Design. 2010;37:284–304. [Google Scholar]
- Shih CM, Telford SR, Spielman A. Effect Of Ambient-Temperature On Competence Of Deer Ticks As Hosts For Lyme-Disease Spirochetes. Journal of Clinical Microbiology. 1995;33:958–961. doi: 10.1128/jcm.33.4.958-961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JB. Mosquito Ecology: Field Sampling Methods. Springer; NY: 2008. [Google Scholar]
- Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. Ross, Macdonald, and a Theory for the Dynamics and Control of Mosquito-Transmitted Pathogens. PLoS Pathog. 2012;8:e1002588. doi: 10.1371/journal.ppat.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Perkins TA, Reiner RC, Barker CM, Niu TC, Chaves LF, Ellis AM, George DB, Le Menach A, Pulliam JRC, Bisanzio D, Buckee C, Chiyaka C, Cummings DAT, Garcia AJ, Gatton ML, Gething PW, Hartley DM, Johnston G, Klein EY, Michael E, Lloyd AL, Pigott DM, Reisen WK, Ruktanonchai N, Singh BK, Stoller J, Tatem AJ, Kitron U, Godfray HCJ, Cohen JM, Hay SI, Scott TW. Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2014;108:185–197. doi: 10.1093/trstmh/tru026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. Journal of Clinical Investigation. 2004:113. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugumaran R, Larson SR, DeGroote JP. Spatio-temporal cluster analysis of county-based human West Nile virus incidence in the continental United States. International Journal of Health Geographics. 2009;8:19. doi: 10.1186/1476-072X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick WJ. Nature, Nurture and Evolution of Intra-Species Variation in Mosquito Arbovirus Transmission Competence. International Journal of Environmental Research and Public Health. 2013;10:249–277. doi: 10.3390/ijerph10010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ, Gething PW, Smith DL, Hay SI. Urbanization and the global malaria recession. Malaria Journal. 2013:12. doi: 10.1186/1475-2875-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatem AJ, Hay SI, Rogers DJ. Global traffic and disease vector dispersal. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6242–6247. doi: 10.1073/pnas.0508391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco C, Ruiz M, McLafferty S. Mosquito politics: Local vector control policies and the spread of West Nile Virus in the Chicago region. Health & Place. 2010;16:1188–1195. doi: 10.1016/j.healthplace.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Thomas MB, Blanford S. Thermal biology in insect-parasite interactions. Trends in Ecology & Evolution. 2003;18:344–350. [Google Scholar]
- Townroe S, Callaghan A. British Container Breeding Mosquitoes: The Impact of Urbanisation and Climate Change on Community Composition and Phenology. Plos One. 2014:9. doi: 10.1371/journal.pone.0095325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PM, Waller LA. Effects of landscape fragmentation and climate on Lyme disease incidence in the northeastern United States. Ecohealth. 2013;10:394–404. doi: 10.1007/s10393-013-0890-y. [DOI] [PubMed] [Google Scholar]
- Uspensky I. Tick pests and vectors (Acari: Ixodoidea) in European towns: Introduction, persistence and management. Ticks and Tick-Borne Diseases. 2014;5:41–47. doi: 10.1016/j.ttbdis.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Ceballos LA, Cecere MC, Gurtler RE. Seasonal variations of microclimatic conditions in domestic and peridomestic habitats of Triatoma infestans in rural northwest Argentina. Acta Tropica. 2002;84:229–238. doi: 10.1016/s0001-706x(02)00204-8. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Ceballos LA, Kitron U, Gurtler RE. Active dispersal of natural populations of Triatoma infestans (Hemiptera : Reduviidae) in rural northwestern Argentina. Journal of Medical Entomology. 2004;41:614–621. doi: 10.1603/0022-2585-41.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk ST, Xu G, Stull JW, Rich SM. Correlation between Tick Density and Pathogen Endemicity, New Hampshire. Emerging Infectious Diseases. 2009;15:585–587. doi: 10.3201/eid1504.080940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC. Urbanization and geographic expansion of zoonotic arboviral diseases: mechanisms and potential strategies for prevention. Trends in Microbiology. 2013;21:360–363. doi: 10.1016/j.tim.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Coffey LL, Nussenzveig R, Ortiz D, Smith D. Vector competence. In: Gillespie SH, Smith L, Osbourn A, editors. Microbe-vector interactions in vector-borne diseases. Cambridge University Press; United Kingdom: 2004. pp. 139–180. [Google Scholar]
- WHO; W.H. Organization, editor. Malaria. 2013. World Malaria Report 2013; p. 284. [Google Scholar]
- Wilcox BA, Gubler DJ. Disease Ecology and the Global Emergence of Zoonotic Pathogens. Environmental Health and Preventive Medicine. 2005;10:263–272. doi: 10.1007/BF02897701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SC, Ward JS, Worthley TE, Stafford KC. Managing Japanese Barberry (Ranunculales: Berberidaceae) Infestations Reduces Blacklegged Tick (Acari: Ixodidae) Abundance and Infection Prevalence With Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) Environmental Entomology. 2009;38:977–984. doi: 10.1603/022.038.0404. [DOI] [PubMed] [Google Scholar]
- Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonham MJ, Lewis MA, Renclawowicz J, Van den Driessche P. Transmission assumptions generate conflicting predictions in host-vector disease models: a case study in West Nile virus. Ecology Letters. 2006;9:706–725. doi: 10.1111/j.1461-0248.2006.00912.x. [DOI] [PubMed] [Google Scholar]
- Zouache K, Fontaine A, Vega-Rua A, Mousson L, Thiberge J-M, Lourenco-De-Oliveira R, Caro V, Lambrechts L, Failloux A-B. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proceedings of the Royal Society B: Biological Sciences. 2014:281. doi: 10.1098/rspb.2014.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]